Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization, Pulsed Electric Field Treatment, and Anaerobic Digestion
Abstract
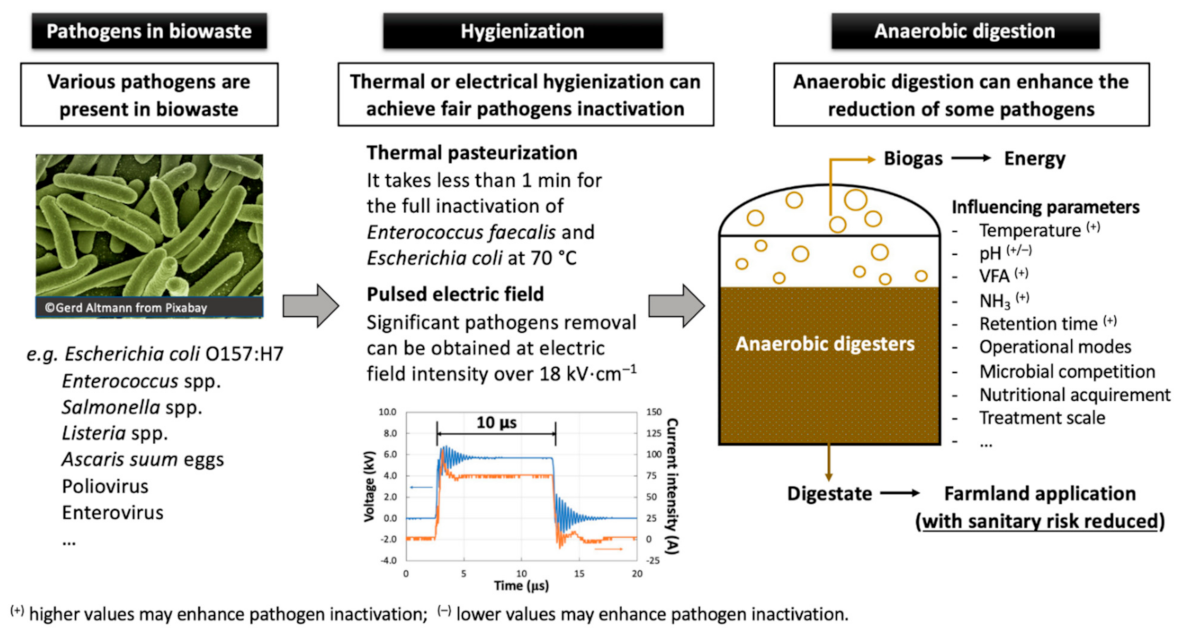
1. Introduction
2. Materials and Methods
2.1. Suspension Preparation
2.1.1. Indicator Bacteria Incubation
2.1.2. Mixed Animal By-Products
2.2. Thermal Pasteurization
2.2.1. Thermal Treatment Protocol
2.2.2. Culturable Bacteria Count
2.2.3. Inactivation Kinetics Modeling
2.3. PEF Treatmennt as Alternative Hygienization
2.3.1. PEF Treatment Protocol
2.3.2. Culturable Bacteria Count
2.3.3. Estimation of Critical Electric Field Intensity
2.4. Microbial Inactivation during AD
2.5. Data Processing
3. Results
3.1. Resistance to Thermal Treatment
3.1.1. Thermal Inactivation Kinetics
3.1.2. Curves Modeling and Activation Energy
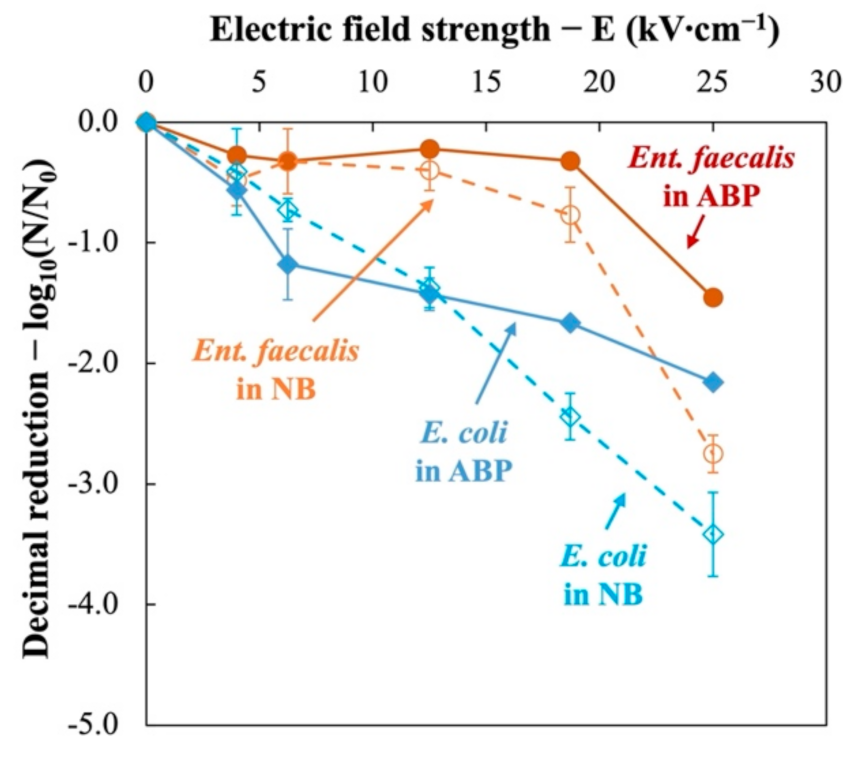
3.2. Resistance to PEF Treatment—Critical Electric Field Strength
3.3. Resistance to AD Process
4. Discussion
4.1. Mechanisms
4.2. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ABP | Animal by-products |
| AD | Anaerobic digestion |
| Adjusted R2 | Adjusted coefficient of determination |
| ARG | Antibiotic resistance genes |
| ASFV | African Swine Fever Virus |
| BGP | Biogas plants |
| BMP | Biochemical methane potential |
| CFU | Colony-forming unit |
| EU | European Union |
| G+ | Gram-positive bacteria |
| G– | Gram-negative bacteria |
| HRT | Hydraulic retention time |
| LCA | Life cycle assessment |
| MAD | Mesophilic anaerobic digestion |
| MPN | Most probable number |
| MSW | Municipal solid waste |
| NB | Nutrient broth |
| PEF | Pulsed electric field |
| RMSE | Root mean squared errors |
| SSE | Sum of squared errors |
| TAD | Thermophilic anaerobic digestion |
| US EPA | the United States Environmental Protection Agency |
| US FDA | the United States Food and Drug Administration |
| VFA | Volatile fatty acids |
| VS | Volatile solids |
| WAS | Waste activated sludge |
| WWTP | Wastewater treatment plant |
References
- Rekleitis, G.; Haralambous, K.-J.; Loizidou, M.; Aravossis, K. Utilization of Agricultural and Livestock Waste in Anaerobic Digestion (A.D): Applying the Biorefinery Concept in a Circular Economy. Energies 2020, 13, 4428. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Synge, B.A.; Moore, A. Levels of Zoonotic Agents in British Livestock Manures. Lett. Appl. Microbiol. 2004, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Le Maréchal, C.; Druilhe, C.; Repérant, E.; Boscher, E.; Rouxel, S.; Roux, S.L.; Poëzévara, T.; Ziebal, C.; Houdayer, C.; Nagard, B.; et al. Evaluation of the Occurrence of Sporulating and Nonsporulating Pathogenic Bacteria in Manure and in Digestate of Five Agricultural Biogas Plants. Microbiol. Open 2019, 8, e872. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yu, Z.; Shao, L.; Zhou, Y.; Lü, F. Fate of Antibiotics and Antibiotic-Resistance Genes in a Full-Scale Restaurant Food Waste Treatment Plant: Implications of the Roles beyond Heavy Metals and Mobile Genetic Elements. J. Environ. Sci. 2019. [Google Scholar] [CrossRef]
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Overview of Hygienization Pretreatment for Pasteurization and Methane Potential Enhancement of Biowaste: Challenges, State of the Art and Alternative Technologies. J. Clean. Prod. 2019, 236, 117525. [Google Scholar] [CrossRef]
- Lewis, D.; Gattie, D. Pathogen Risks from Applying Sewage Sludge to Land. Environ. Sci. Technol. 2002, 286A–293A. [Google Scholar] [CrossRef]
- Maynaud, G.; Pourcher, A.-M.; Ziebal, C.; Cuny, A.; Druilhe, C.; Steyer, J.-P.; Wéry, N. Persistence and Potential Viable but Non-Culturable State of Pathogenic Bacteria during Storage of Digestates from Agricultural Biogas Plants. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef][Green Version]
- Coelho, J.J.; Prieto, M.L.; Dowling, S.; Hennessy, A.; Casey, I.; Woodcock, T.; Kennedy, N. Physical-Chemical Traits, Phytotoxicity and Pathogen Detection in Liquid Anaerobic Digestates. Waste Manag. 2018, 78, 8–15. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal by-Products Regulation). Off. J. Eur. Union 2009, 52. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 142/2011 of 25 February 2011 Implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council Laying down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Implementing Council Directive 97/78/EC as Regards Certain Samples and Items Exempt from Veterinary Checks at the Border under That Directive. Off. J. Eur. Union 2011, 54. [Google Scholar] [CrossRef]
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. A Review of Hygienization Technology of Biowastes for Anaerobic Digestion: Effect on Pathogen Inactivation and Methane Production. Chem. Eng. Trans. 2018, 70, 529–534. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Processing of Foods and Biomass Feedstocks by Pulsed Electric Energy; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Garner, A.L. Pulsed Electric Field Inactivation of Microorganisms: From Fundamental Biophysics to Synergistic Treatments. Appl. Microbiol. Biotechnol. 2019, 1–13. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, L.-H.; Bekhit, A.E.-D.A.; Yang, J.; Hou, Z.-P.; Wang, Y.-Z.; Dai, Q.-Z.; Zeng, X.-A. A Review of Sublethal Effects of Pulsed Electric Field on Cells in Food Processing. J. Food Eng. 2018, 223, 32–41. [Google Scholar] [CrossRef]
- Liu, X.; Lendormi, T.; Le Fellic, M.; Lemée, Y.; Lanoisellé, J.-L. Hygienization of Mixed Animal By-Product Using Pulsed Electric Field: Inactivation Kinetics Modeling and Recovery of Indicator Bacteria. Chem. Eng. J. 2019, 368, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Lendormi, T.; Le Fellic, M.; Lemée, Y.; Lanoisellé, J.-L. Hygienization of Mixed Animal By-Product Using Pulsed Electric Field in a Continuous Treatment System: Synergistic Effect with Ohmic Heating on the Inactivation of Indicator Bacteria. Waste Manag. 2020, 118, 18–26. [Google Scholar] [CrossRef]
- Grim, J.; Malmros, P.; Schnürer, A.; Nordberg, Å. Comparison of Pasteurization and Integrated Thermophilic Sanitation at a Full-Scale Biogas Plant—Heat Demand and Biogas Production. Energy 2015, 79, 419–427. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y. Is Anaerobic Digestion a Reliable Barrier for Deactivation of Pathogens in Biosludge? Sci. Total Environ. 2019, 668, 893–902. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Khatib, L.A.; Hill, V.R.; Alocilja, E.; Pillai, S. Pathogens in Animal Wastes and the Impacts of Waste Management Practices on Their Survival, Transport and Fate. In Animal Agriculture and the Environment: National Center for Manure and Animal Waste Management White Papers; Rice, J.M., Caldwell, D.F., Humenik, F.J., Eds.; ASABE: St. Joseph, MI, USA, 2006; pp. 609–666. [Google Scholar] [CrossRef]
- Sahlström, L. A Review of Survival of Pathogenic Bacteria in Organic Waste Used in Biogas Plants. Bioresour. Technol. 2003, 87, 161–166. [Google Scholar] [CrossRef]
- Tallon, P.; Magajna, B.; Lofranco, C.; Leung, K.T. Microbial Indicators of Faecal Contamination in Water: A Current Perspective. Water Air Soil Pollut. 2005, 166, 139–166. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Mañas, P.; Condón, S.; Pagán, R. Effect of Environmental Factors and Cell Physiological State on Pulsed Electric Fields Resistance and Repair Capacity of Various Strains of Escherichia coli. Int. J. Food Microbiol. 2008, 124, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Le Jean, G.; Abraham, G.; Debray, E.; Candau, Y.; Piar, G. Kinetics of Thermal Destruction of Bacillus Stearothermophilus Spores Using a Two Reaction Model. Food Microbiol. 1994, 11, 229–241. [Google Scholar] [CrossRef]
- Abraham, G.; Debray, E.; Candau, Y.; Piar, G. Mathematical Model of Thermal Destruction of Bacillus Stearothermophilus Spores. Appl. Environ. Microbiol. 1990, 56, 3073–3080. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Laboratory Methods—Bacteriological Analytical Manual (BAM). Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm2006949.htm (accessed on 28 April 2017).
- US DA. Laboratory Guidebook—Most Probable Number Procedure and Tables. 2014. Available online: https://www.fsis.usda.gov/news-events/publications/microbiology-laboratory-guidebook (accessed on 29 March 2021).
- Van Boekel, M.A.J.S. On the Use of the Weibull Model to Describe Thermal Inactivation of Microbial Vegetative Cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]
- Huang, K.; Yu, L.; Liu, D.; Gai, L.; Wang, J. Modeling of Yeast Inactivation of PEF-Treated Chinese Rice Wine: Effects of Electric Field Intensity, Treatment Time and Initial Temperature. Food Res. Int. 2013, 54, 456–467. [Google Scholar] [CrossRef]
- Zhang, Q.; Monsalve-González, A.; Qin, B.-L.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Saccharomyces cerevisiae in Apple Juice by Square-Wave and Exponential-Decay Pulsed Electric Fields. J. Food Process Eng. 1994, 17, 469–478. [Google Scholar] [CrossRef]
- Peleg, M. A Model of Microbial Survival after Exposure to Pulsed Electric Fields. J. Sci. Food Agric. 1995, 67, 93–99. [Google Scholar] [CrossRef]
- Liu, X. Hygiénisation par Technologie Électrique des déchets alimentaires en vue de leur méthanisation. Ph.D. Thesis, Université Bretagne Sud (Université Bretagne Loire), Lorient, France, 2019. [Google Scholar]
- Heinz, V.; Alvarez, I.; Angersbach, A.; Knorr, D. Preservation of Liquid Foods by High Intensity Pulsed Electric Fields—Basic Concepts for Process Design. Trends Food Sci. Technol. 2001, 12, 103–111. [Google Scholar] [CrossRef]
- Olsen, J.E.; Jørgensen, J.B.; Nansen, P. On the Reduction of Mycobacterium paratuberculosis in Bovine Slurry Subjected to Batch Mesophilic or Thermophilic Anaerobic Digestion. Agric. Wastes 1985, 13, 273–280. [Google Scholar] [CrossRef]
- Elmerdahl Olsen, J.; Errebo Larsen, H. Bacterial Decimation Times in Anaerobic Digestions of Animal Slurries. Biol. Wastes 1987, 21, 153–168. [Google Scholar] [CrossRef]
- Shih, J.C.H. Ecological Benefits of Anaerobic Digestion. Poult. Sci. 1987, 66, 946–950. [Google Scholar] [CrossRef]
- Forshell, L.P. Survival of Salmonellas and Ascaris suum eggs in a Thermophilic Biogas Plant. In Environment and Animal Health. Proceedings of the 6th International Congress on Animal Hygiene, Skara, Sweden, 14–17 June 1988; Sveriges Lantbruksuniversitet: Skara, Sweden, 1988; Volume II, pp. 612–618. [Google Scholar]
- Kearney, T.E.; Larkin, M.J.; Frost, J.P.; Levett, P.N. Survival of Pathogenic Bacteria during Mesophilic Anaerobic Digestion of Animal Waste. J. Appl. Bacteriol. 1993, 75, 215–219. [Google Scholar] [CrossRef]
- Lund, B.; Jensen, V.F.; Have, P.; Ahring, B. Inactivation of Virus during Anaerobic Digestion of Manure in Laboratory Scale Biogas Reactors. Antonie Leeuwenhoek 1996, 69, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Paavola, T.; Syväsalo, E.; Rintala, J. Co-Digestion of Manure and Biowaste According to the EC Animal By-Products Regulation and Finnish National Regulations. Water Sci. Technol. 2006, 53, 223–231. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J. Evaluation of Thermophilic Anaerobic Digestion Processes for Full-Scale Class A Biosolids Disinfection at Hyperion Treatment Plant. Biotechnol. Bioeng. 2007, 97, 19–39. [Google Scholar] [CrossRef]
- Bagge, E.; Persson, M.; Johansson, K.-E. Diversity of Spore-forming Bacteria in Cattle Manure, Slaughterhouse Waste and Samples from Biogas Plants. J. Appl. Microbiol. 2010, 109, 1549–1565. [Google Scholar] [CrossRef]
- Massé, D.; Gilbert, Y.; Topp, E. Pathogen Removal in Farm-Scale Psychrophilic Anaerobic Digesters Processing Swine Manure. Bioresour. Technol. 2011, 102, 641–646. [Google Scholar] [CrossRef]
- Pandey, P.K.; Soupir, M.L. Escherichia coli Inactivation Kinetics in Anaerobic Digestion of Dairy Manure under Moderate, Mesophilic and Thermophilic Temperatures. AMB Expr. 2011, 1, 18. [Google Scholar] [CrossRef]
- Manser, N.D.; Wald, I.; Ergas, S.J.; Izurieta, R.; Mihelcic, J.R. Assessing the Fate of Ascaris suum Ova during Mesophilic Anaerobic Digestion. Environ. Sci. Technol. 2015, 49, 3128–3135. [Google Scholar] [CrossRef]
- Mazzone, P.; Corneli, S.; Di Paolo, A.; Maresca, C.; Felici, A.; Biagetti, M.; Ciullo, M.; Sebastiani, C.; Pezzotti, G.; Leo, S.; et al. Survival of Mycobacterium avium Subsp. Paratuberculosis in the Intermediate and Final Digestion Products of Biogas Plants. J. Appl. Microbiol. 2018, 125, 36–44. [Google Scholar] [CrossRef]
- Tápparo, D.C.; Viancelli, A.; do Amaral, A.C.; Fongaro, G.; Steinmetz, R.L.R.; Magri, M.E.; Barardi, C.R.M.; Kunz, A. Sanitary Effectiveness and Biogas Yield by Anaerobic Co-Digestion of Swine Carcasses and Manure. Environ. Technol. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.; Waters, N.R.; Brennan, F.; Auer, A.; Fenton, O.; Richards, K.; Bolton, D.J.; Pritchard, L.; O’Flaherty, V.; Abram, F. Toward Assessing Farm-Based Anaerobic Digestate Public Health Risks: Comparative Investigation with Slurry, Effect of Pasteurization Treatments, and Use of Miniature Bioreactors as Proxies for Pathogen Spiking Trials. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef]
- Jepsen, S.-E.; Krause, M.; Grüttner, H. Reduction of Fecal Streptococcus and Salmonella by Selected Treatment Methods for Sludge and Organic Waste. Water Sci. Technol. 1997, 36, 203–210. [Google Scholar] [CrossRef]
- De Luca, G.; Zanetti, F.; Fateh-Moghadm, P.; Stampi, S. Occurrence of Listeria monocytogenes in Sewage Sludge. Zent. Hyg. Umweltmed. 1998, 201, 269–277. [Google Scholar]
- Aitken, M.D.; Sobsey, M.D.; Blauth, K.E.; Shehee, M.; Crunk, P.L.; Walters, G.W. Inactivation of Ascaris suum and Poliovirus in Biosolids under Thermophilic Anaerobic Digestion Conditions. Environ. Sci. Technol. 2005, 39, 5804–5809. [Google Scholar] [CrossRef]
- Aitken, M.D.; Sobsey, M.D.; Shehee, M.; Blauth, K.E.; Hill, V.R.; Farrell, J.B.; Nappier, S.P.; Walters, G.W.; Crunk, P.L.; Van Abel, N. Laboratory Evaluation of Thermophilic-Anaerobic Digestion to Produce Class A Biosolids. 2. Inactivation of Pathogens and Indicator Organisms in a Continuous-Flow Reactor Followed by Batch Treatment. Water Environ. Res. 2005, 77, 3028–3036. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J.; Oh, S.; Fan, S.; Kearney, R.J.; Abkian, V.; Haug, R.T. Thermophilic-Anaerobic Digestion to Produce Class A Biosolids: Initial Full-Scale Studies at Hyperion Treatment Plant. Water Environ. Res. 2006, 78, 170–180. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J.; Fan, S.; Abkian, V.; Minamide, T.; Kearney, R.J.; Haug, R.T. Full-Scale Class A Biosolids Production by Two-Stage Continuous-Batch Thermophilic Anaerobic Digestion at the Hyperion Treatment Plant, Los Angeles, California. Water Environ. Res. 2006, 78, 2244–2252. [Google Scholar] [CrossRef]
- Lloret, E.; Salar, M.J.; Blaya, J.; Pascual, J.A. Two-Stage Mesophilic Anaerobic–Thermophilic Digestion for Sludge Sanitation to Obtain Advanced Treated Sludge. Chem. Eng. J. 2013, 230, 59–63. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Imporzano, G.; Garuti, G.; Negri, M.; Adani, F. Sanitation Ability of Anaerobic Digestion Performed at Different Temperature on Sewage Sludge. Sci. Total Environ. 2014, 466–467, 888–897. [Google Scholar] [CrossRef]
- Engeli, H.; Edelmann, W.; Fuchs, J.; Rottermann, K. Survival of Plant Pathogens and Weed Seeds during Anaerobic Digestion. Water Sci. Technol. 1993, 27, 69–76. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Cops, S.; Coosemans, J. The Fate of Plant Pathogens and Seeds During Anaerobic Digestion and Aerobic Composting of Source Separated Household Wastes. Compost Sci. Util. 2002, 10, 204–216. [Google Scholar] [CrossRef]
- Termorshuizen, A.J.; Volker, D.; Blok, W.J.; ten Brummeler, E.; Hartog, B.J.; Janse, J.D.; Knol, W.; Wenneker, M. Survival of Human and Plant Pathogens during Anaerobic Mesophilic Digestion of Vegetable, Fruit, and Garden Waste. Eur. J. Soil Biol. 2003, 39, 165–171. [Google Scholar] [CrossRef]
- Schnürer, A.; Schnürer, J. Fungal Survival during Anaerobic Digestion of Organic Household Waste. Waste Manag. 2006, 26, 1205–1211. [Google Scholar] [CrossRef]
- Wagner, A.O.; Gstraunthaler, G.; Illmer, P. Survival of Bacterial Pathogens during the Thermophilic Anaerobic Digestion of Biowaste: Laboratory Experiments and in Situ Validation. Anaerobe 2008, 14, 181–183. [Google Scholar] [CrossRef]
- Rounsefell, B.D.; O’Sullivan, C.A.; Chinivasagam, N.; Batstone, D.; Clarke, W.P. Fate of Pathogen Indicators in a Domestic Blend of Food Waste and Wastewater through a Two-Stage Anaerobic Digestion System. Water Sci. Technol. 2013, 67, 366–373. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Paluszak, Z.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Pińkowska, H. Pathogen Reduction Potential in Anaerobic Digestion of Organic Fraction of Municipal Solid Waste and Food Waste. Molecules 2020, 25, 275. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Yamamoto, Y.; Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Iwasaki, M.; Ihara, I.; Tangtaweewipat, S.; Umetsu, K. The Survival of Pathogenic Bacteria and Plant Growth Promoting Bacteria during Mesophilic Anaerobic Digestion in Full-Scale Biogas Plants. Anim. Sci. J. 2019, 90, 297–303. [Google Scholar] [CrossRef]
- Magnus, C.A.; Ingledew, W.M.; McCurdy, A.R. Thermal Resistance of Streptococci Isolated from Pasteurized Ham. Can. Inst. Food Sci. Technol. J. 1986, 19, 62–67. [Google Scholar] [CrossRef]
- Cunault, C.; Pourcher, A.M.; Burton, C.H. Using Temperature and Time Criteria to Control the Effectiveness of Continuous Thermal Sanitation of Piggery Effluent in Terms of Set Microbial Indicators. J. Appl. Microbiol. 2011, 111, 1492–1504. [Google Scholar] [CrossRef]
- Bischof, J.C. Thermal Stability of Proteins. Ann. N.Y. Acad. Sci. 2005, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, S.H.; Dennehy, C.; Lawlor, P.G.; Hu, Z.H.; Wu, G.X.; Zhan, X.M.; Gardiner, G.E. Inactivation of Pathogens in Anaerobic Digestion Systems for Converting Biowastes to Bioenergy: A Review. Renew. Sustain. Energy Rev. 2020, 120, 109654. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Gómez, N.; Mañas, P.; Raso, J.; Pagán, R. Pulsed Electric Fields Cause Bacterial Envelopes Permeabilization Depending on the Treatment Intensity, the Treatment Medium PH and the Microorganism Investigated. Int. J. Food Microbiol. 2007, 113, 219–227. [Google Scholar] [CrossRef]
- Sharma, P.; Bremer, P.; Oey, I.; Everett, D.W. Bacterial Inactivation in Whole Milk Using Pulsed Electric Field Processing. Int. Dairy J. 2014, 35, 49–56. [Google Scholar] [CrossRef]
- Martín, O.; Qin, B.L.; Chang, F.J.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Escherichia coli in Skim Milk by High Intensity Pulsed Electric Fields. J. Food Process Eng. 1997, 20, 317–336. [Google Scholar] [CrossRef]
- Grahl, T.; Märkl, H. Killing of Microorganisms by Pulsed Electric Fields. Appl. Microbiol. Biotechnol. 1996, 45, 148–157. [Google Scholar] [CrossRef]
- Ho, S.Y.; Mittal, G.S.; Cross, J.D.; Griffiths, M.W. Inactivation of Pseudomonas fluorescens by High Voltage Electric Pulses. J. Food Sci. 1995, 60, 1337–1340. [Google Scholar] [CrossRef]
- Donsì, G.; Ferrari, G.; Pataro, G. Inactivation Kinetics of Saccharomyces cerevisiae by Pulsed Electric Fields in a Batch Treatment Chamber: The Effect of Electric Field Unevenness and Initial Cell Concentration. J. Food Eng. 2007, 78, 784–792. [Google Scholar] [CrossRef]
- Popat, S.C.; Yates, M.V.; Deshusses, M.A. Kinetics of Inactivation of Indicator Pathogens during Thermophilic Anaerobic Digestion. Water Res. 2010, 44, 5965–5972. [Google Scholar] [CrossRef]
- Cunault, C. Développement d’une Méthode D’hygiénisation Thermique des Effluents Au Moyen D’échangeurs de Chaleur (Application Au Lisier Porcin). Ph.D. Thesis, Université Rennes, Rennes, France, 2012. [Google Scholar]
- He, P.; Zhou, Y.; Shao, L.; Huang, J.; Yang, Z.; Lü, F. The Discrepant Mobility of Antibiotic Resistant Genes: Evidence from Their Spatial Distribution in Sewage Sludge Flocs. Sci. Total Environ. 2019, 697, 134176. [Google Scholar] [CrossRef]
- Derongs, L.; Druilhe, C.; Ziebal, C.; Le Maréchal, C.; Pourcher, A.-M. Characterization of Clostridium perfringens Isolates Collected from Three Agricultural Biogas Plants over a One-Year Period. IJERPH 2020, 17, 5450. [Google Scholar] [CrossRef]
- Orlando, M.-Q.; Borja, V.-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Luste, S.; Luostarinen, S. Anaerobic Co-Digestion of Meat-Processing by-Products and Sewage Sludge—Effect of Hygienization and Organic Loading Rate. Bioresour. Technol. 2010, 101, 2657–2664. [Google Scholar] [CrossRef]
- Liu, X.; Souli, I.; Chamaa, M.-A.; Lendormi, T.; Sabourin, C.; Lemée, Y.; Boy, V.; Chaira, N.; Ferchichi, A.; Morançais, P.; et al. Effect of Thermal Pretreatment at 70 °C for One Hour (EU Hygienization Conditions) of Various Organic Wastes on Methane Production under Mesophilic Anaerobic Digestion. AIMS Environ. Sci. 2018, 5, 117–129. [Google Scholar] [CrossRef]
- Chamaa, M.A. Couplage de la Méthanisation et des Électrotechnologies: Intentisification de la Production de Biogaz et du Séchage du Digestat. Ph.D. Thesis, Université Bretagne Sud, Lorient, France, 2017. [Google Scholar]

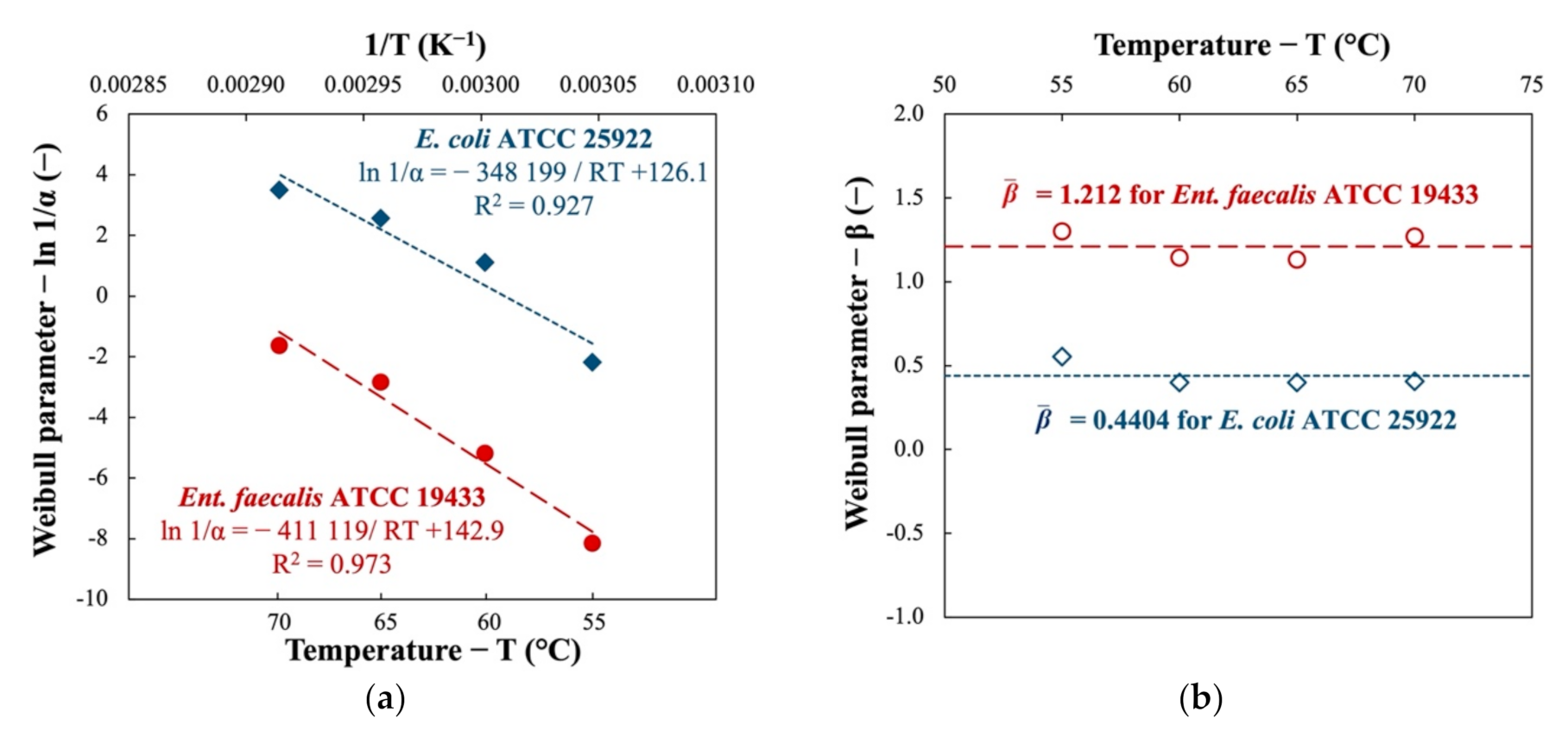

| Temperature T (°C) | Weibull Model | |||||
|---|---|---|---|---|---|---|
| α (s) | β (–) | Adjusted R2 (–) | SSE (–) | RMSE (–) | 5-D Values (s) | |
| Enterococcus faecalis ATCC 19433 | ||||||
| 55 | 3 463 | 1.300 | 0.798 | 0.214 | 0.124 | 22 689 |
| 60 | 177.7 | 1.144 | 0.927 | 0.844 | 0.306 | 1 505 |
| 65 | 17.06 | 1.134 | 0.977 | 0.377 | 0.217 | 147 |
| 70 | 5.097 | 1.271 | 0.922 | 1.11 | 0.429 | 34.8 |
| Escherichia coli ATCC 25922 | ||||||
| 55 | 8.902 | 0.5546 | 0.878 | 2.07 | 0.588 | 728 |
| 60 | 0.3277 | 0.4000 | 0.878 | 2.67 | 0.578 | 147 |
| 65 | 0.0769 | 0.4000 | 0.815 | 1.17 | 0.542 | 34.6 |
| 70 | 0.0300 | 0.4068 | 0.888 | 0.829 | 0.455 | 12.2 |
| Bacteria | Fermi’s Model | ||
|---|---|---|---|
| kc (kV∙cm−1) | Ec (kV∙cm−1) | R2 (–) | |
| Ent. faecalis | 2.12 | 18.0 | 0.81 |
| E. coli | 4.70 | ~1.0 | 0.84 |
| Substrates | AD Conditions | Experimental Scale | Indicator Microorganism | Observations 5 | References |
|---|---|---|---|---|---|
| Bovine Slurry | MAD 1 at 35 °C for 10–28 d | Lab batch | Mycobacterium paratuberculosis | 4 log10 depending on VS 2 | [34] |
| TAD at 53–55 °C for < 3 h | Lab batch | Mycobacterium paratuberculosis | 4 log10 | ||
| Bovine and swine slurry | MAD at 35 °C for 13 d | Lab continuous | Salmonella typhimurium | 4 log10 | [35] |
| MAD at 35 °C for 9 d | Escherichia coli O8 | 3.7 log10 | |||
| MAD at 35 °C for 2 d | Staphylococcus aureus | 5 log10 | |||
| MAD at 35 °C for 8 d | Enterococcus faecalis | 3.6 log10 | |||
| Chicken manure | MAD at 35 °C | Lab batch | Fecal coliforms and salmonella | 50% inactivation | [36] |
| TAD 3 at 50 °C | Fecal coliforms and salmonella | Full inactivation | |||
| MAD | oocysts of Eimeria tenella | 1–2 log10 | |||
| TAD | oocysts of Eimeria tenella | 3 log10 | |||
| Animal manure | TAD for 24 h | BGP continuous | Salmonella and Ascaris suum eggs | Full inactivation | [37] |
| Animal waste | MAD for 140 d | Full-scale continuously stirred digester | Escherichia coli | 0.33 log10 | [38] |
| Salmonella typhimurium | 0.73 log10 | ||||
| Yersinia enterocolitica | 1.39 log10 | ||||
| Listeria monocytogenes | 0.88 log10 | ||||
| Campylobacter jejuni | 0.05 log10 | ||||
| Manure | MAD at 35 °C for ~35 d | Lab batch | Fecal enterococci | 4 log10 | [39] |
| MAD at 35 °C for ~9 d | Bovine enterovirus | 4 log10 | |||
| MAD at 35 °C | Porcine parvovirus | No effect | |||
| TAD at 55 °C for 3.8 h | Porcine parvovirus | 4 log10 | |||
| TAD at 55 °C for 2.3 h | Bovine enterovirus | 4 log10 | |||
| TAD at 55 °C for 148 h | Fecal enterococci | 4 log10 | |||
| Manure and biowaste | MAD at 35 °C for months | Continuously stirred 5-L digester | Salmonella spp. | Full inactivation | [40] |
| TAD at 55 °C for months | Salmonella spp. | Full inactivation | |||
| MSW 4 and ABP | MAD at high level of NH3 | Semi-continuous reactors | Enterococcus faecalis | 6 log10 | [41] |
| Salmonella typhimurium | >5 log10 | ||||
| ABP | MAD or TAD | Full-scale BGP | Bacillus spp. | Slight decrease | [42] |
| Clostridium spp. | No effect | ||||
| Swine manure | Psychrophilic AD | Farm-scale sequencing batch reactors | Total coliforms | 6 log10 | [43] |
| Salmonella spp. | 3 log10 | ||||
| Campylobacter spp. | 3 log10 | ||||
| Yersinia enterocolitica | 4 log10 | ||||
| Clostridium perfringens | No sig. effect | ||||
| Enterococcus spp. | No sig. effect | ||||
| Dairy manure | AD at 25, 37, and 52.5 °C for 60, 40, and 4 d | Lab batch | Escherichia coli | ~7, 4 and 8 log10 | [44] |
| Swine manure | MAD at 35 °C | Lab batch | Ascaris suum eggs | Full inactivation after 24 h | [45] |
| Dairy slurry and manure | MAD for 11 months | Farm-scale digester | Mycobacterium avium | 88% inactivation | [46] |
| MAD for 11 months | 2-stage BGP | Mycobacterium avium | Full inactivation | ||
| Swine carcasses and manure | AD at 24 °C | Lab batch | Escherichia coli | Full inactivation after 25–30 d | [47] |
| Salmonella Senftenberg | Full inactivation after 10 d | ||||
| Phage PhiX–174 | No sig. effect | ||||
| Phage MS2 and PCV2 | 1.5 log10 and 3 log10 after 30 d | ||||
| MAD at 37 °C | Lab batch | Escherichia coli | Full inactivation after 8–10 d | ||
| Salmonella Senftenberg | Full inactivation after 10 d | ||||
| Phage PhiX-174 | 1.8 log10 after 30 d | ||||
| Phage MS2 & PCV2 | Full inactivation & 3 log10 after 30 d | ||||
| Animal slurry | MAD at 37 °C for 28 d | Lab batch | Total coliforms | >3 log10 | [48] |
| Escherichia coli | >3 log10 | ||||
| Enterococci | >2 log10 | ||||
| Animal manure | MAD at 27–41 °C for 40–70 d | 5 Farm BGP | Escherichia coli | 0.7–2.5 log10 | [3] |
| Enterococci | −0.49 6 to +1.17 7 log10 | ||||
| Clostridium perfringens total | –0.80 to +0.07 log10 | ||||
| Clostridium perfringens spores | 0.15–0.83 log10 | ||||
| Thermotolerant Campylobacter | –1.40 to +0.30 log10 | ||||
| Listeria monocytogenes | Almost full inactivation | ||||
| Salmonella spp. | Almost full inactivation | ||||
| Clostridium botulinum total | Very low level | ||||
| Clostridioides difficile total | Very low level |
| Substrates | AD Conditions | Experimental Scale | Indicator Microorganisms | Observations | References |
| WAS 1 | MAD 2 | Several WWTP 3 | Fecal enterococci | 1–1.5 log10 | [49] |
| Sewage sludge | MAD | WWTP | Listeria monocytogenes | Sensitive to anaerobic conditions | [50] |
| Biosolids | TAD 4 for 0.5–6 h | Lab batch | Ascaris suum eggs | ~4 log10 | [51] |
| TAD for 0.03–2 h | Lab batch | Poliovirus | ~6 log10 | ||
| Biosolids | TAD | Continuous flow reactor | Salmonella spp. | Not detected for most samples | [52] |
| Fecal enterococci | 3.68–4.89 log10 | ||||
| Somatic and male-specific coliphages | 0–2.36 log10 | ||||
| Ascaris suum eggs | >2 log10 | ||||
| Poliovirus | 2.07–5.76 log10 | ||||
| Biosolids | TAD | Full scale | Microbial indicators for Biosolids | Meet US Class-A biosolids criteria | [53] |
| Primary sludge and farm biosolids | TAD | Two-stage continuous-batch full-scale digester | Microbial indicators for Biosolids | Meet US Class-A biosolids criteria | [54] |
| Sewage sludge | MAD or TAD | Lab batch | Total coliforms | 1.9 or 6.3 log10 | [55] |
| Escherichia coli | 1.69 or 5.38 log10 | ||||
| Clostridium perfringens spores | 0.48 or 0.93 log10 | ||||
| Salmonella spp. | Full inactivation | ||||
| Sewage sludge | AD at 35 or 55 °C for 60 d | Lab batch | Fecal coliforms | 5.13 log10 or 5.09 log10 | [56] |
| Salmonella spp. | Full inactivation | ||||
| Helminths eggs | Full inactivation |
| Substrates | AD Conditions | Experimental Scale | Indicator Microorganisms | Observations | References |
|---|---|---|---|---|---|
| Cabbage roots | MAD 1 at 35 °C for 2 weeks | Lab batch | Plasmodiophora brassicae | No effect | [57] |
| TAD 2 at 55 °C for 2 weeks | Lab batch | Plasmodiophora brassicae | 3 log10 | ||
| Household wastes | Solid phase TAD at 52 °C for 12 h | Lab batch | Ralstonia solanacearum | 8 log10 | [58] |
| Green waste | MAD for 21 d | 300-L batch reactor | Fusarium oxysporum f. sp. asparagi | 2.9 log10 | [59] |
| Ralstonia solanacearum | 6 log10 | ||||
| Salmonella typhimurium | 7 log10 | ||||
| Sclerotium cepivorum | No effect | ||||
| Enterobacteriaceae | 3 log10 | ||||
| Household wastes | MAD at 35 °C and TAD at 55 °C | 45-L semi-continuous digester | Several strains of fungi | 2–7 log10 depending on strains | [60] |
| Biowaste | TAD for 24 h | Full-scale 750-m3 BGP | Listeria monocytogenes Salmonella enterica Escherichia coli Campylobacter jejuni | Full inactivation | [61] |
| Full inactivation | |||||
| Full inactivation | |||||
| Full inactivation | |||||
| Food waste and blackwater | Two-stage AD (TAD + MAD) | Lab batch | Escherichia coli | 6.4 log10 after TAD | [62] |
| regenerated by 4 log10 after MAD | |||||
| Clostridium perfringens | No sig. effect | ||||
| Coliphage | 1.4 log10 | ||||
| MSW and food waste | TAD at 54 °C | Full scale 1 500-m3 BGP | Salmonella Senftenberg W775, Enterococcus spp., and Ascaris suum | Full inactivation | [63] |
| Anaerobic digestate | MAD at 38 °C for > 30 d | Full-scale BGP | Campylobacter spp. | reduction of 98.7% | [64] |
| Bacillus spp. | reduction of 25.3–38.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization, Pulsed Electric Field Treatment, and Anaerobic Digestion. Energies 2021, 14, 1938. https://doi.org/10.3390/en14071938
Liu X, Lendormi T, Lanoisellé J-L. Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization, Pulsed Electric Field Treatment, and Anaerobic Digestion. Energies. 2021; 14(7):1938. https://doi.org/10.3390/en14071938
Chicago/Turabian StyleLiu, Xiaojun, Thomas Lendormi, and Jean-Louis Lanoisellé. 2021. "Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization, Pulsed Electric Field Treatment, and Anaerobic Digestion" Energies 14, no. 7: 1938. https://doi.org/10.3390/en14071938
APA StyleLiu, X., Lendormi, T., & Lanoisellé, J.-L. (2021). Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization, Pulsed Electric Field Treatment, and Anaerobic Digestion. Energies, 14(7), 1938. https://doi.org/10.3390/en14071938








