Abstract
Liquid carbon dioxide (L-CO2) phase-transition blasting technology (LCPTB) has caused wide concern in many fields, but there is a lack of research on the initiation of the carbon dioxide fracturing pipe. Studies regarding the carbon dioxide fracturing pipe initiation are critical for controlling and optimizing the LCPTB. Therefore, in this article, a series of exploratory experiments of carbon dioxide blasting were carried out to investigate the qualitative and quantitative relationships between the carbon dioxide fracturing pipe initiation and the three key variables (the filling mass of liquid carbon dioxide (L-CO2) (X1), the amount of chemical heating material (X2) and the thickness of the constant-stress shear plate (X3)). The failure mechanisms of three variables on the phase-transition blasting process of a carbon dioxide fracturing pipe was analyzed qualitatively based on experiment temperature, strain curve and failure form of constant-stress shear plate. An empirical model between the carbon dioxide fracturing pipe initiation (Y) and the three key variables (X1, X2, X3) was obtained after processing experiment result data quantitatively. Based on the phase-transition and blasting process of carbon dioxide, two methods, the Viral–Han–Long (VHL) equation of gas state (EOS) and the strength-failure method were used to calculate the blasting pressure and determine the failure mode of the fracturing pipe. The proposed blasting empirical model can be used to optimize the structural design of carbon dioxide fracturing pipes, guide on-site carbon dioxide blasting operations and further achieve the best blasting effect of LCPTB, so this work can enable LCPTB to be better applied to practical projects.
1. Introduction
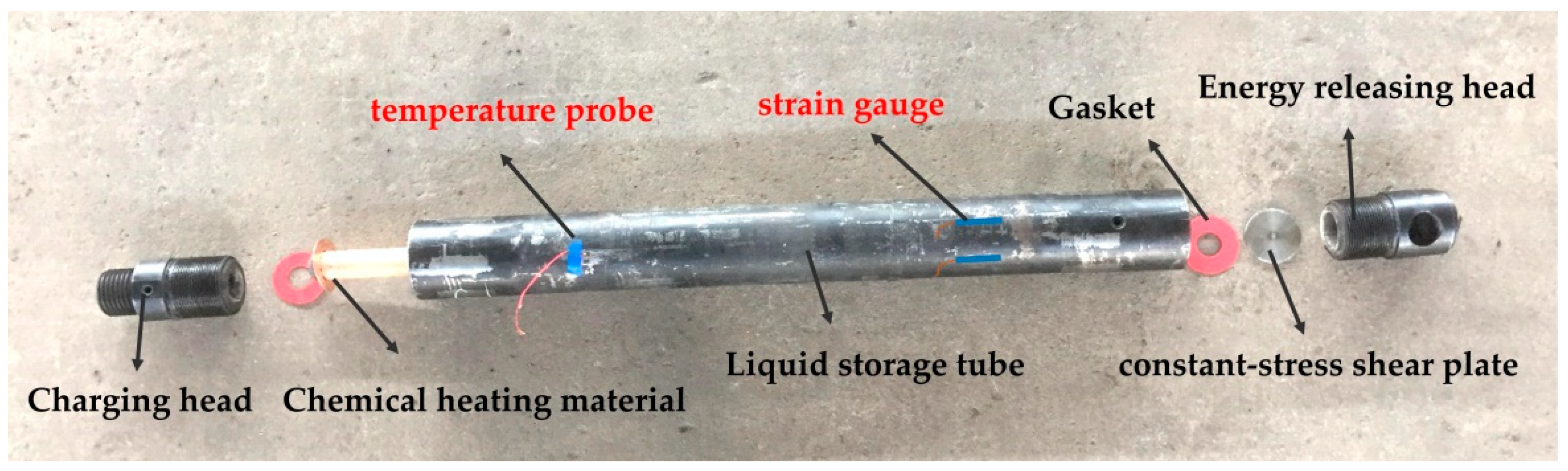
Liquid carbon dioxide (L-CO2) phase-transition blasting technology (LCPTB) has caused wide concern in many fields, and utilizes the conversion characteristics between carbon dioxide gas and liquid phases for blasting fracturing [1]. The fracturing pipe is the core of the entire LCPTB system, which is used to realize the phase-transition blasting process of L-CO2 (LCPTB). As shown in Figure 1, the carbon dioxide fracturing pipe is mainly composed of a charging head, activator (chemical heating material), liquid storage tube, constant-stress shear plate, gasket and energy releasing head. The activator is a composite device, which is used to store the chemical heating material. Under the stimulation of the instantaneous high pressure generated by a remote detonator, the chemical heating material is ignited to realize the heating of the fracturing pipe. After absorbing a large amount of thermal energy generated by the activator, L-CO2 gasifies quickly and its volume instantly expands more than 600 times [2]. When the gas pressure reaches the ultimate strength of the constant-stress shear plate, the constant-stress shear plate will be destroyed. After that, the high-energy carbon dioxide gas is released from the front end of the energy releasing head, and the blasting occurs and causes the rock mass crack, then the high-pressure gas migration makes fractures secondary development and expansion. The blasting products are gaseous CO2 and water vapor, with no sparks or flames, and the releasing pressure and energy can be controlled and modified easily according to user requirements [3]. Therefore, the LCPTB has the significant advantages in controllable blasting pressure, uniform seam formation, being economical and pollution-free, and having intelligent control. Meanwhile, it overcomes the disadvantages of heavy harm, serious pollution, and high cost in the chemical blasting process. As such, the blasting technology has caused a wide concern in geotechnical engineering [4], municipal transportation [5], geological engineering [6], gaseous industry [7], geothermal engineering [8,9,10,11] and other fields [12,13].
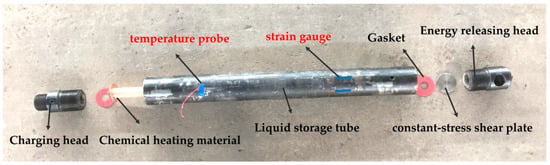
Figure 1.
The structure of carbon dioxide fracturing pipe.
LCPTB is also known as the cardox tube system, which was proposed by a British company in 1914, and it began to be used during the early 1950s [14,15,16]. In 1989, it was introduced into licensed coal mines in South Wales and recently was successfully used on a shaft sinking project in granite rock [17]. Patrick [18] and Caldwell, T [19] contrasted the advantages of CO2 blasting relative to traditional chemical blasting. Then, in 1998, Singh simply described a rock breaking project with this blasting technology at quarrying in Turkey. At the end of the 20th century, LCPTB was introduced to the coal mining industry and has been greatly applied in the enhancement of coal seam permeability [2,3,4,7,20,21]. Nowadays, with the help of field experiment, laboratory simulation, comparative analysis, numerical simulation and other scientific research methods, the blasting fracturing process [22,23,24,25], blasting fracturing energy [26,27,28,29,30], blasting fracturing mechanism [22,26,31] and blasting phase-transition process of the LCPTB were discussed in detail [23,30,31,32,33]. The blasting process of the LCPTB can be accurately controlled by the carbon dioxide fracturing pipe, and many scholars have also focused on this research. Chen et al. [17] designed experimental equipment to monitor shock wave pressure during the LCPTB, which is convenient for in-depth study of the LCPTB mechanism. Based on in-depth research on gas blasting, Hu et al. [6] proposed a new CO2 static pneumatic fracturing technology. Li et al. [34] proposed novel liquid carbon dioxide rock-breaking technology and designed the relative device. Finally, the technology was successfully applied in rock excavation at a metro station construction site.
In summary, most of the LCPTB research focuses on phase-transition mechanism [22,26,31], rock blasting [32,35], blasting antireflection [2,3,4,7,20,21] and blasting fracture propagation [11,20,36,37]. However, there is a lack of research on the initiation of the fracturing pipe and especially research on the relationship among controllable blasting parameters, blasting pressure and blasting effect in the LCPTB. The filling mass of L-CO2, the amount of chemical heating material and the thickness of the constant-stress shear plate are three key variables which directly determine the fracturing pipe initiation. These three key variables have mainly relied on practical experience, which is not completely scientific and reliable. Studies regarding the carbon dioxide fracturing pipe initiation are critical for controlling and optimizing the LCPTB. Furthermore, the fracturing pipe initiation directly affects the effect of blasting and fracturing. The successful blasting of the fracturing pipe is conducive to improving blasting efficiency, saving blasting costs, and achieving optimal blasting benefits. Therefore, it is necessary to study the relationship between the fracturing pipe initiation and the three key variables. Hence, in this article, according to the results of carbon dioxide blasting experiments, the quantitative and qualitative relationships among the three key variables (X1, X2, X3) and the fracturing pipe initiation (Y) were explored. The calculation models of blasting pressure were used to determine the failure mode of the fracturing pipe. The proposed blasting empirical model can be used to optimize the structural design of carbon dioxide fracturing pipes, guide on-site carbon dioxide blasting operations, and further achieve the best blasting effect of LCPTB. This will enable the LCPTB to be better applied to practical projects, so this work has great practical guiding significance.
2. Experimental Methodology
2.1. Experimental Apparatus
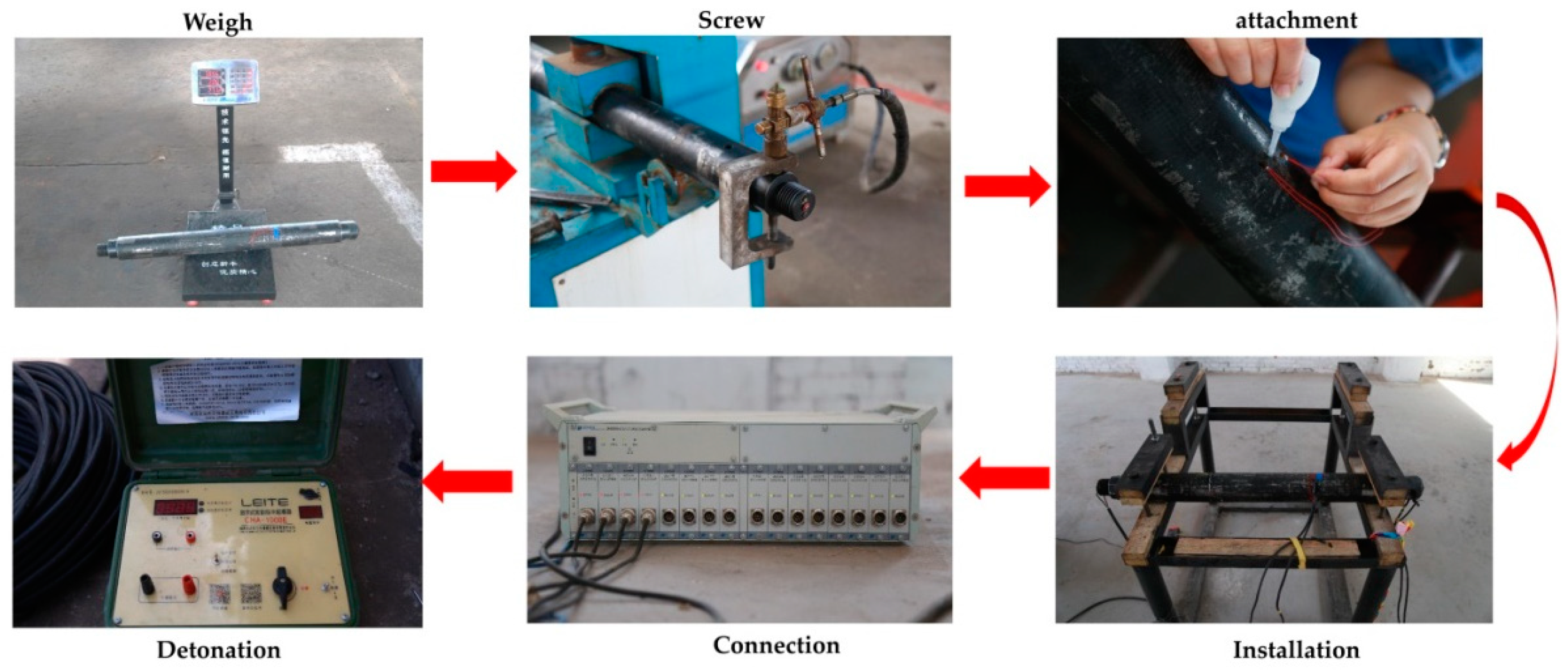
A carbon dioxide blasting experiment system consists of a filling system, blasting system and detection system. The filling system is used to complete the transfer of L-CO2 from the storage container to the fracturing pipe, and is equipped with a carbon dioxide storage tank, a filling machine and a fracturing pipe screwing machine. The blasting system is the key to successful experiment, and it is also the location of the three key variables in the experiment, including fracturing pipe and high-energy pulse detonator. The detection system plays the role of recording real-time temperature and strain changes during the experiment, and consists of a 8852K.J.T. thermocouple temperature meter and DH5956 strain dynamic measuring instrument. The fracturing pipe-95 was used in the experiment (Figure 1), with a length of 910 mm, an outer diameter of 95 mm, an inner diameter of 65 mm and a volume of 1.93 L. The diameter of the constant-stress shear plate used in the experiment was 42 mm, and the material was 45 steel. The physical diagram of the carbon dioxide blasting experiment system is shown in Figure 2.

Figure 2.
Carbon dioxide blasting experiment system.
2.2. Experimental Setup
In this article, 17 groups of exploratory experiments on carbon dioxide blasting were carried out in a 10 × 10 m abandoned boiler room. The experimental object was the carbon dioxide fracturing pipe, which has no action object. The experiments were used to explore the empirical model between the three key variables and the fracturing pipe initiation. During the experiment, the thickness of constant-stress shear plate and the amount of chemical heating material are considered as specific variables. The constant-stress shear plate of 4.0 mm, 4.5 mm and 5.0 mm, and the amount of chemical heating material of 250 g and 300 g were designed, respectively. The filling mass of L-CO2 is an undetermined variable, which needs to be adjusted according to the field experiment situation. At the same time, considering the experiment cost and sealing requirements, the experiment adopted the method of attaching the temperature measurement probe to the outside of the liquid storage tube, and used the stress-strain method to measure the pressure value indirectly. The strain gauge and temperature gauge were used to record the strain and temperature data of the entire experiment, and the remote intelligent control of the detonator was adopted in the blasting field to make this experiment safe and operable.
2.3. Experimental Process
The experimental filling-blasting process is shown in Figure 3. Firstly, the thickness of constant-stress shear plate and the amount of chemical heating material used in the experiments were selected, the internal volume V of Fracturing pipe was measured by water injection, and the mass (m) of the fracturing pipe before filling was measured. Secondly, the filling mass of L-CO2 (M) in the experiment was measured, and the L-CO2 storage tank and L-CO2 charging machine were used to complete the filling of L-CO2. Furthermore, after meeting the air tightness requirements of a fracturing pipe, the experimental site was arranged, and the strain gauge and the temperature probe were fixed as shown in Figure 1. Finally, the detonating wire was connected to high-energy pulse detonator, and the detonator was switched on to realize blasting.
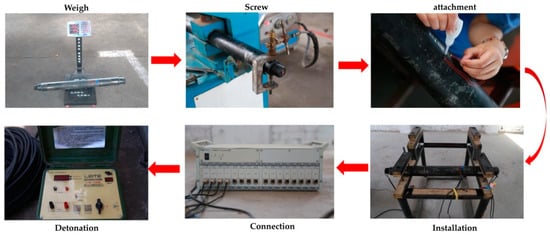
Figure 3.
The experimental filling-blasting process.
3. Results and Discussion
Among the 17 groups of carbon dioxide blasting experiments, 10 groups were successfully blasted, while 7 groups failed, and the specific values of the experiment variables are shown in Table 1. The constant-stress shear plate of the successful blasting fracturing pipe was broken into fragments and showed different degrees of distortion. However, the failed blasting fracturing pipe did not fracture, and showed a convex shape with different widths.

Table 1.
The values of the experimental variables.
3.1. Variables that Affect the Phase-Transition of Liquid Carbon Dioxide (L-CO2)
CO2 is a strong sublimation substance, which generally exists in a gaseous state in nature. Studies have shown that it can exist as liquid form when the pressure exceeds 5.1 times the atmospheric pressure [3]. When the temperature is higher than 31.4 °C and the pressure exceeds 7.385 MPa, the interface between liquid phase and gas phase of carbon dioxide disappears and carbon dioxide transforms into the supercritical state [21], which has the dual properties of a high density liquid and high diffusion gas.
In the carbon dioxide blasting experiments, chemical heating material supplied thermal energy to the system and raised the temperature of the system, then L-CO2 was vaporized. The filling mass carbon dioxide determines the expansion pressure of L-CO2 phase-transition, and the two key variables jointly control the entire CO2 phase-transition process and directly affect the blasting effect of the fracturing pipe. Exploring the effects of the above two variables on the phase-transition of CO2 is helpful to reasonably determine the combination parameters, improve the efficiency of blasting operations, optimize the structure design of the fracturing pipe, and achieve the best blasting effect.
- (1)
- The amount of chemical heating material
The thickness of the constant-stress shear plate and the filling mass of L-CO2 remain unchanged, only the amount of chemical heating material was changed from 250 g to 300 g. The fracturing pipe is likely to be detonated from failure to success. Experiment 1 and 11, 9 and 17 were used to analyze in this paper, and the experimental parameters are shown in Table 1. The constant-stress shear plate of experiment 1 is not damaged, the gas pressure makes it protrude outwards, and the maximum convex width is 10.4 mm. The constant-stress shear plate of experiment 11 is broken into fragments, and the fragments show varying degrees of distortion (Figure 4).

Figure 4.
Displays after experiments of constant-stress shear plate (the maximum width of convex area of each constant-stress shear plate is shown in the figures).
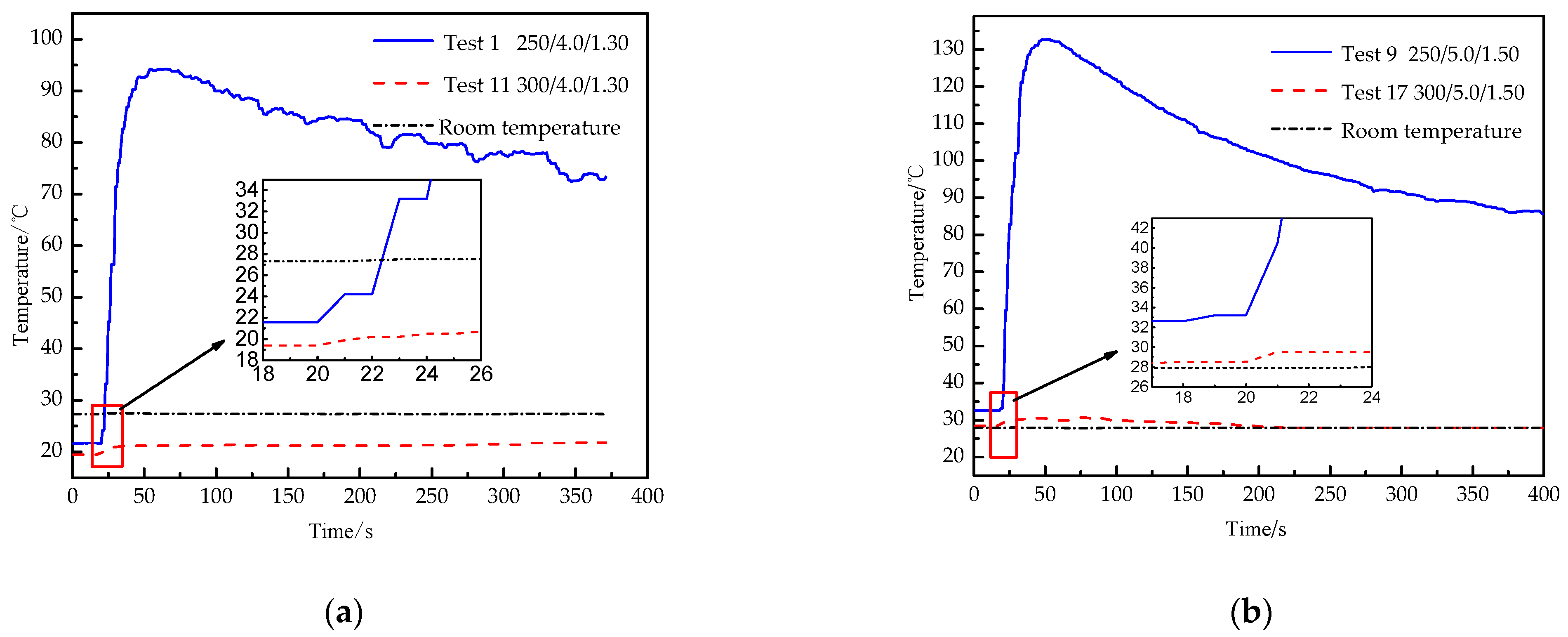
Temperature can be used to reveal the mechanism and effect of the activator. Figure 5 shows the real-time temperature curves of experiments 1, 11, 9 and 17, in which 250/4.0/1.30 means the amount of chemical heating material is 250 g, the thickness of constant-stress shear plate is 4.0 mm, and the filling mass of L-CO2 is 1.30 kg.
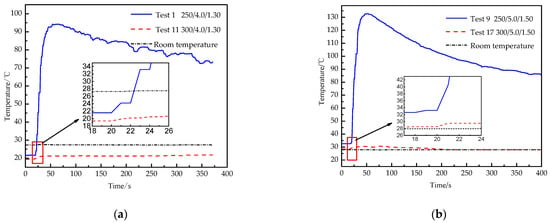
Figure 5.
Real-time temperature curve of texts. (a). Real-time temperature curve of experiments 1 and 11; (b). real-time temperature curve of experiments 9 and 17.
The temperature of experiment 1 increases by 4.36 times from 21.6 °C to 94.1 °C, and then gradually decreases to room temperature. The temperature of experiment 11 increases by 1.10 times from 19.4 °C to 21.4 °C, then gradually parallels to room temperature, and room temperature stabilizes at 27.4 °C. The temperature of experiment 9 increases by 4.07 times from 32.6 °C to 132.8 °C, and then gradually decreases to room temperature. The temperature of experiment 17 increases by 1.08 times from 28.4 °C to 30.8 °C, then gradually decreases to room temperature, and room temperature stabilizes at 27.9 °C. There is an obvious difference between successful blasting and failure blasting. When the blasting is successful, most of the thermal energy is taken away by the gas rushed out from the energy releasing head, so that the temperature of the outer wall is too late to rise. By contrast, when the blasting fails, high-pressure gas accumulates in the tube, and the gas slowly leaks out from the convex deformed part of the constant-stress shear plate, and the outer wall is fully heated, so that the temperature of the outer wall rises sharply.
The temperature curves of experiment 1 and experiment 9 fluctuate greatly, with local oscillation, and increase rapidly before the curve highest point. The local oscillation of the temperature curves is mainly due to the local uneven heating of the outer wall and the softening of thermometry probe rubber induced by high temperature. The temperature curves of experiment 11 and experiment 17 increase slightly and the growth rate of the latter is slower than the former, the increase does not exceed 11%. It is further illustrated that the 300 g of the amount of chemical heating material can fully gasify L-CO2, and a large amount of thermal energy is taken away through the gas rushing out, while the thermal energy absorbed by the outer wall is less.
In conclusion, the increase of the amount of chemical heating material will increase the amount of heat released, which aggravates the phase-transition reaction process of carbon dioxide and increases the blasting pressure, and finally, the blasting effect of the fracturing pipe is improved.
- (2)
- Carbon dioxide mass
The thickness of constant-stress shear plate and the amount of chemical heating material remain unchanged, and only the filling mass of L-CO2 was changed. The experiment was divided into two groups, and the first group included experiments 4, 5, 6 and 7. The results show that the experiments were not blasted when the filling mass was 1.30 kg and 1.40 kg, while others blasted successfully. The second group included experiment 8, 9 and 10. The results show the experiments were not blasted when the filling mass was 1.20 kg and 1.50 kg, and the other one blasted successfully.
The experiment parameters are shown in Table 1, and only the first group of experiments are researched and analyzed in this paper. The constant-stress shear plates of experiment 4 (250/4.5/1.30) and experiment 5 (250/4.5/1.40) are not broken, and the maximum outward convex width is 10.4 mm and 10.9 mm, respectively. In experiment 6 (250/4.5/1.50), the constant-stress shear plate was divided into multiple sections, and the fragments show bending deformation. In experiment 7 (250/4.5/1.60), the constant-stress shear plate was directly sheared off (Figure 4). The damage degree of constant-stress shear plate in experiments 4–6 increases in turn, which indicates that the volume of carbon dioxide, the gas formed by L-CO2 phase-transition, increases in turn. The blasting pressure increases in turn, so that the experimental phenomena are more obvious.
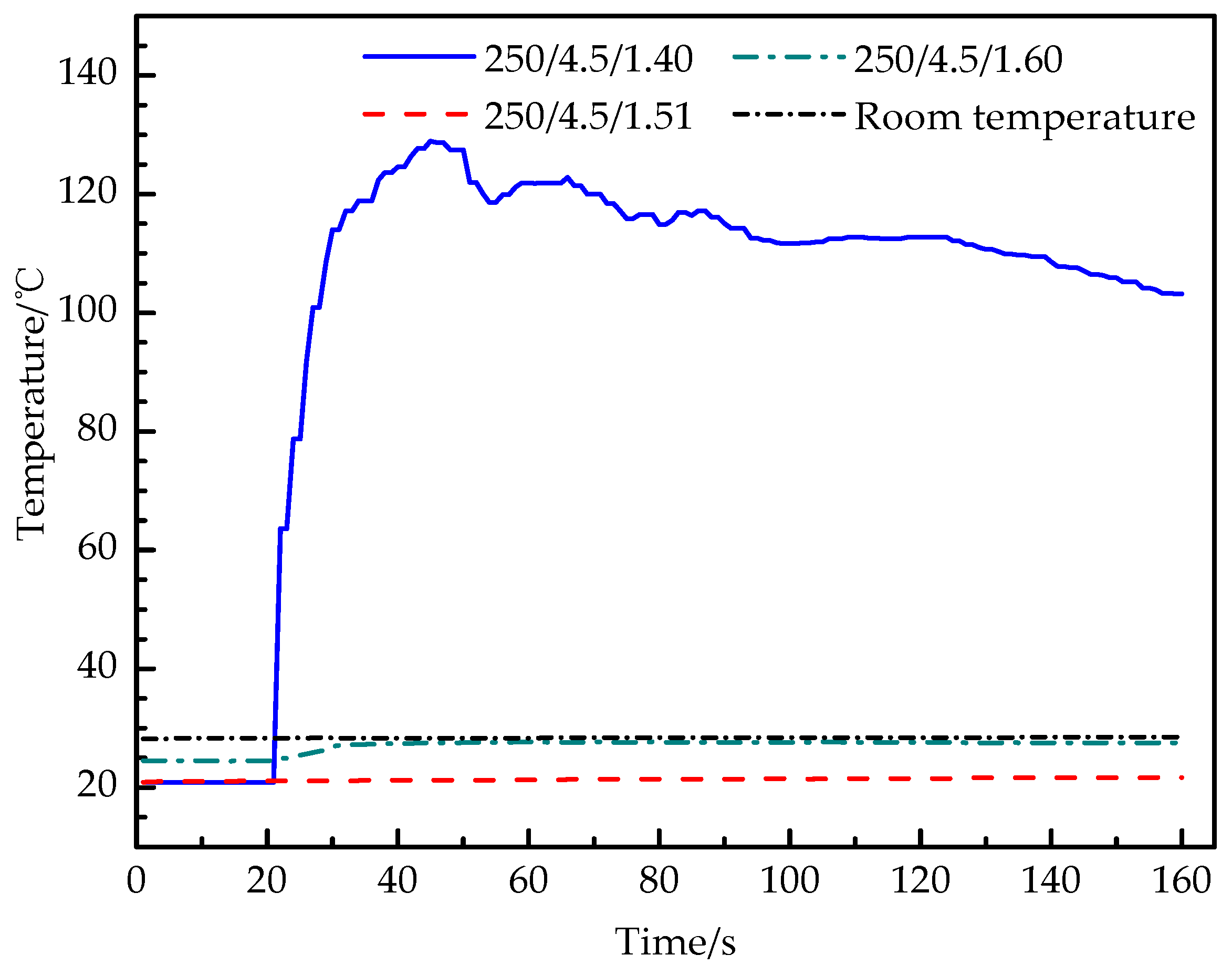
Figure 6 shows the real-time temperature curves of the outer wall in experiment 5, 6 and 7, and the temperature curves of experiment 6 and 7 are basically parallel to the room temperature line and lower than the room temperature line. The temperature of experiment 6 increases from 21 °C to 21.7 °C. Experiment 7 increases from 24.5 °C to 27.6 °C and experiment 5 increases rapidly from 20.9 °C to 129.4 °C, with a rising rate of 5.4 °C /s, then decreases gradually. The temperature curve shows that the increase of the filling mass of carbon dioxide will improve the intensity of the carbon dioxide phase-transition reaction in the tube, and a large amount of L-CO2 gasifies and expands. When the expansion pressure in the tube reaches the yield strength of the constant-stress shear plate, the blasting is successful.
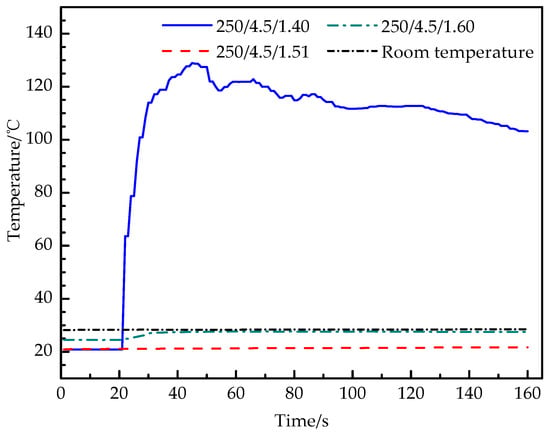
Figure 6.
Temperature–time curve of experiments 5, 6 and 7.
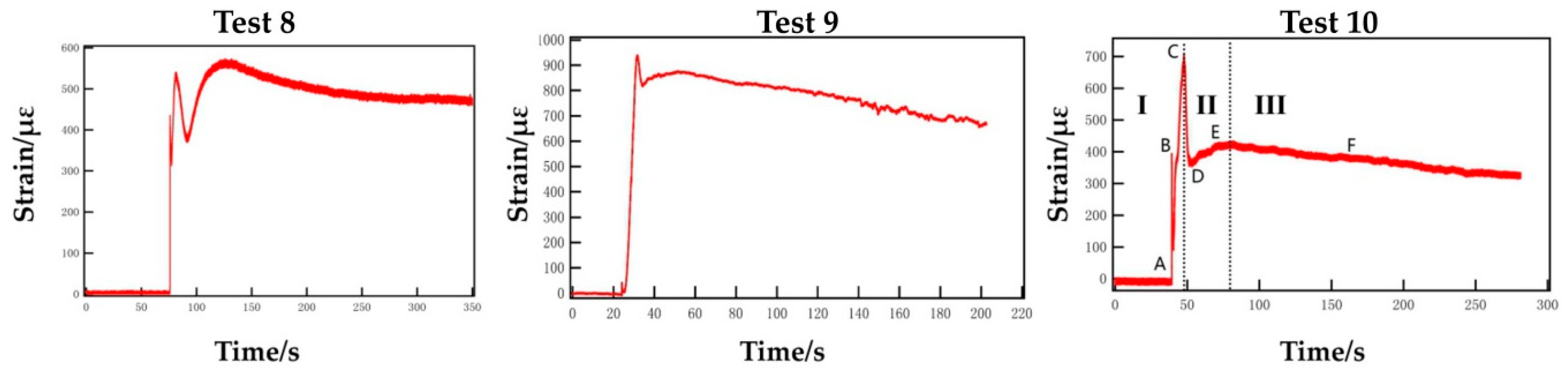
Figure 7 shows the strain variation trend of the fracturing pipe outer wall with time in experiments 8, 9 and 10. The paper is a simple analysis of the strain-time diagram of the successful blasting experiment 10 (250/5.0/1.60). Before the activator is electrified, the strain value swings back and forth in a very small range due to the electromagnetic interference of surrounding environment. After the activator is electrified, the curve of strain versus time can be divided into three stages in general. The first (I) stage is ABC (strain rising stage), the curve initially rises rapidly from point A to point B, then experiences a slight decrease and, finally, rises rapidly again until the curve reaches the highest at point C. The stage takes a short time and the curve rises quickly. A large amount of heat is released instantly when the activator is activated, which causes a slight thermal strain of the strain gauge. The first high point B appears, then L-CO2 absorbs heat and expands externally. After that, the high-pressure gas continues to act on the outer wall, and the deformation of the strain gauge increases exponentially, until reaching the highest point C.
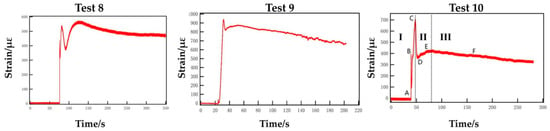
Figure 7.
Strain–time curve of experiments 8, 9 and 10.
The second (II) stage is CDE (“V”-shaped change stage); the curve drops quickly from the highest point C to the point D, then rises slowly to the point E again, and the point E is lower than the highest point C, which manifests a “V” shape. The rapid release of high-pressure gas makes the pressure in the tube drop sharply, and the deformation of the strain gauge recovers to a certain extent. However, because of the restriction of the symmetrical outlet hole of the energy releasing head and the closed design of the front-end, the release speed of high-pressure gas is reduced. The low-speed gas that is not fully released from the front end, the blockage of partially reflected gas and the squeezing effect of subsequent high-pressure gas make the internal pressure rise temporarily. Then the deformation of the strain gauge increases, thus forming a “V”-shaped trend.
The third (III) stage is EF (strain recovery stage), the curve decreases slowly, and gradually tends to be stable with a low decline and a long time. After the blasting is completed, the high-pressure gas fully overflows, and the pressure in the tube gradually returns to normal atmospheric. Then the deformation of the strain gauge is also gradually recovered, but the plastic deformation cannot be recovered, so the curve declines and gradually tends to a stable value.
In conclusion, the increase in the amount of chemical heating material will increase the amount of heat released, which aggravates the phase-transition reaction process of carbon dioxide and increases the blasting pressure, and finally the blasting effect of the fracturing pipe is improved.
3.2. Variables that Affect the Damage of the Fracturing Pipe-Constant-Stress Shear Plate
The constant-stress shear plate is equivalent to the control valve of the blasting energy releasing channel, and controls whether the whole system is successfully blasted. When the pressure in the tube is greater than the yield strength of the constant-stress shear plate, the constant-stress shear plate is destroyed, then the high-pressure gas rushes out quickly, and the blasting is successful. Otherwise, the blasting fails. The thickness of the constant-stress shear plate is directly related to its yield strength, and indirectly determines the blasting pressure released by the whole blasting system. The research on the influence of the thickness of the constant-stress shear plate on the blasting performance of the fracturing pipe has greatly guiding significance, which can optimize the structural design of carbon dioxide fracturing pipes, guide on-site carbon dioxide blasting operations and further achieve the best blasting effect of LCPTB.
The paper analyzes the experimental phenomena and data of experiment 3.6.9, experiment 9 (250/5.0/1.50) fails to blast, the constant-stress shear plate remains intact as a whole, and the maximum convex width of the central part is 11.3 mm (Figure 4). The constant-stress shear plates of experiment 6 (250/4.5/1.50) and experiment 3 (250/4.0/1.50) have different degrees of bending and torsion deformation, and the degree of deformation and fracture in experiment 6 is greater than that in experiment 3. The main reasons are as follows: firstly, L-CO2 gasifies and expands the instant when activator is excited, which makes the internal pressure of the fracturing pipe rise sharply, and the blasting occurs when the yield strength of the constant-stress shear plate is less than the internal pressure of the fracturing pipe. Secondly, at the moment of blasting, the constant-stress shear plate is simultaneously sheared and stretched. Coupled with the special structure of the front energy releasing head, the fragments are initially pushed by internal pressure to hit the front end of the energy releasing head, then pushed out of the tube by carbon dioxide airflow. So this reciprocating process aggravates the distortion of the fragments. Finally, when the amount of chemical heating material and the filling mass of L-CO2 are fixed, the maximum gas released pressure that can be generated inside the tube is determined. Therefore, the lower the failure pressure required for the constant-stress shear plate and the shorter the blasting time, the lower the degree of distortion of the fragments.
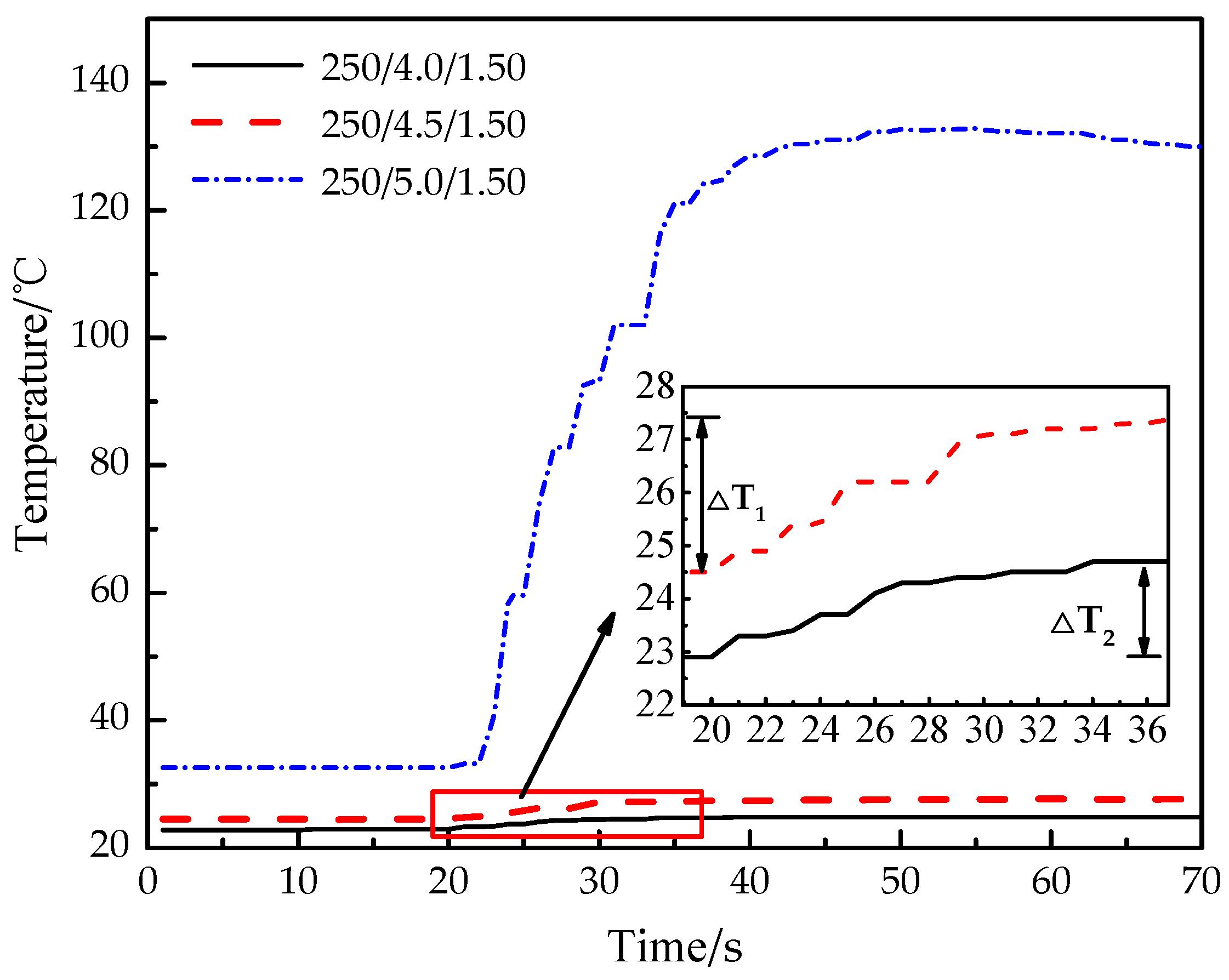
As shown in Figure 8, the temperature of experiments 3 and 6 increase slowly, and the temperature difference of experiment 6 (ΔT1) is 52.6% larger than that of experiment 3 (ΔT2). The temperature of experiment 3 (250/4.0/1.50) increases from 22.9 °C to 24.8 °C, which takes 19 s and the growth rate is 0.10 °C/s. The temperature of experiment 6 (250/4.5/1.50) increases from 24.5 °C to 27.4 °C, which takes 17 s and the growth rate is 0.17 °C/s. The temperature of experiment 9 (250/5.0/1.50) increases obviously, and the maximum value is much higher than that in experiments 3 and 6. The temperature of experiment 9 increases from 32.6 °C to 132.8 °C, which takes 35 s and the growth rate is 2.86 °C/s. The main reasons are as follows: with the increase of the thickness of the constant-stress shear plate, the constant-stress shear plate needs greater gas pressure to cause damage. The carbon dioxide gas inside the tube continues to absorb heat and heat up, and the continuous process of CO2 phase-transition becomes longer, then the temperature transferred to the outer wall becomes higher.
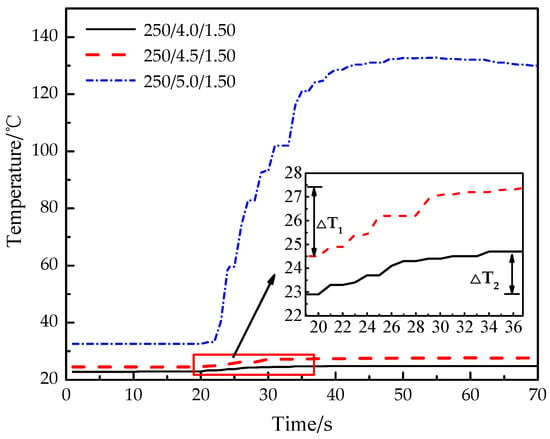
Figure 8.
Real-time temperature curve of experiments 3, 6 and 9.
To sum up, the yield strength of the constant-stress shear plate can be approximately regarded as the actual outlet pressure of the fracturing pipe, and the increase of the thickness of the constant-stress shear plate can significantly improve the blasting pressure of the fracturing pipe. The greater the thickness of the constant-stress shear plate, the greater the gas pressure required. However, when the internal gas pressure tends to the maximum, the thickness of the constant-stress shear plate will also limit the progress of the blasting.
3.3. The Quantitative Relationship between the Variables and Blasting
The filling mass of L-CO2 and the amount of chemical heating material jointly control the internal phase-transition reaction process of the fracturing pipe, and both directly affect the maximum gas pressure that can be released. The thickness of the constant-stress shear plate controls the blasting and energy release process of the fracturing pipe, which directly affects the blasting effect of the fracturing pipe. The process of L-CO2 from phase-transition to blasting is controlled by the three key variables. In order to explain the internal relationship between the successful blasting of the fracturing pipe and the three key variables, whether the fracturing pipe is blasted or not is regarded as the logical dependent variable Y (“1” stands for successful blasting and “0” stands for blasting failure), and the amount of chemical heating material, the thickness of constant-stress shear plate and the filling mass of L-CO2 are defined as independent variables X1, X2, X3, respectively. Then, Fisher discriminant analysis was used to quantify the experimental results, and the mathematical representation equation between Y and X was obtained.
Among them, the coefficients in front of the independent variables X1, X2, X3 respectively represent their weights ω1, ω2, ω3 for the dependent variable Y, and ω4 represent the weight of unconsidered factors for dependent variable Y.
According to the analysis of the equation, it can be seen that the filling mass of L-CO2 (kg) and the amount of chemical heating material (g) have a positive effect on whether the fracturing pipe is blasted. And the influence degree of the filling mass of carbon dioxide is about 231 times higher than the amount of chemical heating material. Yet the thickness (mm) of constant-stress shear plate has a negative effect. In the actual blasting operations, the filling mass of L-CO2 is also the most easily controlled variable, so the filling mass of L-CO2 can be properly increased to ensure the success of blasting. This result is consistent with the analysis of the previous experimental results and the conclusion of the pressure, volume and temperature (PVT) equation of high-pressure gas. The equation can be further explained that the area above the plane (Y > 0) indicates successful blasting, while the area below the plane (Y < 0) represents failed blasting. This result can quantitatively explain the mathematical relationship between the successful blasting and the three key variables, predict whether carbon dioxide blasting operations can be successfully carried out under the relevant blasting parameters, guide the actual on-site blasting work, and improve the efficiency of carbon dioxide blasting.
4. Failure Mode of the Fracturing Pipe
In a prior study of this paper, how the filling mass of L-CO2, the amount of chemical heating material and the thickness of constant-stress shear plate affect the phase-transition blasting process of the carbon dioxide fracturing pipe have been analyzed qualitatively and quantitatively. It can be found that these three key variables also determine the blasting pressure of the whole carbon dioxide fracturing pipe. From the perspective of the phase-transition of L-CO2, most of the CO2 in the tube exists in a gaseous and supercritical state. The comparative state Viral–Han–Long (VHL) equation can be used to quantitatively describe the relationships among pressure, temperature and volume of carbon dioxide in the tube, Then, the theoretical value of internal pressure (P) can be derived as the blasting pressure. From the perspective of the damage of the constant-stress shear plate during the blasting of the fracturing pipe, the constant-stress shear plate is subjected to tension and shear simultaneously, and then the blasting pressure P acting on the constant-stress shear plate is determined by the breaking strength when the constant-stress shear plate is broken. At the same time, only when the pressure of the high-pressure gas released by the phase-transition of L-CO2 is greater than the ultimate yield strength of the constant-stress shear plate, the fracturing pipe is successfully blasted.
- (1)
- Strength-failure method
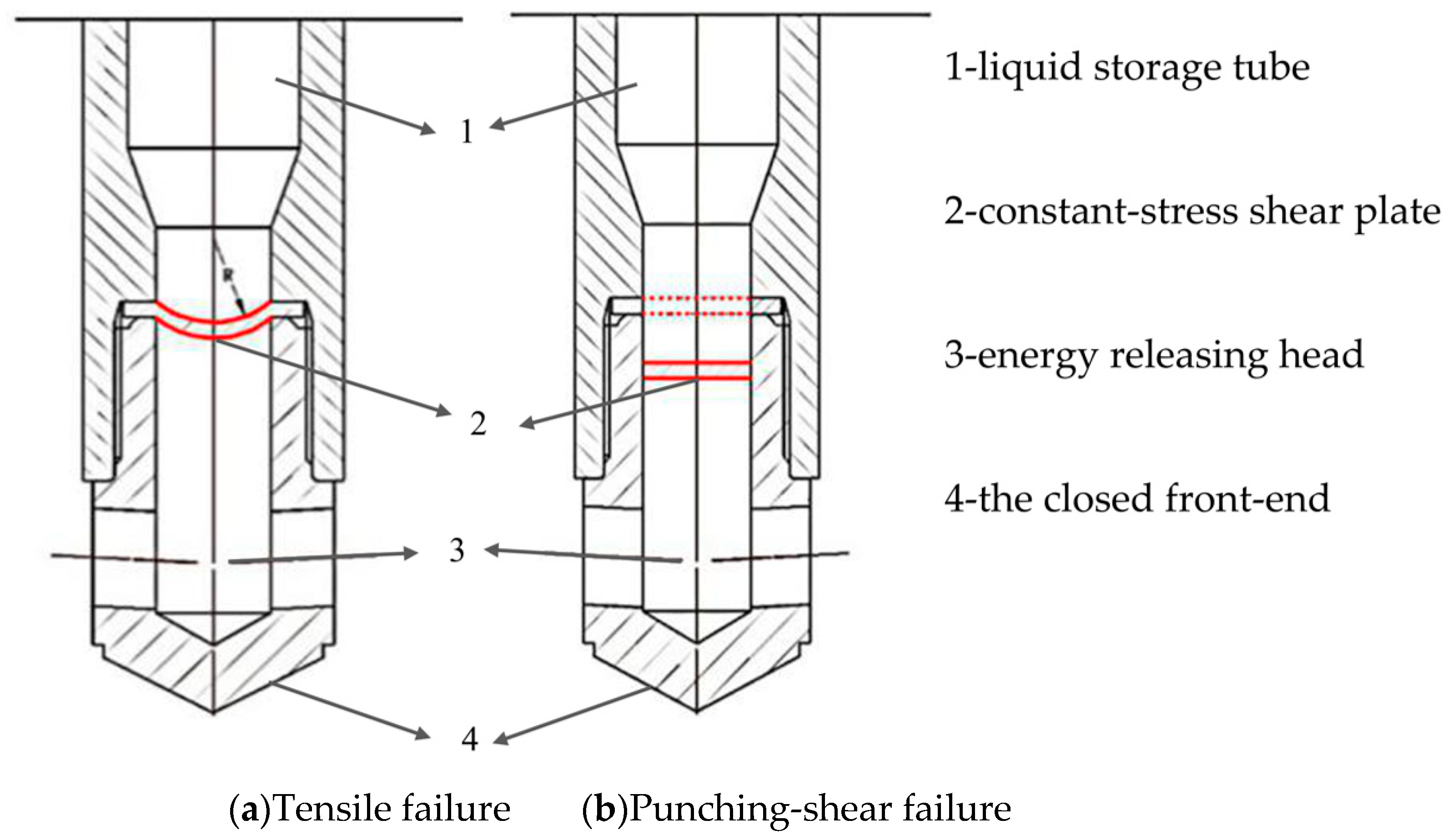
As shown in Figure 9, there are two failure modes of the constant-stress shear plate. (1) Tensile failure with non-surging internal pressure, (2) Punching-shear failure with surging internal pressure. In this paper, the material of the constant-stress shear plate is 45 steel, which belongs to the flat plate with a diameter of 42 mm, and the diameter of area of thrust surface on which carbon dioxide gas acts is 32 mm.
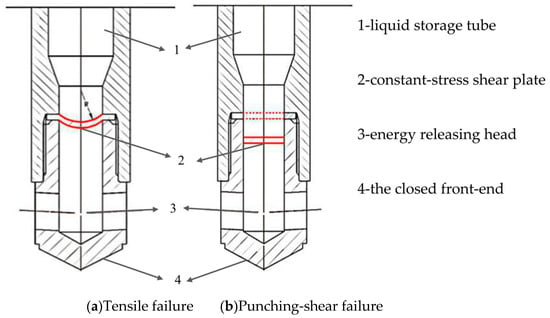
Figure 9.
Schematic diagram of two different failure mode of constant-stress shear plates.
Some simplifications and assumptions for these two failure modes:
(i) Tensile failure with non-surging internal pressure. Before the force is applied, the thickness of the constant-stress shear plate is d0, and after being deformed into a spherical structure, the thickness is d, the height of the spherical crown is h, the radius of the sphere is R, and the actual diameter of the internal pressure acting surface is D. The blasting pressure equation is expressed as follows [38,39]:
(ii) Punching-shear failure with surging internal pressure. Due to the surge in internal pressure, the increase of this pressure is greater than the yield strength of the constant-stress shear plate within milliseconds, then the central part of the constant-stress shear plate is damaged before being deformed, and the whole compressed part of the constant-stress shear plate is sheared off. The blasting pressure equation is expressed as follows [40]:
where is the tensile strength of the constant-stress shear plate, MPa; is the elongation rate in the direction of tensile stress; is the shear strength of the constant-stress shear plate, MPa; Pb is the blasting pressure, MPa.
- (2)
- Viral–Han–Long Equation of Gas State (VHL-EOS)
At present, the gas state equation under high temperature and high-pressure has the forms of Becker–Kistiakowski–Wilson (BKW), Jones–Wilkins–Lee (JWL) [41], Jacobs–Cowperthwaite–Zwisler (JCZ) [42], etc. The VHL equation based on the L-J potential energy function [43], which has been thoroughly studied the thermodynamic state transition of the gas components in explosive products under high temperature and high-pressure [44,45]. It can effectively describe the thermodynamic relationship of PVT of gas in detonation environment [46,47,48], so the theoretical value P of the internal pressure can be derived by taking the internal temperature of tube and the filling mass of L-CO2 as variables in the gas state VHL equation.
The specific form of the VHL equation of state is expressed as follows [49,50]:
where P, V, T are the actual pressure (GPa), volume (ml), and temperature (K) of the gas. n is the number of molar of carbon dioxide gas filled in a fixed volume, mol. R is the molar gas constant 8.3145 × 10−5. The second-order dimensionless virial coefficient B is approximately calculated by the variable step sinbsen quadrature method [49,50,51]. C, D are the third-order and fourth-order dimensionless virial coefficients respectively, and c1-c10, d1-d10 are constants. For the dimensionless virial coefficients above the fifth order, they are represented by a combination function, the values of coefficients e1-e4, a, b and f are the thermodynamic data of non-polar molecules CH4. bCH4 is 67.21. ω.δ is the LJ potential parameter, the VHL equation of state realizes the comparison of the equation of state of other gases and methane through the parameter ω.δ.
The successful blasting experiment 10 (250/5.0/1.60) was taken as an example. By using the above two methods to calculate the blasting pressure and determining the failure mode of the fracturing pipe, the calculation results are shown in Table 2. The shear strength , tensile strength and elongation rate of 45 steel were 355 MPa, 600 MPa, and 16%, respectively. It was concluded that the gas blasting pressure of experiment 10 was 202.56 MPa, which was compared with the blasting pressure of the two failure modes of the constant-stress shear plate calculated by the strength-failure method. The blasting pressure was between the tensile failure stress and the punching-shear failure stress. The constant-stress shear plate was stretched and sheared under the pressure P of the high-pressure gas released by the phase-transition of L-CO2. The constant-stress shear plate will only fail when the pressure P is greater than its ultimate yield strength. Hence, the fracturing pipe of experiment 10 was successfully blasted, and the constant-stress shear plate suffered tensile failure. Finally, the data of experiment 10 were put into the initiation empirical model. The result was Y = 0.77, which was above the plane Y = 0, and indicated that the blasting was successful, which was consistent with the experimental result. Therefore, the new set of blasting empirical model and experience can be considered to guide on-site blasting operations.

Table 2.
Blasting pressure of experiment 10.
Therefore, comparing the blasting pressure calculated by the Viral–Han–Long equation of gas state (VHL-EOS) and the strength-failure method, and when the gas blasting pressure is between the tensile failure stress and the punching-shear failure stress, the constant-stress shear plate will suffer tensile failure. When the gas blasting pressure is greater than the punching-shear failure stress, the constant-stress shear plate will suffer tensile punching-shear failure. Otherwise, the fracturing pipe fails to blast, and the constant-stress shear plate will not be broken.
5. Conclusions
In this paper, a series of exploratory experiments of carbon dioxide blasting were carried out. Based on experiment temperature, strain curve and failure form of constant-stress shear plate, the main conclusions are as follows:
- (1)
- The filling mass of L-CO2 and the amount of chemical heating material jointly control the phase-transition reaction process of carbon dioxide. The thickness of constant-stress shear plate controls the carbon dioxide blasting, so the three variables determine the initiation of the fracturing pipe together.
- (2)
- An empirical model between the carbon dioxide fracturing pipe initiation (Y) and three key variables (the filling mass of liquid carbon dioxide (L-CO2) (X1), the amount of chemical heating material(X2) and the thickness of constant-stress shear plate(X3)) is obtained, which can be used to explain, predict and guide on-site carbon dioxide blasting operations.
- (3)
- Two methods for the blasting pressure of the fracturing pipe are summarized. (a) The comparative state VHL equation is used to quantitatively describe the relationships among pressure, temperature and volume of carbon dioxide in the tube, and then the theoretical value P of the internal pressure is derived as the blasting pressure; (b) the blast stress P acting on the constant-stress shear plate is determined by the yield strength of constant-stress shear plate.
- (4)
- The failure model of the fracturing pipe can be determined by comparing the blasting pressure calculated by the VHL-EOS with the blasting pressure calculated by the strength-failure method.
Author Contributions
J.X. conceived and designed the experiment; H.T. and J.Z. carried out the carbon dioxide experiments; J.X. wrote the manuscript; B.D. and G.C. provided advice on the writing of abstract and conclusions; B.D., H.T., J.Z., G.C. and M.K. made a significant contribution to the revision of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Key Research and Development Programs of China grant number 2019YFB1504201, 2019YFB1504203, and 2019YFB1504204, Experimental Technology Research Project of China University of Geosciences (Wuhan) grant number SKJ2019088, and The Fundamental Research Founds for National University, China University of Geosciences (Wuhan) grant number 1910491A18.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sun, W.; Wang, Y. Numerical Simulation of Rock Fracturing by Carbon Dioxide Phase Transition. In Proceedings of the 51st U.S. Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 28 August 2017; p. 6. [Google Scholar]
- Chen, H.D.; Wang, Z.F.; Chen, X.E.; Chen, X.J.; Wang, L.G. Increasing permeability of coal seams using the phase energy of liquid carbon dioxide. J. CO2 Util. 2017, 19, 112–119. [Google Scholar] [CrossRef]
- Fan, Y.C.; Qin, B.T.; Zhou, Q.; Shi, Q.L.; Wu, J.H. Liquid CO2 phase transition fracturing technology and its application in enhancing gas drainage of coal mines. Adsorpt. Sci. Technol. 2020, 38, 393–412. [Google Scholar] [CrossRef]
- Vidanovic, N.; Ognjanovic, S.; Ilincic, N.; Ilic, N.; Tokalic, R. Application of unconventional methods of underground premises construction in coal mines. Tech. Technol. Educ. Manag. 2011, 6, 861–865. [Google Scholar]
- Spur, G.; Uhlmann, E.; Elbing, F. Dry-ice blasting for cleaning: Process, optimization and application. Wear 1999, 233–235, 402–411. [Google Scholar] [CrossRef]
- Hu, S.B.; Pang, S.G.; Yan, Z.Y. A new dynamic fracturing method: Deflagration fracturing technology with carbon dioxide. Int. J. Fract. 2019, 220, 99–111. [Google Scholar] [CrossRef]
- Hu, G.Z.; He, W.R.; Sun, M. Enhancing coal seam gas using liquid CO2 phase-transition blasting with cross-measure borehole. J. Nat. Gas Sci. Eng. 2018, 60, 164–173. [Google Scholar] [CrossRef]
- Cui, G.D.; Ren, S.R.; Dou, B.; Ning, F.L. Geothermal energy exploitation from depleted high-temperature gas reservoirs by recycling CO2: The superiority and existing problems. Geosci. Front. 2020. [Google Scholar] [CrossRef]
- Pruess, K. Enhanced geothermal systems (EGS) using CO2 as working fluid—A novel approach for generating renewable energy with simultaneous sequestration of carbon. Geothermics 2006, 35, 351–367. [Google Scholar] [CrossRef]
- Brown, D. A hot dry rock geothermal energy concept utilizing supercritical CO2 instead of water. Proc. Twenty-Fifth Workshop Geotherm. Reserv. Eng. 2000. Available online: https://www.researchgate.net/publication/237219291_A_hot_dry_rock_geothermal_energy_concept_utilizing_supercritical_CO2_instead_of_water (accessed on 13 December 2020).
- Stevens, S.H.; Spector, D.; Riemer, P. Enhanced Coalbed Methane Recovery Using CO2 Injection: Worldwide Resource and CO2 Sequestration Potential. In Proceedings of the SPE International Oil and Gas Conference and Exhibition in China, Beijing, China, 1 January 1998; p. 13. [Google Scholar]
- Cui, G.; Pei, S.; Rui, Z.; Dou, B.; Ning, F.; Wang, J. Whole process analysis of geothermal exploitation and power generation from a depleted high-temperature gas reservoir by recycling CO2. Energy 2021, 217, 119340. [Google Scholar] [CrossRef]
- Gross, D.; Ferguson, N.; Amon, S.; Hanenkamp, N. Theoretical Investigation of the Influence of Different Chamber Geometries on the Agglomeration Capacity of Carbon Dioxide. Appl. Mech. Mater. 2017, 871, 169–175. [Google Scholar] [CrossRef]
- Archibald, L.C. Cryogenic Blasting Using Carbon Dioxide Media for the Removal of Organic Coatings. Trans. IMF 1991, 69, 128–132. [Google Scholar] [CrossRef]
- Wilson, H.H. Coal augers: Development and application underground. Trans. Inst. Min. Eng. 1954, 113, 524–539. [Google Scholar]
- Clairet, J. Use of Cardox in coal mining in Sarre. Rev. Ind. Miner. 1952, 33, 846–854. [Google Scholar]
- Weir, P.; Edwards, J.H. Mechanical loading and Cardox revolutionize an old mine. Coal Age 1928, 33, 288–290. [Google Scholar]
- Chen, Y.; Zhang, H.; Zhu, Z.; Ren, T.; Cao, C.; Zhu, F.; Li, Y.P. A new shock-wave test apparatus for liquid CO2 blasting and measurement analysis. Meas. Control 2019, 52, 399–408. [Google Scholar] [CrossRef]
- Patrick. CO2 blasting in Europe. Nucl. Eng. Int. 1995, 36, 193. [Google Scholar]
- Caldwell, T. A Comparison of Non-Explosive Rock Breaking Techniques. Bachelor’s Thesis, School of Engineering, The University of Queensland, Queensland, Australia, 1 January 2004. [Google Scholar]
- He, W.R.; He, F.L.; Zhang, K.; Zhao, Y.Q.; Zhu, H.Z. Increasing Permeability of Coal Seam and Improving Gas Drainage Using a Liquid Carbon Dioxide Phase Transition Explosive Technology. Adv. Civ. Eng. 2018, 2018, 15. [Google Scholar] [CrossRef]
- Vishal, V. Saturation time dependency of liquid and supercritical CO2 permeability of bituminous coals: Implications for carbon storage. Fuel 2017, 192, 201–207. [Google Scholar] [CrossRef]
- Gao, F.; Tang, L.; Zhou, K.; Zhang, Y.; Ke, B. Mechanism Analysis of Liquid Carbon Dioxide Phase Transition for Fracturing Rock Masses. Energies 2018, 11, 2909. [Google Scholar] [CrossRef]
- Ke, B.; Zhou, K.P.; Ren, G.F.; Shi, J.; Zhang, Y.N. Positive Phase Pressure Function and Pressure Attenuation Characteristic of a Liquid Carbon Dioxide Blasting System. Energies 2019, 12, 4134. [Google Scholar] [CrossRef]
- Lak, M.; Fatehi Marji, M.; Yarahmadi Bafghi, A.; Abdollahipour, A. A Coupled Finite Difference-Boundary Element Method for modeling the propagation of explosion-induced radial cracks around a wellbore. J. Nat. Gas. Sci. Eng. 2019, 64, 41–51. [Google Scholar] [CrossRef]
- Lak, M.; Fatehi Marji, M.; Yarahamdi Bafghi, A.R.; Abdollahipour, A. Discrete element modeling of explosion-induced fracture extension in jointed rock masses. J. Min. Environ. 2019, 10, 125–138. [Google Scholar] [CrossRef]
- Ke, B.; Zhou, K.; Xu, C.; Ren, G.; Jiang, T. Thermodynamic properties and explosion energy analysis of carbon dioxide blasting systems. Min. Technol. 2018, 128, 39–50. [Google Scholar] [CrossRef]
- Huang, X.; Li, Q.Y.; Wei, X.A.; Yang, X.X.; Luo, D.Y.; Zeng, H.D.; Wang, H.W. Indoor Test System for Liquid CO2 Phase Change Shock Wave Pressure with PVDF Sensors. Sensors 2020, 20, 2395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.P.; Ke, B.; Li, J.L.; Zhang, Y.N.; Cheng, L. Pressure dynamic response and explosion energy of liquid carbon dioxide blasting system. Blasting 2017, 34, 7. [Google Scholar]
- Wang, Z.F.; Sun, X.M.; Lu, T.K.; Han, Y.B. Experiment research on strengthening gas drainage effect with fracturing technique by liquid CO2 phase transition. J. Henan Polytech. Univ. Nat. Sci. 2015, 34, 1–5. [Google Scholar]
- Zhang, Y.; Deng, J.; Deng, H.; Ke, B. Peridynamics simulation of rock fracturing under liquid carbon dioxide blasting. Int. J. Damage Mech. 2018, 28, 1038–1052. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Deng, J.R.; Ke, B.; Deng, H.W.; Li, J.L. Experimental Study on Explosion Pressure and Rock Breaking Characteristics under Liquid Carbon Dioxide Blasting. Adv. Civ. Eng. 2018, 2018, 7840125. [Google Scholar] [CrossRef]
- Zhou, S.T.; Jiang, N.; He, X.; Luo, X.D. Rock Breaking and Dynamic Response Characteristics of Carbon Dioxide Phase Transition Fracturing Considering the Gathering Energy Effect. Energies 2020, 13, 1336. [Google Scholar] [CrossRef]
- Li, Q.Y.; Chen, G.; Luo, D.Y.; Ma, H.P.; Liu, Y. An experimental study of a novel liquid carbon dioxide rock-breaking technology. Int. J. Rock Mech. Min. Sci. 2020, 128, 104244. [Google Scholar] [CrossRef]
- Qiduo, Z. Rock Breaking Mechanism and Numerical Simulation Analysis of Liquid Carbon Dioxide Blasting; Hebei University of Technology: Tianjin, China, 2018. [Google Scholar]
- Pan, H.Y.; Li, J.W.; Zhang, T.J.; Li, S.G.; Zhang, L. Study on crack propagation of the CO2 presplitting blasting empty hole effect in coal seam. Energy Sci. Eng. 2020, 8, 3898–3908. [Google Scholar] [CrossRef]
- Mingyu, W. Study on Crack Propagation Law of Liquid Carbon Dioxide Phase Transition Blasting and its Application. Master’s Thesis, China University of Mining, Xuzhou, China, 2018. [Google Scholar]
- Yinming, Z.; Liu, L.; Jun, Y. Analysis of rupture disk bursting pressure of a hybrid inflator. Appl. Sci. Technol. 2014, 41, 73–76. [Google Scholar]
- Shengchang, L. Discussion on several main formulas for explosion pressure of explosion-proof membrane. Petro-Chem. Equip Techno 1988, 2, 17–19. [Google Scholar]
- Zewei, W.; Qingyu, Q.; Xiuxia, Y.; Liqing, C. Calculation of burst pressure of bursting membrane. Chem. Equip. Pip. 1980, 4, 1–9. [Google Scholar]
- Cowan, R.D.; Fickett, W. Calculation of the Detonation Properties of Solid Explosives with the Kistiakowski-Wilson Equation of State. J. Chem. Phys. 1956, 24, 932–939. [Google Scholar] [CrossRef]
- Guidry, R.; Mcguire, R.; Lee, E. Parameterization of the BKW and JCZ Equations of State for Explosives; Frank J. Seiler Research Laboratory: Alexandria, VA, USA, 1978. [Google Scholar] [CrossRef]
- Liu, Q.; Han, Y.; Long, X.P.; Duan, Y.L. Prediction of H2O PVT relations at high temperatures by VHL equation of state. AIP Adv. 2019, 9, 8. [Google Scholar] [CrossRef]
- Maillet, J.B.; Bourasseau, E. Ab initio simulations of thermodynamic and chemical properties of detonation product mixtures. J. Chem. Phys. 2009, 131, 9. [Google Scholar] [CrossRef]
- Kopyshev, V.P.; Medvedev, A.B.; Khrustalev, V.V. Equation of state of explosion products on the basis of a modified Van der Waals model. Combust. Explos. 2006, 42, 76–87. [Google Scholar] [CrossRef]
- Son, E.E. Current investigations of thermophysical properties of substances (based on recent publications in the journal High Temperature). High. Temp. 2013, 51, 351–368. [Google Scholar] [CrossRef]
- Fegley, B., Jr. Practical chemical thermodynamics for geoscientists. Acad. Press 2013, 48, 712–713. [Google Scholar]
- Al-Jawad, M.S.; Hassan, O.F. Comprehensive Model for Flash Calculations of Heavy Oils Using the Soave—Redlich—Kwong Equation of State. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 1 January 2012; p. 14. [Google Scholar]
- Han, Y.; Guo, X.L.; Long, X.P. High Temperature and High Pressure Equation of State of Carbon Dioxide. Chin. J. Energetic Mater. 2016, 24, 0462–0468. [Google Scholar] [CrossRef]
- Hirschfelder, J.O.; Curtiss, C.F.; Bird, R.B. Molecular Theory of Gases and Liquids; Wileg: New York, NY, USA, 1954; p. 34. [Google Scholar]
- Han, Y.; Long, X.P.; Huang, Y.M.; Jiang, Z.H. Effect of L-J or Exp-6 Potential Function on Calcula tion of Reduced Second Viral Coefficient. Chin. J. Energ. Mater. 2009, 17, 574–577. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).