3.2. Locomotives Powered by Hydrogen Fuel Cells
Currently, there is strong interest in fuel-cell-powered locomotives for sustainable transportation. In
Table 1, a review of the developments during the years 2002–2016 is presented.
Rail transport plays a very important role in providing land transport services. The technological process that is used for rail transport has a significant impact on the rail economy. Consequently, it is also important for the national economy. The condition for the proper development of rail transport is the successive replacement of steam traction by electric traction. The evolution of electric traction depends on the increase in the rate of electrification and modernization of traction. Railway electrification is one of the basic elements of the modernization of railway transport, and it determines the capacity and costs of transportation. Its introduction allows a significant reduction in the operating costs of rail transport and energy and fuel savings [
14].
The dual-mode vehicle can use both diesel and electric propulsion. The new locomotives are powered by electricity on electrified sections of the line, saving fuel and reducing maintenance costs. The locomotive can be switched to diesel mode on non-electrified sections. Thanks to the dual-mode concept, operators can increase their environmental contribution throughout the life cycle of the locomotive.
It is also possible that diesel trains could run on electrified components along partially electrified lines. Vectron Dual Mode combines the best features of two areas: it is equipped with both a powerful diesel engine and an electric drive. Where there is an overhead catenary, the locomotive runs quietly without emissions, saving fuel and maintenance costs. The Vectron Dual Mode is a sustainable, environmentally friendly and cost-effective alternative to conventional locomotives, as highlighted by Sabrina Soussan, Siemens Mobility. Germany’s rail network is currently around 60 percent electrified, and new locomotives from Siemens Mobility can also operate between electrified sections, eliminating the need to change locomotives. This allows agglomerations and metropolises, where rail networks are often electrified, to reduce emissions.
Such trains include regional passenger trains, shunting or mainline locomotives. According to numerous trade magazines, for some prototypes of FCH (fuel cell hydrogen trains—hydrail) passenger trains, in recent years, their operating principle was not only developed but also tested. The interest in this technology demonstrated by many public transport authorities (PTAs), both in the EU and abroad, has led to procurement in Austria and Germany for small vehicle doughs to be put into service between 2021 and 2023. The goal is to use fuel and hydrogen in the railway environment. Current special technology allows for a zero-emission and cost-competitive solution to replace diesel trains.
The EU’s goal is a decarbonization method for the production of hydrogen and 33% of green hydrogen in the energy mix in 2050, integrating the EU energy system into a more circular energy system, where energy is not wasted and where energy efficiency is paramount. An important element is also the decline in the prices of RES (renewable energy sources) installations, which will increase their share in the energy mix. Another important aspect is also the promotion of renewable and low-emission fuels, including hydrogen, where electrification and decarbonization are difficult, e.g., in heavy transport and industry. The key sectors of the Polish hydrogen strategy are: transport (urban, rail, truck), energy storage (using the surplus from renewable energy peaks to produce hydrogen), energy-intensive industry (replacing coal/natural gas with hydrogen) and heating (hydrogen mixed with natural gas, from electrolysis). Today, attention is also drawn to the European Common Interest Projects (IPCEI), which are one of the EU’s key instruments to strengthen the potential of European industry in the context of global economic competition. The IPCEI procedure allows Member States to subsidize international projects with high development potential whose value chain is organized in the EU. Within the framework of IPCEI, the entire value chain is designed, i.e., hydrogen production, transport, storage, hydrogen cells for vehicles, industrial use [
15,
16,
17,
18,
19,
20].
Examples of challenges in the areas of climate, energy and mobility include strengthening the European value chain for the near-zero carbon footprint of hydrogen and fuel cells, which offers a major decarbonization pathway for energy, transport and industry. On many aspects, we should also mention a joint venture for fuel cells and hydrogen technologies that supports research, technological development and demonstration activities aimed at accelerating the market introduction of hydrogen technologies, using their potential as an instrument to achieve carbon clean energy [
21,
22,
23,
24,
25,
26,
27].
An alternative to many environmental problems is hydrogen technology (
Figure 2).
The use of waste (low-cost materials) and naturally occurring raw materials can help to implement the concepts of “Green Chemistry”. The obtained hydrogen may be directly utilized in both hydrogen cell systems and internal combustion engines due to the lack of further conversions. The ability to control the microstructure and morphology allows us to obtain a material with specific properties.
Hydrogen in the industry is already broadly used and the resulting technological achievements are becoming more and more interesting (
Table 2).
In addition to the use of hydrogen in the food, chemical and metallurgical industries, it is widely used to produce fuel. In relation to its mass, it has a very low heat of combustion (141.9 MJ/kg) and a huge calorific value of 120 MJ/kg. For the above-mentioned reasons, this element has become the basic component of rocket engine exhaust mixtures, and today, it is beginning to dominate the automotive market. The term “hydrogen economy” has become widely used, which carries information about the positive ecological and economic aspects of using this element as an energy source. Due to the development of civilization, the need to supply energy is constantly increasing. Despite the high hydrogen content in the universe, natural deposits do not exist. Scientists are currently seeking the most efficient way to obtain this highly energetic element, which will meet, above all, the economic requirements, such as low price, simple construction of the necessary devices and high efficiency of the synthesis process.
Hydrogen is one of the most efficient fuels, and its combustion produces only water vapor, which has a positive effect on the Earth’s atmosphere. By using this type of energy source, it is possible to reduce greenhouse gas emissions and the amount of acid rainfall. However, ecological problems still exist during the hydrogen production process itself. The solution is biohydrogen. The prefix “bio” comes from the biological methods of producing this gas. However, an amendment should be made to the strict classification of methods. This high-energy gas is also generated in biomass gasification and biogas conversion processes, which are based on chemical processes. Microorganisms take part in bioprocesses, so the two above-mentioned chemical methods can be included in the biological methods of hydrogen synthesis due to the bacterial activity in the inevitable stages of creating the substrates for ecological fuel.
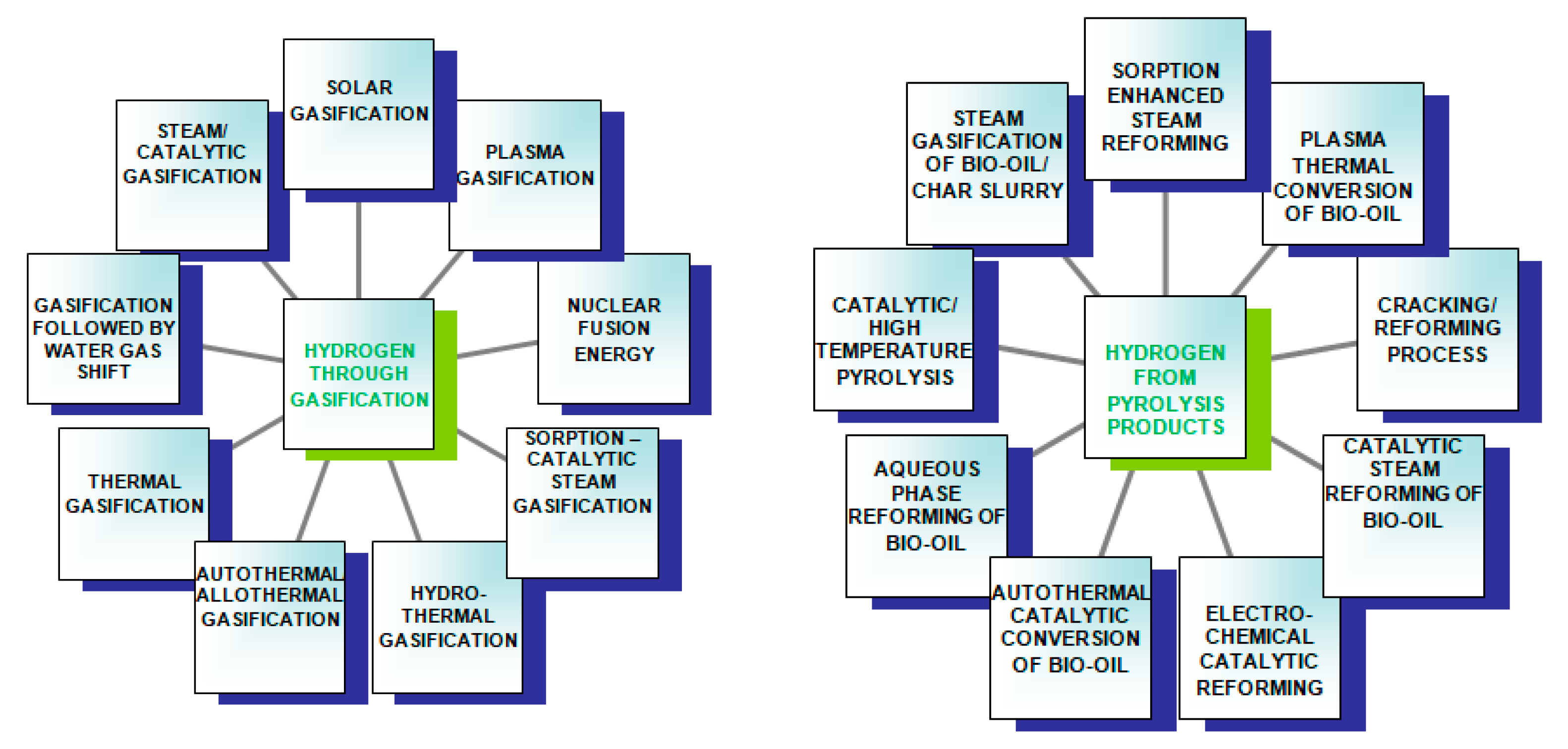
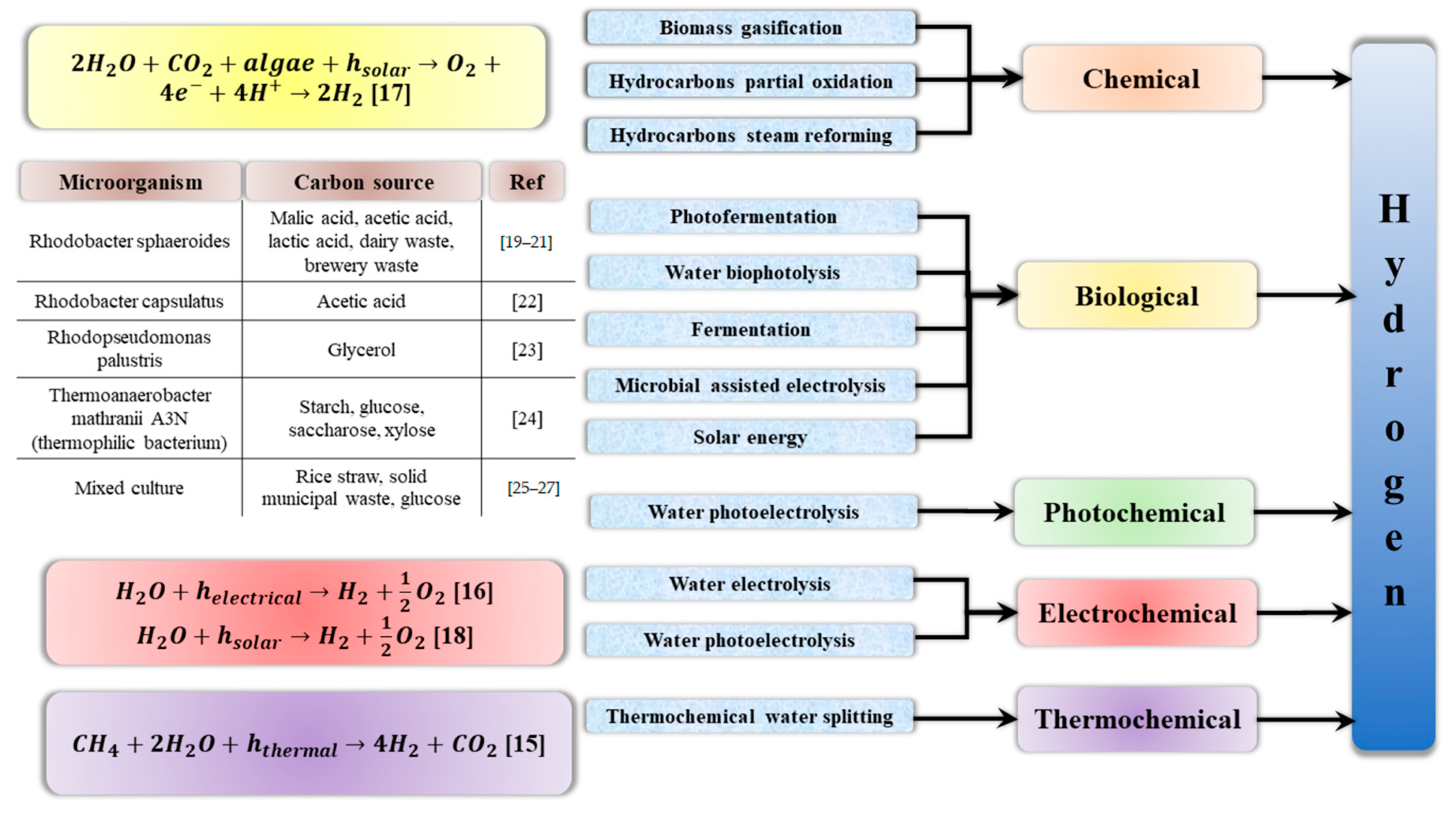
A diagram of the hydrogen production methods is shown in
Figure 3.
Although industrial hydrogen can be obtained from water, hydrocarbons and even biomass, currently, as much as 48% of its production is based on methane-reforming using steam, 30% comes from refining crude oil, 18% is released during coal transformations, e.g., in the Haber–Bosch method, and only 4% is the product of water electrolysis [
29,
30,
31,
32,
33]. Its importance as an energy source grows year by year. The most popular methods of obtaining hydrogen are, unfortunately, still based mainly on exhaustible deposits, such as crude oil. Additionally, fossil fuels are causing global warming to increase by emitting greenhouse gases.
In this respect, biotechnology holds promise, offering both economic and ecological solutions. The production of hydrogen by microorganisms, known as the biosynthesis process, could represent a turning-point in the field of automotive and environmental protection.
Hydrogen synthesis by biodegradation and biotransformation methods usually involves the use of anaerobic bacteria, photosynthetic bacteria, etc., to convert organic material into gas. If this organic degradation of wastewater or other biomaterials can be carried out for energy generation, we achieve double success. The entire process is fully in line with the principles of environmental protection and recycling and can additionally be economically efficient [
34]. H
2 biosynthesis occurs in various types of biochemical changes; in this respect, renewable energy sources are important. Renewable sources are never-ending and carry many more benefits than losses. For these reasons, biotechnologists are currently focusing on increasing the efficiency of biohydrogen synthesis. Gasification of biomass entails the necessity of separating hydrogen from other products formed in the reaction, the use of catalysts and high pressure or temperature. In the process of water electrolysis, high-purity hydrogen is produced, but this method is quite energy-intensive and therefore very expensive and unprofitable [
35]. The use of bacteria to synthesize hydrogen can solve ecological and economic problems, allowing the cheap and fast production of high-purity H
2. The conditions that are necessary for the synthesis of this gas by microorganisms are not too high. The optimal room temperature is usually the room temperature and the natural atmospheric pressure, as well as water conditions. The methods of biohydrogen synthesis known so far include: direct photolysis of water with the use of cyanobacteria or algae, decomposition of organic compounds using sulfur-free purple bacteria and fermentation or bioelectrochemical processes, such as bioelectrolysis [
33]. Hybrid combinations of the above-mentioned methods are also used. The greatest economic predisposition is shown by biological methods such as biophotolysis or dark fermentation, as well as the use of coupled dark and photofermentation [
36].
Ecological methods of producing energy and processing organic matter are becoming more and more important in industry. One of the more interesting methods of obtaining biohydrogen as an alternative energy source is the fermentations described in the previous chapters: light and dark, especially compressed together. Another efficient way to synthesize this high-energy gas is biomass processing. Biomass is a general term that defines the organic material formed during the photosynthesis of green plants, including algae, trees and crops [
37]. The use of biomass for the production of hydrogen is highly profitable due to the use of already existing organic matter as a raw material. An important aspect is also the fact that waste is utilized in biomass conversion processes, during which energy is produced. Agricultural crops (sugar beet and oilseed crops) are used as sources of biomass, ligno-cellulosic waste such as wood and wood waste, aquatic plants such as algae and aquatic weeds, industrial or municipal waste and animal waste [
38]. It is worth noting that many types of agri-food waste contain starch or cellulose. The complex nature of these carbohydrates can adversely affect their biodegradability. Starch is hydrolyzed to glucose and maltose by acidic or enzymatic hydrolysis, and these carbohydrates are further converted into organic acids and then into hydrogen gas. Agricultural waste containing cellulose requires more extensive pretreatment. It should be ground and, prior to fermentation, delignified by mechanical or chemical means [
39].
Graphene itself is not magnetic, but in combination with hydrogen, it represents an additional source of magnetic energy.
Magnetic moments are formed during hydrogenation, when atoms attach to the carbon in the honeycomb carbon layers. Honeycombs are subnetted: the moments align ferromagnetically (blue arrows) when two hydrogen atoms are on the same subnetwork and antiferromagnetically when they are on the opposite lattice (orange arrow). It is the hydrogen atoms that induce magnetism in graphene, which is applicable to the production of universal magnetic ordering within a one-dimensional material. Graphene itself is not magnetic, but in combination with hydrogen, represents an additional source of magnetic energy. It is possible to manipulate the movement of hydrogen by activating and deactivating the magnetic field. When we have a variable magnetic field (movement on a micro scale), a vortex electric field and a reverse one (Maxwell’s theses) are always created around the field, which becomes a source of electricity. Each movement of electrons in a conductor is accompanied by an alternating magnetic field. These fields interpenetrate and move in space—these are electromagnetic waves that travel at the speed of light. Since the arrangement of these hydrogens can be manipulated, the magnetic field can be activated further; that is, the efficiency of the adsorption and desorption process on the same halfnets can be increased if an adsorption and desorption process occurs instead of the absorption process.
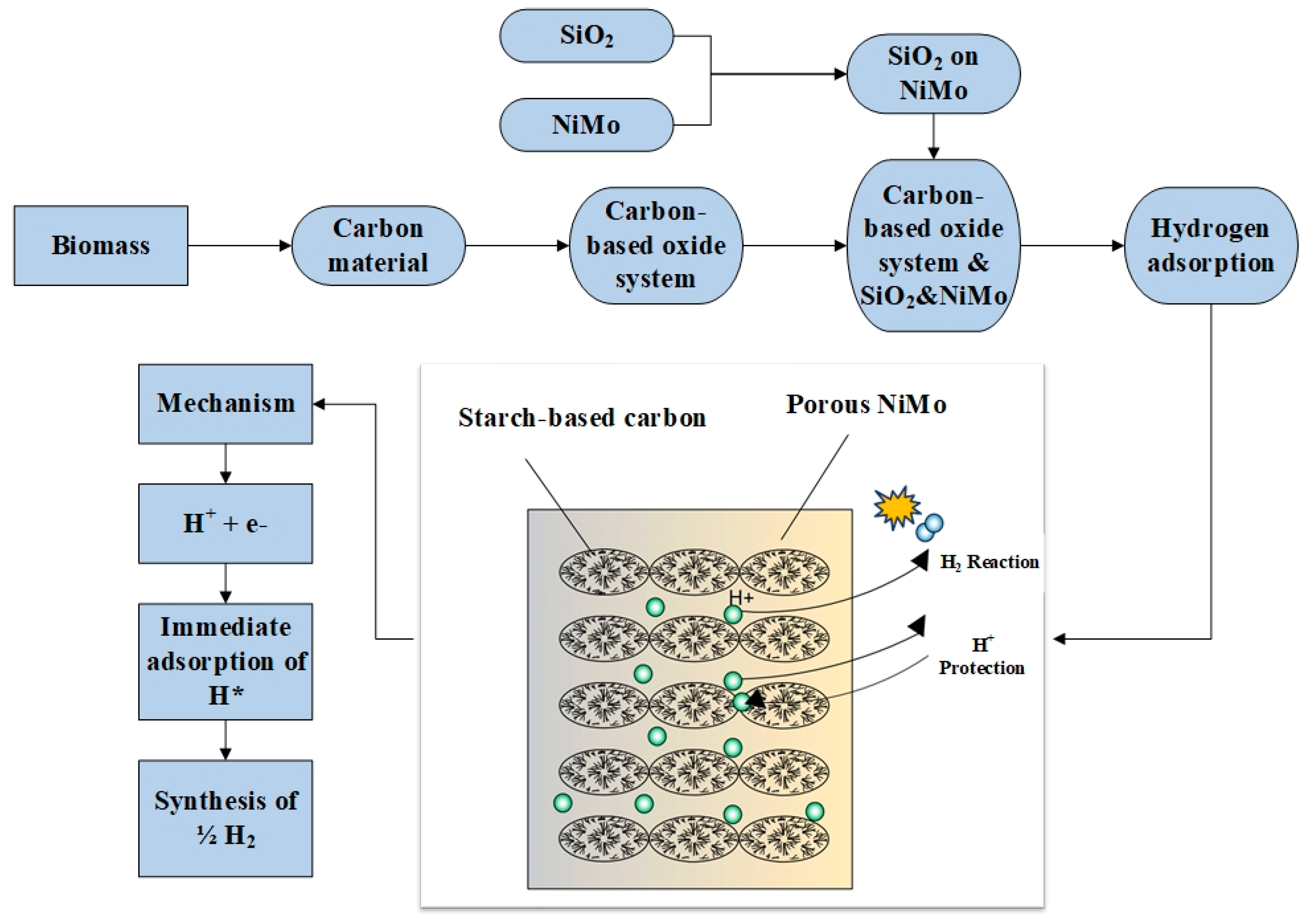
The planned process will be conducted so that adsorption and desorption do not require high temperatures and are as close to room temperature as possible. The diagram is a simplification to visualize one of the materials used. The desired structure is honeycomb clusters. One of the methods of obtaining hydrogen is the adsorption process (
Figure 4).
In the adsorption method, it is proposed to use an acid electrolyte, which may be sulfuric acid (VI), which is the source of the H
+ proton.
Figure 4 shows the structure of the electrochemical system: NiMo composition is responsible for the porosity; the deposited carbon acts as an adsorbent; the non-porous area of the carbon protects against corrosion from electrolyte protons, while silica, along with NiMo and the graphene structure, is responsible for increasing porosity. The method’s innovations include the use of natural carbon, the high activity of the system and the differentiation of functional groups by the oxygen content in the structure. The advantages of the method are protection against NiMo dissolution in the electrolyte, preservation of activity at the proton source and, above all, efficient hydrogen production.
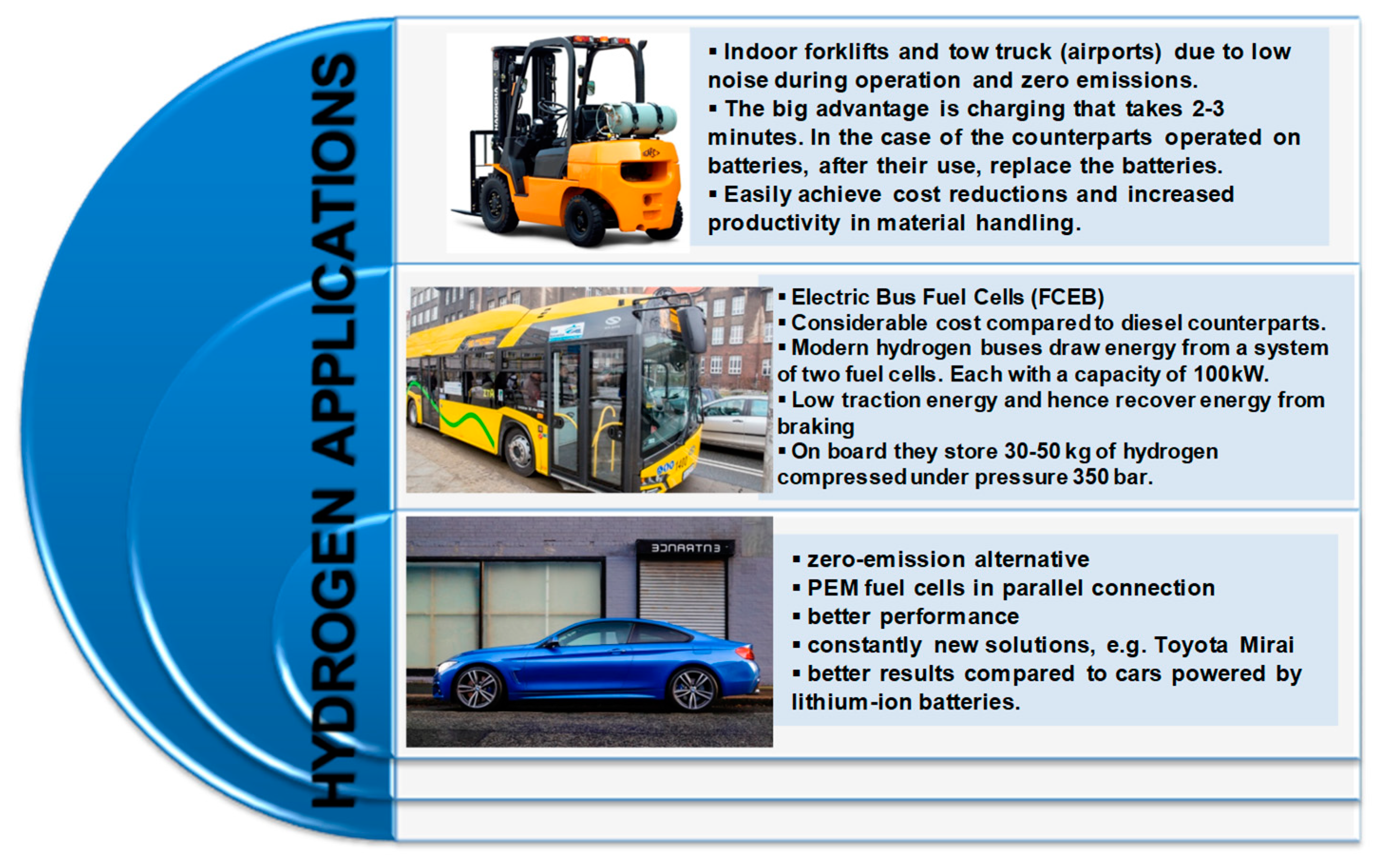
Figure 5 shows hydrogen applications; the most common are aircraft, planes, on-board power supply, electric locomotives, indoor forklifts and electrobuses.
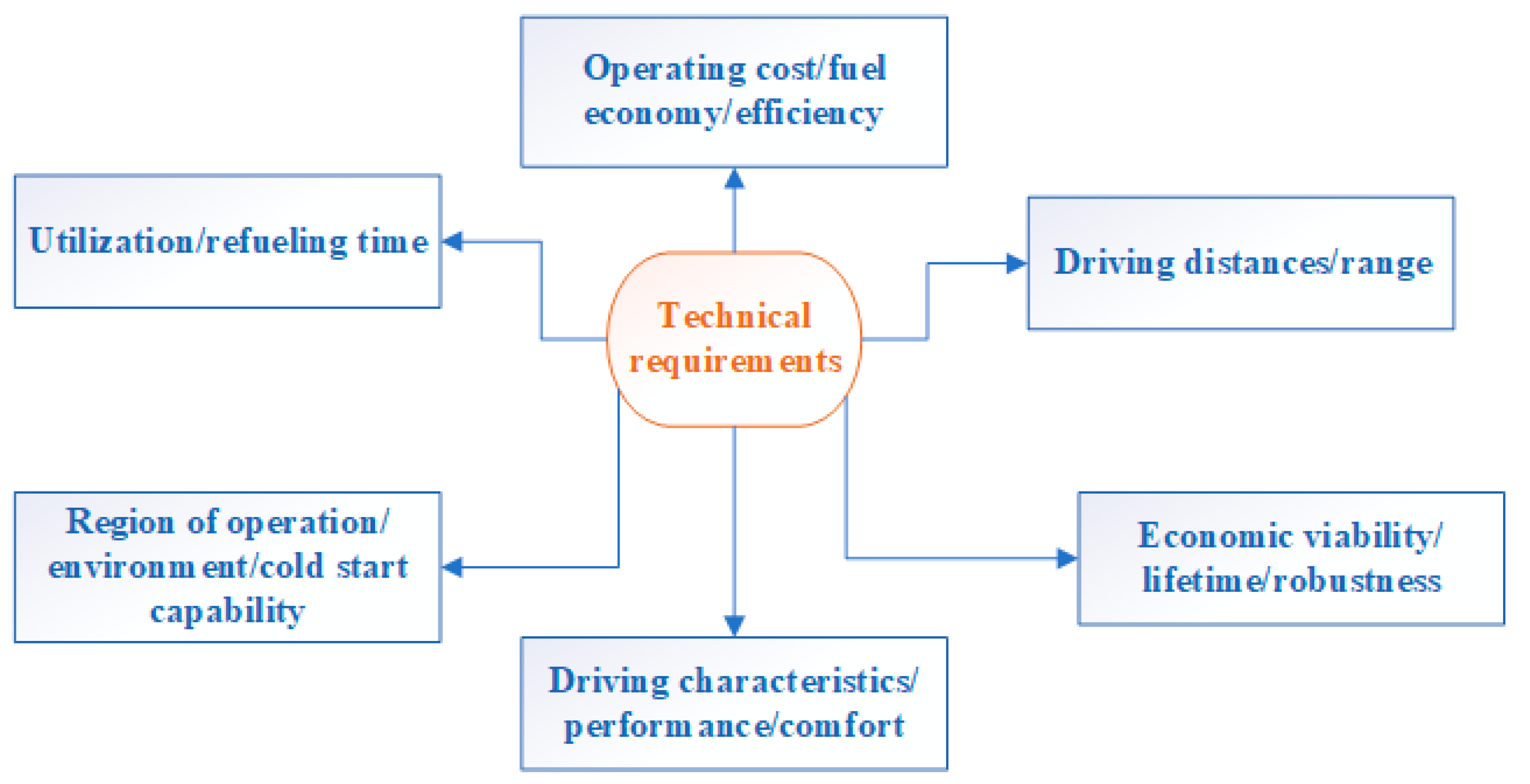
The eco-revolution in the automotive industry has been taking place for several years. Manufacturers, forced by restrictive EU regulations on the permissible level of exhaust emissions and the changing expectations of conscious customers, are working intensively on the development and laborious introduction of alternative power sources for passenger cars and trucks to the world markets [
40,
41,
42]. The problem is still the limited functionality of hybrid vehicles and a modest network of fast charging points dedicated to electric cars. Hybrid cars are most efficient only in urban conditions. Where frequent starts, stops and long and slow driving in traffic jams are common, the cooperation of the combustion engine and electric engine offers the greatest benefits and results. Electric energy stored in batteries during braking, operation of an internal combustion unit or charging from a household socket is used only for covering several or several dozen kilometers in city traffic in zero-emission mode.
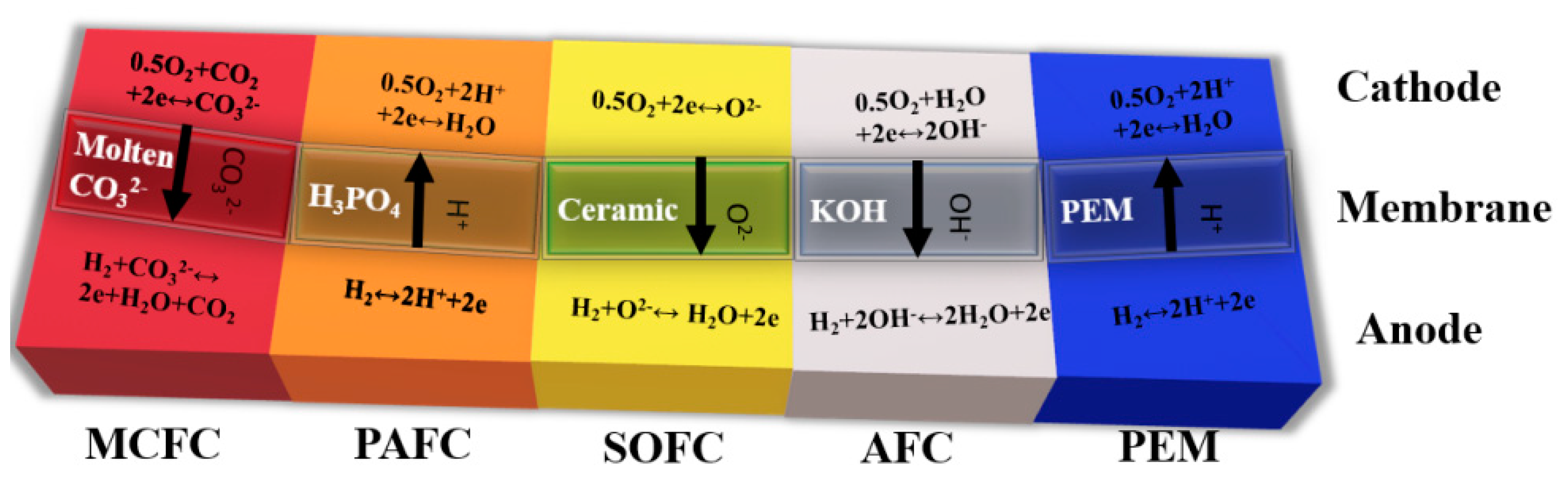
One type of alternative fuel propulsion system is the so-called hybrid systems. These use fuel cells instead of an internal combustion engine. The division of fuel cells reflects the type of electrolyte used. Thus, it determines at which temperatures the reactions in the cell occur. Fuel cells differ in the structure of the materials used in their construction and the efficiency of energy generated depending on the type of fuel. Any type of cell has both advantages and disadvantages.
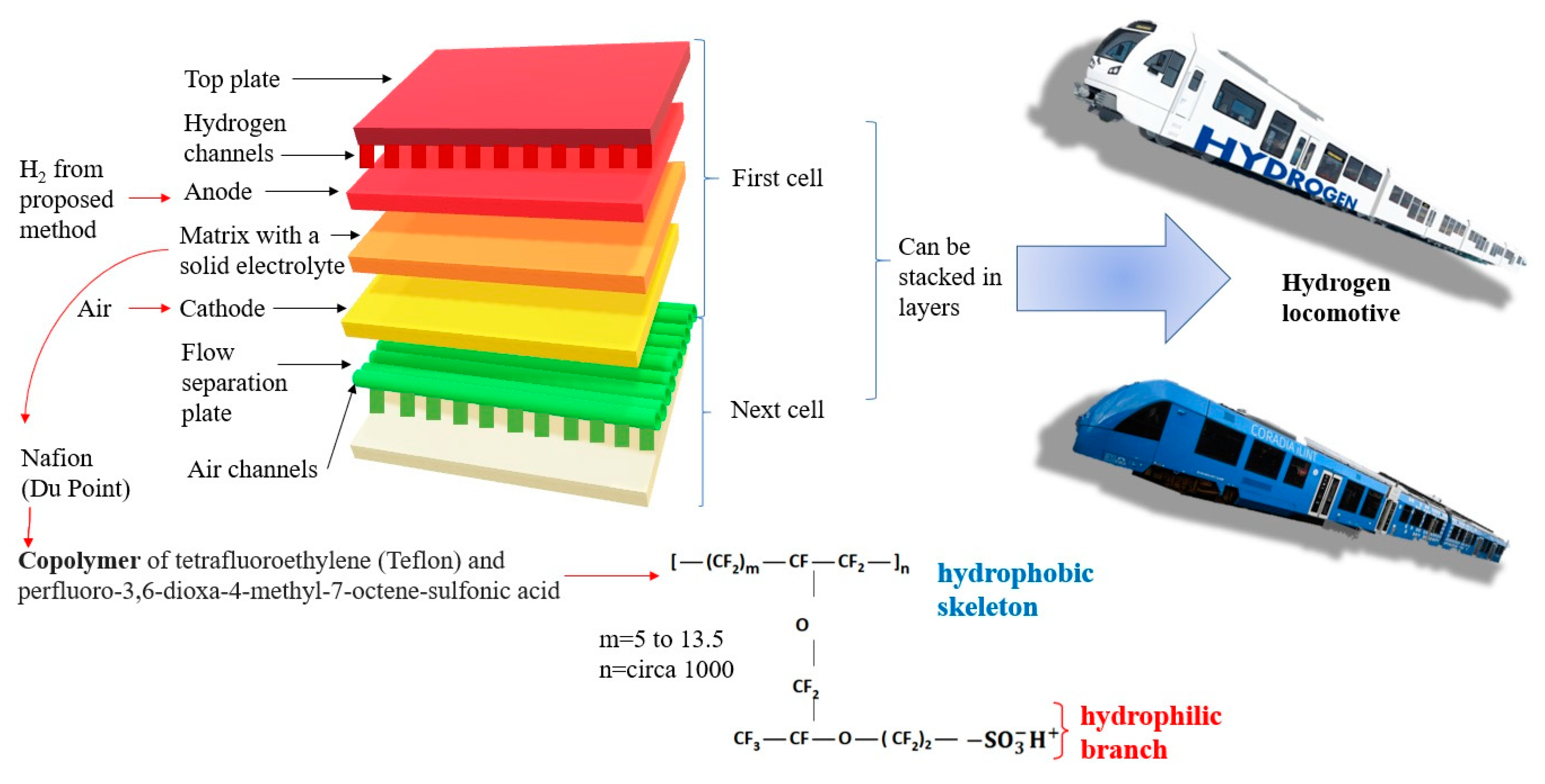
PEM fuel cells (proton exchange membrane or polymer electrolyte membrane) are driven by hydrogen with high purity. The fuel cell consists of two electrodes: anode and cathode. The electrodes are separated by electrolyte in liquid or solid form. Classification criterion cells are a type of electrolyte and, in the case of a PEM cell, the electrolyte is a special membrane that allows only positive hydrogen ions (protons) to pass through, hence the abbreviation PEM, from the English name Proton Exchange Membrane Fuel Cell. The electrolyte allows the flow of cations but prevents the flow of electrons. The chemical reaction that takes place in the cell consists of breaking the hydrogen into a proton and an electron at the anode, and then joining the reactants at the cathode. Electrochemical processes are accompanied by the flow of an electron from the anode to the cathode, bypassing the impermeable membrane. The electrochemical reaction of hydrogen and oxygen produces electricity, water and heat. The fuel—pure hydrogen or hydrogen mixed with other gases—is fed continuously to the anode, and the oxidant—pure oxygen or a mixture (air)—is fed continuously to the cathode. Nafion, which is a polymeric material, acts as a membrane. It is characterized by high efficiency in electricity production (up to 65%) and a slightly exothermic process. These aspects result from the low reaction temperature in the chamber of −60–100 °C.
In an alkaline fuel cell (AFC), the electrolyte is an aqueous alkaline solution, most commonly potassium hydroxide (KOH). The cell, in which 85% KOH solution is used, works at a temperature of 250 °C, while if the concentration of KOH solution is 35–50%, the cell operating temperature is lower, at 120 °C. The catalyst of the reaction can be nickel (Ni), silver (Ag), tungsten compounds, metal oxides or precious metals (e.g., platinum). The anode is a mesh or porous nickel with a maximum pore diameter of 16 μm on the electrolyte side and 30 μm on the gas side, while the cathode is porous lithium nickel oxide NiO with Ag. The fuel is hydrogen (H2) and the oxidant is oxygen (O2). Efficiency is 50% at an ambient temperature of 20 °C.
DMFC (Direct Methanol Fuel Cell) has a polymer membrane such as PEM. The difference lies in the construction of the anode. In the DMFC cell, internal reforming of methanol is allowed. Here, the as-obtained hydrogen is used to power up the cell. There are also no difficulties with fuel storage. It is also practical for mobile use due to the low reaction temperature (approx. 80 °C). The DMFC cell has lower efficiency compared to the PEM cell (equal to 40%).
PAFC (Phosphoric Acid Fuel Cell) cells are used to form electricity and heat cogeneration systems. The efficiency of electricity production is the same as in the DMFC. Moreover, the water vapor produced may be converted into heat. The electrolyte in the PAFC cell is phosphoric acid (H3PO4). The advantage of the cells is high tolerance to carbon monoxide. This allows the application of different fuels (however, the desulphurization process of fuel is significant).
In MCFC (Molten Carbonate Fuel Cell), the electrolyte is Li/K carbonate (in a molten state). These are high-temperature fuel cells that operate at temperatures of 600 °C and above. Molten Carbonate Fuel Cells (MCFCs) are currently being developed for natural gas, biogas (produced by anaerobic fermentation or gasification of biomass) and coal-based power plants for electrical utility, industrial and military applications. MCFCs are high-temperature fuel cells that use an electrolyte consisting of a molten mixture of a carbonate salt suspended in a porous, chemically inert ceramic matrix of beta-alumina solid electrolyte. Since they operate at extremely high temperatures of 650 °C and above, base metals can be used as anode and cathode catalysts, reducing operation costs.
In SOFC cells (Solid Oxide Fuel Cell), the separator is created with oxide ceramics. The temperature range of operation is high and amounts to 650–1000 °C. As a result, in cogeneration systems, they achieve high efficiency of electricity and heat (up to 85%). The disadvantage is the start-up and shutdown time of the cells, which translates into their use in stationary CHP systems (heat and energy cogeneration). SOFCs show a high tolerance to fuel pollutants: carbon monoxide and sulfur compounds.
Considering the operational parameters of the cells in terms of their application as the power supply systems of transport drive systems, it is important to consider high efficiency in generating electricity and the short start and stop times of the cell.
Among the presented fuel cells, PEM and AFC cells have properties that allow them to be used in future drive modes of transport. Their efficiency in generating energy is in the range of 35% to 70%, and the working temperature for PEM cells is 60–100 °C and for AFC cells 100–250 °C (
Table 3). These cells are powered by hydrogen; therefore, if we wish to use methanol as the fuel supplying the cell, it is necessary to use additional external reforming systems. In terms of energy parameters and the possibility of supplying various fuels, SOFC cells are an interesting solution. In their application, however, there is an operational problem related to the required continuity of work.
In recent years, the process of burning fossil fuels has been criticized due to the emission of greenhouse gases, especially carbon dioxide. The energy industry is seeking alternative, environmentally friendly energy sources. The combustion of hydrogen in heat engines does not generate harmful emissions of carbon monoxides and hydrocarbons (apart from the inevitable burning of some of the lubricating oil), but still produces nitrogen oxides due to the high combustion temperature. One solution to the problem of nitrogen oxide emissions is the use of fuel cells capable of converting the chemical energy of hydrogen into electricity without combustion in the working chamber. To properly control the working process of the fuel cell, it is necessary to properly control not only the fuel (hydrogen) dosing but also the oxygen (air) dosing.
Currently, the most promising and most intensively developed type of fuel cells is a PEM (Proton Exchange Membrane) cell, which provides results an order of magnitude greater than other fuel cells, with the exception of the highly advanced Alkaline Fuel Cells (AFC) used in space technology. PEM cells can be powered by pure hydrogen or reformed hydrocarbon fuel. Cold weather capability allows them to get started quickly. The very small thickness of the membrane–electrode assembly allows for a compact construction of this type of cell. Another advantage is the lack of chemically aggressive fluids that can cause corrosion and the fact that the cell can operate in any geometric orientation. These advantages confirm the possibility of using PEM fuel cells for both automotive and portable applications.
The high involvement of research teams has resulted in a reduction of costs so that PEM cells are currently the cheapest of all types of cells.
PEM fuel cell stacks are also used in commercial car drives. The acid membrane, which has the ability to conduct protons, consists of a Nafion material. As there are electrochemical reactions in the fuel cell, it is important to use a catalyst (platinum black). The platinum black catalyst material is deposited on carbon paper or fabrics that are positioned on both sides of the cell. The disadvantage of the platinum anode is the aggressive reaction of carbon monoxide with the catalyst—it lowers the catalytic properties.
Figure 6 shows the reactions taking place both at the cathode and at the anode (hydrogen gas is fed to the anode of the fuel cell, and humidified air passes through the cathode).
The disadvantages of virtually all types of fuel cells include:
The production of fuel cells is expensive due to the use of expensive construction materials with specific properties. Cheap substitutes for these materials are difficult to find;
Fuel production technology is expensive and complex and requires additional energy;
When using fuels other than hydrogen, the operating efficiency gradually decreases. This is caused by electrolyte degradation and poisoning of catalysts.
The great challenge in this type of fuel cell is heat and water management. A significant increase in the temperature of the cell itself was observed as a result of the occurrence exothermic reactions. PEM fuel cells are widely used in the transportation and automotive industries.
SOFCs have the advantage that they can use diesel fuel to power them without having to process fuel to obtain pure hydrogen. The electrical efficiency of a conventional on-board generator in a vehicle is around 10–15%. The electrical efficiency of the SOFC cell is currently around 30%, which is twice the standard solution. Emission of harmful compounds and noise are lower than in the case of operation of propulsion systems powered by internal combustion engines. The overall efficiency of the system is higher due to the heat generated by the operation of the fuel cell. One of the solutions obtained as part of the work carried out in this area is a study presented jointly by AVL and NISSAN as an SOFC Auxiliary Power Unit (SOFC APU). This is a solution based on the use of the SOFC as a battery charging system (with a maximum energy of 24 kWh) Li-Ion in a vehicle with its own ms of up to 3.5 tonnes. The e-NV200 model uses a 5 kW SOFC cell powered by ethanol or a mixture of ethanol and water (55% water, 45% ethanol). As a result of internal reforming of the supplied fuel, hydrogen is obtained, which feeds the cell. The range of a vehicle equipped with such a support system solution is around 600 km. The disadvantage of this solution is the production of carbon monoxide during methanol reforming. Its presence is associated with the efficiency of the methanol steam reforming process and is around 70–80%. The presence of carbon monoxide is unfavorable due to the impact on the cathode surface, which, when switching off the cell, can lead to its so-called oxide treatment, which, with the repeated process of starting and stopping the cell, causes deterioration of its efficiency. Ascend Energy together with the manufacturer of SOFC Atrex Energy cells offer a tubular fuel cell for ATVs (all-terrain vehicles). The cell uses a conventional hydrocarbon fuel such as natural gas, propane or LPG without an external reformer system. No hydrogen storage is therefore required in the vehicle. The cell operates at a temperature of 600–800 °C, generating 4.5 kW.
The components of a polymer stack fuel cell are presented in
Figure 7. This figure also shows the structure of Nafion, which is the most commonly used solid electrolyte and PEM membrane.
Despite the global energy demand, the willingness to protect the environment from pollution has made hydrogen a realistic alternative to traditional fossil fuels. Hydrogen is the most abundant element in the universe, and therefore is an endless and renewable energy source [
43,
44]. In addition, hydrogen may be obtained from renewable and sustainable resources, making it environmentally hopeful. Hydrogen is also produced from biomass and can be a low-cost approach thanks to the use of supercritical water gasification [
45] and fermentation methods [
43]. This fuel could also be obtained using solar energy [
46,
47,
48]. Hydrogen has the highest gravimetric energy density (among non-nuclear fuels) and can easily be transformed into heat, mechanical and electrical energy [
49]. A promising way to use electricity and heat without impacting the environment is their use in both stationary and automotive applications. This paves the way for new possibilities in the field of sustainable energy use worldwide [
49,
50,
51,
52].
It is also worth mentioning other technical solutions which have been widely analyzed.
In [
53], the authors presented an energy management strategy for hybrid electric buses (HEB). The main topic was thermal stability and driving costs, while the degradation of the lithium-ion battery (LIB) was also focused on. There is an emphasis on multilateral indicators, so the least costly and comprehensive pathways are presented to focus on increasing penalties and the cost of LIB degradation and overheating.
The authors of [
54] propose an energy management strategy based on machine learning for HEB. The Deep Deterministic Policy Gradient (DDPG) algorithm was used to increase the cold start efficiency and optimize HEB power allocation. It is linked to the expert help system. As in [
1], penalties are applied for overheating and degradation of the battery. This method is used in various road missions to show that it is superior in terms of maintaining adequate performance, efficiency and optimization.
It should be noted that there are many other modern methods of energy management in HEB. This includes a combination of deep support learning (DRL) and transfer learning (TL). This is to remove DRL defects. In the first step, the optimization of the hybrid tracked vehicle is modeled. This is done by placing complex components of the driveline. An energy management strategy (EMS) needs a two-tier control framework. There are two levels: higher and lower. Higher requires the Deep Deterministic Policy Gradient (DDPG) algorithm. With this method, it is possible to control the EMS at different speed intervals. The TL method is used to create the appropriate drive cycle by transforming neural networks (lower level). After a series of tests, it was confirmed that the submitted control policy with DRL and TL enabled is able to increase energy efficiency and improve the efficiency of the entire system [
55].
3.4. Gasification
Biomass is a source of primary energy consisting of all substances of plant and/or animal origin that are biodegradable and whose use for energy purposes is not restricted by law. Biomass is used often for the generation of heat and biofuels.
Taking into account the current trends in technologies using biomass for energy purposes (relatively low conversion efficiency), it can be assumed that, in the future, the use of cogeneration (electricity and heat production in one process) and trigeneration (electricity, heat and cold energy production in one process) can significantly improve the management of biomass resources.
Biomass is primarily used to produce heat and biofuels. Biomass gasification generally refers to the thermochemical conversion of solid fuels from biomass using a gasifying agent (e.g., vapors, air (partial oxidation) or CO
2) to a mixture of flammable gaseous products including H
2, CH
4, CO and CO
2. Gasification is one of the most efficient ways of extracting energy from fuel sources and converting it into a usable form by converting solids partially or completely into gases. It is an energy conversion process that has been explored as an alternative solution to environmental problems related to energy production [
65]. Gasification of biomass is the earliest and most economical way to produce renewable H
2. Biomass is used for energy purposes in the processes of direct combustion, co-combustion of solid biofuels, converted into liquid fuels or subject to gasification. The conversion of biomass into energy carriers can be achieved by one of three methods: physical, chemical and biochemical. Depending on the dominant output of this process, which may be gas, liquid fuel or solid fuel, this process is called incineration, co-incineration, gasification, pyrolysis or a biochemical process. Combustion is used for the production of thermal energy as well as for the production of electricity and is the most common and, at the same time, the simplest form of obtaining energy from biomass. Worldwide, as much as 90% of energy obtained from biomass is generated in the combustion process, and biomass can be burned in all states of matter. In order for wood to be burned as efficiently as possible, while meeting environmental standards, it is recommended that it proceed in three phases: drying and degassing of wood material, resulting in the formation of wood gas, wood gas combustion at a temperature of 1200 °C, gas afterburning and heat dissipation in exchanger.
For ecological, efficient combustion of biomass in order to obtain energy, specifically designed boilers are used, equipped with combustion chambers with fixed or movable grates and characterized by an increased heat exchange surface. The effectiveness of the combustion process depends on the amount of air supplied. In modern boilers, the combustion air is supplied in the form of so-called primary and secondary air. Primary air is mixed with fuel and used in the gasification and combustion of charcoal. Secondary air, on the other hand, is used for the combustion of volatile substances and it does not mix with the primary air. Combustion installations can be used to utilize various types of biomass, including lump wood, wood chips, sawdust and straw. Due to the physico-chemical properties of biomass, appropriate technological solutions should be used, tailored to the specific type of fuel. The greatest complications are the differences in the proportions of solid and volatile compounds in wood and fossil fuels (hard coal, brown coal, coke) and high biomass moisture.
Gasification of biomass is a two-stage pyrolysis method. It is obviously followed by gasification. Pyrolysis is the decomposition of biomass raw material under the influence of heat. This step, also known as degassing, is endothermic. In this process, 75% to 90% of the volatile materials in the form of gaseous and liquid hydrocarbons is generated. The remaining non-volatile material with a high carbon content is called char [
66] (
Figure 9).
The volatile hydrocarbons and char are subsequently converted to syngas in the second step—gasification. A few of the major reactions involved in this step are listed here [
66]. In the presence of an oxidizing agent at high temperature, the large polymeric molecules of biomass decompose into lighter molecules and eventually to permanent gases (CO, H
2, CH
4 and lighter hydrocarbons), ash, char and tar and minor contaminants. Char and tar are the result of the incomplete conversion of biomass. Several methods of biomass gasification are described in the following sections.
Miller et al. [
67] created the entire system for the locomotive (Vehicle Projects LLC) described earlier. The proposed system layout is shown in
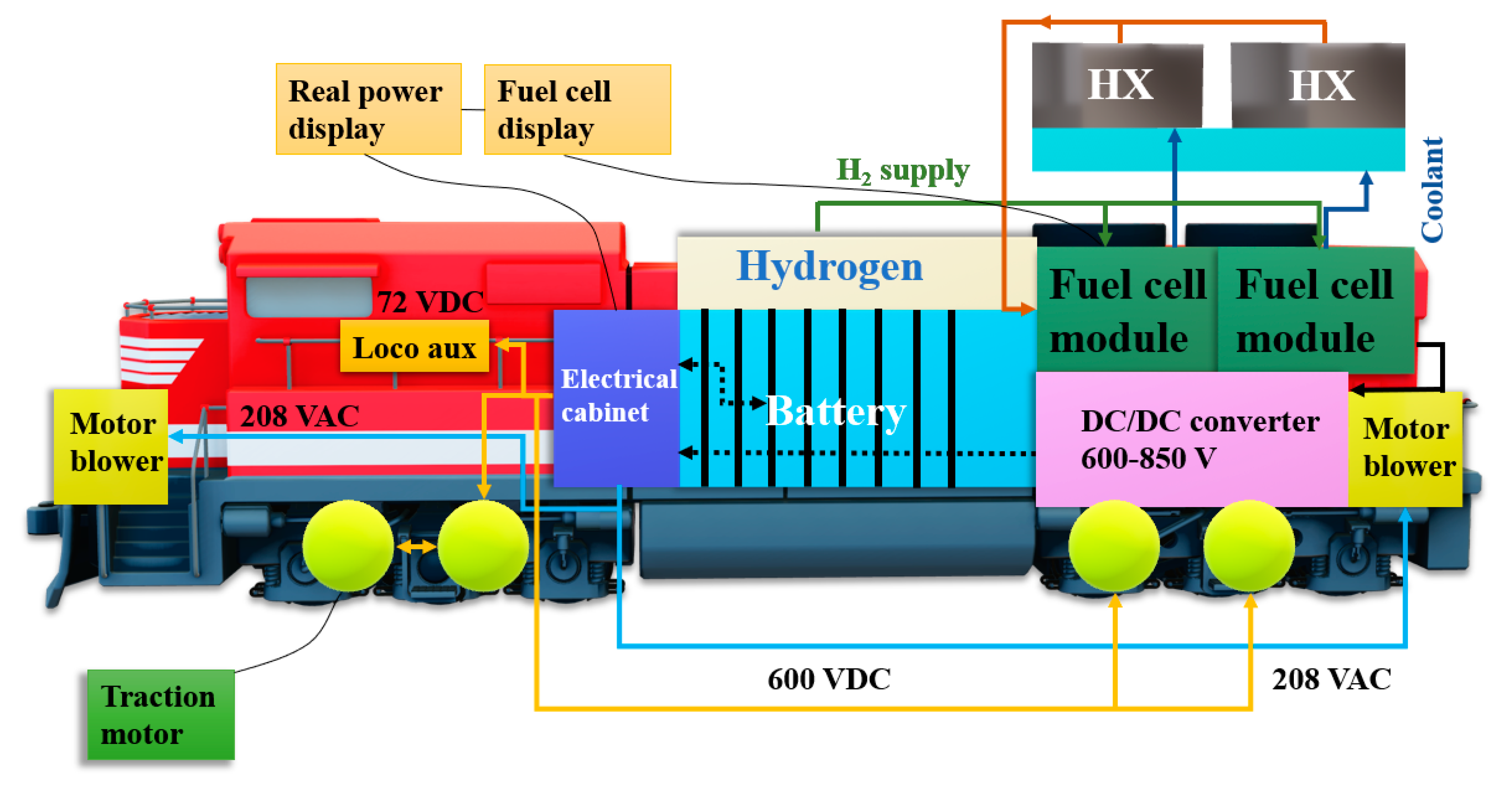
Figure 8. First of all, the work cycle of the switch locomotive was taken into account in order to investigate the power demand as a function of time during various modes of the locomotive operation.
In the next stage, the vehicle system was addressed with subsystems embedded in the vehicle, hydrogen storage and cooling, and finally, the control system was focused on. It demonstrated 300 kW gross and 624 VDC.
Hoffrichter et al. [
68] focused on hydrogen propulsion for use in building a hybrid locomotive. The idea involved the use of PEM fuel cell technology, which used hydrogen fuel to generate the power used to power batteries or traction motors. Lead acid batteries were used. The prototype was operated and tested at various speeds from 2 to 10 km/h. It was found that the efficiency of the power system was in the range of 2–40%. The locomotive prototype was designed with a power system that used fuel cell energy with near-average energy demand and battery power when power demand was high.
Peng et al. [
69] also focused their research on a hydrogen-powered locomotive. Their research, however, was aimed at reducing the environmental impact of traditional technologies.
Tests of the developed prototype were carried out in China.
The advantage is that they are not harmful to the environment and have relatively low infrastructure costs.
It is well known that hydrogen fuel storage uses readily available equipment and proven safety systems. First, by mounting the two modules, the core is mounted above the traction battery (see
Figure 10). Each has seven carbon/aluminum tanks with specific specifications. Fuel is supplied by the system in cyclic operation for around 9 h. The system is designed to have a tank with a relief valve, a pressure limiting device (PRD), a thermocouple (or other temperature sensor) and a solenoid valve. A safety element has been introduced so that when the conduit between the tank and the distribution manifold is broken, the tank’s overflow valve will close. Limited operation of the battery at elevated temperature, which may self-ignite, has also been noticed. Hence, the thermally activated PRDs will vent the tank contents through a vent line directed upwards and away from the vehicle. Temperature sensors have been installed to warn of excessive temperature.
A collector is installed in each module. It is primarily fed from each tank. At the next stage, the modular collectors contain individual pressure sensors, which are connected to the main distribution line [
67]. The main distribution line connects to the fuel line and then goes to the filter, pressure regulator, additional electronic solenoid valve, pressure sensor and additional PRD. The introduction of an additional solenoid valve facilitates the possibility of switching off (pressure sensor verifies the operation of the regulator). The diagram of emergency installation of the cut-off device is similar to that in diesel locomotives, i.e., it is easier for tank personnel to switch off the fuel system.