State of Art of Using Biofuels in Spark Ignition Engines
Abstract
1. Introduction
2. Biofuels for Spark Ignition Engines
2.1. Ethanol
2.2. Methanol
2.3. Butanol
2.4. Acetone
3. Comparison between Single Biofuel Blends in SIE
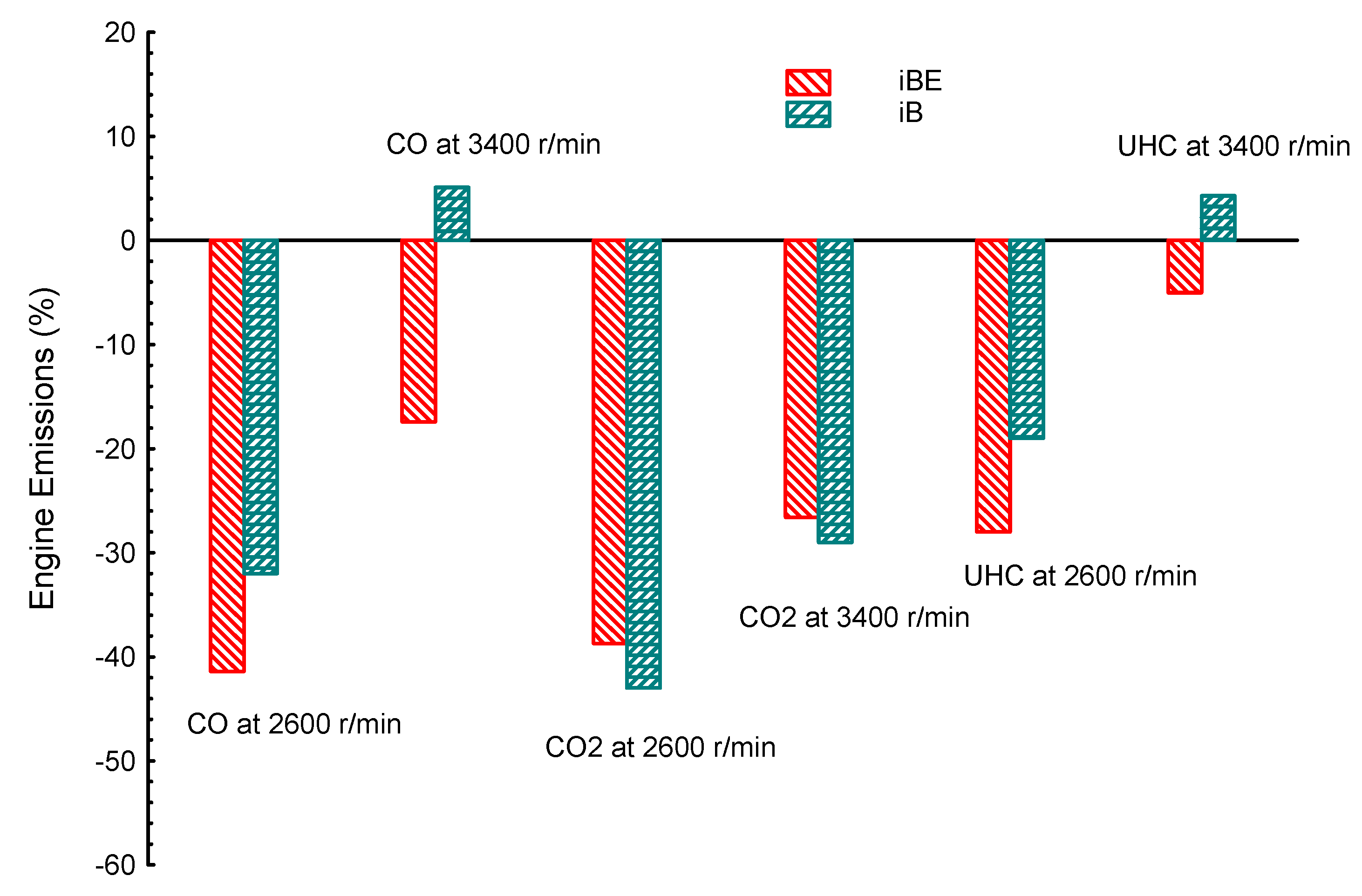
4. Comparison between Dual Biofuel Blends in SIE
5. Comparison between Single and Dual Biofuel Blends in SIE
6. Benefits and Weaknesses of Using Biofuels in SIE
7. Future Biofuels for SIE
8. A Proposed Method to Reduce Engine Pollutant Emissions
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Midttun, A.; Piccini, P.B. Facing the climate and digital challenge: European energy industry from boom to crisis and transformation. Energy Policy 2017, 108, 330–343. [Google Scholar] [CrossRef]
- Celik, A.N.; Ozgür, E. Review of Turkey’s photovoltaic energy status: Legal structure, existing installed power and comparative analysis. Renew. Sustain. Energy Rev. 2020, 134, 110344. [Google Scholar] [CrossRef]
- Elfasakhany, A.; Bai, X.S. Numerical and experimental studies of irregular-shape biomass particle motions in turbulent flows. Eng. Sci. Technol. 2019, 22, 249–265. [Google Scholar] [CrossRef]
- Alpanda, S.; Alva, A.P. Oil crisis, energy-saving technological change and the stock market crash of 1973–74. Rev. Econ. Dyn. 2010, 13, 824–842. [Google Scholar]
- Ajanovic, A.; Haas, R. On the future prospects and limits of biofuels in Brazil, the US and EU. Appl. Energy 2014, 135, 730–737. [Google Scholar] [CrossRef]
- Thompson, W.; Whistance, J.; Meyer, S. Effects of US biofuel policies on US and world petroleum product markets with consequences for greenhouse gas emissions. Energy Policy 2011, 39, 5509–5518. [Google Scholar] [CrossRef]
- Kay, A.; Ackrill, R. Governing the transition to a biofuels economy in the US and EU: Accommodating value conflicts, implementing uncertainty. Policy Soc. 2012, 31, 295–306. [Google Scholar] [CrossRef]
- Martinez, C.L.M.; Saari, J.; Melo, Y.; Cardoso, M.; Almeida, G.M.; Vakkilainen, E. Evaluation of thermochemical routes for the valorization of solid coffee residues to produce biofuels: A Brazilian case. Renew. Sustain. Energy Rev. 2021, 137. [Google Scholar] [CrossRef]
- Usmani, R.A. Potential for energy and biofuel from biomass in India. Renew. Energy 2020, 155, 921–930. [Google Scholar] [CrossRef]
- Sindelar, S.; Aradhey, A. India Biofuels Annual 2017. In USDA Foreign Agriculture Service; New Delhi, India, 2017. Available online: https://www.fas.usda.gov/data/india-biofuels-annual-1 (accessed on 25 November 2020).
- Buchspies, B.; Kaltschmitt, M.; Neuling, U. Potential changes in GHG emissions arising from the introduction of biorefineries combining biofuel and electrofuel production within the European Union–A location specific assessment. Renew. Sustain. Energy Rev. 2020, 134, 0395. [Google Scholar] [CrossRef]
- Bórawski, P.; Borawska, A.B.; Szymanska, E.J.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of renewable energy sources market and biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Z.; Zhao, F.; Ren, J.; Chang, S.; Rong, K.; Du, J. Biofuel for vehicle use in China: Current status, future potential and policy implications. Renew. Sustain. Energy Rev. 2018, 82, 645–653. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Optimization of palm oil biodiesel blends and engine operating parameters to improve performance and PM morphology in a common rail direct injection diesel engine. Fuel 2020, 260, 6326. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Elfasakhany, A. Powder biomass fast pyrolysis as in combustion conditions: Numerical prediction and validation. Renew. Energy Focus 2018, 27, 78–87. [Google Scholar] [CrossRef]
- Elfasakhany, A. Modeling of Pulverised Wood Flames. Ph.D. Thesis, Fluid Mechanics Department, Lund University, Lund, Sweden, 2005. [Google Scholar]
- Elfasakhany, A.; Bai, X.S. Modeling of Pulverised Wood Combustion: A Comparison of Different Models. Prog. Comp. Fluid Dyn. 2006, 6, 188–199. [Google Scholar] [CrossRef]
- Elfasakhany, A.; Klason, T.; Bai, X.S. Modeling of Pulverised Wood Combustion Using a Functional Group Model. Combust. Theory Modeling 2008, 12, 883–904. [Google Scholar] [CrossRef]
- Elfasakhany, A. Modeling of Secondary Reactions of Tar (SRT) Using a Functional Group Model. Int. J. Mech. Eng. Tech. 2012, 3, 123–136. [Google Scholar]
- Sangeeta; Moka, S.; Pande, M.; Rani, M.; Gakhar, R.; Sharma, M.; Rani, J.; Bhaskarwar, A.N. Alternative fuels: An overview of current trends and scope for future. Renew. Sustain. Energy Rev. 2014, 32, 697–712. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Ahmedb, A.S.; Ani, F.N. Review on bioethanol as alternative fuel for spark ignition engines. Renew. Sustain. Energy Rev. 2016, 56, 820–835. [Google Scholar] [CrossRef]
- Elfasakhany, A. Alcohols as Fuels in Spark Ignition Engines: Second Blended Generation; Lambert Academic Publishing: Bahnhofstrabe, Germany, 2017; ISBN 978–3–659–97691–9. [Google Scholar]
- Elfasakhany, A. Benefits and Drawbacks on the Use Biofuels in Spark Ignition Engines; Lambert Academic Publishing: Beau Bassin, Mauritius, 2017; ISBN 978–620–2–05720–2. [Google Scholar]
- Elfasakhany, A.; Tao, L.X.; Bai, X.S. Transport of pulverized wood particles in turbulent flow: Numerical and experimental studies. Energy Procedia 2014, 61, 1540–1543. [Google Scholar] [CrossRef]
- Elfasakhany, A.; Tao, L.; Espenas, B.; Larfeldt, J.; Bai, X.S. Pulverised Wood Combustion in a Vertical Furnace: Experimental and Computational Analyses. Appl. Energy 2013, 112, 454–464. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lu, C. Development perspectives of promising lignocellulose feedstocks for production of advanced generation biofuels: A review. Renew. Sustain. Energy Rev. 2021, 136, 0445. [Google Scholar] [CrossRef]
- McDowall, W.; Eames, M. Forecasts, scenarios, visions, backcasts and roadmaps to the hydrogen economy: A review of the hydrogen futures literature. Energy Pol. 2006, 34, 1236–1250. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines–a review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Bezerra, T.L.; Ragauskas, A.J. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuel. Bioprod. Biorefin. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Hall, L.M.H.; Buckley, A.R. A review of energy systems models in the UK: Prevalent usage and categorisation. Appl. Energy 2016, 169, 607–628. [Google Scholar]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.; Rahman, S.A.; Mahmudul, H. Energy scenario and biofuel policies and targets in ASEAN countries. Renew. Sustain. Energy Rev. 2015, 46, 51–61. [Google Scholar] [CrossRef]
- Azad, A.K.; Rasul, M.; Khan, M.M.K.; Sharma, S.C.; Hazrat, M. Prospect of biofuels as an alternative transport fuel in Australia. Renew. Sustain. Energy Rev. 2015, 43, 331–351. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Feroldi, M.; Araújo, A.V.; Borges, M.N.; Meier, T.W.; Feiden, A.; Teleken, J.G. Biofuels in Brazilian aviation: Current scenario and prospects. Renew. Sustain. Energy Rev. 2015, 43, 1063–1072. [Google Scholar] [CrossRef]
- Alizadeh, R.; Peter, D.; Soltanisehat, L.L. Outlook on biofuels in future studies: A systematic literature review. Renew. Sustain. Energy Rev. 2020, 134, 0326. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A. Biofuels Historical Perspectives and Public Opinions; CRC Press; Taylor and Francis Group: Boca Raton, FL, USA, 2016; ISBN 9781315370743. [Google Scholar]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Nadaleti, W.C.; Przybyla, G.; Filho, P.B. Analysis of emissions and combustion of typical biofuels generated in the agroindustry sector of Rio Grande do Sul State–Brazil: Bio75, syngas and blends. J. Clean. Prod. 2019, 208, 988–998. [Google Scholar] [CrossRef]
- Santos, I.T. Confronting governance challenges of the resource nexus through reflexivity: A cross-case comparison of biofuels policies in Germany and Brazil. Energy Res. Soc. Sci. 2020, 65, 1464. [Google Scholar]
- Puricelli, S.; Cardellini, G.; Grosso, M. A review on biofuels for light-duty vehicles in Europe. Renew. Sustain. Energy Rev. 2021, 137, 0398. [Google Scholar] [CrossRef]
- Liew, W.H.; Hassim, M.H.; Ng, D.K.S. Review of evolution, technology and sustainability assessments of biofuel production. J Clean. Prod. 2014, 71, 11–29. [Google Scholar] [CrossRef]
- Carneiro, M.L.N.M.; Pradelle, F.; Braga, S.L.; Gomes, M.S.P.; Martins, A.R.F.A.; Turkovic, F. Potential of biofuels from algae: Comparison with fossil fuels, ethanol and biodiesel in Europe and Brazil through life cycle assessment (LCA). Renew. Sustain. Energy Rev. 2017, 73, 632–653. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Oehlschlaeger, M.A.; Wang, H.; Sexton, M.N. Prospects for biofuels: A review. J. Therm. Sci. Eng. Appl. 2013, 5, 1006. [Google Scholar] [CrossRef]
- Souza, L.L.P.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Renó, M.L.G.; Venturini, O.J. Comparative environmental life cycle assessment of conventional vehicles with different fuel options, plug-in hybrid and electric vehicles for a sustainable transportation system in Brazil. J. Clean. Prod. 2018, 203, 444–468. [Google Scholar] [CrossRef]
- Bhargavi, G.; Rao, N.P.; Renganathan, S. Review on the extraction methods of crude oil from all generation biofuels in last few decades. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Recent Advances in Materials, Mechanical and Civil Engineering, Hyderabad, India, 1–2 June 2017; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 330. [Google Scholar] [CrossRef]
- Awudu, I.; Zhang, J. Uncertainties and sustainability concepts in biofuel supply chain management: A review. Renew. Sustain. Energy Rev. 2012, 16, 1359–1368. [Google Scholar] [CrossRef]
- Menten, F.; Chèze, B.; Patouillard, L.; Bouvart, F. A review of LCA greenhouse gas emissions results for advanced biofuels: The use of meta-regression analysis. Renew. Sustain. Energy Rev. 2013, 26, 108–134. [Google Scholar] [CrossRef]
- Gohain, M.; Khalifa, H.M.; Eldiehy, S.H.; Bardhan, P.; Laskar, K.; Phukon, H.; Mandal, M.; Kalita, D.; Deka, D. Bio-ethanol production: A route to sustainability of fuels using bio-based heterogeneous catalyst derived from waste. Process Saf. Environ. Prot. 2021, 146, 190–200. [Google Scholar] [CrossRef]
- Neitzel, T.; Lima, C.S.; Biazi, L.E.; Collograi, K.C.; Costa, A.C.; Santos, L.V.; LutzIenczak, J. Impact of the Melle-Boinot process on the enhancement of second-generation ethanol production by Spathaspora passalidarum. Renew. Energy 2020, 160, 1206–1216. [Google Scholar] [CrossRef]
- Abdollahipoor, B.; Shirazi, S.A.; Kenneth, F.; Bret, R.; Windom, C. Near-azeotropic volatility behavior of hydrous and anhydrous ethanol gasoline mixtures and impact on droplet evaporation dynamics. Fuel Process. Technol. 2018, 181, 166–174. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, N.; Prasad, R. Anhydrousethanol: A renewable source of energy. Renew. Sustain. Energy Rev. 2010, 14, 1830–1844. [Google Scholar] [CrossRef]
- Han, J.; Somers, L.M.T.; Cracknell, R.; Mohan, V.R.R. Experimental investigation of ethanol/diesel dual-fuel combustion in a heavy-duty diesel engine. Fuel 2020, 275, 7867. [Google Scholar] [CrossRef]
- Polat, S. An experimental investigation on combustion, performance and ringing operation characteristics of a low compression ratio early direct injection HCCI engine with ethanol fuel blends. Fuel 2020, 277, 8092. [Google Scholar] [CrossRef]
- Colorado, A.; McDonell, V. Surface stabilized combustion technology: An experimental evaluation of the extent of its fuel-flexibility and pollutant emissions using low and high calorific value fuels. Appl. Therm. Eng. 2018, 136, 206–218. [Google Scholar] [CrossRef]
- Duc, K.N.; Tien, H.N.; Duy, V.N. Performance enhancement and emission reduction of used motorcycles using flexible fuel technology. J. Energy Inst. 2018, 91, 145–152. [Google Scholar] [CrossRef]
- Elfasakhany, A. The effects of ethanol–gasoline blends on performance and exhaust emission characteristics of spark ignition engines. Int. J. Automot. Eng. 2014, 4, 608–620. [Google Scholar]
- Garcia, G.; Arriola, E.; Chen, W.H.; De Luna, M.D. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Mina, J.Z.; Restrepo, A.; Romero, C.; Quintero, H. Exergy analysis of a diesel engine converted to spark ignition operating with diesel, ethanol, and gasoline/ethanol blends. Sustain. Energy Technol. Assess. 2020, 42, 0803. [Google Scholar]
- Sakthivel, P.; Subramanian, K.A.; Mathai, R. Experimental study on unregulated emission characteristics of a two-wheeler with ethanol-gasoline blends (E0 to E50). Fuel 2020, 262, 6504. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Zhang, D.; Dong, F.; Liu, X.; Yang, Y.; Huang, H.; Wang, Y.; Wang, Q.; Zheng, Z. Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle. Energies 2019, 12, 1845. [Google Scholar] [CrossRef]
- Guo, T.; Duan, X.; Liu, Y.; Liu, J.; Zhou, X.; Li, Y.; Lai, M.C.; Guo, G. A comparative experimental study on emission characteristics of a turbocharged gasoline direct-injection (TGDI) engine fuelled with gasoline/ethanol blends under transient cold-start and steady-state conditions. Fuel 2020, 277, 8153. [Google Scholar] [CrossRef]
- Iodice, P.; Senatore, A.; Langella, G.; Amoresano, A. Effect of ethanol–gasoline blends on CO and HC emissions in last generation SI engines within the cold-start transient: An experimental investigation. Appl. Energy 2016, 179, 182–190. [Google Scholar] [CrossRef]
- Amine, M.; Ezis, N.; Barakat, A.Y. Volatility criteria of isomerate-enriched gasoline-ethanol blends. Egypt. J. Pet. 2020, 29, 227–233. [Google Scholar] [CrossRef]
- Fan, Q.; Qi, Y.; Wang, Y.; Wang, Z. Investigation into pressure dependence of flame speed for fuels with low and high octane sensitivity through blending ethanol. Combust. Flame 2020, 212, 252–269. [Google Scholar] [CrossRef]
- Roth, P.; Yang, J.; Peng, W.; Karavalakis, G. Intermediate and high ethanol blends reduce secondary organic aerosol formation from gasoline direct injection vehicles. Atmos. Environ. 2020, 220, 7064. [Google Scholar] [CrossRef]
- Michael, J.W.; James, M.H.; Felix, H.B. Maize, sweet sorghum, and high biomass sorghum ethanol yield comparison on marginal soils in Midwest USA. Biomass Bioenergy 2017, 107, 164–171. [Google Scholar]
- Cardona, E.; Rios, J.; Peña, J.; Peñuela, M.; Rios, L. King Grass: A very promising material for the production of second generation ethanol in tropical countries. Biomass Bioenergy 2016, 95, 206–213. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Li, H.; Liu, T.; Sang, S.; Chen, S.; Duan, L.; Zeng, L.; Xiang, W.; Gong, J. Chemical looping oxidative steam reforming of methanol: A new pathway for auto-thermal conversion. Appl. Catal. B Environ. 2020, 269, 8758. [Google Scholar] [CrossRef]
- Mansfield, A. A revised chemical kinetic mechanism for methanol combustion in sub and supercritical water. J. Supercrit. Fluids 2020, 166, 5023. [Google Scholar] [CrossRef]
- Butera, G.; Fendt, S.; Jensen, S.H.; Lasse, A.J.; Clausen, R. Flexible methanol production units coupling solid oxide cells and thermochemical biomass conversion via different gasification technologies. Energy 2020, 208, 8432. [Google Scholar] [CrossRef]
- Wu, Y.; Panigrahy, S.; Sahu, A.B.; Bariki, C.; Beeckmann, J.; Liang, J.; Mohamed, A.E.; Dong, S.; Tang, C.; Pitschc, H.; et al. Understanding the antagonistic effect of methanol as a component in surrogate fuel models: A case study of methanol/n-heptane mixtures. Combust. Flame 2021, 226, 229–242. [Google Scholar] [CrossRef]
- Andersson, J.; Krüger, A.; Grönkvist, S. Methanol as a carrier of hydrogen and carbon in fossil-free production of direct reduced iron. Energy Convers. Manag. 2020, 7, 0051. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Lee, J.K. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour. Technol. 2021, 323, 4550. [Google Scholar] [CrossRef]
- Cantera, S.; Andrea, I.S.; Lebrero, R.; Encina, P.A.G.; Stams, A.J.M.; Muñoz, R. Multi-production of high added market value metabolites from diluted methane emissions via methanotrophic extremophile. Bioresour. Technol. 2018, 267, 401–407. [Google Scholar] [CrossRef]
- Fei, Q.; Guarnieri, M.T.; Tao, L.; Laurens, L.M.L.; Dowe, N.; Pienkos, P.T. Bioconversion of natural gas to liquid fuel: Opportunities and challenges. Biotechnol. Adv. 2014, 32, 596–614. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, L.; Escobar, N.; Stolten, D.; Burauel, P.; Stolten, D. Life cycle assessment of a small-scale methanol production system: A Power-to-Fuel strategy for biogas plants. J. Clean. Prod. 2020, 271, 2476. [Google Scholar] [CrossRef]
- Wei, J.; Feng, H.; Liu, H.; Zhu, H.; Yue, Z.; Yao, M. Analysis of knocking combustion with methanol/iso-octane and ethanol/iso-octane blends in a spark-ignition engine. Fuel 2021, 284, 8979. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Xu, C.; Badawy, T.; Sahu, A.; Jiang, C. Methanol as an octane booster for gasoline fuels. Fuel 2019, 284, 76–84. [Google Scholar] [CrossRef]
- Prasad, B.S.N.; Pandey, J.K.; Kumar, G.N. Impact of changing compression ratio on engine characteristics of an SI engine fueled with equi-volume blend of methanol and gasoline. Energy 2020, 191, 6605. [Google Scholar]
- Yilmaz, I.; Taştan, M. Investigation of hydrogen addition to methanol-gasoline blends in an SI engine. Int. J. Hydrog. Energy 2018, 43, 20252–20261. [Google Scholar]
- Elfasakhany, A. Investigation on performance and emissions characteristics of an internal combustion engine fuelled with petroleum gasoline and a hybrid methanol–gasoline fuel. Int. J Eng. Tech. 2013, 13, 24–43. [Google Scholar]
- Sharma, N.; Patel, C.; Tiwari, N.; Agarwal, A.K. Experimental investigations of noise and vibration characteristics of gasoline-methanol blend fuelled gasoline direct injection engine and their relationship with combustion characteristics. Appl. Therm. Eng. 2019, 158, 3754. [Google Scholar] [CrossRef]
- Liu, F.; Hua, Y.; Wu, H.; Lee, C.F.; Shi, Z. Experimental and kinetic studies of soot formation in methanol-gasoline coflow diffusion flames. J. Energy Inst. 2019, 92, 38–50. [Google Scholar] [CrossRef]
- Oßwald, P.; Güldenberg, H.; Höinghaus, K.K.; Yang, B.; Yuan, T.; Qi, F. Combustion of butanol isomers—A detailed molecular beam mass spectrometry investigation of their flame chemistry. Combust. Flame 2010, 158, 2–15. [Google Scholar] [CrossRef]
- Rochón, E.; Cortizo, G.; Cabot, M.I.; Cubero, M.T.G.; Coca, M.; Ferrari, M.D.; Lareo, C. Bioprocess intensification for isopropanol, butanol and ethanol (IBE) production by fermentation from sugarcane and sweet sorghum juices through a gas stripping-pervaporation recovery process. Fuel 2020, 281, 8593. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, S.; Sen, R.; Das, D. Concomitant hydrogen and butanol production via co-digestion of organic wastewater and nitrogenous residues. Int. J. Hydrog. Energy 2020, 45, 24477–24490. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched–chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar]
- Díaz, V.H.G.; Willis, M.J.; Stosch, M.; Tost, G.O.; Rubio, O.P. Assessing the energy requirements for butanol production using fermentation tanks-in-series operated under vacuum. Renew. Energy 2020, 160, 1253–1264. [Google Scholar] [CrossRef]
- Sheng, y.; Wu, J.; Zhao, L.; Wu, C.; Zian, Q.I.; Cao, G. Optimization of Culture Conditions for Enhanced Butanol Production by a High Butanol Tolerant Clostridium Beijerinckii F-6. Energy Procedia 2019, 158, 471–476. [Google Scholar] [CrossRef]
- Wu, M.; Wang, M.; Liu, J.; Huo, J. Assessment of potential life–cycle energy and greenhouse gas emission effects from using corn–based butanol as a transportation fuel. Biotechnol. Prog. 2008, 24, 1204–1214. [Google Scholar] [CrossRef]
- Farkade, H.S.; Pathre, A.P. Experimental investigation of methanol, ethanol and butanol blends with gasoline on SI engine. Int. J. Emerg. Tech. Adv. Eng. 2012, 2, 205–215. [Google Scholar]
- Deng, B.; Yang, J.; Zhang, D.; Feng, R.; Fu, J.; Liu, J.; Li, K.; Liu, X. The challenges and strategies of butanol application in conventional engines: The sensitivity study of ignition and valve timing. Appl. Energy 2013, 108, 248–260. [Google Scholar] [CrossRef]
- Deng, B.; Fu, J.; Zhang, D.; Yang, J.; Feng, R.; Liu, J.; Li, K.; Liu, X. The heat release analysis of bio-butanol/gasoline blends on a high speed SI (spark ignition) engine. Energy 2013, 60, 230–241. [Google Scholar] [CrossRef]
- Szwaja, S.; Naber, J.D. Combustion of n-butanol in a spark–ignition IC engine. Fuel 2010, 89, 1573–1582. [Google Scholar] [CrossRef]
- Elfasakhany, A. Experimental study on emissions and performance of an internal combustion engine fueled with gasoline and gasoline/n-butanol blends. Energy Convers. Manag. 2014, 88, 277–283. [Google Scholar] [CrossRef]
- Dernotte, J.; Rousselle, C.M.; Halter, F.; Seers, P. Evaluation of butanol–gasoline blends in a port fuel–injection, spark–ignition engine. Oil Gas Sci. Technol. Rev. 2010, 65, 345–351. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Liu, J.; Han, Z.; Zhong, Z. Test Investigations of Gasoline Engine Fueled with Butanol–Gasoline Blends; SAE Technical Paper: Warrendale, PA, USA, 2009; p. 01-1891. [Google Scholar]
- Yang, J.; Wang, Y.; Feng, R. The Performance Analysis of an Engine Fueled with Butanol–Gasoline Blend; SAE Technical Paper: Warrendale, PA, USA, 2011; p. 01-1191. [Google Scholar]
- Wigg, B.; Coverdill, R.; Lee, C.; Kyritsis, D. Emissions characteristics of neat butanol fuel using a port fuel–injected, spark–ignition engine; SAE Technical Paper: Warrendale, PA, USA, 2011; p. 01-0902. [Google Scholar]
- Martin, P.; Martin, M.; Michal, V.L. Effect of Higher Content n-Butanol Blends on Combustion, Exhaust Emissions and Catalyst Performance of an Unmodified SI Vehicle Engine; SAE Technical Paper: Warrendale, PA, USA, 2012; p. 01-1594. [Google Scholar]
- Dagaut, P.; Togbe, C. Oxidation kinetics of butanol–gasoline surrogate mixtures in a jet–stirred reactor: Experimental and modeling study. Fuel 2008, 87, 3313–3321. [Google Scholar] [CrossRef]
- Dagaut, P.; Togbe, C. Experimental and modeling study of the kinetics of oxidation of butanol–n-heptane mixtures in a jet–stirred reactor. Energy Fuel 2009, 23, 3527–3535. [Google Scholar] [CrossRef]
- Venugopal, T.; Ramesh, A. Experimental studies on the effect of injection timing in a SI engine using dual injection of n-butanol and gasoline in the intake port. Fuel 2014, 115, 295–305. [Google Scholar] [CrossRef]
- Yacoub, Y.; Bara, R.; Gautam, M. The performance and emission characteristics of C1–C5 alcohol–gasoline blends with matched oxygen content in a single cylinder spark ignition engine. Proc. Inst. Mech. Eng. Part A Power Energy 1998, 212, 363–379. [Google Scholar] [CrossRef]
- Gu, X.; Huang, Z.; Cai, J.; Gong, J.; Wu, X.; Lee, C.F. Emission characteristics of a spark–ignition engine fuelled with gasoline–n-butanol blends in combination with EGR. Fuel 2012, 93, 611–617. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Choi, B. Emission characteristics of exhaust gases and nanoparticles from a diesel engine with biodiesel–diesel blended fuel (BD20). J. Mech. Sci. Technol. 2009, 23, 2555–2564. [Google Scholar] [CrossRef]
- Cairns, A.; Stansfield, P.; Fraser, N.; Blaxill, H.; Gold, M.; Rogerson, J.; Goodfellow, C. A Study of Gasoline–Alcohol Blended Fuels in an Advanced Turbocharged DISI Engine; SAE Technical Paper: Warrendale, PA, USA, 2009; p. 01-0138. [Google Scholar]
- Niass, T.; Amer, A.A.; Xu, W.; Vogel, S.R.; Hortmann, K.K.; Adomeit, P.; Brassat, A. Butanol Blending–A Promising Approach to Enhance the Thermodynamic Potential of Gasoline–Part 1; SAE Technical Paper: Warrendale, PA, USA, 2011; p. 01-1990. [Google Scholar]
- Gautam, M.; Martin, D.W. Combustion characteristics of higher alcohol/gasoline blends. Proc. Inst. Mech. Eng. 2000, 214, 497–511. [Google Scholar] [CrossRef]
- Gautam, M.; Martin, D.W. Emission characteristics of higher–alcohol/gasoline blends. Proc. Inst. Mech. Eng. 2000, 214, 165–182. [Google Scholar] [CrossRef]
- Williams, J.; Goodfellow, C.; Lance, D.; Ota, A.; Nakata, K.; Kawatake, K. Impact of Butanol and Other Bio-Components on the Thermal Efficiency of Prototype and Conventional Engines; SAE Technical Paper: Warrendale, PA, USA, 2009; p. 01-1908. [Google Scholar]
- Cooney, C.; Wallner, T.; McConnell, S.; Gillen, J.C.; Abell, C.; Miers, S.A. Effects of Blending Gasoline With Ethanol and Butanol on Engine Efficiency and Emissions Using a Direct-Injection, Spark-Ignition Engine. In Proceedings of the ASME 2009 Internal Combustion Engine Division Spring Technical Conference, Milwaukee, Wisconsin, USA, 3–6 May 2009; ICES2009–76155. ASME: Milwaukee, WI, USA, 2009. [Google Scholar]
- Mittal, N.; Athony, R.L.; Bansal, R.; Kumar, C.R. Study of performance and emission characteristics of a partially coated LHR SI engine blended with n-butanol and gasoline. Alex. Eng. J. 2013, 52, 285–293. [Google Scholar] [CrossRef]
- Pereira, J.S.; Aleiferis, P.G.; Richardson, D.; Wallace, S. Characteristics of Ethanol, Butanol, Iso-Octane and Gasoline Sprays and Combustion from a Multi–Hole Injector in a DISI Engine; SAE Technical Paper: Warrendale, PA, USA, 2008; pp. 01–1591. [Google Scholar]
- Broustail, G.; Halter, F.; Seers, P.; Moréac, G.; Rousselle, C.M. Comparison of regulated and non–regulated pollutants with iso-octane/butanol and iso-octane/ethanol blends in a portfuel injection spark–ignition engine. Fuel 2012, 94, 251–261. [Google Scholar] [CrossRef]
- Venugopal, T.; Ramesh, A. Effective utilisation of butanol along with gasoline in a spark ignition engine through a dual injection system. Appl. Therm. Eng. 2013, 59, 550–558. [Google Scholar] [CrossRef]
- Broustail, G.; Seers, P.; Halter, F.; Moréac, G.; Rousselle, C.M. Experimental determination of laminar burning velocity for butanol and ethanol isooctane blends. Fuel 2011, 90, 1–6. [Google Scholar] [CrossRef]
- Lavoie, G.A.; Blumberg, P.N. A fundamental model for predicting fuel consumption, NOx and HC emissions of the conventional spark–ignited engine. Combust. Sci. Technol. 1980, 21, 225–258. [Google Scholar] [CrossRef]
- Hu, E.J.; Huang, Z.H.; Liu, B.; Zheng, J.J.; Gu, X.L. Experimental study on combustion characteristics of a spark–ignition engine fueled with natural gas–hydrogen blends combining with EGR. Int. J. Hydrog. Energy 2009, 34, 1035–1044. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw–Hill: New York, NY, USA, 1988; ISBN 978–0070286375. [Google Scholar]
- Wallner, T.; Miers, S.A.; McConnell, S. A comparison of ethanol and butanol as oxygenates using a direct–injection, spark–ignition engine. J. Eng. Gas Turb. Power 2009, 131, 1–9. [Google Scholar] [CrossRef]
- Feng, R.; Yang, J.; Zhang, D.; Deng, B.; Fu, J.; Liu, J.; Liu, X. Experimental study on SI engine fuelled with butanol–gasoline blend and H2O addition. Energy Convers Manag. 2013, 74, 192–200. [Google Scholar] [CrossRef]
- Wigg, B.R. A Study on the Emissions of Butanol Using a Spark Ignition Engine and Their Reduction Using Electrostatically Assisted Injection. Master’s Thesis, University of Illinois at Urbana–Champaign, Urbana, IL, USA, 2011. [Google Scholar]
- Elfasakhany, A. Experimental investigation on SI engine using gasoline and a hybrid iso-butanol/gasoline fuel. Energy Convers Manag. 2015, 95, 398–405. [Google Scholar] [CrossRef]
- Irimescu, A. Performance and fuel conversion efficiency of a spark ignition engine fueled with iso-butanol. Appl. Energy 2012, 96, 477–483. [Google Scholar] [CrossRef]
- Alasfour, F.N. Butanol—A single–cylinder engine study: Availability analysis. Appl. Therm. Eng. 1997, 17, 537–549. [Google Scholar] [CrossRef]
- Alasfour, F.N. Nox emission from a spark ignition engine using 30% iso-butanol/gasoline blend: Part 2—Ignition timing. Appl. Therm. Eng. 1998, 18, 609–618. [Google Scholar] [CrossRef]
- Alasfour, F.N. Effect of using 30% iso-butanol–gasoline blend on hydrocarbon emissions from a spark–ignition engine. Energy Sources 1999, 21, 379–394. [Google Scholar] [CrossRef]
- Kelkar, A. Comparative Study of Methanol, Ethanol, Isopropanol and Butanol as Motor Fuels, Either Pure or Blended with Gasoline. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 1988. [Google Scholar]
- Bata, R.; Elrod, A.; Lewandowski, T. Evaluation of butanol as an alternative fuel. In Proceedings of the Energy–Sources Technology Conferences and Exhibition, Houston, TX, USA, 22–25 January 1989. [Google Scholar]
- Rice, R.W.; Sanyal, A.K.; Elrod, A.C.; Bata, R. Exhaust–gas emissions of butanol, ethanol, and methanol–gasoline blends. J. Eng. Gas Turbines Power 1991, 113, 377–381. [Google Scholar] [CrossRef]
- He, B.Q.; Wang, X.; Hao, M.; Yan, G.; Xiao, H. A study on emission characteristics of an EFI engine with ethanol blended gasoline fuels. Atmos. Environ. 2003, 37, 949–957. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, W.J.; Lin, S.L.; Wang, L.C. Green energy: Water–containing acetone–butanol–ethanol diesel blends fueled in diesel engines. Appl. Energy 2013, 109, 182–191. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, W.J.; Wu, T.S.; Wu, C.Y.; Chen, S.J. Use of water containing acetone–butanol–ethanol for NOx–PM (nitrogen oxide–particulate matter) trade–off in the diesel engine fueled with biodiesel. Energy 2014, 64, 678–687. [Google Scholar] [CrossRef]
- Lin, S.L.; Lee, W.J.; Lee, C.F.; Chen, S.J. Energy savings and emission reduction of nitrogen oxides, particulate matter, and polycyclic aromatic hydrocarbons by adding water–containing acetone and neat soybean oil to a diesel–fueled engine generator. Energy Fuels 2010, 24, 4522–4533. [Google Scholar] [CrossRef]
- Elfasakhany, A. Performance and emissions analysis on using acetone–gasoline fuel blends in spark–ignition engine. Eng. Sci. Tech. JESTECH 2016, 19, 1224–1232. [Google Scholar] [CrossRef][Green Version]
- Chen, R.H.; Chiang, L.B.; Wu, M.H.; Lin, T.H. Gasoline displacement and NOx reduction in an SI engine by aqueous alcohol injection. Fuel 2010, 89, 604–610. [Google Scholar] [CrossRef]
- Liaquat, A.M.; Kalam, M.A.; Masjuki, H.H.; Jayed, M.H. Potential emissions reduction in road transport sector using biofuel in developing countries. Atmos. Environ. 2010, 44, 3869–3877. [Google Scholar] [CrossRef]
- Srinivasan, C.; Saravanan, C.G. Study of combustion characteristics of an SI engine fuelled with ethanol and oxygenated fuel additives. J. Sustain. Energy Environ. 2010, 1, 85–91. [Google Scholar]
- Irimescu, A. Fuel conversion efficiency of a port injection engine fueled with gasoline–isobutanol blends. Energy 2011, 36, 3030–3035. [Google Scholar] [CrossRef]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Separation techniques in butanol production: Challenges and developments. Biomass Bioenergy 2014, 60, 222–246. [Google Scholar] [CrossRef]
- Gu, X.; Li, Q.; Huang, Z. Laminar burning velocities and flame instabilities of butanol isomers–air mixtures. Comb. Flame 2010, 157, 2318–2325. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Comb. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Szukzyk, K.R. Which is better transportation fuel–butanol or ethanol? Int. J. Energy Environ. 2010, 1, 501–512. [Google Scholar]
- Elfasakhany, A. Investigations on performance and pollutant emissions of spark–ignition engines fueled with n-butanol–, iso-butanol–, ethanol–, methanol–, and acetone–gasoline blends: A comparative study. Renew. Sustain. Energy Rev. 2017, 71, 404–413. [Google Scholar] [CrossRef]
- Elfasakhany, A. Gasoline engine fueled with bioethanol-bio-acetone-gasoline blends: Performance and emissions exploration. Fuel 2020, 274, 7825. [Google Scholar] [CrossRef]
- Elfasakhany, A. Dual and Ternary Biofuel Blends for Desalination Process: Emissions and Heat Recovered Assessment. Energies 2021, 14, 61. [Google Scholar] [CrossRef]
- Elfasakhany, A. Performance and emissions of spark–ignition engine using ethanol–methanol–gasoline, n-butanol–iso-butanol–gasoline and iso-butanol–ethanol–gasoline blends: A comparative study. Eng. Sci. Tech. JESTECH 2016, 19, 2053–2059. [Google Scholar] [CrossRef]
- Elfasakhany, A. Exhaust emissions and performance of ternary iso-butanol–bio-methanol–gasoline and n-butanol–bio-ethanol–gasoline fuel blends in spark-ignition engines: Assessment and comparison. Energy 2018, 158, 830–844. [Google Scholar] [CrossRef]
- Elfasakhany, A. Investigations on the effects of ethanol–methanol–gasoline blends in a spark–ignition engine: Performance and emissions analysis. Eng. Sci. Tech. JESTECH 2015, 18, 713–719. [Google Scholar]
- Elfasakhany, A.; Mahrous, A.F. Performance and emissions assessment of n-butanol–methanol–gasoline blends as a fuel in spark–ignition engines. Alex. Eng. J. 2016, 55, 3015–3024. [Google Scholar] [CrossRef]
- Balaji, D.; Govindarajan, P.; Venkatesan, J. Influence of isobutanol blend in spark ignition engine performance operated with gasoline and ethanol. Int. J. Eng. Sci. Tech. 2010, 2, 2859–2868. [Google Scholar]
- Siwale, L.; Kristóf, L.; Bereczky, A.; Mbarawa, M.; Kolesnikov, A. Performance, combustion and emission characteristics of n-butanol additive in methanol–gasoline blend fired in a naturally–aspirated spark ignition engine. Fuel Proc. Tech. 2014, 118, 318–326. [Google Scholar] [CrossRef]
- Nazzal, I.T. Experimental study of gasoline—Alcohol blends on performance of internal combustion engine. Eur. J. Sci. Res. 2011, 52, 16–22. [Google Scholar]
- Elfasakhany, A. Experimental study of dual n-butanol and iso-butanol additives on spark–ignition engine performance and emissions. Fuel 2016, 163, 166–174. [Google Scholar] [CrossRef]
- Elfasakhany, A. Engine performance evaluation and pollutant emissions analysis using ternary bio-ethanol–iso-butanol–gasoline blends in gasoline engines. J. Clean. Prod. 2016, 139, 1057–1067. [Google Scholar] [CrossRef]
- Minato, R. Advantage of ethanol fuel for gas generator cycle air turbo ramjet engine. Aerosp. Sci. Technol. 2016, 50, 161–172. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Chen, Z.; Shen, Y.; Zhang, H.; Wang, S.; Qi, J.; Zhu, Z.; Wang, Y.L.; Gao, J. Comprehensive analysis of environmental impacts and energy consumption of biomass-to-methanol and coal-to-methanol via life cycle assessment. Energy 2020, 204, 7961. [Google Scholar] [CrossRef]
- Veza, I.; Said, M.F.M.; Latiff, Z.A. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 2021, 144, 5919. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.L.L.; Zhuang, L.; Lu, J.; Xu, B. A feasibility analysis for alkaline membrane direct methanol fuel cell: Thermodynamic disadvantages versus kinetic advantages. Electrochem. Commun. 2003, 5, 662–666. [Google Scholar]
- Elfasakhany, A. Biofuels in Automobiles: Advantages and Disadvantages: A Review. Curr. Altern. Energy 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Maclean, H.L.; Lave, L.B. Evaluating automobile fuel/propulsion system technologies. Prog. Energy Combust. Sci. 2003, 29, 1–69. [Google Scholar] [CrossRef]
- Alasfour, F.N. Nox emission from a spark ignition engine using 30% iso-butanol/gasoline blend: Part 1—Preheating inlet air. Appl. Therm. Eng. 1998, 18, 245–256. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2021, 766, 4319. [Google Scholar] [CrossRef]
- Buttes, A.G.; Jeanneret, B.; Kéromnès, A.; Moyne, L.; Pélissier, S. Energy management strategy to reduce pollutant emissions during the catalyst light-off of parallel hybrid vehicles. Appl. Energy 2020, 266, 4866. [Google Scholar]
- Kozina, A.; Radica, G.; Nižetić, S. Analysis of methods towards reduction of harmful pollutants from diesel engines. J. Clean. Prod. 2020, 262, 1105. [Google Scholar] [CrossRef]
- Elfasakhany, A. Reducing automobile pollutant emissions and re−using some of such emissions as a fuel. Ciência Técnica. J. 2017, 32, 160–176. [Google Scholar]
- Elfasakhany, A.; Alsehli, M.; Saleh, B.; Aly, A.A.; Bassuoni, M. Renewable Pulverized Biomass Fuel for Internal Combustion Engines. Processes 2020, 8, 465. [Google Scholar] [CrossRef]



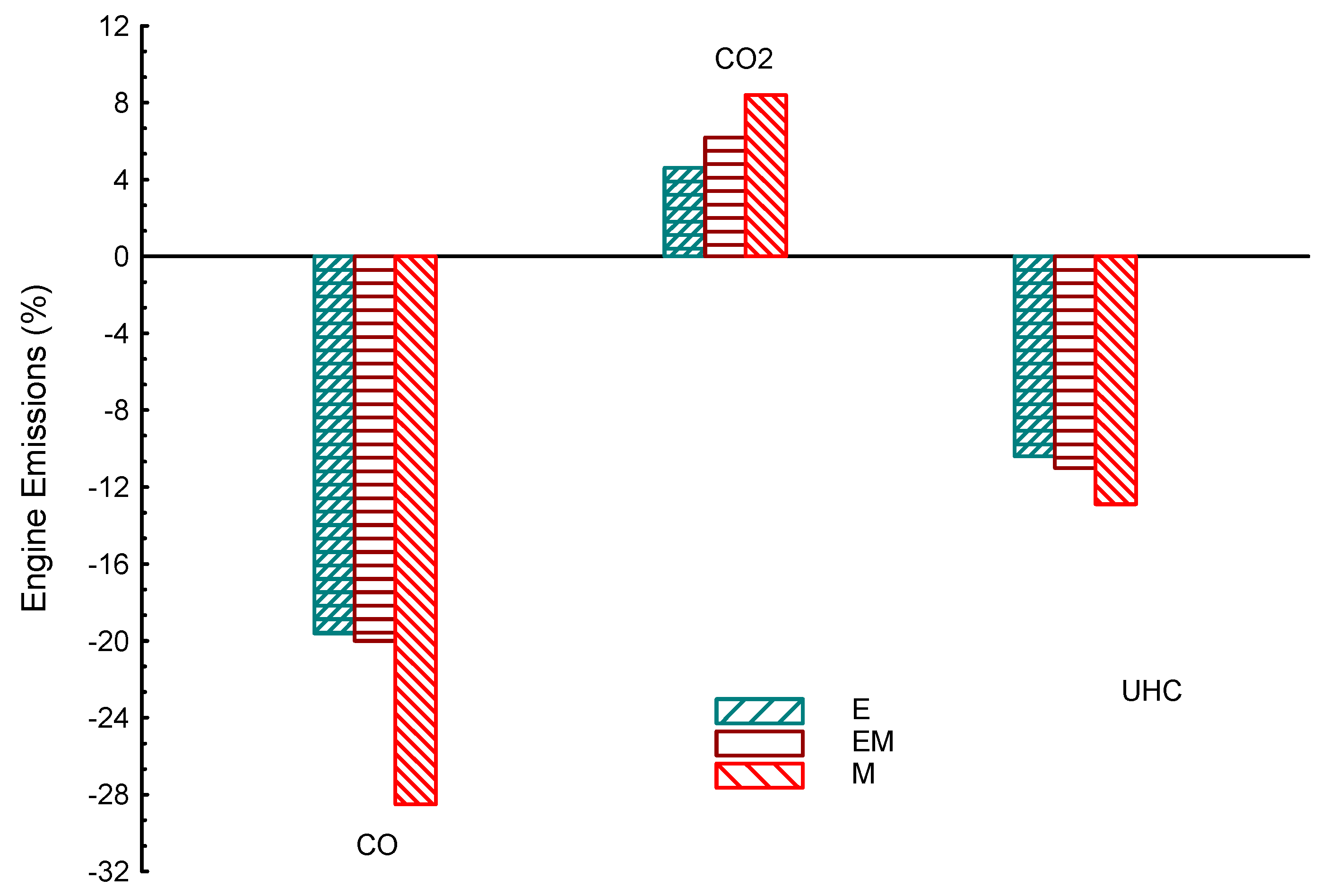
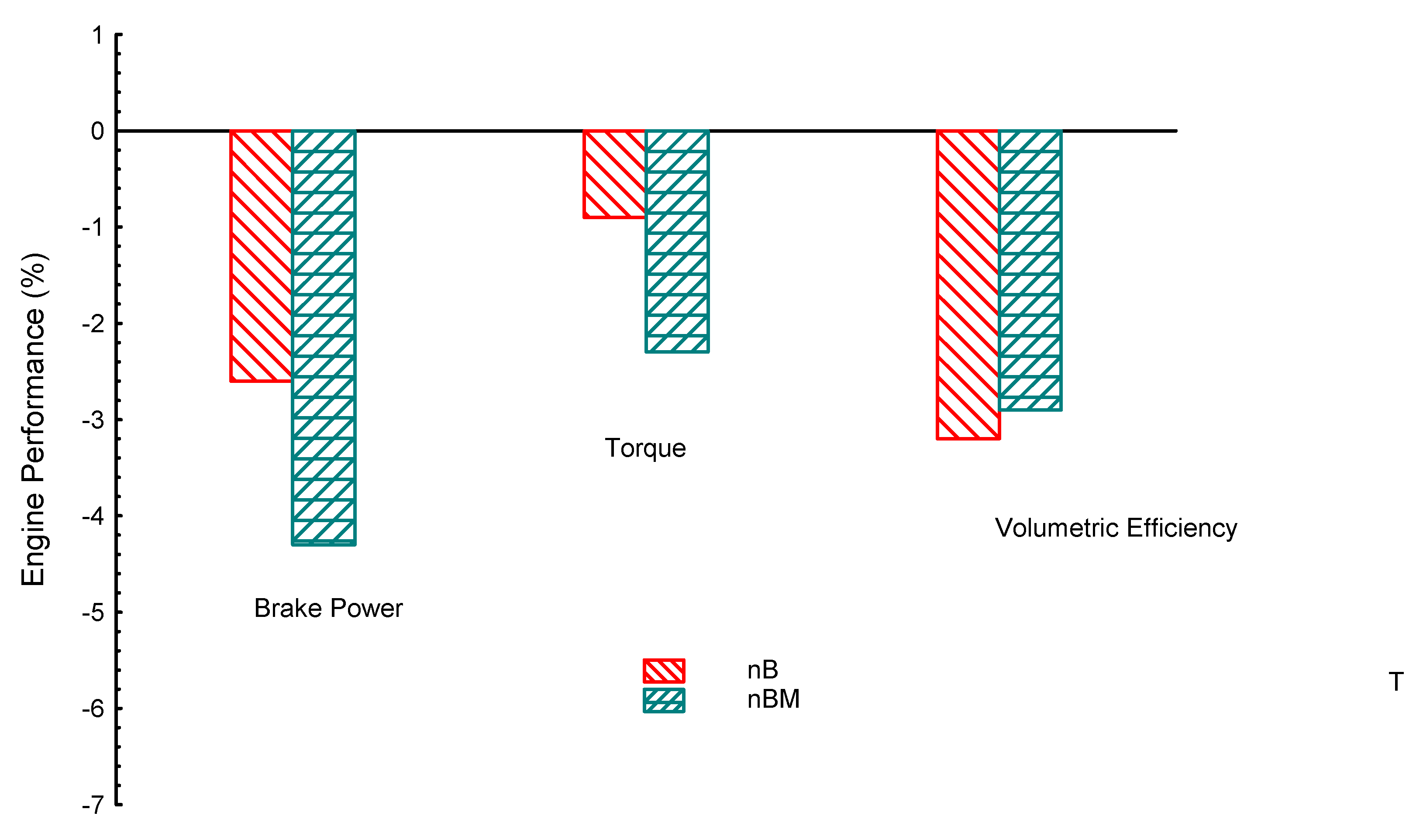

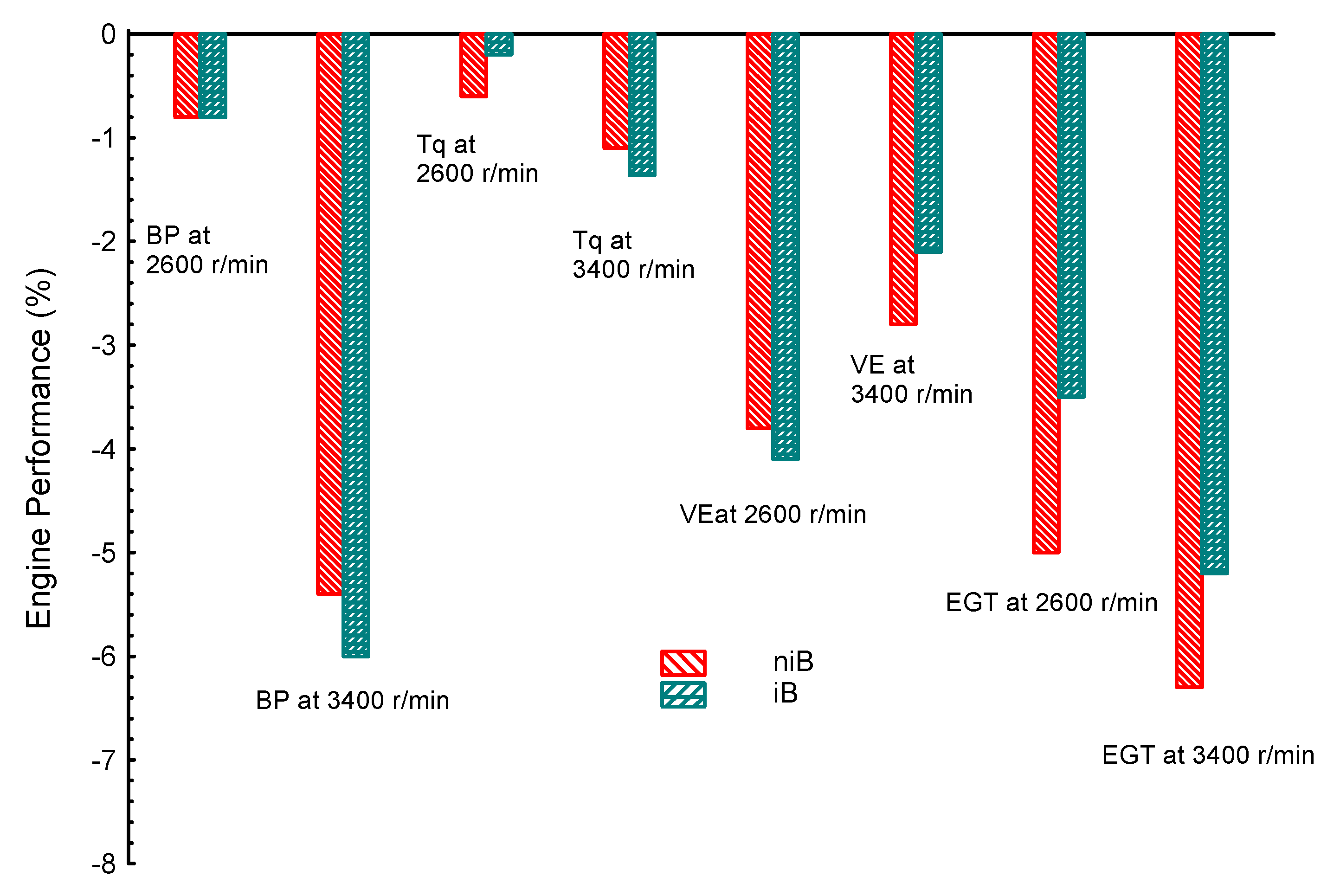
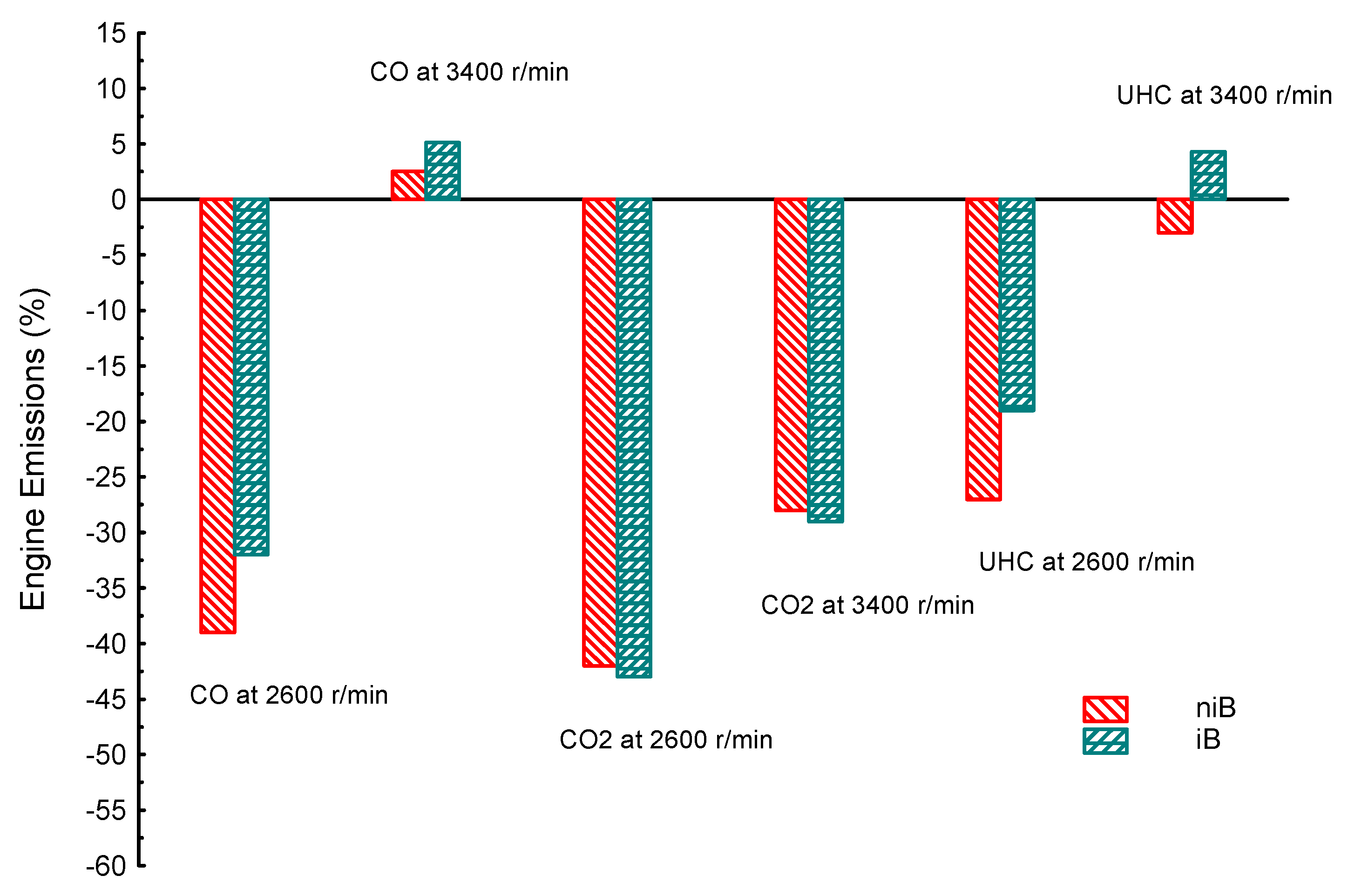

| Property | Gasoline | Ethanol | Methanol | I-Butanol | N-Butanol | Acetone |
|---|---|---|---|---|---|---|
| Chemical formula | C8H15 | C2H5OH | CH3OH | C4H9OH | C4H9OH | C3H5OH |
| Composition (C,H,O)(%) | 86,14,0 | 52, 13, 35 | 37.5, 12.5, 50 | 65, 13.5, 21.5 | 65, 13.5, 21.5 | 62, 10.5, 27.5 |
| Lower H. V (MJ/kg) | 43.5 | 27.0 | 20.1 | 33.3 | 33.1 | 29.6 |
| Heat of evap. (kJ/kg) | 223.2 | 725.4 | 920.7 | 474.3 | 582 | 501.7 |
| Stoichiometric A/F ratio | 14.6 | 9.0 | 6.4 | 11.1 | 11.2 | 9.54 |
| Oxygen content, mass % | 0.0 | 34.7 | 49.9 | 21.6 | 21.6 | 27.6 |
| Density (kg/m3) | 760 | 790 | 796 | 802 | 810 | 791 |
| Saturation pressure | 31 | 13.8 | 31.69 | 2.3 | 2.27 | 53.4 |
| Flash point (°C) | –45 to –38 | 21.1 | 11.1 | 28 | 35 | 17.8 |
| Auto-ignition temp. (°C) | 420 | 434 | 470 | 415 | 385 | 560 |
| Boiling point (°C) | 25–215 | 78.4 | 64.5 | 108 | 117.7 | 56.1 |
| Solubility in water | <0.1 | Fully miscible | Fully miscible | 10.6 | 7.7 | Miscible |
| Vapor toxicity | Moderate | Very toxic | Toxic | Moderate | Moderate | Low |
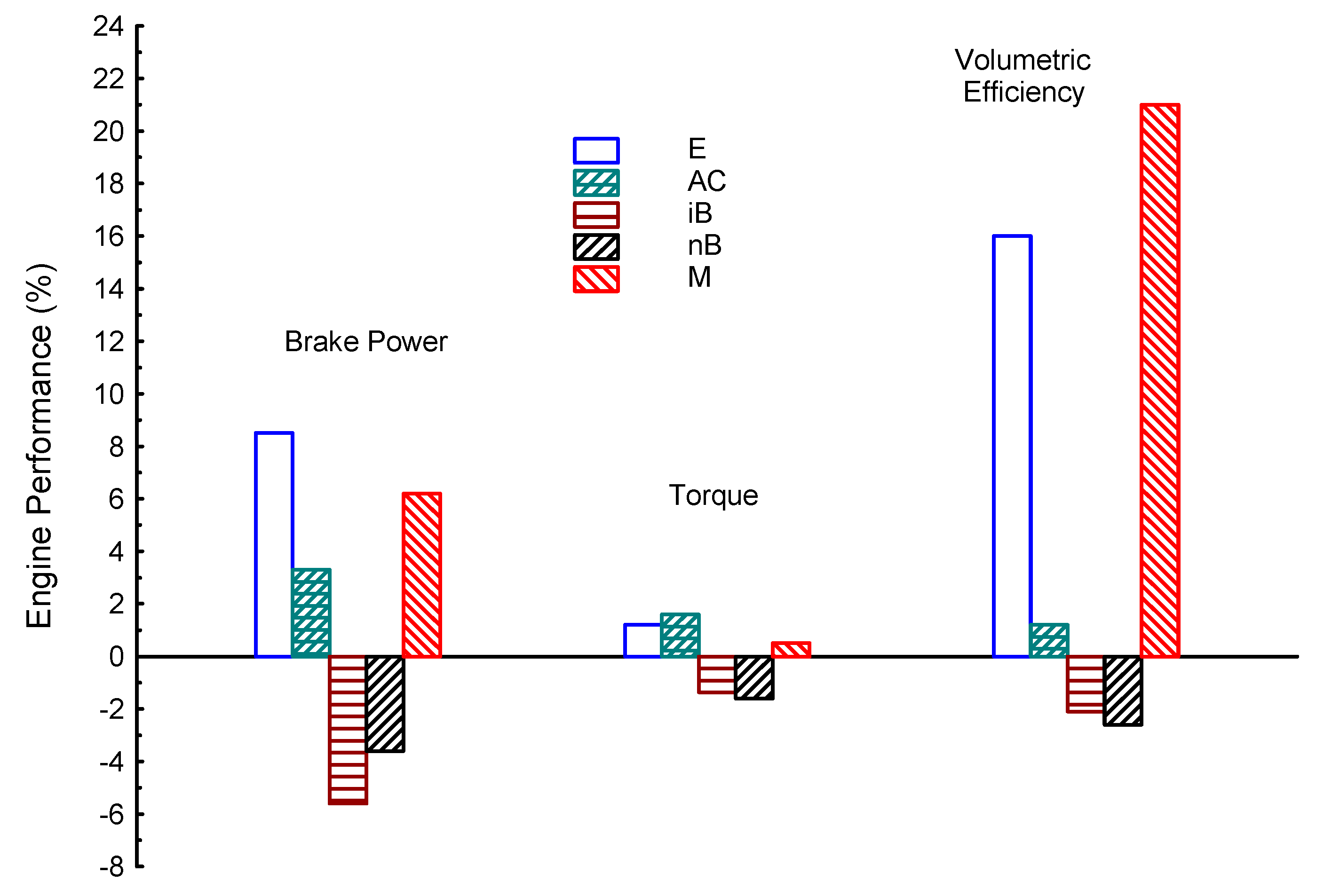
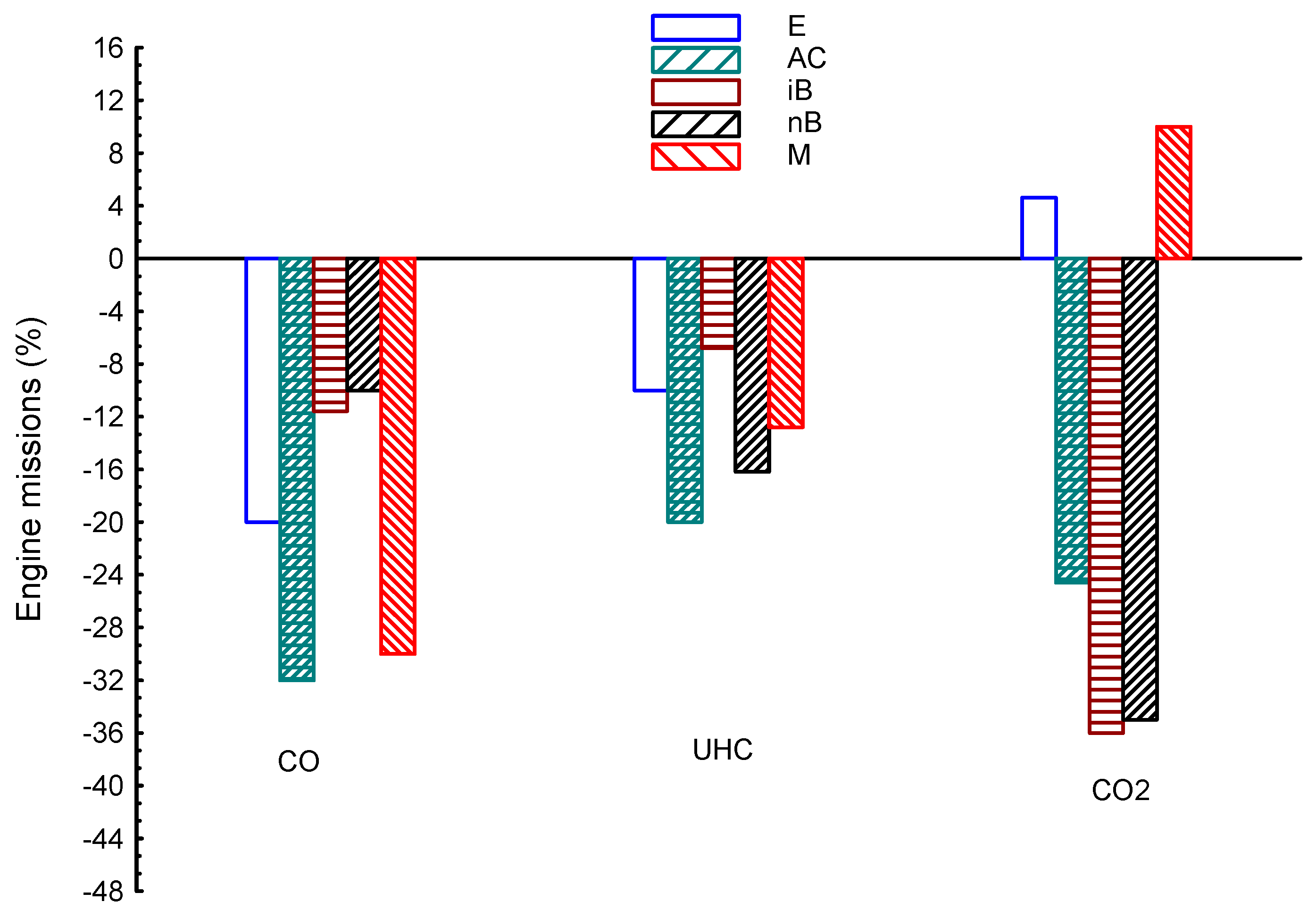
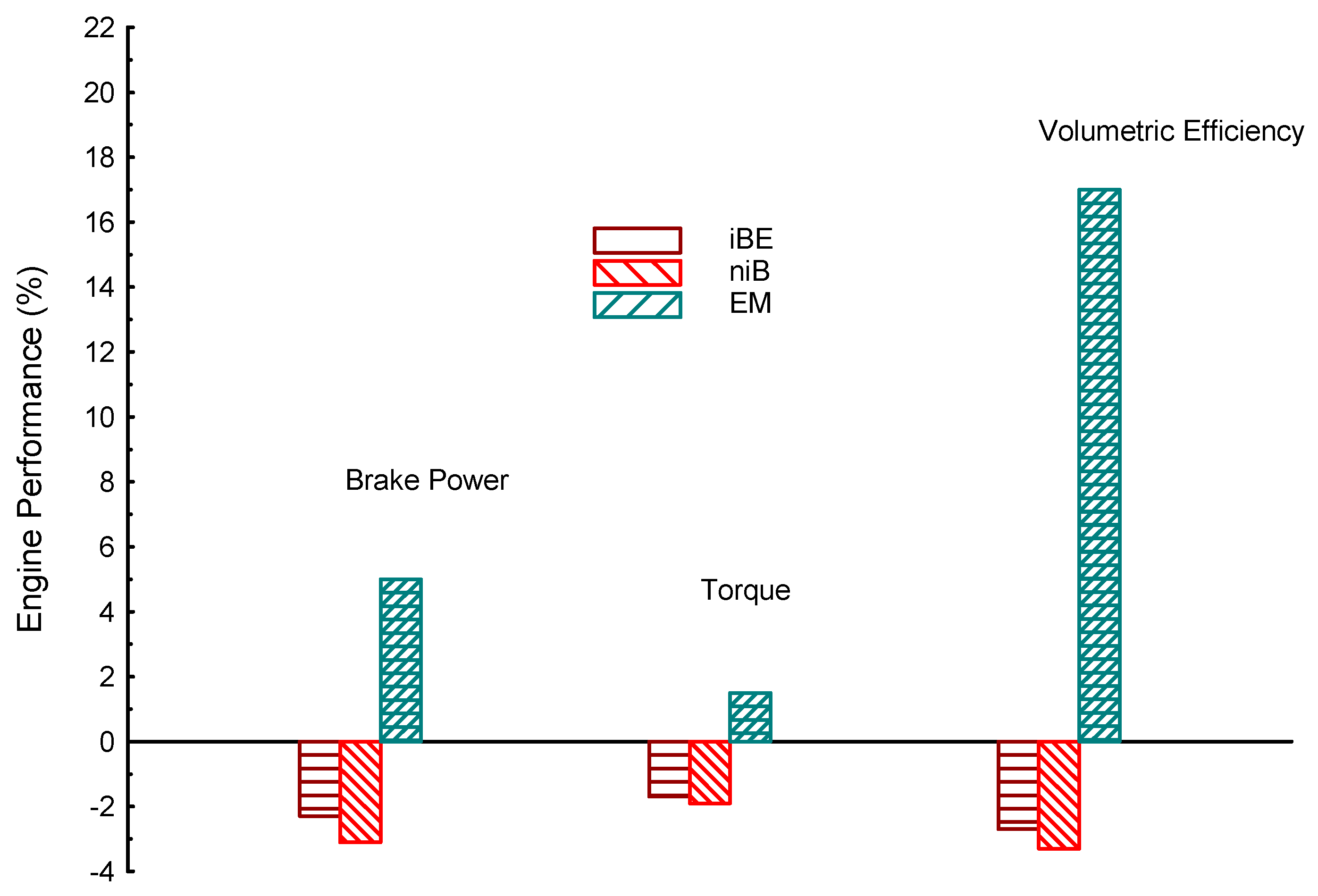
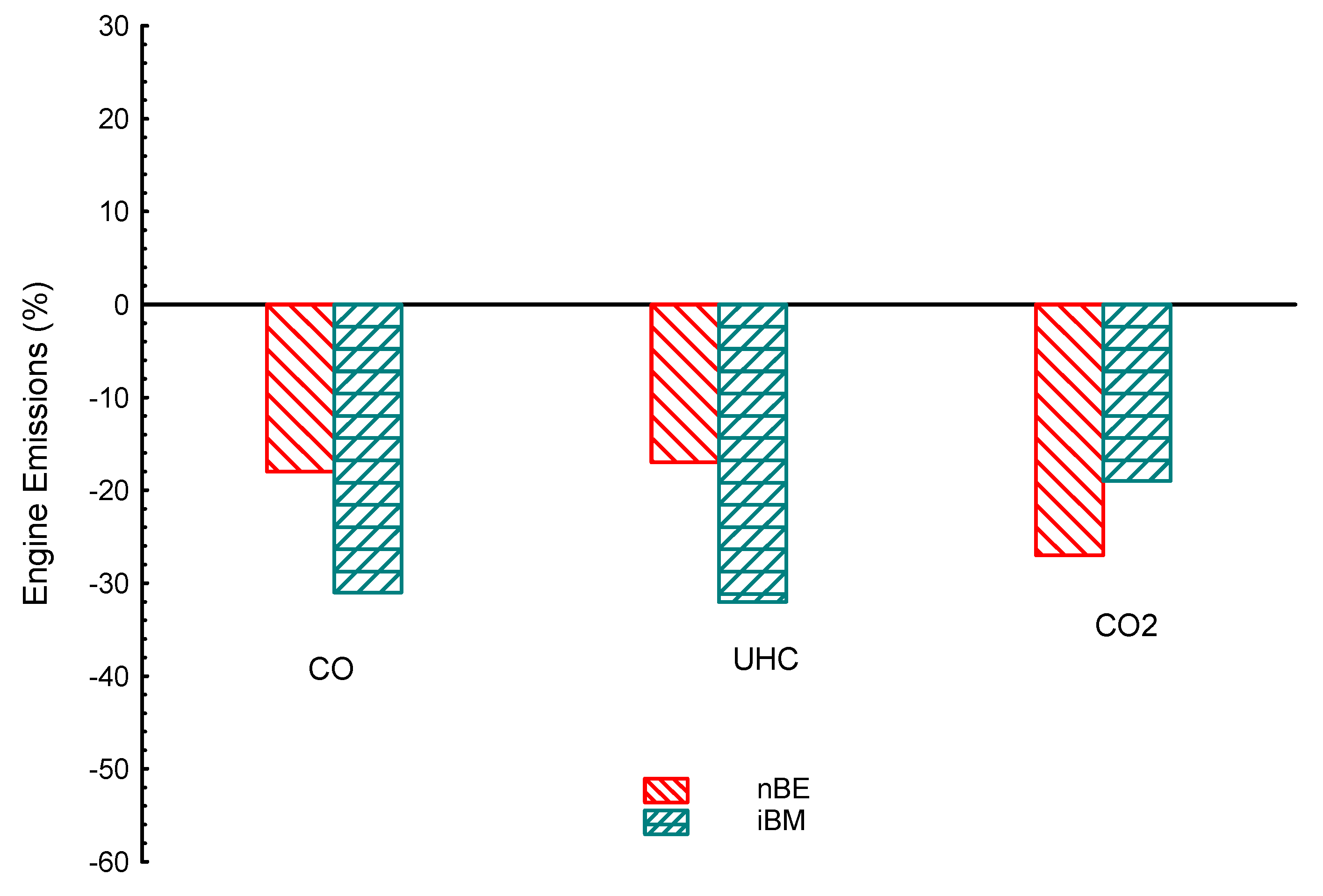
| Properties | BP | Tq | VE | CO | UHC | CO2 | |
|---|---|---|---|---|---|---|---|
| Biofuel | |||||||
| E | 8.5 | 1.2 | 16 | −20 | −10 | 4.6 | |
| AC | 3.3 | 1.6 | 1.2 | −32 | −20 | −24.6 | |
| iB | −5.6 | −1.36 | −2.1 | −11.6 | −6.8 | −36 | |
| nB | −3.6 | −1.6 | −2.6 | −10 | −16.2 | −35 | |
| M | 1 | 0.5 | 21 | −30 | −12.8 | 10 | |
| iBE | −2.3 | −1.7 | −2.7 | −14 | −14 | −14 | |
| niB | −3.1 | −1.9 | −3.3 | −5 | −13.5 | −18 | |
| EM | 5 | 1.5 | 17 | −21 | −18 | 6.3 | |
| nBM | −4.3 | −2.3 | −2.9 | 10 | 3.7 | −5.8 | |
| iBM | −2.6 | −0.43 | −2.4 | −31 | −32 | −19 | |
| nBE | −5.2 | −1.9 | −2.4 | −18 | −17 | −27 | |
| Properties | Bio-Ethanol | Bio-Methanol | I-Butanol | N-Butanol | Acetone |
|---|---|---|---|---|---|
| Performance | Ben. | Ben. | Ben. | Ben. | Ben. |
| Emissions | Ben. | Ben. | Ben. | Ben. | Ben. |
| Oxygen content | Ben. | Ben. | Ben. | Ben. | Ben. |
| Hydrogen content | Wek. | Wek. | Wek. | Wek. | Wek. |
| Carbon content | Wek. | Wek. | Wek. | Wek. | Wek. |
| Absorption with water | Wek. | Wek. | Ben. | Ben. | Ben. |
| Boiling Temp. | Wek. | Wek. | Ben. | Ben. | Wek. |
| Vapor toxicity | Ben. | Ben. | Wek. | Wek. | Ben. |
| Heating value | Wek. | Wek. | Wek. | Wek. | Wek. |
| Autoignition | Ben. | Ben. | Wek. | Wek. | Ben. |
| Corrosion | Wek. | Wek. | Ben. | Ben. | Wek. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfasakhany, A. State of Art of Using Biofuels in Spark Ignition Engines. Energies 2021, 14, 779. https://doi.org/10.3390/en14030779
Elfasakhany A. State of Art of Using Biofuels in Spark Ignition Engines. Energies. 2021; 14(3):779. https://doi.org/10.3390/en14030779
Chicago/Turabian StyleElfasakhany, Ashraf. 2021. "State of Art of Using Biofuels in Spark Ignition Engines" Energies 14, no. 3: 779. https://doi.org/10.3390/en14030779
APA StyleElfasakhany, A. (2021). State of Art of Using Biofuels in Spark Ignition Engines. Energies, 14(3), 779. https://doi.org/10.3390/en14030779




