3.2. Coupled Effect of Light Intensity and Carbon Source on Biomass Productivity of C. sorokiniana
In this phase, microalgal growth was optimised using different parameters, as described in
Section 2.2. Feng et al. [
17] reported a decrease in biomass productivity at a high nitrate concentration. Nitrate in high concentrations may be toxic to microalgae species. Whereas, Blair et al. [
18] observed no toxic effect of nitrate on the biomass productivity of
Chlorella vulgaris. In the present study, nitrate was used as the sole source of nitrogen, and no such detrimental effect of nitrate on biomass productivity was observed. Six different combinations of the parameters used in this study are described in
Table 1.
The biomass productivity of both the algal strains of
C. sorokiniana was found to be dependent on the light intensity and carbon sources (
Table 3a,b).
For the algal strain isolated from the modified BG-11 medium (
C. sorokiniana 1): High biomass productivity of 39.8 mg/L/day was found in the growth medium containing 100 mg/L of glucose (
Table 3a). It was observed that low intensity light might affect the growth rate. Hence, for the subsequent studies, the light intensity was increased to 221 µmol/s/m
2 from 140 µmol/s/m
2. According to Sacristan de Alva et al. [
19], adequate light intensity contributes to enhanced cell growth, as the light energy increases CO
2 assimilation and enhances the cell dry weight.
Biomass productivity of
C. sorokiniana 1 increased with the increase in light intensity, and the estimated biomass productivity was 56 mg/L/day in a 50 mg/L glucose dose. Optimum light stimulates the synthesis of critical components required for the cell division of
Chlorella strains [
20].
Supplementation of growth media with the inorganic carbon dose of 75 mg/L/day increased the biomass productivity further to 153 mg/L/day. The growth rate and lipid production could be controlled via regulating the availability of essential nutrients and trace elements [
21,
22]. Various ions, such as iron and magnesium, are essential for cellular mechanisms, including photosynthesis, cell division, respiration, intracellular transportation, and protein synthesis in microalgae [
23,
24]. However, in the present study, supplementation of metal ions (iron) with organic and inorganic carbon had a detrimental effect on biomass productivity, as it decreased to 24 mg/L/day from 153 mg/L/day (
Table 3a). Wan et al. [
25] reported that if the iron concentration in a medium is more than 55 mg/L, it can reduce the biomass growth of
Chlorella sp. However, in our study, we observed a toxic effect of iron at a much lower dose than the one reported by Wan et al. [
25]. Besides iron, daily doses of inorganic carbon without the addition of organic carbon also reduced the biomass productivity to 43 mg/L/day from 153 mg/L/day (
Table 3a). According to TSS measurements, the combined effects of the organic carbon and inorganic carbon in the presence of a high light intensity showed higher biomass productivity. Sun et al. [
26] observed a positive effect of NaHCO
3 on the biomass productivity of algae. However, the addition of CO
2, along with NaHCO
3 further improved biomass productivity. Hence, it is most likely that a supply of CO
2 or organic carbon was the key behind the improved biomass productivity.
For the algal strain isolated from Knop’s medium (
C. sorokiniana 2): This strain showed higher biomass productivity compared to
C. sorokiniana 1. The highest biomass productivity of 160 mg/L/day was recorded in the growth medium containing 50 mg/L of glucose under a light intensity of 140 µmol/s/m
2. In the 100 mg/L of glucose dose, the biomass productivity decreased to 106 mg/L/day, from 160 mg/L/day (
Table 3b).
Light intensity was increased from 140 µmol/s/m
2 to 221 µmol/s/m
2 to improve biomass productivity. With a 50 mg/L glucose dose, the estimated biomass productivity was 163 mg/L/day. An increase in light intensity could not improve biomass productivity further. However, the addition of an inorganic carbon source along with organic carbon (glucose) enhanced biomass productivity by 10 mg/L (biomass productivity: 170 mg/L/day). On the other hand, daily doses of only inorganic carbon (75 mg/L/day) in the growth media resulted in a biomass productivity of 90 mg/L/day (
Table 3b). Hence, both carbon sources (inorganic and organic) are required for enhanced biomass productivity. This observation indicates that organic carbon helps the algal cells to reduce their dependency on light for inorganic carbon assimilation, and to channel the harvested light energy in the form of ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate) for cell growth [
27]. Lin and Wu [
28] reported that glucose enhanced the acetyl CoA/malonyl CoA for cell synthesis. These observations also suggest that
Chlorella sp. utilises the energy from light in the non-glucose added mode, and uses the organic carbon source available for cell anabolism to obtain a remarkably higher growth rate [
29]. As carbon uptake is the main mechanism for energy conversion and utilisation by microalgae, the consumption rate of carbon would be directly affected by nutrient supply, and further controls microalgal biomass production [
30,
31].
In addition to the carbon source, various trace elements also affect cell metabolism. Various ions, such as Ca
2+, Fe
3+, and Cu
2+ are key factors involved in the physiological metabolism of algae, and increase the biomass yield and oil content at low concentration [
24,
32]. However, in the present study (
Table 3b), the addition of ferric ion showed a detrimental effect on biomass productivity (74 mg/L/day of biomass production).
The most common growth mode for microalgae is autotrophic cultivation using CO
2 and light, while in the heterotrophic mode, algae only utilise organic compounds without light illumination [
30,
33]. However, according to the results observed in the present study, the combined effects of light intensity, and organic and inorganic carbon resulted in better algal biomass productivity for both the algal strains. Lin and Wu [
28] also reported that the specific growth rate of
C. vulgaris under mixotrophic growth conditions was significantly higher than the sum of those from photoautotrophic and heterotrophic growth, illustrating synergistic growth mechanisms. Mixotrophy comprises a dual system in which the autotrophic process utilises light energy and converts the light energy into chemical energy via photosynthesis. The heterotrophic process facilitates catabolism of organic compounds to obtain the energy essential for cell division [
29,
31]. On the other hand, carbon dioxide from cellular respiration becomes available for re-utilisation in photosynthesis under light conditions [
30]. Therefore, the external CO
2 supply is reduced.
A comparative study of biomass productivity was carried out to evaluate the attractiveness of the present strain’s biomass productivity, compared to the other strains reported in the literature (
Figure 1). The biomass productivity in this study was higher than the average biomass productivity of previous studies, which indicates that our study showed better results. If we evaluate only the biomass productivity of
Chlorella sp., our study shows improved biomass production. It ultimately demonstrates that hydrolysate medium is an attractive growth medium for algal biomass production. One more advantage of using hydrolysate medium is that it is cheap compared to other growth media, and is produced from a waste source.
3.3. Effect of Monosaccharide Supplementation to the Hydrolysate Growth Medium on C. sorokiniana Biomass Productivity
In the previous experiment, various doses of organic and inorganic carbon were added to understand their effects on biomass productivity. The hydrolysate prepared from waste biomass contains organic carbon in the form of C5 and C6 sugars. The hydrolysate also contains various forms of nitrogen, especially ammonium nitrogen. Hence, the effects of C5 sugars (xylose) and ammonium–N were evaluated based on biomass productivity of the two algal strains.
Ammonium is a growth substrate for many oleaginous microalgae [
34]. Microalgae usually prefer ammonium over other nitrogen sources for intracellular metabolic activities [
46]. In general, when nitrogen is supplied as nitrate, conversion of nitrate to ammonium consumes a considerable amount of energy compared to the uptake of ammonium [
9]. Hence, uptake of nitrogen as nitrate is energy intensive and may reduce algal growth. Moreover, Rani et al. [
12] observed higher uptake of ammonium–nitrogen by
Chlorella sorokiniana1 compared to nitrate. So, in further study, the growth medium was supplemented with two different nitrogen sources, i.e., Nitrate–N (60 mg/L) and Ammonium–N (15 mg/L). Phosphate–P was kept at 15 mg/L. A sufficient P supply ensures unhindered ATP production, which ultimately ensures algal growth [
47,
48,
49].
Microalgae have a much higher growth potential when they use organic carbon for growth [
50]. The use of exogenous carbon sources with either heterotrophic (without light) or mixotrophic (with light) culture modes has been reported to increase the biomass of several microalgal species [
51,
52,
53]. The biomass growth rate and lipid content of
Chlorella strains can be raised by supplementing the culture medium with organic carbon sources [
35]. Various organic carbon sources, including polysaccharides, disaccharides, monosaccharides, starch, and ammonium acetate have been used for microalgal cultivation, but systematic comparisons of data between primary monosaccharides are limited. Hence, in this study, the effect of two different monosaccharides, i.e., glucose (C6 sugar) and xylose (C5 sugar) was analysed with respect to the optical density of media along with light/dark treatments to develop a cultivation mode that enhances biomass production. Monosaccharides and inorganic carbon sources were added every 24 h to the growth medium (with a concentration of 5 mg/L/day and 75 mg/L/day, respectively, details are given in
Table 2). The light intensity was also kept constant at 221 µmol/s/m
2, which was optimum according to the first phase of the study, as explained in
Section 3.2.
For
C. sorokiniana 1: The highest biomass productivity (38 mg/L/day) was observed in the growth medium supplemented with glucose along with an inorganic carbon source (
Table 4a). Biomass productivity in the growth medium supplemented with xylose was found to be low (13 mg/L/day). In the absence of monosaccharides (control), the cultures displayed the typical green colour. Whereas, supplementation of xylose in the growth medium resulted in the discolouration of the culture, which could be due to the decline in the microalgal biomass. However, supplementation with glucose turned the microalgal culture to a deep green colour as compared to the control, which showed an increase in algal biomass and chlorophyll content (data not shown).
Some researchers, like Ribeiro et al. [
54] and Derner et al. [
55], have reported that the mixotrophic action of
Chlorella sp. is possibly due to the photoperiod cycles, as the light availability is directly related to the inorganic carbon consumption. Similarly, in this study, the 12 h:12 h light–dark cycle was responsible for the uptake of NaHCO
3. In the mixotrophy, organic carbon such as glucose, provides auxiliary energy in the form of Acetyl-CoA, NADPH and other intermediates for the cellular biosynthesis [
26,
54].
For
C. sorokiniana 2: The highest biomass productivity (187 mg/L/day) was found in the growth medium supplemented with glucose (10 mg/L/day) along with an inorganic carbon source (
Table 4b), whereas in the growth medium supplemented with xylose, the biomass productivity was found to be less (134 mg/L/day). Biomass productivity was estimated to be 150 mg/L/day when the growth media was supplemented with inorganic carbon (Na
2CO
3) only. The biomass productivity under inorganic carbon supplement was higher than the one reported in the presence of xylose + Na
2CO
3, and lower than that observed under the glucose + Na
2CO
3 supplement. Hence, from the different mixtures of organic and inorganic carbon, it was observed that C
5 sugar reduced the growth of the algal strain, whereas C6 sugar increased the biomass productivity (
Table 4b). Our results are corroborated by the findings of Chai et al. [
35], who investigated the effect of glucose, galactose, fructose, and xylose on the growth of
Chlorella sorokiniana UTEX 1230. They reported that supplementation with fructose promoted
C. sorokiniana UTEX 1230 growth to a much lesser extent compared to glucose. Supplementation with galactose had no effect, and supplementation with xylose inhibited growth. Xylose supplementation had no effect on the growth of
C. sorokiniana UTEX 1230 during the dark phase, whereas xylose significantly inhibited the algal growth under light conditions [
35].
As compared to the strain isolated from the BG-11 medium (C. sorokiniana 1), algae isolated from Knop’s medium (C. sorokiniana 2) showed improved biomass productivity. After observing such an encouraging result, a one-time glucose dose was also tried, to check its role in biomass productivity. An initial dose of 200 mg/L glucose increased the biomass productivity of the strain to 204 mg/L. For a low glucose dose of 10 mg/L/day, the lower dry weight production suggested that a lower content of glucose might have been assimilated and converted into energy directly, leading to cell growth. A low glucose dose could not improve the production of the storage compounds that can increase biomass dry weight. Thus, both glucose and inorganic sources contributed to the algal growth, and likely had a cumulative effect. Whereas, xylose had a negative impact on algal growth.
Several researchers have evaluated the effect of monosaccharide supplementation on the biomass productivity of various strains of
Chlorella sp. Woodworth et al. [
56] found that the biomass productivity of
C. vulgaris cultured under mixotrophic conditions (with glucose supplementation of 20 g/L) was higher compared to autotrophic and heterotrophic cultures. Similarly, Li et al. [
57] investigated the impact of glucose on
C. protothecoides, and applied a two-step process in which glucose was added in the first step and then removed in the second. Glucose addition in the process was found to influence biomass productivity and lipid accumulation, including the chlorophyll content, and protein and starch levels. Hawkins et al. [
58] and Chai et al. [
35] reported that
C. sorokiniana utilised glucose as the primary monosaccharide compared to other sugars such as xylose, galactose, and fructose. Glucose, being the primary metabolic fuel, is more stable than other monosaccharides, and less susceptible to the formation of nonspecific glycol conjugates [
35]. A perusal of the literature showed that the effect of glucose on biomass productivity of several
Chlorella sp. matched with our results obtained in the present investigation. Therefore, between C5 and C6 sugars, C6 sugar (Glucose) was found to be the better monosaccharide for the growth of both
Chlorella sorokiniana 1 and
Chlorella sorokiniana 2, in terms of biomass productivity.
3.4. Effects of Different Parameters on C. sorokiniana Lipid Productivity and FAME Composition
After optimising the different cultivation conditions, the microalgal biomass was grown in a 3 L photobioreactor using the best parameters for growth obtained from our previous study, i.e., the combination of glucose (50 mg/L) and inorganic C-source (75 mg/L) at 221 μmol/s/m
2 light intensity. As the N and P content was high in the growth medium, it is not possible to produce value-added products in the form of lipid and carbohydrate. However, increasing lipid accumulation by subjecting them to suboptimal conditions negatively affected lipid productivity due to the low growth [
59]. Hence, in this study, after growth in a nutrient-sufficient condition (6 days), the algal biomass was transferred to a nutrient-amended medium for the value-added product formation (7 days). A similar strategy was also adopted previously by various researchers [
60,
61]. As described in
Section 2.2, the nutrient-sufficient medium in this study consisted of glucose (50 mg/L), inorganic C-source (75 mg/L/day), nitrate-N (60 mg/L), NH
4-N (15 mg/L), and phosphate -P (15 mg/L). The study was conducted under 12 h:12 h light–dark phase.
The biomass productivity in the photobioreactor under the nutrient sufficient condition was found to be 73 mg/L/day for
C. sorokiniana 1 (
Table 5), which was lower than the one obtained during our previous study (
Table 3a). Nitrogen and phosphorus contents were measured on the 7th day, and the nitrate-N decreased to 0.082 mg/L from 60 mg/L. Ammonium–N content was found to be 0.019 from the initial amount of 15 mg/L, and phosphate-P content decreased to 0.837 mg/L from 15 mg/L. For
C. sorokiniana 2, biomass productivity was found to be 267 mg/L/day (
Table 5). The nitrogen and phosphorus contents were measured on the 7th day, and the nitrate-N decreased to 1.66 mg/L from 60 mg/L. Ammonium–N content decreased to 0 from the initial amount of 15 mg/L, and phosphate-P content decreased to 1.2 mg/L from 15 mg/L.
The second part of this study emphasises the enhancement of lipid accumulation by transferring the algal biomass into the nutrient amended conditions. Two different media were prepared (details are given in
Section 2.3.2). The first nutrient amended medium (referred to as N amended) contained an initial amount of 5 mg/L ammonium–N, with no phosphate-P in the medium. The other nutrient amended medium (referred to as P- amended) had an initial amount of 3 mg/L phosphate-P, without a nitrogen source. The carbon sources were regularly added (5 mg/L of glucose and 125 mg/L NaHCO
3).
The biomass productivity was found to be higher in the P-amended medium for both the algal strains.
C. sorokiniana 1 registered an 82.9 mg/L/day biomass productivity (
Table 5), and
C. sorokiniana 2 showed a biomass productivity of 178 mg/L/day (
Table 5). Whereas, in the N-amended medium, biomass productivity was found to be 58.5 mg/L/day (
Table 5) and 94.3 mg/L/day, for
C. sorokiniana 1 and
C. sorokiniana 2, respectively. Contrary to biomass productivity, the lipid accumulation was higher in the N- amended medium than the P-amended medium for both the algal strains (
Table 6 and
Table 7).
For
C. sorokiniana 1: lipid accumulation increased to its highest (17.2%) on the 5th day in the N-amended medium. Whereas, in the P-amended medium, the highest (9.9%) lipid production was observed on the 3rd day (
Table 6).
Lipid productivity is one of the indicators for selecting an algal strain for biodiesel production. Hence, lipid productivity for both strains was estimated. It was observed that for C. sorokiniana 1, lipid productivity (taking into account biomass productivity in the nutrient sufficient condition) was 20 and 13 mg/L/day for the N- and P-amended media, respectively. On the other hand, C. sorokiniana 2, registered lipid productivity of 30 and 33 mg/L/day for the N- and P-amended media, respectively. Hence, although lipid production was low in the Knop’s medium strain, because of high biomass productivity in the P-amended medium, this strain showed higher lipid productivity than the other strain.
Shi et al. [
62] studied the marine algae
Micractinium inermum, which could grow in phototrophic, mixotrophic, and heterotrophic conditions, and showed lipid productivity as high as 0.68 g/L/day in the heterotrophic condition. Similarly, a
Chlorella vulgaris strain studied by a research group at the University of Minnesota showed that the strain could grow in three modes, i.e., autotrophy, mixotrophy, and heterotrophy. The largest lipid productivity, however, was observed in the mixotrophic mode (0.23 g/L/day), and the largest lipid content was observed when the culture was fed solely by glucose (40% lipid content) [
63]. In autotrophic and heterotrophic modes, the lipid content varied from 8–25%. Similarly, Gladue and Maxey [
64] observed that in the presence of acetate,
Chlorella vulgaris could produce 0.027 g/L/day lipid. Maurya et al. [
36] used hydrolysate produced from algal biomass as a feed for producing algal biomass (
Chlorella vulgaris). In the present study, supplementation of algal hydrolysate increased the lipid content in the algal cells (depending on the concentration of algal hydrolysate in the synthetic media), and lipid content varied from 23% to 32%. However, lipid productivity (0.007 g/L/day) was very low compared to the other studies. To evaluate the attractiveness of the present strains, the lipid productivity of other species reported in the literature was collected, and is presented in
Figure 2. An average productivity of 100 mg/L/day was estimated for the lipid productivity obtained from the literature. However, the high productivity was because of a few species of
Scenedesmus and
Micractinium. These species showed extremely high lipid productivity (500–2000 mg/L/day) [
65]. However, if one considered only the species of
Chlorella, the average lipid productivity was 49 mg/L/day, whereas species of
Nannochloropsis registered lipid productivity of 120 mg/L/day. Various species of
Botryococcus showed average lipid productivity of 32 mg/L/day. Hence, the strains used in this study showed below-average lipid productivity compared to the average lipid productivity observed in the literature (
Figure 2). However, most of the species showed lipid productivity ranging between 5–50 mg/L/day. Hence, to understand the attractiveness of the present strain as a biodiesel feedstock, a frequency distribution of the lipid productivity was plotted (data are not shown). The frequency distribution revealed that the most common lipid productivity obtained from the literature was between 20 to 40 mg/L/day. Hence, the analysis showed that the present strains showed a lipid productivity that is frequently encountered for most of the species, and these species showed a higher than average biomass productivity.
Beside lipid productivity and lipid content, lipid composition and individual fatty acid components also affect biodiesel quality. Hence, fatty acid profiles of individual strains were also evaluated in detail.
For
C. sorokiniana 1, in the N-amended medium (5-N), the highest saturated fatty acids (SFA) of 9.8%, and total FAME content of 11.56% were reported on the 5th day. The total FAME content increased by 5% as compared to day 0, amongst which polyunsaturated fatty acid (PUFA) increased by 3% (
Table S4, Supplementary Information). Lipid content started decreasing after 5 days, as only 9.3% and 5% of lipid production was estimated on the 7th day in the N-amended medium (7-N) and P-amended medium (7-P), respectively (
Figure 3). The SFA comprised C16:0 (Palmitic acid), C18:0 (Stearic acid), and C20:0 (Eicosanoic acid). In the present study, C16:0 constituted a significant fraction of SFA, whereas C18:1 and C18:2 were the major fractions of unsaturated fatty acid.
Contrary to lipid accumulation, the highest carbohydrate content was recorded in the P-amended medium at the end of the study (22%).
For
C. sorokiniana 2: Lipid accumulation on the initial day (referred to as 0 day) was 10.87% (
Figure 3) of which 72% represents the SFA. After that, the lipid accumulation increased in both the media. On the 3rd day, (referred to as 3-N and 3-P) accumulated lipid was 13.43% and 15.09% in the N-amended medium and P-amended medium, respectively (
Table 7). While, 92% of the total FAME composition consisted of SFA (
Table S5, supplementary information). The highest lipid content was observed on the 5th day, with 16.88% and 14.07% in the N-amended medium (referred as 5-N) and P-amended medium (referred as 5-P), respectively (
Figure 3). In the N-amended medium (5th day), the content of PUFA was 42% of the total FAME composition, and was found to be the highest compared to the other days. SFA content was estimated to be 56% of the total FAME, and the rest was monounsaturated fatty acid (MUFA). However, in the P-amended medium, 88% of the total FAME was SFA, and the rest were MUFA and PUFA. Lipid content declined after 5 days as only 12.51% and 6.92% of lipid was estimated on 7th day in the N-amended medium (7-N) and P-amended medium (7-P), respectively (
Table 7). In the N-amended medium (5th day), the total FAME content was the highest compared to the other days, but the composition of SFA alone was less. Whereas in the P-amended media, the SFA alone comprised the maximum FAME composition, whereas MUFA and PUFA were only about 1–2%. At the end of the study, the carbohydrate content was estimated to be higher in the N-amended medium (12.7 %) than the P-amended medium (3.13%). This result was found to be contrary to the algae isolated from the BG-11 medium.
According to the biodiesel standard EN 14214, IS 15607 and ASTM D-6751, the linolenic methyl ester (produced from C18:3 FA) content of biodiesel must be less than 12%, and the polyunsaturated methyl ester (at least four double bonds) content must be less than 1% [
77]. In the present study, for both the algal strains, the proportion of linolenic acid was 0, which satisfied the standard of biodiesel. Compared to the present strains, Huang et al. [
29] reported that marine algae
Phaeodactylum tricornutum showed enhanced lipid productivity under nitrogen deprived conditions. Whereas,
Isochrysis zhangjiangensis showed enhanced lipid productivity under phosphorus deficient conditions, which is in accordance with our observations. Fu et al. [
78] observed that phosphorus supplementation enhanced the synthesis of saturated fatty acids, while curtailing the unsaturated fatty acid production pathways. Zhu et al. [
79] and Singh et al. [
80] also discussed in their work that all nitrogen limited conditions led to an increasing proportion of C18:1. Whereas, Chu et al. [
11] observed that nitrogen deficiency increased unsaturated fatty acids, including mono and poly unsaturated fatty acids. Supplementing the media with P increased lipid production. Both N and P limitation affected the lipid productivity.
In the present study, it was observed that for the algal strain isolated from Knop’s medium, i.e.,
C. sorokiniana 2, when grown in nutrient amended medium, especially P-amended medium, the major fraction of the saturated fatty acid was C16:0. At the same time, a minor fraction of C14:0 was also observed. However, for the other strain (BG-11), no C14:0 fatty acid was detected (
Table 6 and
Table 7).
C. sorokiniana 1 accumulated more unsaturated fatty acid in the N-amended media than that in the P-amended media. Whereas,
C. sorokiniana 2 accumulated almost 80% saturated fatty acids in both media. A considerable portion of C18:0 was also detected in
C. sorokiniana 2, making it more suitable for producing biodiesel compared to the other strain (BG-11).
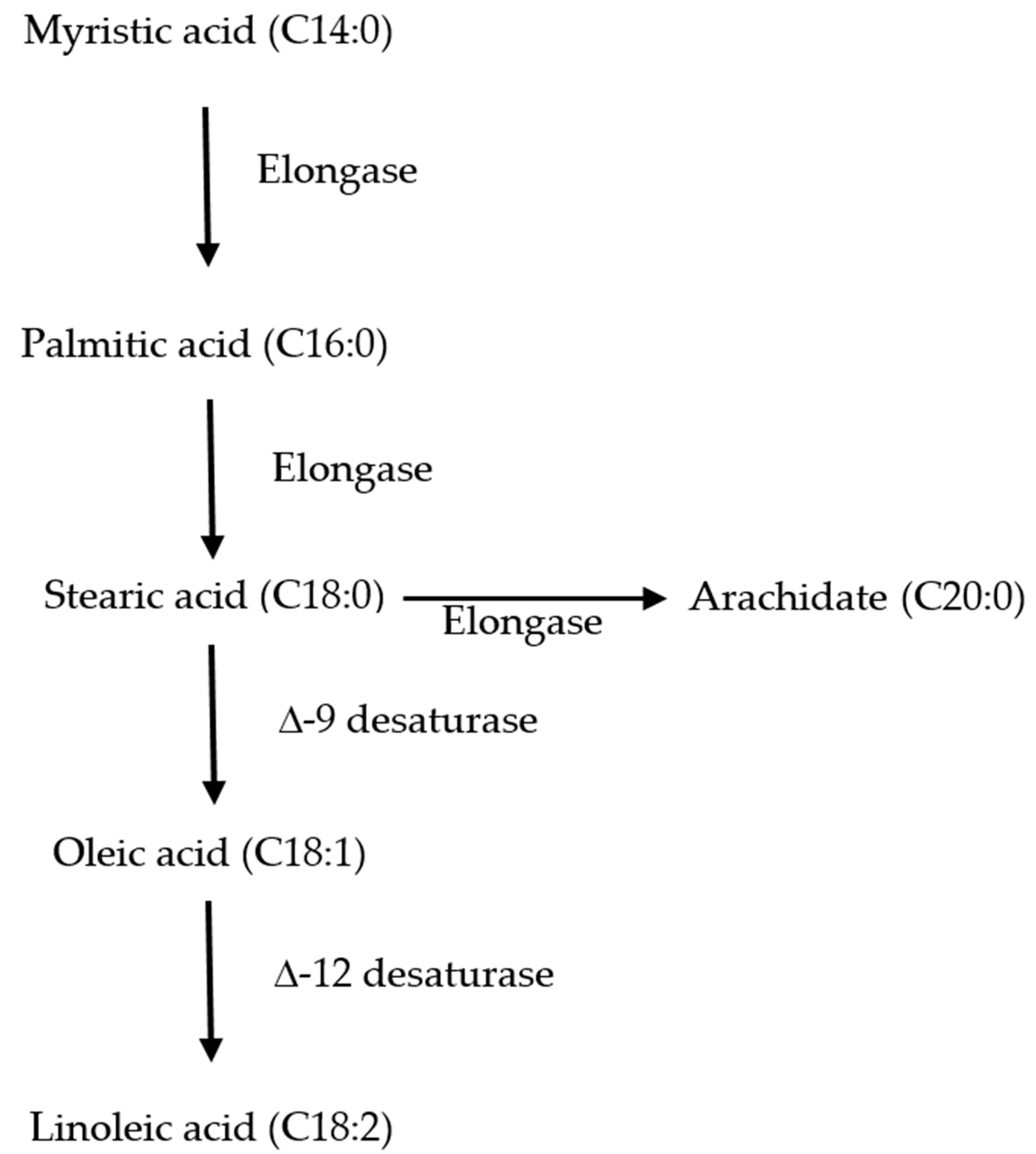
From a review of this study, it was observed that the constituents of FAME were different in the two strains, and the most striking difference was found with respect to the saturated and unsaturated fatty acids. To better understand the inherent difference in fatty acid production, kinetic modeling of the fatty acid profile of these strains was carried out. Before presenting a kinetic model, one needs to know the various fatty acids which are being used to produce neutral lipids and free fatty acids. In this study, C14:0 (myristic acid) was the first fatty acid produced, which later elongated to C16:0 and C18:0. These saturated fatty acids were produced from shorter chain fatty acids by the delta elongase enzyme (
Figure 4). The saturated fatty acids were later converted to unsaturated fatty acids, and delta desaturase enzyme was used to catalyse the conversion. However, depending on the position of the unsaturated bond, different desaturase enzymes act on a particular fatty acid. For simulating such a conversion, it was assumed that the kinetics were first order (
Section 2.4).
C. sorokiniana 1 produced C16:0, C18:0, C 20:0 as SFA, and C18:1 and C18:2 as the unsaturated fatty acid. Hence, C16:0 worked as the precursor for the production of other fatty acids, and the rate of production of C16:0 was assumed as zero-order kinetics. Other rate constants were modeled as first order (details of the model are provided in
Section 2.4.). For
C.sorokiniana 2, C14:0 appeared to act as a precursor for producing other higher-order chain fatty acids.
From a perusal of the kinetic coefficients obtained from the models, no definite trend was observed. Some of the kinetic coefficients were higher in
C. sorokiniana 1, compared to
C. sorokiniana 2. The maximum kinetic coefficient for
C. sorokiniana 1 and
C. sorokiniana 2 was for the conversion of C16:0 to C18:0, and C18:0 to C18:1 (K
3). Prima facie, it seems that the conversion was a bit higher in
C. sorokiniana 1 compared to
C. sorokiniana 2. Similarly, some of the kinetic coefficients were quite different in the N-amended medium compared to the P-amended medium. Therefore, to understand the statistical difference among the kinetic coefficients, one-way ANOVA followed by Tukey’s test for multiple comparison of means were undertaken [
16]. From a perusal of the ANOVA, it was observed that the kinetic coefficients for
C. sorokiniana 2 were statistically significant (p = 0.05). Later, Tukey’s test [
16] revealed that K
3 was statistically significant compared to other kinetic coefficients (
Table 8). However, no such difference was observed between kinetic coefficients obtained using the N-amended and P-amended media. Similarly, no such statistical significance was observed between kinetic coefficients obtained for
C. sorokiniana 1 and
C. sorokiniana 2. Hence, the statistical analysis revealed that the kinetics of lipid production for the strains are the same, even though there were some differences observed in lipid quantity and lipid profile between the two strains. It can also be deduced that the C18:0 production and conversion to C18:1 were quite fast. Hence, the rate-limiting steps for lipid production were most likely the production of C14:0 and C16:0, and their conversion (for
C. sorokiniana 2).
To better understand the lipid production, the biochemical processes responsible for lipid production were also examined. Lipid production is a stress-induced phenomenon. Under nitrogen depleted conditions, adenosine monophosphate (AMP) can be decomposed into inosine monophosphate (IMP) and NH
4+, and catalysed by AMP deaminase as a way to release ammonia. The decrease of AMP, a co-substrate for isocitrate dehydrogenase catalysing the transformation of isocitrate to α-ketoglutarate, will disturb the Kreb’s cycle [
81] and, as a result, the mitochondrion accumulates isocitrate that remains imbalanced with citrate. Excess non-metabolised citrate is then transported to the cytoplasm via the citrate–malate shuttle to be converted into acetyl-CoA by ATP-citrate lyase (ACL), which is an additional pathway in oleaginous microorganisms. Under conditions of nitrogen exhaustion, the flow of carbon in oleaginous microorganisms is transferred towards the accumulation of citric acid, which is later transformed to acetyl–CoA, a precursor for fatty acid synthesis. The present study appears to support these observations, as nitrogen amended media seemed to trigger the lipid accumulation in both the strains.
When the availability of nitrogen is restricted, the synthesis of proteins and nucleic acids is curtailed. Carbon is used for protein, and nucleic acid synthesis used for lipid synthesis, and its concentration increases. Therefore, enhancing the activity of acetyl-CoA synthetase (ACS) and reducing the activity of isocitrate dehydrogenase (ICDH), resulted in more acetyl–CoA to participate in the lipid biosynthetic pathway, which was suggested to be beneficial for lipid accumulation [
26]. Acetyl–CoA carboxylase (ACCase) converts acetyl–CoA into malonyl-CoA, the first, and rate-limiting, step of fatty acid biosynthesis [
26]. Phosphoenolpyruvate can be converted into oxaloacetate by phosphoenolpyruvate carboxylase (PEPC), which decreases the flow of carbon towards lipid biosynthesis in microalgae. Accordingly, the inhibition of PEPC may be beneficial for lipid production.
In the P-amended medium, phosphorus was stored in the form of Poly-P, providing energy to participate in the synthesis of cellular materials, which enhanced cell growth and lipid accumulation, similar to ATP provided in the anabolic processes under nitrogen starvation [
11,
79]. According to the previous literature, the following potential mechanisms may be involved during the presence of phosphorus: (1) nitrogen limitation causes upregulation of malic enzyme, generating NADPH, which leads to lipid accumulation [
37]; (2) upregulated glutamate dehydrogenase promotes the tricarboxylic acid cycle that offers intermediates and energy for lipid biosynthesis [
46]; and (3) abundant phosphorus intake causes the downregulation of the ADP (Adenosine diphosphate)–glucose pyrophosphorylase activity of
Chlorella sp., inhibiting starch synthesis, and regulating carbon assimilation towards the lipid synthesis pathway [
79].
In the present study, nitrogen deficiency certainly inhibited the TCA (Tri-carboxylic acid) cycle [
81] for energy production, and a part of the organic carbon participated in the lipid and starch production. It was observed that the presence of phosphorus promotes the fatty acid synthesis, and restricts the conversion of saturated fatty acids to unsaturated fatty acids.