A Novel Design Portable Plugged-Type Soil Microbial Fuel Cell for Bioelectricity Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soil Sampling
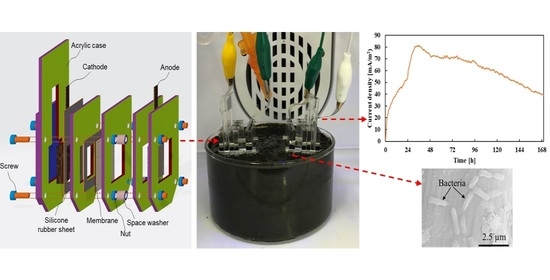
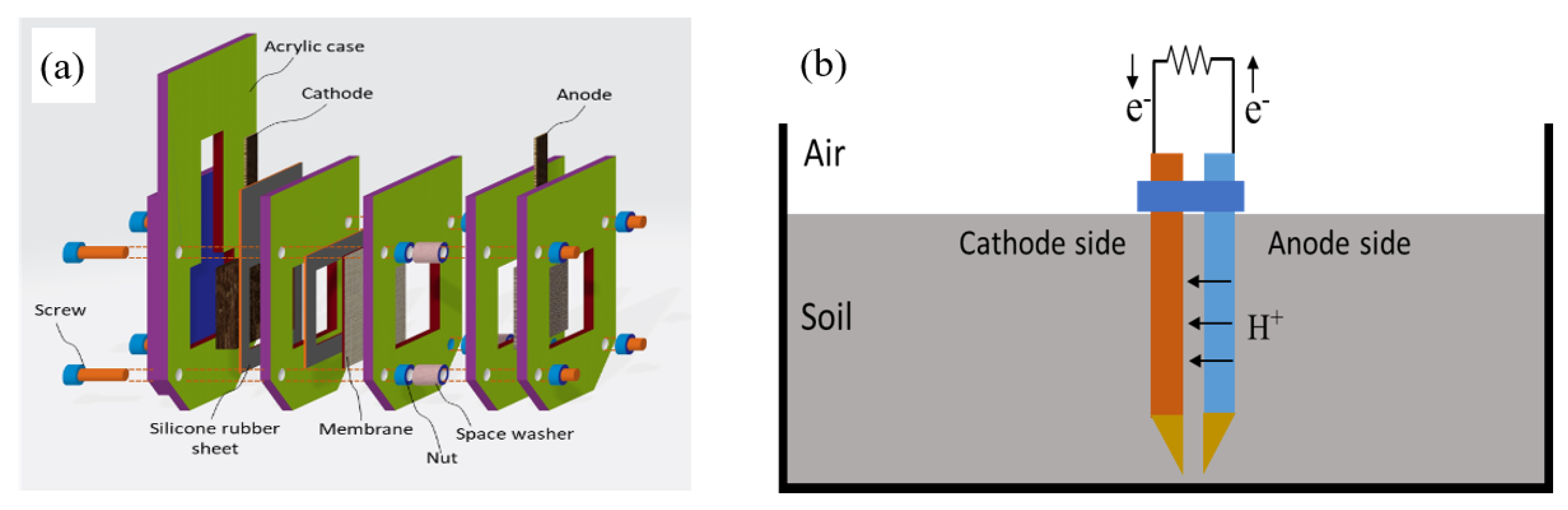
2.3. Plugged-Type Soil Microbial Fuel Cell (PSMFC) Configuration and Operation
2.4. Analysis and Calculation
3. Results and Discussion
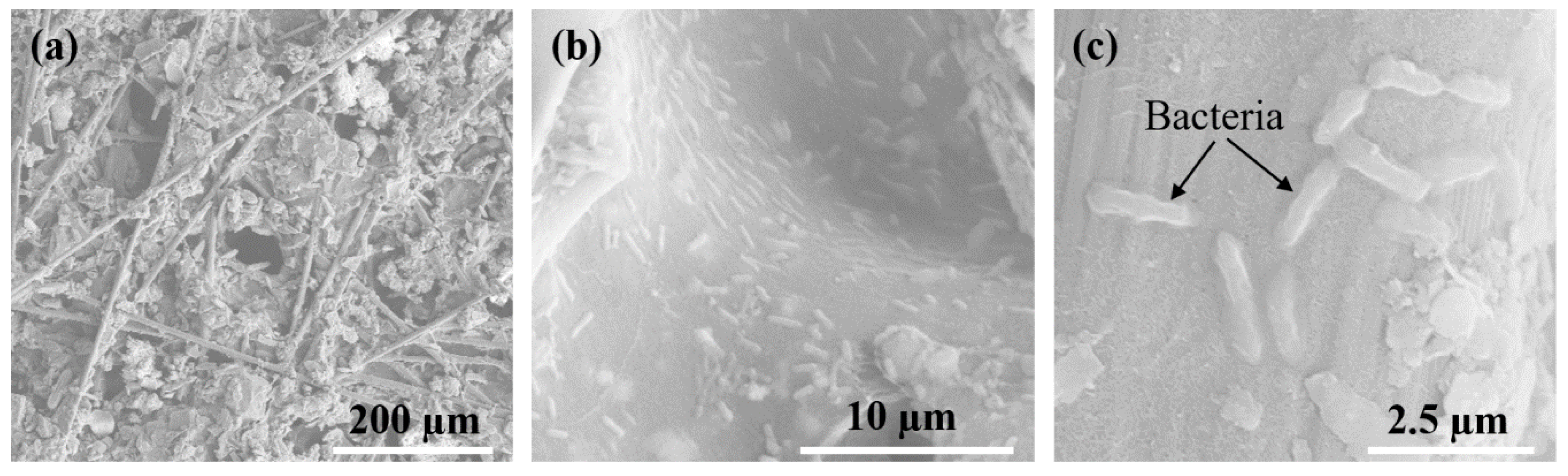
3.1. Characterization of Anode Surface Morphology and Biofilm Formation
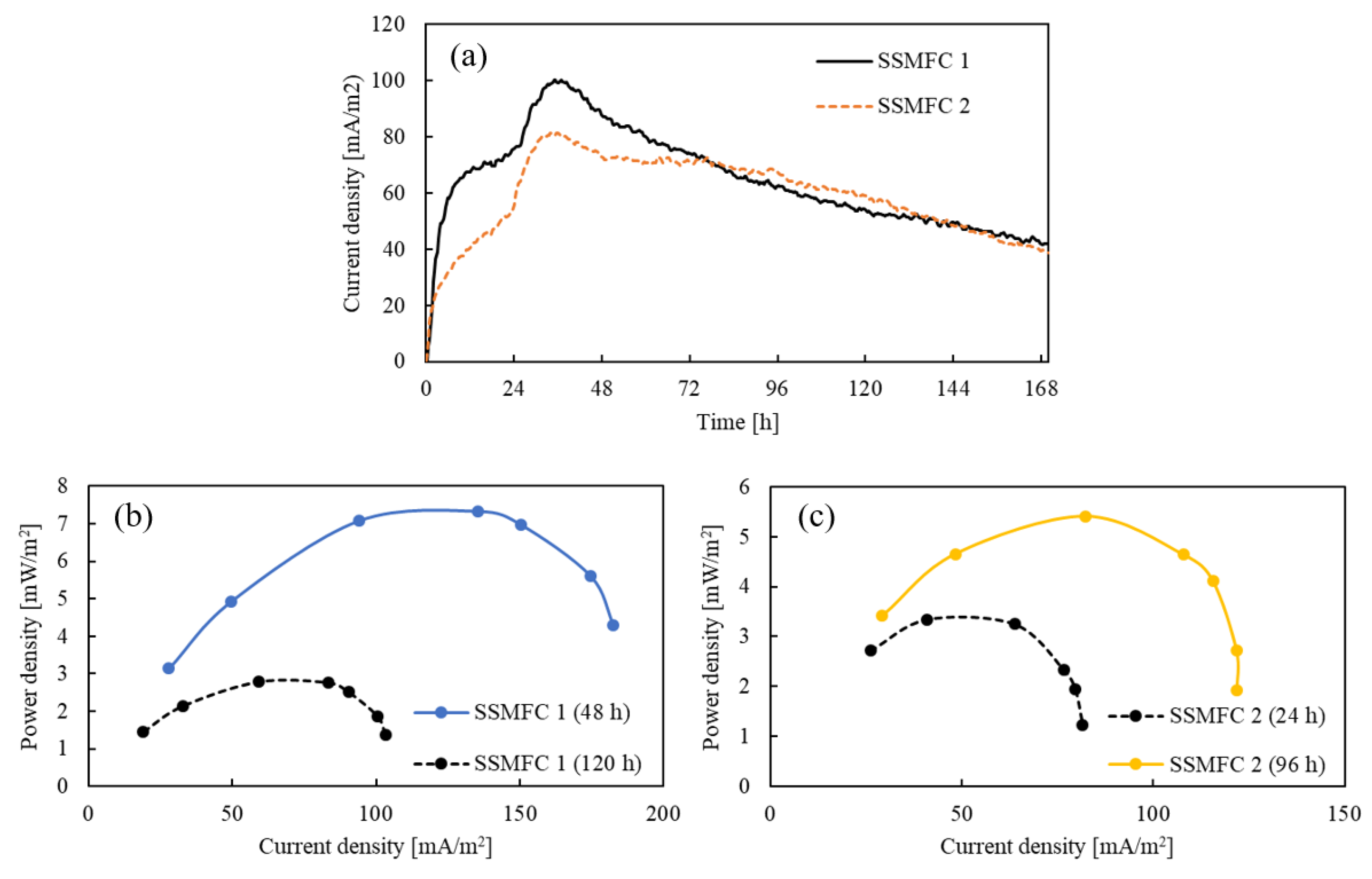
3.2. PSMFC Performance and the Effect of Different Cathodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigo, J.; Boltes, K.; Esteve-Nuñez, A. Microbial-electrochemical bioremediation and detoxification of dibenzothiophene-polluted soil. Chemosphere 2014, 101, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sherafatmand, M.; Ng, H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Jiang, Y.; Alvarado-Morales, M.; Treu, L.; Angelidaki, I.; Zhang, Y. Electricity generation and microbial communities in microbial fuel cell powered by macroalgal biomass. Bioelectrochemistry 2018, 123, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, Q.; Chen, Y.; Yin, J.; Duan, T. Enhanced Performance of a Microbial Fuel Cell with a Capacitive Bioanode and Removal of Cr (VI) Using the Intermittent Operation. Appl. Biochem. Biotechnol. 2016, 180, 1372–1385. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Wang, H.; Long, X.; Li, X. Simultaneous enhancement of heavy metal removal and electricity generation in soil microbial fuel cell. Ecotoxicol. Environ. Saf. 2020, 192, 110314. [Google Scholar] [CrossRef]
- Yu, D.; Bai, L.; Zhai, J.; Wang, Y.; Dong, S. Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta 2017, 168, 210–216. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhao, Q.; Wan, L.; Li, Y.; Zhou, Q. Carbon fiber enhanced bioelectricity generation in soil microbial fuel cells. Biosens. Bioelectron. 2016, 85, 135–141. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Zhou, Q.; Zhang, Z.; Chen, C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol. Bioeng. 2012, 109, 426–433. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RCS Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ren, Z.J.; Zhang, Y.; Li, N.; Zhou, Q. Sand amendment enhances bioelectrochemical remediation of petroleum hydrocarbon contaminated soil. Chemosphere 2015, 141, 62–70. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, Y.; Cheng, L.; Liu, J.; Li, F.; Gao, B.; Zhou, Q. Extended petroleum hydrocarbon bioremediation in saline soil using Pt-free multianodes microbial fuel cells. RSC Adv. 2014, 4, 59803–59808. [Google Scholar] [CrossRef]
- Lu, L.; Yazdi, H.; Jin, S.; Zuo, Y.; Fallgren, P.H.; Ren, Z.J. Enhanced bioremediation of hydrocarbon-contaminated soil using pilot-scale bioelectrochemical systems. J. Hazard. Mater. 2014, 274, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Wen, Q.; Zheng, H.; Xu, H.; Qi, L. Electricity generation, energy storage, and microbial-community analysis in microbial fuel cells with multilayer capacitive anodes. Energy 2019, 189, 116342. [Google Scholar] [CrossRef]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally Sustainable Fabrication of Ag@g-C3N4 Nanostructures and Their Multifunctional Efficacy as Antibacterial Agents and Photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Environmentally sustainable biogenic fabrication of AuNP decorated-graphitic g-C3N4 nanostructures towards improved photoelectrochemical performances. RSC Adv. 2018, 8, 13898–13909. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.E.; Khan, M.M.; Min, B.K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1723. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Ansari, S.A.; Lee, J.H.; Lee, J.; Cho, M.H. Mixed Culture Electrochemically Active Biofilms and their Microscopic and Spectroelectrochemical Studies. ACS Sustain. Chem. Eng. 2014, 2, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.; Mohamed, A.; Ha, P.T.; Bloom, J.; Ewing, T.; Arias-Thode, M.; Chadwick, B.; Beyenal, H. The Influence of Energy Harvesting Strategies on Performance and Microbial Community for Sediment Microbial Fuel Cells. J. Electrochem. Soc. 2017, 164, H3109–H3114. [Google Scholar] [CrossRef]
- Jayakumar, A.R. An assessment on polymer electrolyte membrane fuel cell stack components. Appl. Phys. Chem. Multidiscip. Approaches 2018, 3, 23–49. [Google Scholar]
- Li, S.; Cheng, C.; Thomas, A. Carbon-Based Microbial-Fuel-Cell Electrodes: From Conductive Supports to Active Catalysts. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Taguchi, K. Enhancing the performance of E. coli-powered MFCs by using porous 3D anodes based on coconut activated carbon. Biochem. Eng. J. 2019, 151, 107357. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.-U.-D.; Nguyen, D.-T.; Taguchi, K. A Novel Design Portable Plugged-Type Soil Microbial Fuel Cell for Bioelectricity Generation. Energies 2021, 14, 553. https://doi.org/10.3390/en14030553
Nguyen H-U-D, Nguyen D-T, Taguchi K. A Novel Design Portable Plugged-Type Soil Microbial Fuel Cell for Bioelectricity Generation. Energies. 2021; 14(3):553. https://doi.org/10.3390/en14030553
Chicago/Turabian StyleNguyen, Hoang-Uyen-Dung, Dang-Trang Nguyen, and Kozo Taguchi. 2021. "A Novel Design Portable Plugged-Type Soil Microbial Fuel Cell for Bioelectricity Generation" Energies 14, no. 3: 553. https://doi.org/10.3390/en14030553
APA StyleNguyen, H.-U.-D., Nguyen, D.-T., & Taguchi, K. (2021). A Novel Design Portable Plugged-Type Soil Microbial Fuel Cell for Bioelectricity Generation. Energies, 14(3), 553. https://doi.org/10.3390/en14030553