Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Delignification Treatments
2.2.1. Aqueous Sodium Hydroxide Process (ASP)
2.2.2. Alkali-Catalyzed Glycerol Organosolv (AGO)
2.2.3. Ethanol Organosolv Process (EO)
2.3. Lignin Precipitation
2.4. Analytical Methods
Quantification of Carbohydrates and Lignin
2.5. Chemical Characterization of Lignin and Lignin-Derived Compounds
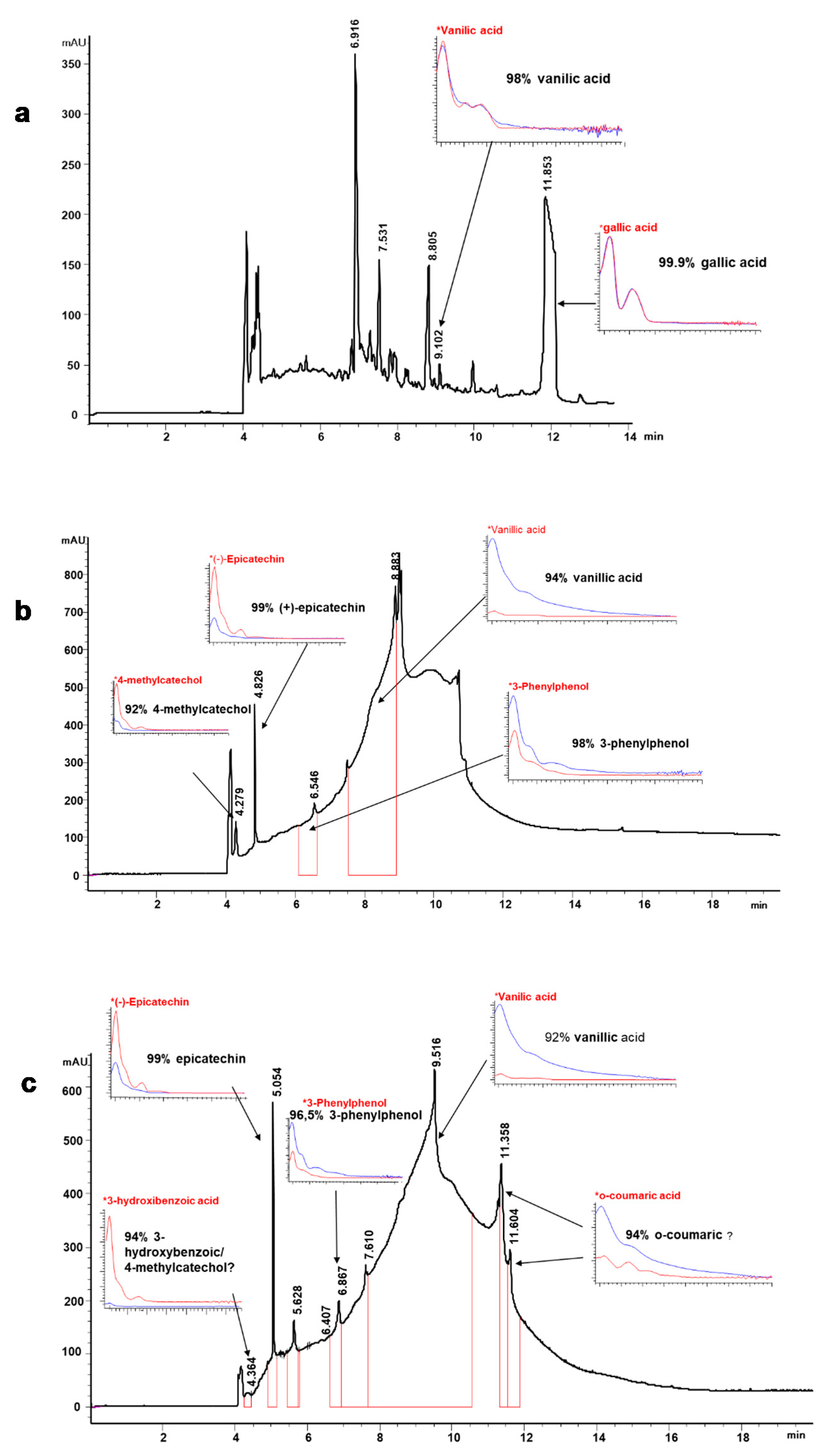
2.5.1. Phenolic Profile by Capillary Zone Electrophoresis (CZE)
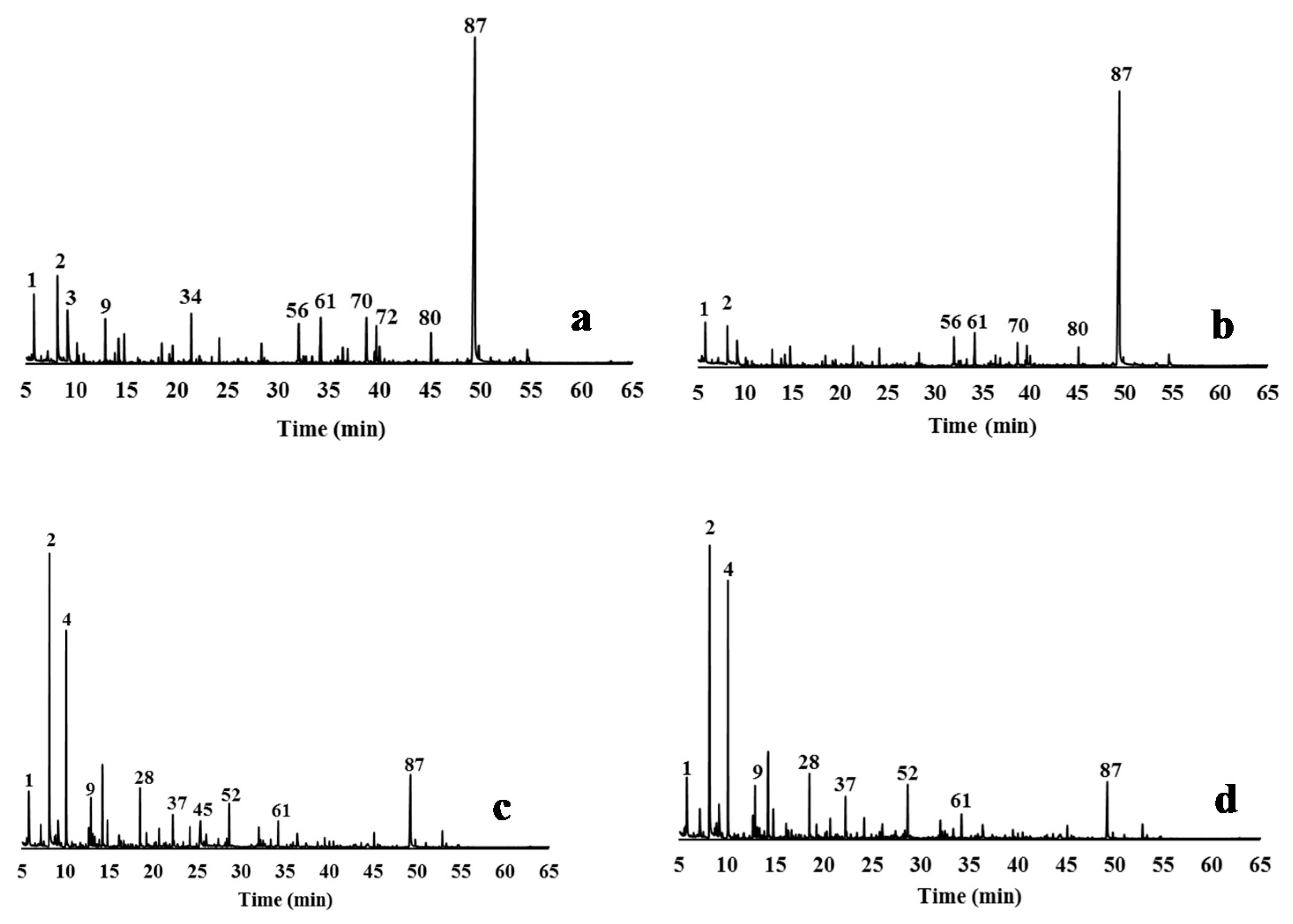
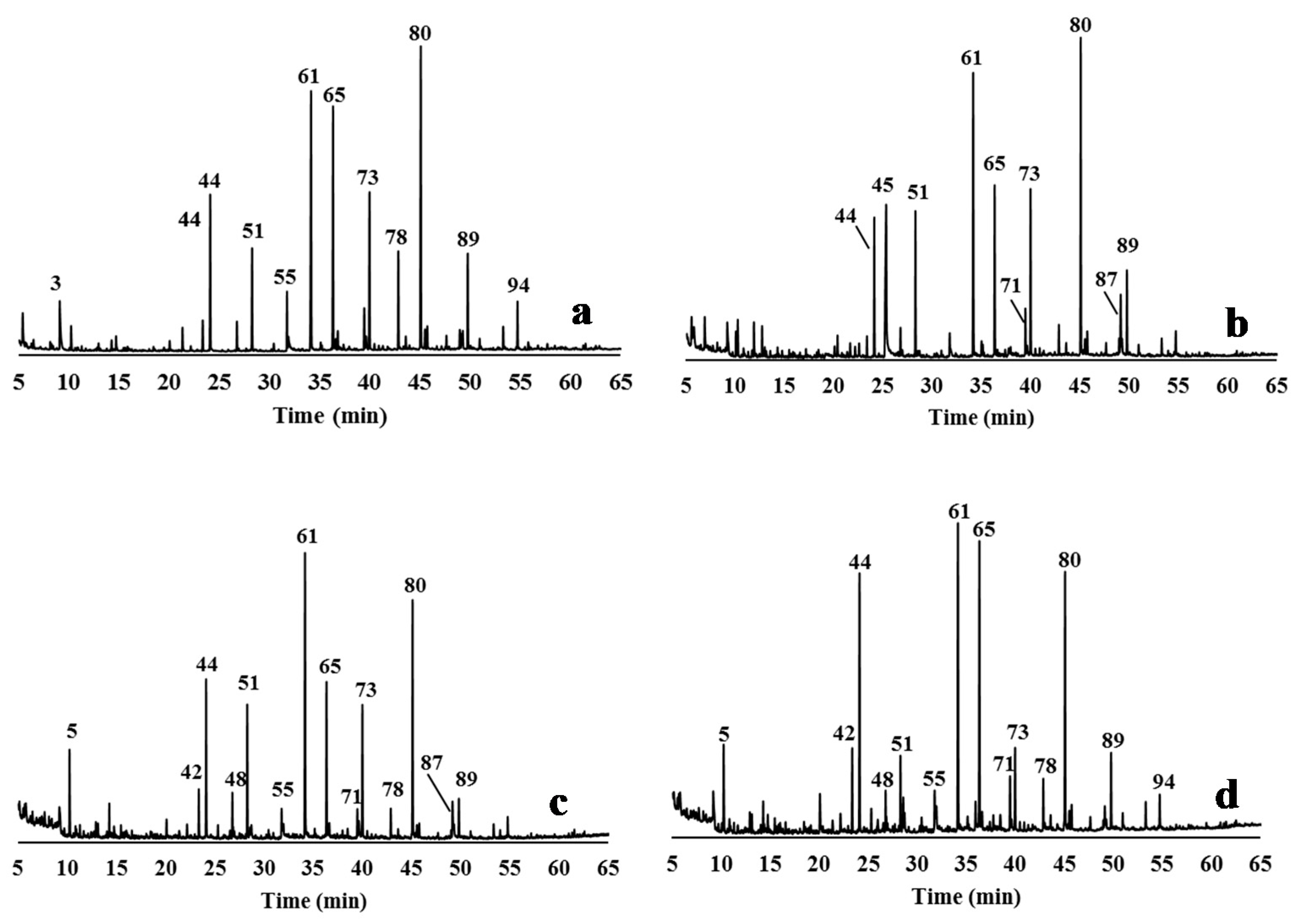
2.5.2. Pyrolysis Experiments
2.6. Enzymatic Hydrolysis
3. Results and Discussion
3.1. Chemical Composition of the Delignified Solids and Delignification Yield
3.2. Enzymatic Hydrolysis
3.3. Phenolic Composition of Lignin Liquors
3.4. Characterisation of Delignified Solids and Isolated Lignins by Py-GC/MS Pyrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Patón, D.; Azocar, P.; Tovar, J. Growth and productivity in forage biomass in relation to the age assessed by dendrochronology in the evergreen shrub Cistus ladanifer (L.) using different regression models. J. Arid Environ. 1998, 38, 221–235. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass. In Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830; Pacific Northwest National Laboratory: Richland, WA, USA, 2007; Volume 2. Available online: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-16983.pdf (accessed on 2 April 2020).
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins: Analysis, Properties, and Applications. Bioenergy Res. Adv. Appl. 2014, 315–336. [Google Scholar] [CrossRef]
- Smolarski, N. High-Value Opportunities for Lignin: Unlocking Its Potential Lignin Potential; Frost & Sullivan: San Antonio, TX, USA, 2012; pp. 1–15. [Google Scholar]
- Erdocia, X.; Prado, R.; Corcuera, M.Á.; Labidi, J. Effect of different organosolv treatments on the structure and properties of olive tree pruning lignin. J. Ind. Eng. Chem. 2014, 20, 1103–1108. [Google Scholar] [CrossRef]
- Sridach, W. The environmentally benign pulping process of non-wood fibers. Suranaree J. Sci. Technol. 2010, 17, 105–123. [Google Scholar]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 7 December 2011. [Google Scholar]
- Cybulska, I.; Brudecki, G.; Schmidt, J.E.; Tomsen, M.H. Organosolv fractionation of palm tree residues. Energy Procedia 2015, 75, 742–747. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Moniz, P.; Serralheiro, C.; Matos, C.T.; Boeriu, C.G.; Frissen, A.E.; Duarte, L.C.; Roseiro, L.B.; Pereira, H.; Carvalheiro, F. Membrane separation and characterisation of lignin and its derived products obtained by a mild ethanol organosolv treatment of rice straw. Process Biochem. 2018, 65, 136–145. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Sun, R.C.; Fowler, P.; Baird, M.S. Comparative study of organosolv fignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef]
- Alriols, M.G.; Tejado, A.; Blanco, M.; Mondragon, I.; Labidi, J. Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chem. Eng. J. 2009, 148, 106–114. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Benjelloun-Mlayah, B.; Huijgen, W.J.J.; de Wild, P.J.; Gosselink, R.J.A.; Gerritsma, J.; Courtin, C.M. Biorefining of wheat straw using an acetic and formic acid based organosolv fractionation process. Bioresour. Technol. 2014, 156, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery residues from Cistus ladanifer (rockrose) as feedstock for the production of added-value phenolic compounds and hemicellulosic oligosaccharides. Bioenergy Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Hydrothermal treatments of Cistus ladanifer industrial residues obtained from essential oil distilleries. Waste Biomass Valorization 2019, 10, 1303–1310. [Google Scholar] [CrossRef]
- Pérez, P.; Saúl, L.; Ciria, M.P. Distribución Geográfica, Caracterización Ecológica y Evaluación de Cistus laurifolius y Cistus ladanifer. Estudios Sobre el Matorral Como Recurso Energético; VidaRural: Lisboa, Portugal, 2011; pp. 66–70. [Google Scholar]
- Carrión-Prieto, P.; Martín-Ramos, P.; Maria, T.M.R.; Hernández-Navarro, S.; Garrido-Laurnaga, F.; Eusébio, M.E.S.; Martín-Gil, J. Vibrational and thermal studies of essential oils derived from Cistus ladanifer and Erica arborea shrubs. Nat. Prod. Commun. 2017, 12, 119–122. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 1–19. [Google Scholar] [CrossRef]
- Biolandes. Cistus Labdanum in Andalusia. Available online: https://www.biolandes.com/en-cistus-labdanum.php?voyage=o&lg=en (accessed on 1 February 2021).
- Moniz, P.; Pereira, H.; Quilhó, T.; Carvalheiro, F. Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind. Crops Prod. 2013, 50, 145–153. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report No. NREL/TP-510-42618; NREL: Golden, CO, USA, 2008; pp. 1–15.
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Faix, O.; Fortmann, I.; Bremer, J.; Meier, D. Thermal degradation products of wood. Holz Roh Werkst 1991, 49, 213–219. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D. Pyrolysis-Gc-Ms Characterization of Forage Materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass: Laboratory analytical procedure (LAP); Technical Report No. NREL/TP-510-42629; NREL: Golden, CO, USA, 2008.
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Berghem, L.E.R.; Pettersson, L.G.; AxiÖ-Fredriksson, U.-B. The mechanism of enzymatic cellulose degradation. Eur. J. Biochem. 1975, 53, 55–62. [Google Scholar] [CrossRef]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, M.; Rezende, C.A.; Bernardinelli, O.D.; Pereira, N.; Curvelo, A.A.S.; de Azevedo, E.R.; Guimarães, F.E.G.; Polikarpov, I. Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crops Prod. 2018, 113, 64–74. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Hu, T.; Zhao, X.; Liu, D. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: Substrate digestibility, fermentability and structural features. Appl. Energy 2015, 150, 224–232. [Google Scholar] [CrossRef]
- Sun, F.F.; Wang, L.; Hong, J.; Ren, J.; Du, F.; Hu, J.; Zhang, Z.; Zhou, B. The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour. Technol. 2015, 187, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hundt, M.; Schnitzlein, K.; Schnitzlein, M.G. Alkaline polyol pulping and enzymatic hydrolysis of hardwood: Effect of pulping severity and pulp composition on cellulase activity and overall sugar yield. Bioresour. Technol. 2013, 136, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Novo, L.P.; Gurgel, L.V.A.; Marabezi, K.; Curvelo, A.A.d.S. Delignification of sugarcane bagasse using glycerol-water mixtures to produce pulps for saccharification. Bioresour. Technol. 2011, 102, 10040–10046. [Google Scholar] [CrossRef]
- Meighan, B.N.; Lima, D.R.S.; Cardoso, W.J.; Baêta, B.E.L.; Adarme, O.F.H.; Santucci, B.S.; Pimenta, M.T.B.; de Aquino, S.F.; Gurgel, L.V.A. Two-stage fractionation of sugarcane bagasse by autohydrolysis and glycerol organosolv delignification in a lignocellulosic biorefinery concept. Ind. Crops Prod. 2017, 108, 431–441. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Zhai, S.; Liang, C.; Huang, C.; Lai, C.; Yong, Q. Improving enzymatic hydrolysis efficiency of wheat straw through sequential autohydrolysis and alkaline post-extraction. Bioresour. Technol. 2018, 251, 374–380. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Wang, Y.; Min, D.; Yong, Q. Associating cooking additives with sodium hydroxide to pretreat bamboo residues for improving the enzymatic saccharification and monosaccharides production. Bioresour. Technol. 2015, 193, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Nagula, K.N.; Pandit, A.B. Process intensification of delignification and enzymatic hydrolysis of delignified cellulosic biomass using various process intensification techniques including cavitation. Bioresour. Technol. 2016, 213, 162–168. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Liu, J.; Wang, Q.; Wang, C.; Yin, Z.; Wu, C. Lactic acid production from Sophora flavescens residues pretreated with sodium hydroxide: Reutilization of the pretreated liquor during fermentation. Bioresour. Technol. 2017, 241, 915–921. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.K.; Kim, H.Y.; Jeong, H.S.; Kim, J.Y.; Yeo, H.; Choi, I.G. Effect of ethanol organosolv pretreatment factors on enzymatic digestibility and ethanol organosolv lignin structure from Liriodendron tulipifera in specific combined severity factors. Renew. Energy 2016, 87, 599–606. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H. Organosolv pretreatment by crude glycerol from oleochemicals industry for enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2008, 99, 5474–5479. [Google Scholar] [CrossRef]
- Martín, C.; Puls, J.; Saake, B.; Schreiber, A. Effect of glycerol preatreatment on component recovery and enzymatic hydrolysis of sugarcane bagasse. Cell. Chem. Technol. 2011, 45, 487–494. [Google Scholar]
- Pan, X.J.; Gilkes, N.; Saddler, J.N. Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Zha, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Arroyo, S.; Barrajon-Catalan, E.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a Cistus ladanifer aqueous extract. Phytochem. Anal. 2010, 21, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a source of chemicals: Structural and chemical characterization. Biomass Convers. Biorefin. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Govindasami, T.; Pandey, A.; Palanivelu, N.; Pandey, A. Synthesis, characterization and antibacterial activity of biologically important vanillin related hydrazone derivatives. Int. J. Org. Chem. 2011, 1, 71–77. [Google Scholar] [CrossRef]
- Tsuda, H.; Uehara, N.; Iwahori, Y.; Asamoto, M.; Ligo, M.; Nagao, M.; Matsumoto, K.; Ito, M.; Hirono, I. Chemopreventive effects of β-carotene, α-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo[4,5-f] qumoline in the rat. Jpn. J. Cancer Res. 1994, 85, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Vanillin Market Size Worth $724.5 Million by 2025 Growth Rate: 7.0%. Available online: https://www.grandviewresearch.com/press-release/global-vanillin-market (accessed on 10 October 2020).
- Bernatova, I. Biological activities of (−)−epicatechin and (−)−epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Braunhut, S.J.; Bruno, F.; McIntosh, D.; Samuelson, L.; Kumar, J. Biocatalytically oligomerized epicatechin with potent and specific anti-proliferative activity for human breast cancer cells. Molecules 2008, 13, 2704–2716. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Zaid, M.A. Insulin-like effect of (−)epicatechin on erythrocyte membrane acetylcholinesterase activity in type 2 diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2001, 28, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Rahuman, A.A.; Bagavan, A.; Geetha, K.; Kamaraj, C.; Elango, G. Evaluation of medicinal plant extracts and isolated compound epicatechin from Ricinus communis against Paramphistomum cervi. Parasitol. Res. 2012, 111, 1629–1635. [Google Scholar] [CrossRef]
- Payton, F.; Bose, R.; Alworth, W.L.; Kumar, A.P.; Ghosh, R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem. Pharmacol. 2011, 81, 1211–1218. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Chai, X.; Luo, Y.; Guo, T.; Ying, H. Regulation of ρ -coumaric acid tolerance in Clostridium beijerinckii by disturbing the intracellular electron transport chain. Process Biochem. 2018, 68, 43–52. [Google Scholar] [CrossRef]
- Grabar, T.B.; Zhou, S.; Shanmugam, K.T.; Yomano, L.P.; Ingram, L.O. Methylglyoxal bypass identified as source of chiral contamination in L(+) and D(−)−lactate fermentations by recombinant Escherichia coli. Biotechnol. Lett. 2006, 28, 1527–1535. [Google Scholar] [CrossRef]
- Lourenço, A.; Pereira, H. Chapter 3: Compositional Variability of Lignin in Biomass. In Lignin Trends Applications; Poletto, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Micco, V.; Aronne, G. Anatomical features, monomer lignin composition and accumulation of phenolics in 1-year-old branches of the Mediterranean Cistus ladanifer L. Bot. J. Linn. Soc. 2007, 361–371. [Google Scholar] [CrossRef]
- Tsutsumi, B.Y.; Kondo, R.; Sakai, K.; Imamura, H. The Difference of Reactivity between Syringyl Lignin and Guaiacyl Lignin in Alkaline Systems. Holzforschung 1995, 49, 423–428. [Google Scholar] [CrossRef]
- Lourenço, A.; Gominho, J.; Marques, A.V.; Pereira, H. Variation of lignin monomeric composition during kraft pulping of Eucalyptus globulus heartwood and sapwood. J. Wood Chem. Technol. 2013, 33, 1–18. [Google Scholar] [CrossRef]
- Oudia, A.; Mészáros, E.; Jakab, E.; Simões, R.; Queiroz, J.; Ragauskas, A.; Novák, L. Analytical pyrolysis study of biodelignification of cloned Eucalyptus globulus (EG) clone and Pinus pinaster Aiton kraft pulp and residual lignins. J. Anal. Appl. Pyrolysis 2009, 85, 19–29. [Google Scholar] [CrossRef]
- Li, S.; Lyons-Hart, J.; Banyasz, J.; Shafer, K. Real-time evolved gas analysis by FTIR method: An experimental study of cellulose pyrolysis. Fuel 2001, 80, 1809–1817. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Product distribution from fast pyrolysis of glucose-based carbohydrates. J. Anal. Appl. Pyrolysis 2009, 86, 323–330. [Google Scholar] [CrossRef]
- Lourenço, A.; Gominho, J.; Marques, A.V.; Pereira, H. Py-GC/MS(FID) assessed behavior of polysaccharides during kraft delignification of Eucalyptus globulus heartwood and sapwood. J. Anal. Appl. Pyrolysis 2013, 101, 142–149. [Google Scholar] [CrossRef]
- Liao, Y.F.; Wang, S.R.; Ma, X.Q. Study of reaction mechanisms in cellulose pyrolysis. Prepr. Pap. Chem. Soc. Div. Fuel Chem. 2004, 49, 407–411. [Google Scholar]

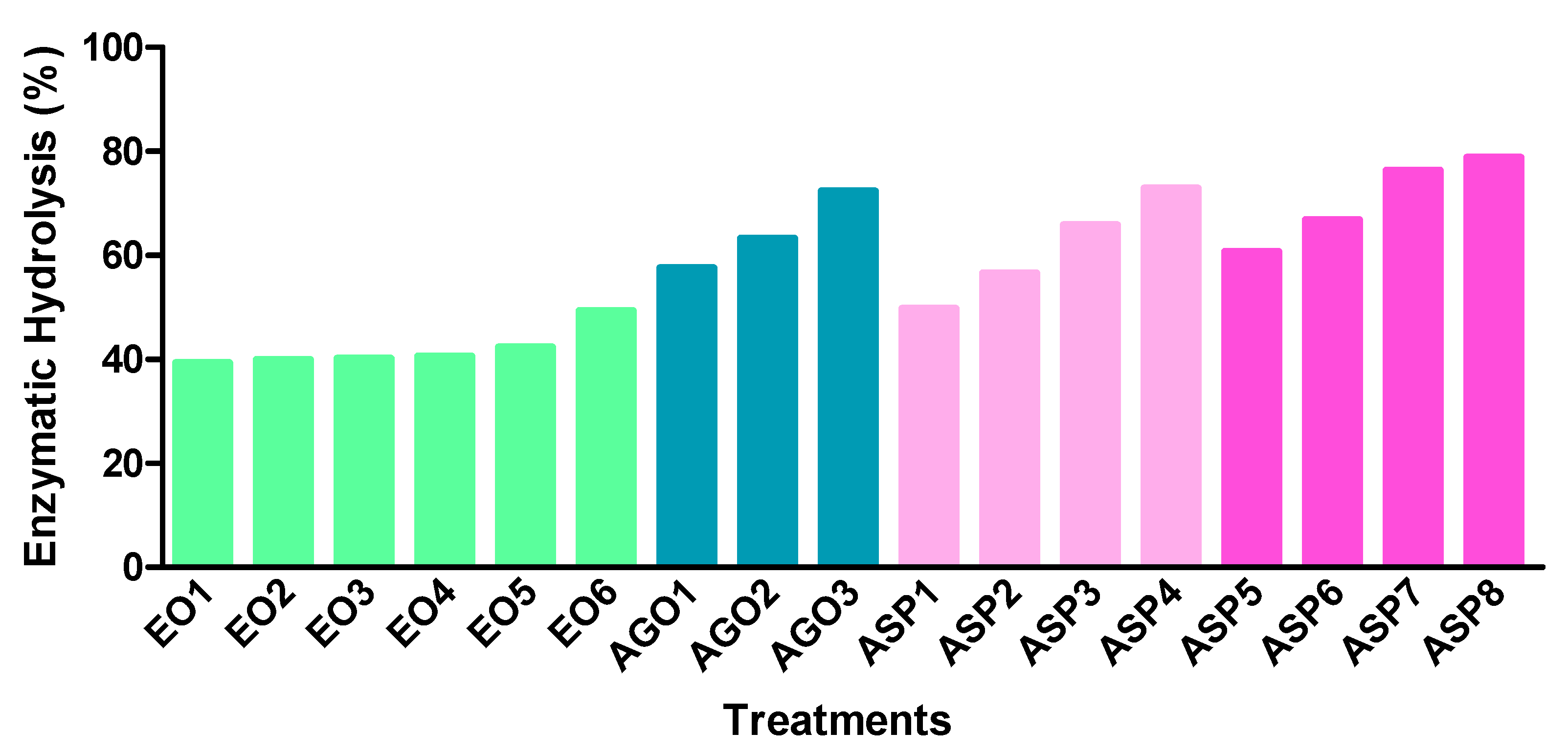
| Treatments | Delignification | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Process | Nr | Reagent | Feedstock | Reactor | Temperature (°C) | Time (h) | pH | Delignification yield (%) | Solid Yield (%) | Klason Lignin (%) | Glucan (%) | Xylan (%) |
| EO | 1 | 50% ethanol | CLRtreat | Parr | 170 | - | 4.4 | 13.3 | 88.2 | 46.0 | 35.2 | 8.8 |
| EO | 2 | 50% ethanol | CLRtreat | Parr | 180 | - | 4.4 | 17.0 | 86.0 | 45.2 | 36.6 | 9.2 |
| EO | 3 | 50% ethanol | CLRtreat | Parr | 190 | - | 4.2 | 20.0 | 85.3 | 43.9 | 39.8 | 8.7 |
| EO | 4 | 50% ethanol | CLRtreat | Parr | 200 | - | 4.2 | 22.4 | 81.1 | 44.7 | 38.9 | 8.3 |
| EO | 5 | 50% ethanol | CLRtreat | Parr | 210 | - | 4.2 | 28.9 | 79.9 | 41.7 | 44.3 | 8.3 |
| EO | 6 | 50% ethanol | CLRtreat | Parr | 220 | - | 4.1 | 21.6 | 80.6 | 46.5 | 40.1 | 5.6 |
| AGO | 1 | 50% glycerol + 1% NaOH | CLRtreat | Autoclave | 130 | 1 | 8.0 | 37.4 | 73.4 | 40.2 | 42.0 | 6.4 |
| AGO | 2 | 50% glycerol + 2% NaOH | CLRtreat | Autoclave | 130 | 1 | 10.5 | 69.1 | 61.0 | 23.9 | 61.0 | 5.1 |
| AGO | 3 | 50% glycerol + 4% NaOH | CLRtreat | Autoclave | 130 | 1 | 11.8 | 76.3 | 60.6 | 18.4 | 67.7 | 4.38 |
| ASP | 1 | 2% NaOH | CLRext | Autoclave | 130 | 1 | 9.9 | 68.8 | 49.0 | 20.2 | 45.7 | 20.0 |
| ASP | 2 | 2% NaOH | CLRext | Autoclave | 130 | 2 | 10.4 | 68.7 | 48.7 | 19.1 | 46.0 | 21.2 |
| ASP | 3 | 4% NaOH | CLRext | Autoclave | 130 | 1 | 12.9 | 77.7 | 39.8 | 16.7 | 51.9 | 21.4 |
| ASP | 4 | 4% NaOH | CLRext | Autoclave | 130 | 2 | 12.8 | 78.3 | 39.7 | 16.2 | 52.8 | 21.5 |
| ASP | 5 | 2% NaOH | CLRtreat | Autoclave | 130 | 1 | 9.7 | 72.8 | 49.3 | 24.1 | 63.5 | 8.4 |
| ASP | 6 | 2% NaOH | CLRtreat | Autoclave | 130 | 2 | 10.5 | 75.2 | 46.8 | 23.1 | 64.4 | 7.7 |
| ASP | 7 | 4% NaOH | CLRtreat | Autoclave | 130 | 1 | 12.9 | 83.9 | 43.0 | 16.3 | 74.0 | 7.6 |
| ASP | 8 | 4% NaOH | CLRtreat | Autoclave | 130 | 2 | 12.8 | 86.7 | 39.8 | 14.6 | 76.8 | 7.2 |
| HydrotermallyTreated | Delignified Solids | Lignin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak nr | Compound | Origin | CLR Treat | EO5 | AGO3 | ASP8 | EO5 | AGO3 | ASP7 | ASP8 |
| 1 | 2-oxo-propanal | c | 3.7 | 3.4 | 3.7 | 3.8 | 0.0 | 1.3 | n.d. | n.d. |
| 2 | hydroxyacetaldehyde | c | 4.5 | 3.0 | 17.2 | 16.7 | 0.0 | n.d. | n.d. | n.d. |
| 3 | acetic acid | c | 3.8 | 2.5 | 2.0 | 2.4 | 3.7 | 1.9 | n.d. | 1.6 |
| 4 | 2-hydroxypropanone | c | 0.8 | 0.5 | 9.3 | 10.8 | 0.0 | 0.9 | n.d. | n.d. |
| 5 | toluene | NDL | 0.3 | 0.4 | 0.2 | n.d. | 1.1 | 1.3 | 4.0 | 2.2 |
| 6 | HOCH=CHOH | c | 0.4 | 0.3 | 0.3 | 0.2 | 0.0. | n.d. | n.d. | n.d. |
| 7 | glycidol | - | n.d. | n.d. | 0.1 | n.d. | 0.0 | 1.2 | n.d. | n.d. |
| 8 | cyclopentanone | c | n.d. | n.d. | n.d. | n.d. | 0.0 | 0.9 | n.d. | n.d. |
| 9 | 3-hydroxypropanal | c | 1.4 | 0.8 | 2.1 | 2.4 | 0.0 | n.d. | n.d. | n.d. |
| 10 | 1,3-dimethyl-benzene | NDL | n.d. | n.d. | n.d. | n.d. | 0.0 | 0.1 | 0.6 | 0.4 |
| 11 | pyrrole | - | 0.1 | 0.1 | n.d. | n.d. | 0.1 | 0.3 | 0.5 | 0.4 |
| 12 | trans 2-methyl-but-2-enal | c | n.d. | n.d. | 0.7 | 0.6 | 0.0 | n.d. | n.d. | n.d. |
| 13 | 1,4-dimethyl-benzene | NDL | n.d. | n.d. | n.d. | n.d. | 0.0 | n.d. | 0.5 | 0.4 |
| 14 | 3H-furan-2-one | c | 0.1 | 0.1 | 0.6 | 0.5 | 0.0 | n.d. | n.d. | n.d. |
| 15 | furan-2-one isomer | c | 0.3 | 0.3 | 0.2 | 0.2 | 0.0 | n.d. | n.d. | n.d. |
| 16 | 3-furaldehyde | c | 0.3 | 0.3 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 17 | CH3-CO-CHOH-CHO | c | 1.0 | 0.4 | 2.4 | 2.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 18 | CHO-CH2-CH2-CHO | c | n.d. | 0.4 | 2.4 | 2.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 19 | styrene | NDL | 0.1 | 0.2 | n.d. | n.d. | 0.4 | 0.3 | 1.3 | 0.6 |
| 21 | furfural | c | 1.2 | 0.7 | 0.8 | 0.8 | 0.2 | 0.0 | 0.0 | 0.0 |
| 22 | 2-cyclopenten-1-one | c | 0.1 | 0.7 | 0.8 | 0.8 | 0.2 | 0.0 | 0.4 | 0.1 |
| 23 | 5-methyl-3H-furan-2-one | c | n.d. | n.d. | 0.4 | n.d. | 0.0 | n.d. | n.d. | n.d. |
| 24 | furfuryl alcohol | c | 0.3 | 0.3 | 0.4 | 0.8 | 0.0 | n.d. | n.d. | n.d. |
| 25 | 4-cyclopentene-1,3-dione | c | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 |
| 26 | similar to 4-cyclopentene-1,3-dione | c | 0.1 | 0.1 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 |
| 27 | dihydro-4-hydroxy-3H-furan-2-one | c | 0.2 | 0.3 | n.d. | n.d. | 0.0 | n.d. | n.d. | n.d. |
| 28 | 2-hydroxy-2-cyclopenten-1-one | c | 0.8 | 0.6 | 2.7 | 2.9 | 0.2 | 0.3 | 0.0 | 0.3 |
| 29 | dihydro-methyl furanone isomer | c | 0.5 | 0.4 | 0.8 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| 30 | 5-methyl-2-furaldehyde | c | 0.2 | 0.2 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 31 | Not identified sugar | c | 0.7 | 0.4 | 0.1 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 |
| 32 | Not identified sugar | c | n.d. | n.d. | n.d. | n.d. | 0.0 | 0.8 | n.d. | 0.3 |
| 33 | 5H-furan-2-one | c | 0.2 | 0.2 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 34 | 4-hydroxy-5,6-dihydro-2H-pyran-2-one | c | 2.1 | 1.3 | 0.2 | 0.2 | 1.1 | 0.3 | 0.0 | 0.3 |
| 35 | 2-ethenyl-1,3-dioxolane-4-methanol | c | n.d. | n.d. | n.d. | n.d. | 0.0 | 0.5 | n.d. | n.d. |
| 36 | 2H-pyran-2-one | c | 0.3 | 0.3 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 37 | 2-hydroxy-3-methyl-2-cyclopenten-1-one | c | 0.2 | 0.2 | 0.9 | 2.1 | 0.0 | n.d. | n.d. | n.d. |
| 38 | methyl-dihydro-2H-pyran-2-one | c | 0.2 | 0.2 | 0.9 | n.d | 0.0 | n.d. | n.d. | n.d. |
| 39 | 2-hydroxy-1-methyl-1-cyclopentene-3-one isomer | c | 0.3 | 0.4 | 0.3 | 0.3 | 0.0 | n.d. | n.d. | n.d. |
| 40 | Not identified sugar | c | n.d. | n.d. | 0.3 | 0.3. | 0.0 | n.d. | n.d. | n.d. |
| 41 | similar to 2-ethenyl-1,3-dioxolane-4-methanol | c | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 |
| 42 | phenol | H | 0.3 | 0.3 | 0.1 | 0.3 | 1.3 | 0.7 | 2.2 | 1.8 |
| 43 | 2-(propan-2-one)-tetrahydrofuran | c | 0.5 | 0.5 | 0.5 | 0.1 | 0.0 | 2.2 | n.d. | n.d. |
| 44 | guaiacol | G | 0.5 | 0.5 | 0.5 | 1.0 | 6.2 | 2.2 | 7.1 | 5.7 |
| 45 | glycerin | O | n.d. | n.d. | 1.1 | n.d. | 0.0 | 12.7 | n.d. | n.d. |
| 46 | o-cresol | H | n.d. | n.d. | 1.1 | n.d. | 0.0 | n.d. | 0.7 | 0.6 |
| 47 | 3-ethyl-2-hydroxy-2-cyclopenten-1-one | c | n.d. | n.d. | 1.1 | 0.3 | 0.0 | n.d. | n.d. | n.d. |
| 48 | p-cresol | H | 0.3 | 0.2 | 0.1 | 0.04 | 1.2 | 0.5 | 2.1 | 0.9 |
| 49 | m-cresol | H | n.d. | n.d. | n.d. | 0.1 | 0.0 | 0.5 | 0.4 | 0.4 |
| 50 | 2-methoxy-6-methylphenol | H | n.d. | n.d. | n.d. | 0.1 | 0.0 | 0.3 | n.d. | 0.3 |
| 51 | 4-methylguaiacol | G | 0.9 | 0.9 | 0.4 | 0.4 | 4.2 | 4.5 | 6.4 | 1.8 |
| 52 | Not identified sugar | c | 0.3 | 0.2 | 2.5 | 3.1 | 0.0 | 0.3 | 0.7 | 1.0 |
| 53 | 2,3-dimethyl-phenol | H | 0.1 | n.d. | 0.1 | 0.1 | 0.0 | 0.2 | 0.9 | 0.6 |
| 54 | ethylphenol | H | n.d. | n.d. | n.d. | n.d. | 0.4 | n.d. | n.d. | n.d. |
| 55 | 4-ethylguaiacol | G | n.d. | 0.1 | 0.2 | 0.2 | 2.2 | 0.7 | 1.4 | 1.1 |
| 56 | Not identified sugar | c | 2.1 | 2.2 | 1.2 | 1.0 | 0.0 | n.d. | n.d. | 0.9 |
| 57 | Similar to dihydro-6-methyl-2H-pyran-3(4H)-one | c | 0.2 | 0.1 | 0.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| 58 | 3,4-anhydro-D-galactosan | c | 0.4 | 0.4 | 0.1 | n.d. | 0.0 | n.d. | n.d. | n.d. |
| 59 | 1,4:3,6-dianhydro-α-D-glucopyranose | c | 0.3 | 0.5 | 0.5 | 0.5 | 0.0 | n.d. | n.d. | n.d. |
| 60 | 2,3-dihydrobenzofuran | H | n.d. | 0.6 | 0.1 | n.d. | 6.8 | 4.9 | n.d. | 4.0 |
| 61 | 4-vinylguaiacol | G | 1.4 | 2.4 | 1.3 | 1.3 | 6.8 | 4.9 | 14.7 | 4.0 |
| 62 | eugenol | G | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 0.4 |
| 63 | 4-propylguaiacol | G | n.d. | n.d. | n.d. | n.d. | 0.2 | n.d. | n.d. | n.d. |
| 64 | 5-hydroxymethylfurfural | c | 0.3 | 0.5 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 65 | syringol | S | 0.8 | 0.7 | 0.7 | 0.5 | 10.8 | 5.5 | 7.5 | 6.9 |
| 66 | indole | P | 0.1 | n.d. | n.d. | n.d. | 0.6 | 0.2 | 0.4 | 0.6 |
| 67 | Not identified sugar | c | 0.6 | 0.5 | n.d. | n.d | 1.0 | n.d. | n.d. | n.d. |
| 68 | dihydro-4-hydroxy-3H-furan-2-one | c | n.d. | n.d. | 0.2 | 0.2 | 0.0 | n.d. | n.d. | n.d. |
| 69 | cis isoeugenol | G | n.d. | n.d. | 0.2 | 0.1 | 0.2 | 0.3 | n.d. | 0.2 |
| 70 | 2-hydroxymethyl-5-hydroxy-2,3-dihydro-4H-pyran-4-one | c | 2.8 | 2.1 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 71 | trans isoeugenol | G | 0.5 | 0.4 | 0.5 | 0.5 | 1.8 | 1.7 | 1.4 | 1.3 |
| 72 | similar to 1,5-Anhydro-arabinofuranose | c | 2.3 | 1.8 | 0.1 | 0.1 | 1.1 | 0.6 | 0.0 | 0.0 |
| 73 | 4-methylsyringol | S | 1.0 | 0.8 | 0.4 | 0.2 | 6.8 | 5.7 | 6.2 | 2.0 |
| 74 | vanillin | G | 0.2 | 0.2 | 0.4 | 0.4 | 0.3 | 0.3 | n.d. | 0.3 |
| 75 | 1-(4-hydroxy-3-methoxyphenyl)-propyne | G | 0.1 | n.d. | 0.1 | n.d. | 0.2 | 0.3 | n.d. | 0.2 |
| 76 | 1-(4-hydroxy-3-methoxyphenyl)-propyne | G | 0.1 | 0.1 | n.d. | n.d. | 0.2 | 0.2 | n.d. | n.d. |
| 77 | homovanillin | G | 0.1 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 78 | 4-ethylsyringol | S | 0.1 | 0.1 | 0.1 | 0.1 | 4.1 | 1.0 | 1.4 | 1.3 |
| 79 | acetoguaiacone | G | 0.1 | 0.1 | 0.3 | 0.3 | 0.6 | 0.4 | 0.6 | 0.4 |
| 80 | 4-vinylsyringol | S | 1.2 | 1.2 | 0.8 | 0.6 | 13.2 | 10.5 | 11.7 | 5.7 |
| 81 | guaiacylacetone | G | 0.1 | 0.1 | 0.2 | 0.2 | 0.9 | 0.6 | 0.8 | 0.5 |
| 82 | 4-allylsyringol | S | 0.1 | 0.1 | 0.1 | 0.1 | 1.0 | 0.4 | 0.4 | 0.3 |
| 83 | 4-propylsyringol | S | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.4 | 0.4 | 0.3 |
| 84 | trans coniferyl alcohol | G | n.d. | n.d. | 0.1 | n.d. | 0.0 | n.d. | n.d. | n.d. |
| 85 | cis 4-propenylsyringol | S | 0.2 | n.d. | 0.1 | n.d. | 0.6 | 0.4 | n.d. | 0.3 |
| 86 | 4-propinylsyringol | S | 0.1 | n.d. | 0.1 | n.d. | 0.8 | 0.7 | 0.4 | 0.3 |
| 87 | 1,6-anhydro-β-D-glucopyranose | c | 42.7 | 51.0 | 7.0 | 4.8 | 0.9 | 3.0 | 2.6 | 0.8 |
| 88 | 4-propinylsyringol | S | n.d. | n.d. | n.d. | n.d. | 0.9 | 0.9 | 0.9 | 0.3 |
| 89 | trans 4-propenylsyringol | S | 0.8 | 0.6 | 0.5 | 0.3 | 3.9 | 2.9 | 1.9 | 1.8 |
| 90 | syringaldehyde | S | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.4 | n.d. | 0.4 |
| 91 | Not identified compound | - | n.d. | n.d. | 1.1 | 1.0 | 0.0 | n.d. | n.d. | n.d. |
| 92 | acetosyringone | S | 0.3 | 0.2 | 0.2 | 0.2 | 1.0 | 0.7 | 0.7 | 0.6 |
| 93 | trans coniferaldehyde | G | n.d. | 0.6 | 0.2 | n.d | 0.0 | n.d. | n.d. | n.d. |
| 94 | syringylacetone | S | 0.2 | 0.2 | 0.2 | 0.1 | 1.9 | 0.9 | 0.9 | 1.0 |
| 95 | propiosyringone | n.d. | n.d. | n.d. | n.d. | n.d. | 0.3 | n.d. | n.d. | n.d. |
| 96 | trans sinapaldehyde | S | 0.2 | n.d. | 0.1 | n.d. | 0.0 | n.d. | n.d. | n.d. |
| S | 5.1 | 4.2 | 3.6 | 2.4 | 44.9 | 30.4 | 32.4 | 21.2 | ||
| G | 4.2 | 5.6 | 4.7 | 4.7 | 24.0 | 16.5 | 32.8 | 15.9 | ||
| H | 0.6 | 1.1 | 1.5 | 0.6 | 9.7 | 7.1 | 6.3 | 8.6 | ||
| NDL | 0.5 | 0.6. | 0.2 | n.d. | 1.5 | 1.7 | 6.4 | 3.6 | ||
| S/G | 1.2 | 0.8 | 0.8 | 0.5 | 1.9 | 1.8 | 1.0 | 1.3 | ||
| S:G:H | 1:0.8:0.1 | 1:1.3:0.3 | 1:1.3:0.4 | 1:2:0.3 | 1:0.5:0.2 | 1:0.5:0.2 | 1:1:0.2 | 1:0.8:0.4 | ||
| Total lignin (% identified area) | 10.4 | 11.5 | 10.0 | 7.7 | 68.7 | 55.7 | 77.9 | 49.3 | ||
| Total carbohydrates (% identified area) | 76.3 | 78.2 | 65.6 | 64.4 | 7.6 | 13.4 | 3.7 | 5.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes. Energies 2021, 14, 1127. https://doi.org/10.3390/en14041127
Alves-Ferreira J, Lourenço A, Morgado F, Duarte LC, Roseiro LB, Fernandes MC, Pereira H, Carvalheiro F. Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes. Energies. 2021; 14(4):1127. https://doi.org/10.3390/en14041127
Chicago/Turabian StyleAlves-Ferreira, Júnia, Ana Lourenço, Francisca Morgado, Luís C. Duarte, Luísa B. Roseiro, Maria C. Fernandes, Helena Pereira, and Florbela Carvalheiro. 2021. "Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes" Energies 14, no. 4: 1127. https://doi.org/10.3390/en14041127
APA StyleAlves-Ferreira, J., Lourenço, A., Morgado, F., Duarte, L. C., Roseiro, L. B., Fernandes, M. C., Pereira, H., & Carvalheiro, F. (2021). Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes. Energies, 14(4), 1127. https://doi.org/10.3390/en14041127











