Effect of the Reactant Transportation on Performance of a Planar Solid Oxide Fuel Cell
Abstract
1. Introduction
2. SOFC Modelling
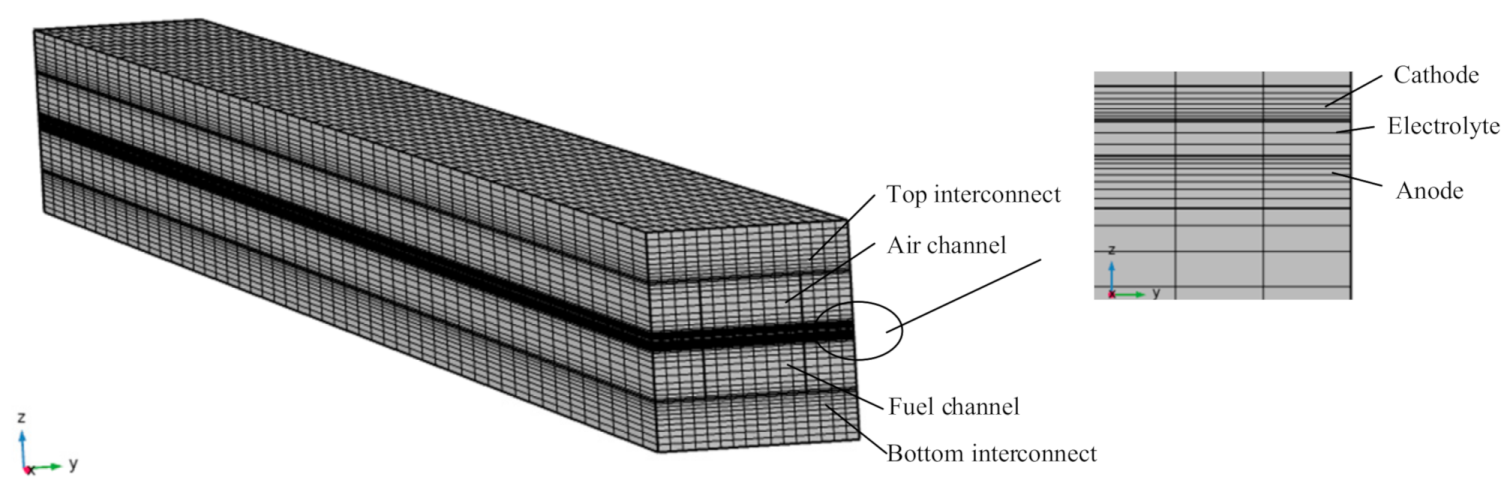
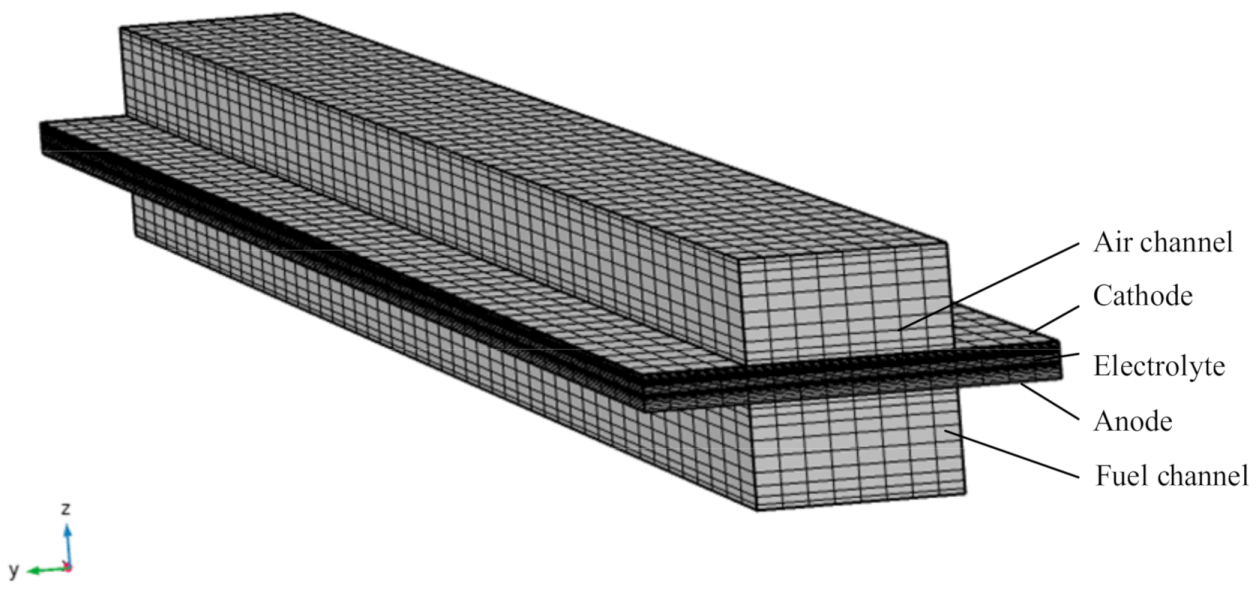
2.1. Numerical Model
2.2. Governing Equations
2.3. Boundary Conditions and Operating Parameters
3. Results and Discussion
3.1. Validation of the Numerical Calculation
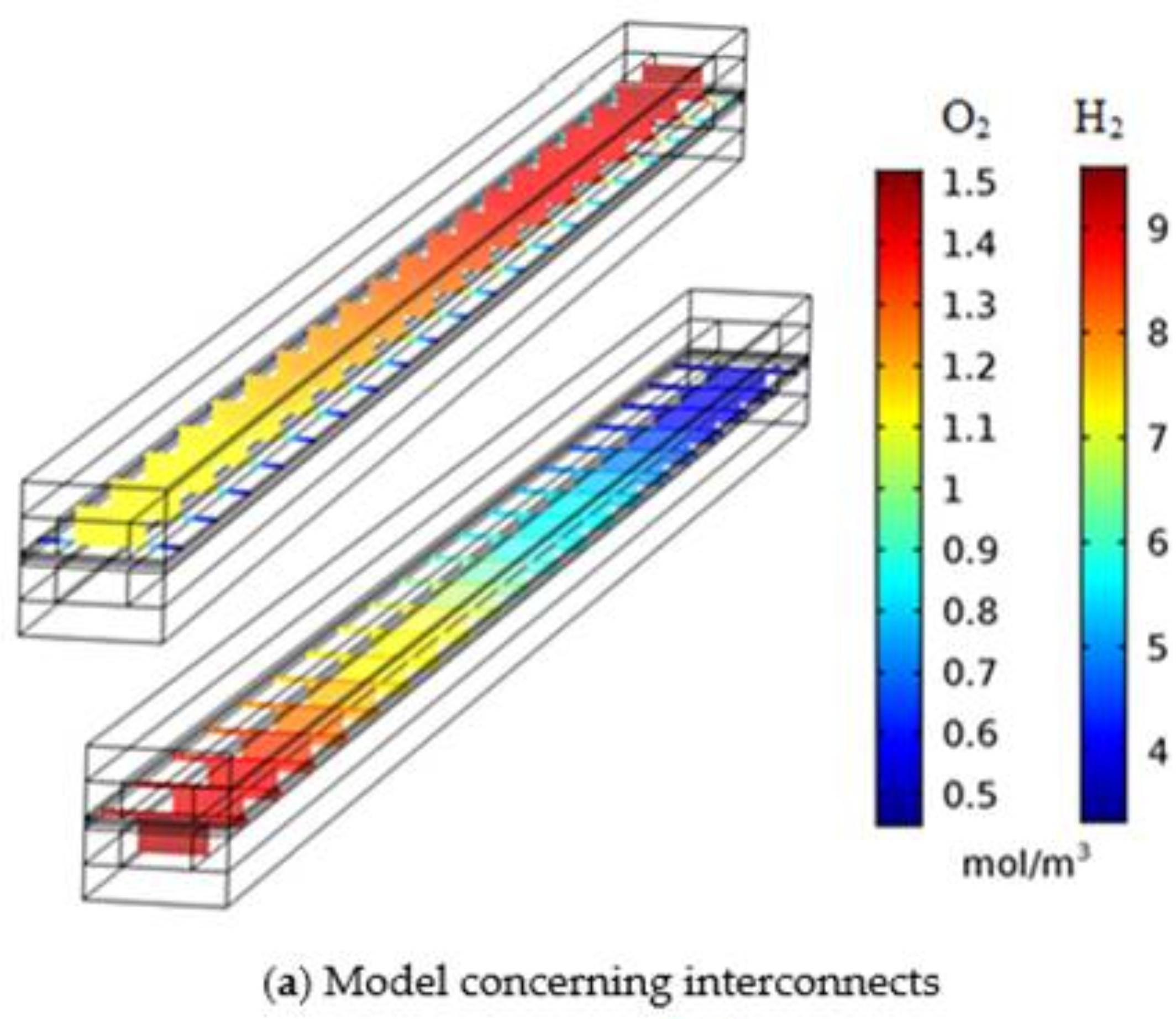
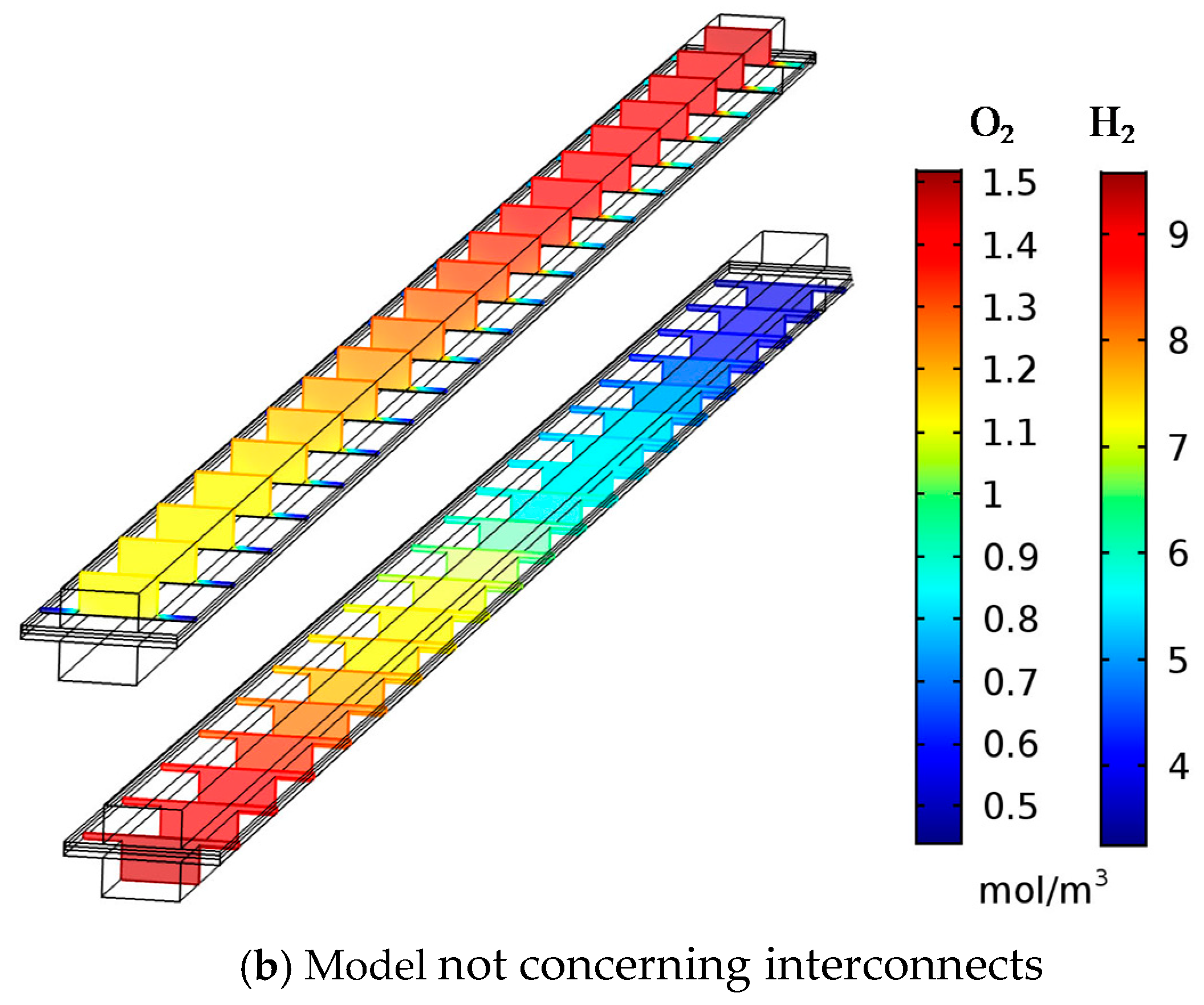
3.2. Effect of Interconnect in Numerical Model and Model Simplification
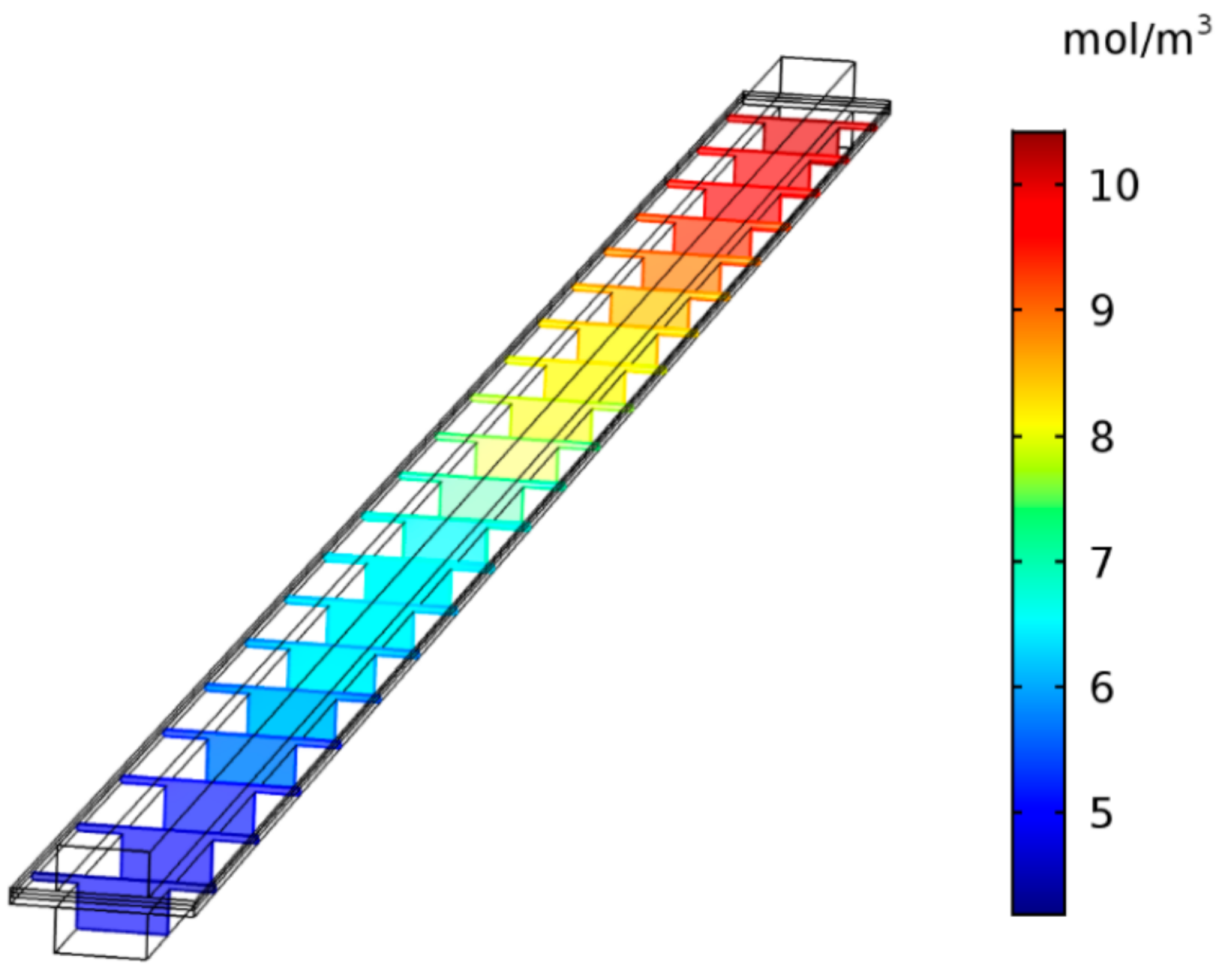
3.3. Effect of Contents of Gas Reactants
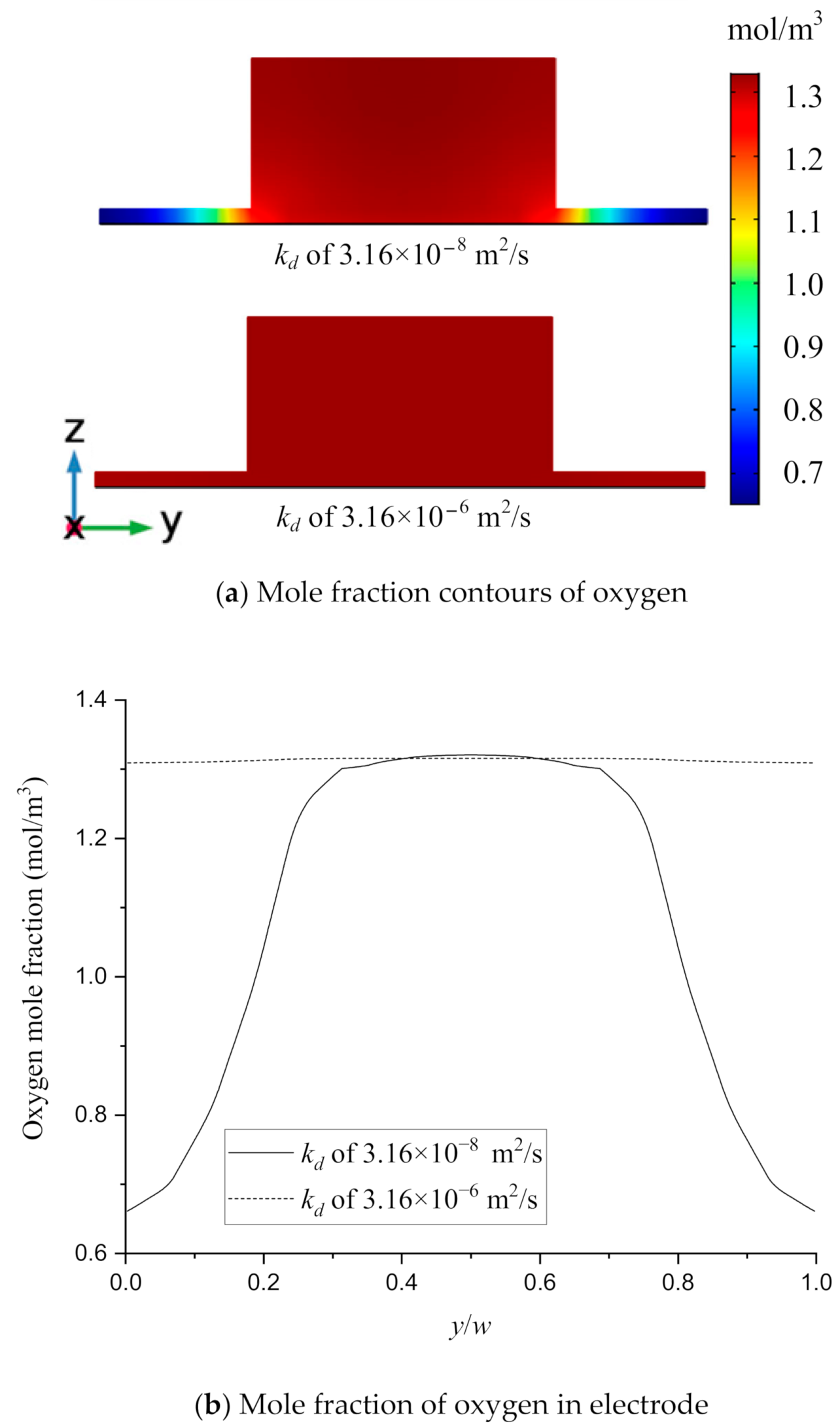
3.4. Effect of the Multi-Reactants Transportation
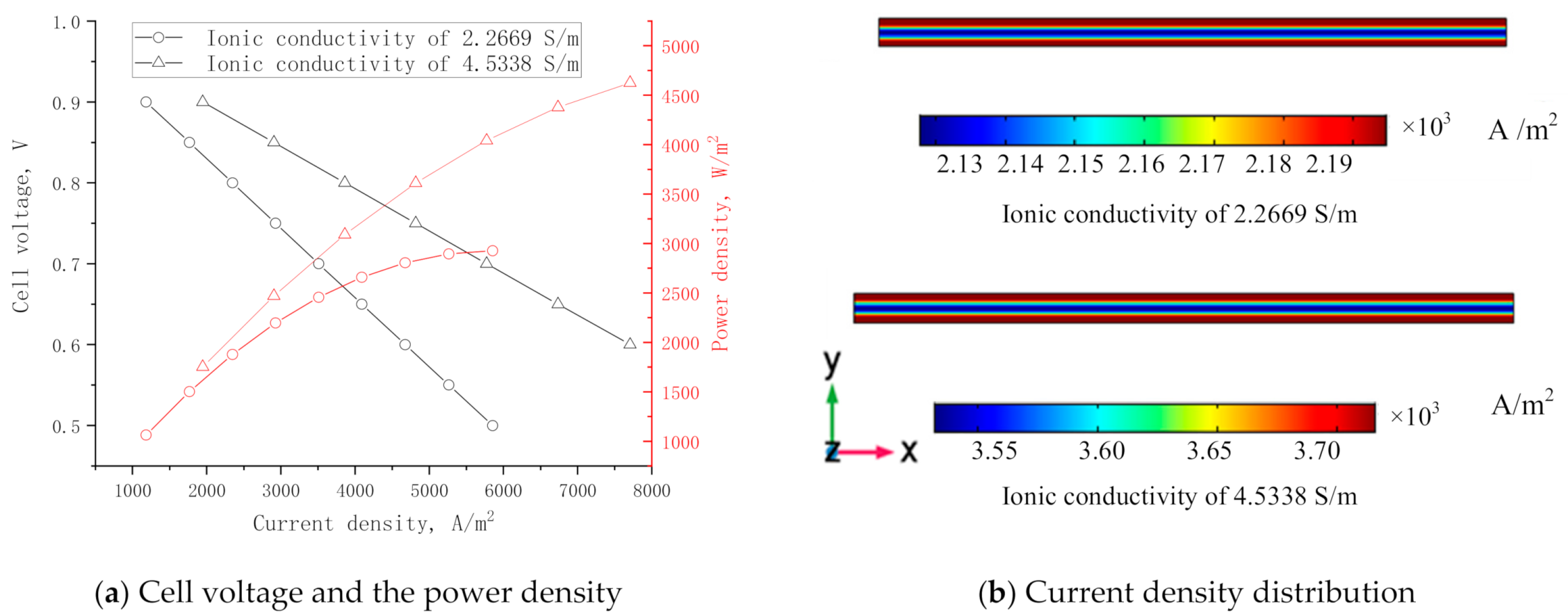
3.5. Effect of Ionic Conduction in Electrolyte
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wachsman, E.D.; Lee, K. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Raj, A.; Sasmito, A.P.; Shamim, T. Numerical investigation of the effect of operating parameters on a planar solid oxide fuel cell. Energy Convers. Manag. 2015, 90, 138–145. [Google Scholar] [CrossRef]
- Liu, S.; Kong, W.; Lin, Z. Three-dimensional modeling of planar solid oxide fuel cells and the rib design optimization. J. Power Sources 2009, 194, 854–863. [Google Scholar] [CrossRef]
- Wu, P.; Shy, S. Cell performance, impedance, and various resistances measurements of an anode-supported button cell using a new pressurized solid oxide fuel cell rig at 1–5 atm and 750–850 °C. J. Power Sources 2017, 362, 105–114. [Google Scholar] [CrossRef]
- Monder, D.S.; Nandakumar, K.; Chuang, K.T. Model development for a SOFC button cell using H2S as fuel. J. Power Sources 2006, 162, 400–414. [Google Scholar] [CrossRef]
- Goldin, G.M.; Zhu, H.; Kee, R.J.; Bierschenk, D.; Barnett, S.A. Multidimensional flow, thermal, and chemical behavior in solid-oxide fuel cell button cells. J. Power Sources 2009, 187, 123–135. [Google Scholar] [CrossRef]
- Corigliano, O.; Fragiacomo, P. Numerical modeling of an indirect internal CO2 reforming solid oxide fuel cell energy system fed by biogas. Fuel 2017, 196, 352–361. [Google Scholar] [CrossRef]
- Hussain, J.; Ali, R.; Akhtar, M.N.; Jaffery, M.H.; Shakir, I.; Raza, R. Modeling and simulation of planar SOFC to study the electrochemical properties. Curr. Appl. Phys. 2020, 20, 660–672. [Google Scholar] [CrossRef]
- Pianko-Oprych, P.; Hosseini, S.M. Dynamic analysis of load operations of two-stage SOFC stacks power generation system. Energies 2017, 10, 2103. [Google Scholar] [CrossRef]
- Dillig, M.; Plankenbühler, T.; Karl, J. Thermal effects of planar high temperature heat pipes in solid oxide cell stacks operated with internal methane reforming. J. Power Sources 2018, 373, 139–149. [Google Scholar] [CrossRef]
- Suzuki, T.; Hasan, Z.; Funahashi, Y.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Impact of anode microstructure on solid oxide fuel cells. Science 2009, 325, 852–855. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Yu, W.; Tanveer, W.H.; Lee, Y.H.; Cho, G.Y.; Park, T.; Zheng, C.; Lee, W.; Cha, S.W. Nickel-based bilayer thin-film anodes for low-temperature solid oxide fuel cells. Energy 2018, 161, 1133–1138. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; Zhu, L.; O’Hayre, R.; Chou, K.-C. Electrochemical performance and stability of La0.5Sr0.5Fe0.9Nb0.1O3-δ symmetric electrode for solid oxide fuel cells. J. Power Sources 2018, 399, 398–405. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Marrero-López, D.; Canales-Vázquez, J.; Irvine, J.T.S. Symmetric and reversible solid oxide fuel cells. RSC Adv. 2011, 1, 1403–1414. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Liu, M.; Tadé, M.O.; Shao, Z. Progress and prospects in symmetrical solid oxide fuel cells with two identical electrodes. Adv. Energy Mater. 2015, 5, 1500188. [Google Scholar] [CrossRef]
- Chelmehsara, M.E.; Mahmoudimehr, J. Techno-economic comparison of anode-supported, cathode-supported, and electrolyte-supported SOFCs. Int. J. Hydrogen Energy 2018, 43, 15521–15530. [Google Scholar] [CrossRef]
- Kupecki, J.; Motylinski, K.; Milewski, J. Dynamic analysis of direct internal reforming in a SOFC stack with electrolyte-supported cells using a quasi-1D model. Appl. Energy 2018, 227, 198–205. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Chang, C.-T.; Jung, G.-B.; Chan, S.-H.; Yeh, C.-C.; Yu, J.-W.; Lee, C.-Y. Improvement on the design and fabrication of planar SOFCs with anode–supported cells based on modified button cells. Renew. Energy 2018, 129, 806–813. [Google Scholar] [CrossRef]
- Hajimolana, S.A.; Hussain, M.A.; Daud, W.A.W.; Soroush, M.; Shamiri, A. Mathematical modeling of solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2011, 15, 1893–1917. [Google Scholar] [CrossRef]
- Kong, W.; Li, J.; Liu, S.; Lin, Z. The influence of interconnect ribs on the performance of planar solid oxide fuel cell and formulae for optimal rib sizes. J. Power Sources 2012, 204, 106–115. [Google Scholar] [CrossRef]
- Aydın, Ö.; Ochiai, T.; Nakajima, H.; Kitahara, T.; Ito, K.; Ogura, Y.; Shimano, J. Mass transport limitation in inlet periphery of fuel cells: Studied on a planar solid oxide fuel cell. Int. J. Hydrogen Energy 2018, 43, 17420–17430. [Google Scholar] [CrossRef]
- Tseronis, K.; Fragkopoulos, I.; Bonis, I.; Theodoropoulos, C. Detailed multi-dimensional modeling of direct internal reforming solid oxide fuel cells. Fuel Cells 2016, 16, 294–312. [Google Scholar] [CrossRef]
- Yahya, A.; Rabhi, R.; Dhahri, H.; Slimi, K. Numerical simulation of heat and mass transfer in solid oxide fuel cell in presence of magnet particles deposited in cathode side. Appl. Phys. A 2018, 124, 464. [Google Scholar] [CrossRef]
- Barzi, Y.M.; Raoufi, A.; Lari, H. Performance analysis of a SOFC button cell using a CFD model. Int. J. Hydrogen Energy 2010, 35, 9468–9478. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, D.Y.; Baek, J.D.; Yoon, Y.-J.; Su, P.-C.; Lee, S.H. Effect of electrolyte thickness on electrochemical reactions and thermo-fluidic characteristics inside a SOFC unit cell. Energies 2018, 11, 473. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Chen, J.S.; Li, T.; Andersson, M. Modeling of solid oxide fuel cells with optimized interconnect designs. Int. J. Heat Mass Transf. 2018, 125, 506–514. [Google Scholar] [CrossRef]
- Khazaee, I.; Rava, A. Numerical simulation of the performance of solid oxide fuel cell with different flow channel geometries. Energy 2017, 119, 235–244. [Google Scholar] [CrossRef]
- Akhtar, N.; Decent, S.P.; Loghin, D.; Kendall, K. A three-dimensional numerical model of a single-chamber solid oxide fuel cell. Int. J. Hydrogen Energy 2009, 34, 8645–8663. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Yoon, K.J.; Son, J.-W.; Lee, J.-H.; Kim, B.-K.; Choi, W.; Hong, J. The effect of fuel utilization on heat and mass transfer within solid oxide fuel cells examined by three-dimensional numerical simulations. Int. J. Heat Mass Transf. 2016, 97, 77–93. [Google Scholar] [CrossRef]
- Yakabe, H.; Ogiwara, T.; Hishinuma, M.; Yasuda, I. 3-D model calculation for planar SOFC. J. Power Sources 2001, 102, 144–154. [Google Scholar] [CrossRef]
- Milewski, J.; Świrski, K.; Santarelli, M.; Leone, P. Advanced Methods of Solid Oxide Fuel Cell Modeling; Springer: London, UK, 2011. [Google Scholar]
- Virkar, A.V. Low-Temperature Anode-Supported High Power Density Solid Oxide Fuel Cells with Nano-Structured Electrodes; University of Utah: Salt Lake City, UT, USA, 2000. [Google Scholar]
- Ma, Z. A Combined Differential and Integral Model for High Temperature Fuel Cells. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2000. [Google Scholar]
- He, Z.; Birgersson, E.; Li, H. Reduced non-isothermal model for the planar solid oxide fuel cell and stack. Energy 2014, 70, 478–492. [Google Scholar] [CrossRef]
- Janardhanan, V.M.; Deutschmann, O. CFD analysis of a solid oxide fuel cell with internal reforming: Coupled interactions of transport, heterogeneous catalysis and electrochemical processes. J. Power Sources 2006, 162, 1192–1202. [Google Scholar] [CrossRef]
- Todd, B.; Young, J. Thermodynamic and transport properties of gases for use in solid oxide fuel cell modelling. J. Power Sources 2002, 110, 186–200. [Google Scholar] [CrossRef]
- Wesselingh, J.; Krishna, R. Mass Transfer in Multicomponent Mixtures; Delft University Press: Delft, The Netherlands, 2000. [Google Scholar]
- Liu, S.; Song, C.; Lin, Z. The effects of the interconnect rib contact resistance on the performance of planar solid oxide fuel cell stack and the rib design optimization. J. Power Sources 2008, 183, 214–225. [Google Scholar] [CrossRef]



| Parameter | Symbol | Value |
|---|---|---|
| Length of cell | Lcell | 100 (mm) |
| Reactant channel height | hch | 1 (mm) |
| Reactant channel width | wch | 2 (mm) |
| Interconnect height | hint | 2 (mm) |
| Cell unit width | wcell | 4 (mm) |
| Anode thickness | ta | 0.15 (mm) |
| Electrolyte thickness | tel | 0.1 (mm) |
| Cathode thickness | tc | 0.1 (mm) |
| Operating pressure | p0 | 1 (atm) |
| Operating temperature | T0 | 800 (°C) |
| Equations | Domain | |
|---|---|---|
| Electrochemical model | Electronic and ionic current densities | |
| Actual potential | ||
| The Butler-Volmer charge transfer kinetics equation | ||
| Mass and momentum transport | Fluid flow in the gas channels, | |
| Electrodes | ||
| Species transport | Material transport | |
| Heat transfer |
| Parameter | Symbol | Value |
|---|---|---|
| Operation temperature | 1073 (K) | |
| Reference diffusivity | 3.16 × 10−8 (m2/s) | |
| Viscosity, fuel | 2.4320 × 10−5 (Pa·s) | |
| Viscosity, oxidant | 4.4574 × 10−5 (Pa·s) | |
| Diffusion volume, H2 | 7.07 × 10−6 (m3/mol) | |
| Diffusion volume, H2O | 12.7 × 10−6 (m3/mol) | |
| Diffusion volume, O2 | 16.6 × 10−6 (m3/mol) | |
| Diffusion volume, N2 | 17.9 × 10−6 (m3/mol) | |
| Porosity, anode and cathode | 0.4 | |
| Tortuosity, anode and cathode | 3.8 | |
| Pore size, anode and cathode | 5 × 10−7 | |
| Permeability, anode and cathode | 1.76 × 10−11 (m2) | |
| Thermal conductivity, anode | 11 (W/m·K) | |
| Thermal conductivity, cathode | 6 (W/m·K) | |
| Thermal conductivity, interconnect | 20 (W/m·K) | |
| Thermal conductivity, electrolyte | 2.7 (W/m·K) | |
| Composition, anode | Ni-YSZ | |
| Composition, cathode | LSM-YSZ | |
| Composition, electrolyte | YSZ | |
| Composition, interconnect | Stainless steel | |
| Density, interconnect | 3030 (kg/m3) | |
| Density, electrolyte | 5160 (kg/m3) | |
| Heat capacity, anode | 450 (J/kg·K) | |
| Heat capacity, cathode | 430 (J/kg·K) | |
| Heat capacity, electrolyte | 470 (J/kg·K) | |
| Heat capacity, interconnect | 550 (J/kg·K) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, X.; Guo, Z.; Wang, K.; Cao, Y. Effect of the Reactant Transportation on Performance of a Planar Solid Oxide Fuel Cell. Energies 2021, 14, 1212. https://doi.org/10.3390/en14041212
Wang Y, Li X, Guo Z, Wang K, Cao Y. Effect of the Reactant Transportation on Performance of a Planar Solid Oxide Fuel Cell. Energies. 2021; 14(4):1212. https://doi.org/10.3390/en14041212
Chicago/Turabian StyleWang, Yongqing, Xingchen Li, Zhenning Guo, Ke Wang, and Yan Cao. 2021. "Effect of the Reactant Transportation on Performance of a Planar Solid Oxide Fuel Cell" Energies 14, no. 4: 1212. https://doi.org/10.3390/en14041212
APA StyleWang, Y., Li, X., Guo, Z., Wang, K., & Cao, Y. (2021). Effect of the Reactant Transportation on Performance of a Planar Solid Oxide Fuel Cell. Energies, 14(4), 1212. https://doi.org/10.3390/en14041212






