The Role of Fluorinated Polymers in the Water Management of Proton Exchange Membrane Fuel Cells: A Review
Abstract
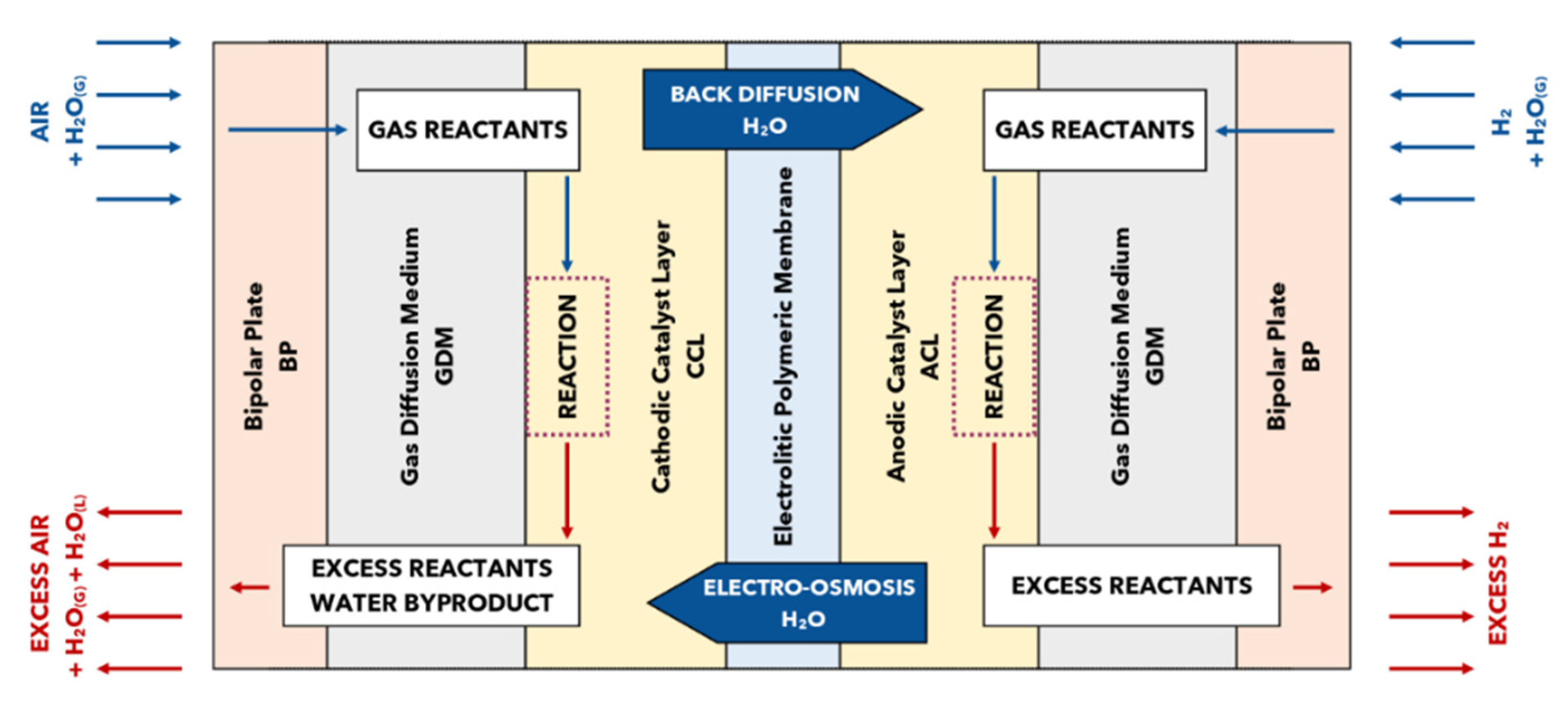
:1. Introduction
2. Materials
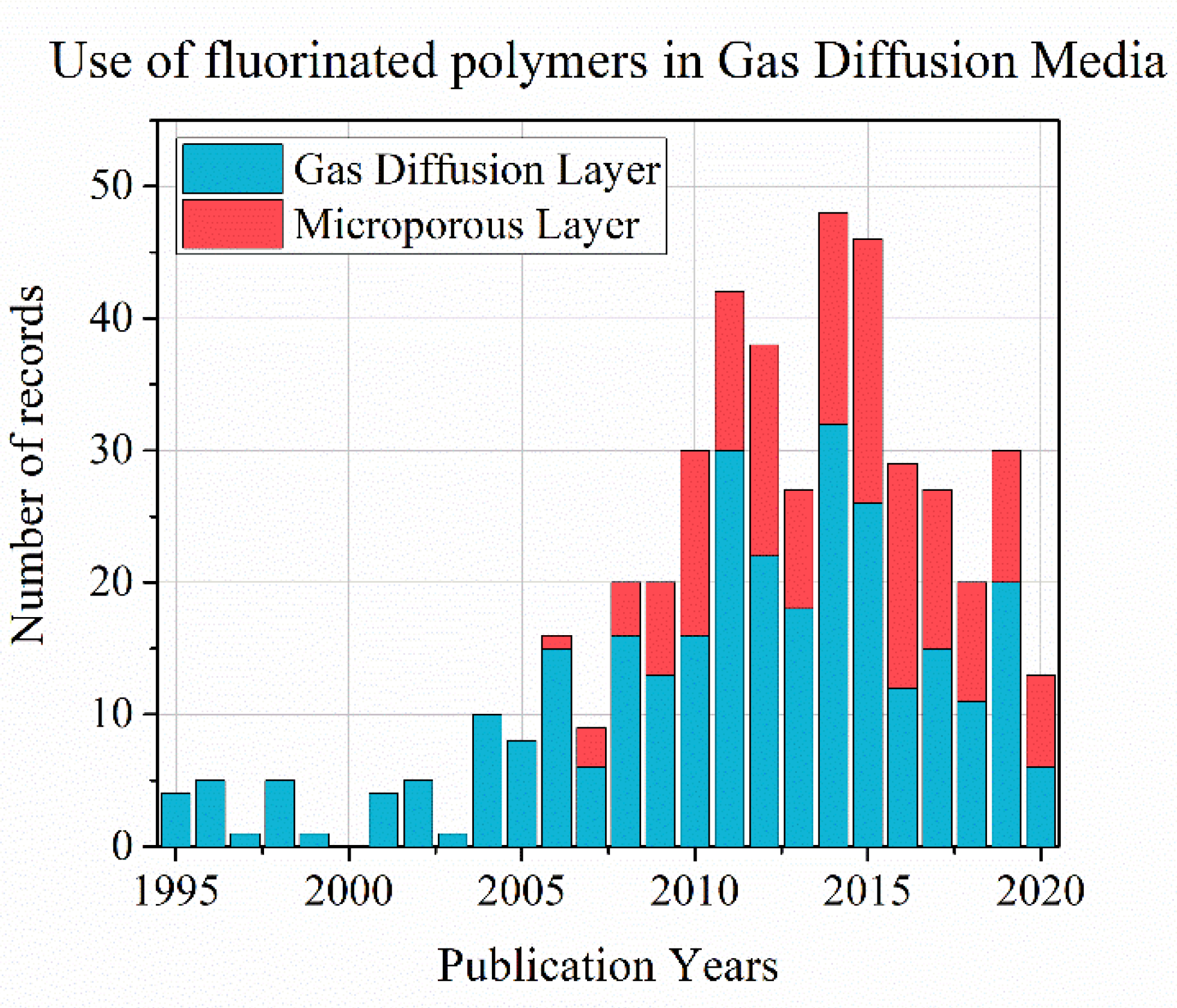
2.1. PTFE
2.1.1. Mass Transport
2.1.2. Electrical Conductivity
- it allows an efficient charge transfer from the GDM to the whole surface of the catalyst layer, thus increasing the number of active sites for reaction.
- it accelerates the collection of electrons at the BP–GDM interface because it promotes the movement of charges from the region below the flow channels to the plates’ ribs.
2.1.3. Thermal Conductivity
2.1.4. Durability
2.2. FEP
- the lower sintering temperature (270 °C vs. 350–360 °C) that reduces the risk of thermal degradation of the carbon support;
- the less abrupt viscosity change of the slurry during sintering at lower temperature that allows the formation of smoother surfaces, which reduces the contact resistances;
- the superior compressive strength, which allows a higher joining pressure during cell assembly, thus lower contact resistances.
2.3. Other Fluorinated Materials
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, V. Materials for Hostile Chemical Environments. In Materials Under Extreme Conditions; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–158. [Google Scholar] [CrossRef]
- Peng, H. Synthesis and Application of Fluorine-Containing Polymers with Low Surface Energy. Polym. Rev. 2019, 59, 739–757. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Ener. 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells–A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Majlan, E.H.; Rohendi, D.; Daud, W.R.W.; Husaini, T.; Haque, M.A. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 89, 117–134. [Google Scholar] [CrossRef]
- Jayakumar, A.; Sethu, S.P.; Ramos, M.; Robertson, J.; Al-Jumaily, A. A technical review on gas diffusion, mechanism and medium of PEM fuel cell. Ionics 2015, 21, 1–18. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. Gas diffusion layer modifications and treatments for improving the performance of proton exchange membrane fuel cells and electrolysers: A review. Int. J. Hydrogen Energy 2017, 42, 28515–28536. [Google Scholar] [CrossRef]
- Ozden, A.; Shahgaldi, S.; Li, X.; Hamdullahpur, F. A review of gas diffusion layers for proton exchange membrane fuel cells—With a focus on characteristics, characterization techniques, materials and designs. Prog. Energy Combust. Sci. 2019, 74, 50–102. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Effects of Microporous Layers in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2005, 152, A677. [Google Scholar] [CrossRef]
- Gostick, J.T.; Ioannidis, M.A.; Fowler, M.W.; Pritzker, M.D. On the role of the microporous layer in PEMFC operation. Electrohem. Commun. 2009, 11, 576–579. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-W.; Popov, B.N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrogen Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Kitahara, T.; Nakajima, H.; Ishikawa, K. Gas Diffusion Layer Coated with a Microporous Layer Containing Hydrophilic CNTs to Enhance PEFC Performance without Humidification Using Anode Gas Recirculation. ECS Trans. 2016, 75, 209–217. [Google Scholar] [CrossRef]
- Kitahara, T.; Nakajima, H.; Inamoto, M.; Shinto, K. Triple microporous layer coated gas diffusion layer for performance enhancement of polymer electrolyte fuel cells under both low and high humidity conditions. J. Power Source 2014, 248, 1256–1263. [Google Scholar] [CrossRef]
- Antonacci, P.; Chevalier, S.; Lee, J.; Ge, N.; Hinebaugh, J.; Yip, R.; Tabuchi, Y.; Kotaka, T.; Bazylak, A. Balancing mass transport resistance and membrane resistance when tailoring microporous layer thickness for polymer electrolyte membrane fuel cells operating at high current densities. Electrochim. Acta 2016, 188, 888–897. [Google Scholar] [CrossRef]
- Mariani, M.; Latorrata, S.; Patrignani, S.; Gallo Stampino, P.; Dotelli, G. Characterization of novel graphene-Based microporous layers for Polymer Electrolyte Membrane Fuel Cells operating under low humidity and high temperature. Int. J. Hydrogen Energy 2020, 45, 7046–7058. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.S.; Oliveira, V.B.; Pinto, A.M.F.R. Experimental study on the membrane electrode assembly of a proton exchange membrane fuel cell: Effects of microporous layer, membrane thickness and gas diffusion layer hydrophobic treatment. Electrochim. Acta 2017, 224, 337–345. [Google Scholar] [CrossRef]
- Ijaodola, O.S.; El-Hassan, Z.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Thompson, J.; Olabi, A.G. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Fan, M.; Duan, F.; Wang, T.; Kang, M.; Zeng, B.; Xu, J.; Anderson, R.; Du, W.; Zhang, L. Effect of Pore Shape and Spacing on Water Droplet Dynamics in Flow Channels of Proton Exchange Membrane Fuel Cells. Energies 2021, 14, 1250. [Google Scholar] [CrossRef]
- Mularczyk, A.; Michalski, A.; Striednig, M.; Herrendörfer, R.; Schmidt, T.J.; Büchi, F.N.; Eller, J. Mass Transport Limitations of Water Evaporation in Polymer Electrolyte Fuel Cell Gas Diffusion Layers. Energies 2021, 14, 2967. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, L.; Chen, L.; Tian, X.; He, T.; Yang, Q. Pore-Scale Modeling of Air–Water Two Phase Flow and Oxygen Transport in Gas Diffusion Layer of Proton Exchange Membrane Fuel Cell. Energies 2021, 14, 3812. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- Lapicque, F.; Belhadj, M.; Bonnet, C.; Pauchet, J.; Thomas, Y. A critical review on gas diffusion micro and macroporous layers degradations for improved membrane fuel cell durability. J. Power Sources 2016, 336, 40–53. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khalid, M.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Walvekar, R.; Kadhum, A.A.H. Additives in proton exchange membranes for low- and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Oberoi, A.S.; Singh, T. Talwinder Factors influencing the performance of PEM fuel cells: A review on performance parameters, water management, and cooling techniques. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Xu, L.; Huang, F.; Ouyang, M. Analytical calculation and evaluation of water transport through a proton exchange membrane fuel cell based on a one-Dimensional model. Energy 2016, 111, 869–883. [Google Scholar] [CrossRef]
- Damian-Ascencio, C.E.; Saldaña-Robles, A.; Hernandez-Guerrero, A.; Cano-Andrade, S. Numerical modeling of a proton exchange membrane fuel cell with tree-Like flow field channels based on an entropy generation analysis. Energy 2017, 133, 306–316. [Google Scholar] [CrossRef]
- Futter, G.A.; Gazdzicki, P.; Friedrich, K.A.; Latz, A.; Jahnke, T. Physical modeling of polymer-Electrolyte membrane fuel cells: Understanding water management and impedance spectra. J. Power Sources 2018, 391, 148–161. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Multi-phase models for water and thermal management of proton exchange membrane fuel cell: A review. J. Power Sources 2018, 391, 120–133. [Google Scholar] [CrossRef]
- Bolwin, K. Preparation of porous electrodes and laminated electrode-Membrane structures for polymer electrolyte fuel cells (PEFC). Solid State Ionics 1995, 77, 324–330. [Google Scholar] [CrossRef]
- da Silva, S.L.A.; Ticianelli, E.A. Studies of the limiting polarization behavior of gas diffusion electrodes with different platinum distributions and hydrophobic properties. J. Electroanal. Chem. 1995, 391, 101–109. [Google Scholar] [CrossRef]
- Bevers, D.; Rogers, R.; von Bradke, M. Examination of the influence of PTFE coating on the properties of carbon paper in polymer electrolyte fuel cells. J. Power Sources 1996, 63, 193–201. [Google Scholar] [CrossRef]
- Paganin, V.A.; Ticianelli, E.A.; Gonzalez, E.R. Development and electrochemical studies of gas diffusion electrodes for polymer electrolyte fuel cells. J. Appl. Electrochem. 1996, 26, 297–304. [Google Scholar] [CrossRef]
- Giorgi, L.; Antolini, E.; Pozio, A.; Passalacqua, E. Influence of the PTFE content in the diffusion layer of low-Pt loading electrodes for polymer electrolyte fuel cells. Electrochim. Acta 1998, 43, 3675–3680. [Google Scholar] [CrossRef]
- Hočevar, S.; Passalacqua, E.; Vivaldi, M.; Patti, A.; Giordano, N. Electrodics at the gas diffusion platinum electrodes—H3PW12O40 proton conducting liquid electrolyte interface. Electrochim. Acta 1996, 41, 2817–2827. [Google Scholar] [CrossRef]
- Staiti, P.; Poltarzewsi, Z.; Alderucci, V.; Maggio, G.; Giordano, N. Solid polymer electrolyte fuel cell (SPEFC) research and development at the institute CNR-TAE of messina. Int. J. Hydrogen Energy 1994, 19, 523–527. [Google Scholar] [CrossRef]
- Gallo Stampino, P.; Latorrata, S.; Molina, D.; Turri, S.; Levi, M.; Dotelli, G. Investigation of hydrophobic treatments with perfluoropolyether derivatives of gas diffusion layers by electrochemical impedance spectroscopy in PEM-FC. Solid State Ionics 2012, 216, 100–104. [Google Scholar] [CrossRef]
- Ong, A.L.; Bottino, A.; Capannelli, G.; Comite, A. Effect of preparative parameters on the characteristic of poly(vinylidene fluoride)-based microporous layer for proton exchange membrane fuel cells. J. Power Sources 2008, 183, 62–68. [Google Scholar] [CrossRef]
- Guilizzoni, M.; Santini, M.; Lorenzi, M.; Knisel, V.; Fest-Santini, S. Micro computed tomography and CFD simulation of drop deposition on gas diffusion layers. J. Phys. Conf. Ser. 2014, 547, 012028. [Google Scholar] [CrossRef] [Green Version]
- Latorrata, S.; Balzarotti, R.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G.; Guilizzoni, M. Design of properties and performances of innovative gas diffusion media for polymer electrolyte membrane fuel cells. Prog. Org. Coatings 2015, 78, 517–525. [Google Scholar] [CrossRef]
- Pai, Y.-H.; Ke, J.-H.; Huang, H.-F.; Lee, C.-M.; Zen, J.-M.; Shieu, F.-S. CF4 plasma treatment for preparing gas diffusion layers in membrane electrode assemblies. J. Power Sources 2006, 161, 275–281. [Google Scholar] [CrossRef]
- Global Fluoropolymers Market 2016-Products, Technologies and Applications. Available online: https://www.globenewswire.com/news-release/2016/06/29/852252/0/en/Global-Fluoropolymers-Market-2016-Products-Technologies-and-Applications.html (accessed on 7 November 2021).
- Park, G.-G.; Sohn, Y.-J.; Yang, T.-H.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Effect of PTFE contents in the gas diffusion media on the performance of PEMFC. J. Power Sources 2004, 131, 182–187. [Google Scholar] [CrossRef]
- Niu, Z.; Bao, Z.; Wu, J.; Wang, Y.; Jiao, K. Two-Phase flow in the mixed-Wettability gas diffusion layer of proton exchange membrane fuel cells. Appl. Energy 2018, 232, 443–450. [Google Scholar] [CrossRef]
- Kakaee, A.H.; Molaeimanesh, G.R.; Elyasi Garmaroudi, M.H. Impact of PTFE distribution across the GDL on the water droplet removal from a PEM fuel cell electrode containing binder. Int. J. Hydrogen Energy 2018, 43, 15481–15491. [Google Scholar] [CrossRef]
- Pan, W.; Chen, Z.; Yao, D.; Chen, X.; Wang, F.; Dai, G. Microstructure and macroscopic rheology of microporous layer nanoinks for PEM fuel cells. Chem. Eng. Sci. 2021, 246, 117001. [Google Scholar] [CrossRef]
- Mortazavi, M.; Tajiri, K. Effect of the PTFE content in the gas diffusion layer on water transport in polymer electrolyte fuel cells (PEFCs). J. Power Sources 2014, 245, 236–244. [Google Scholar] [CrossRef]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Yuan, X.-Z.; Martin, J.; Shen, J.; Pan, M.; Luo, Z. Measurement of water transport rates across the gas diffusion layer in a proton exchange membrane fuel cell, and the influence of polytetrafluoroethylene content and micro-Porous layer. J. Power Sources 2009, 188, 122–126. [Google Scholar] [CrossRef]
- Zamel, N.; Li, X.; Becker, J.; Wiegmann, A. Effect of liquid water on transport properties of the gas diffusion layer of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 5466–5478. [Google Scholar] [CrossRef]
- LaManna, J.M.; Kandlikar, S.G. Determination of effective water vapor diffusion coefficient in pemfc gas diffusion layers. Int. J. Hydrogen Energy 2011, 36, 5021–5029. [Google Scholar] [CrossRef] [Green Version]
- Flückiger, R.; Freunberger, S.A.; Kramer, D.; Wokaun, A.; Scherer, G.G.; Büchi, F.N. Anisotropic, effective diffusivity of porous gas diffusion layer materials for PEFC. Electrochim. Acta 2008, 54, 551–559. [Google Scholar] [CrossRef]
- Hwang, G.S.; Weber, A.Z. Effective-Diffusivity Measurement of Partially-Saturated Fuel-Cell Gas-Diffusion Layers. J. Electrochem. Soc. 2012, 159, F683–F692. [Google Scholar] [CrossRef]
- Biesdorf, J.; Oberholzer, P.; Schmidt, T.J.; Boillat, P. Influence of Hydrophobic Coating of Gas Diffusion Layers on the Performance and Water Transport inside PEFC. ECS Trans. 2014, 64, 467–475. [Google Scholar] [CrossRef]
- Biesdorf, J.; Forner-Cuenca, A.; Schmidt, T.J.; Boillat, P. Impact of Hydrophobic Coating on Mass Transport Losses in PEFCs. J. Electrochem. Soc. 2015, 162, F1243–F1252. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, J.D.; Cheung, P.; Schwartz, D.T. The effects of wetproofing on the capillary properties of proton exchange membrane fuel cell gas diffusion layers. J. Power Sources 2010, 195, 787–793. [Google Scholar] [CrossRef]
- Benziger, J.; Nehlsen, J.; Blackwell, D.; Brennan, T.; Itescu, J. Water flow in the gas diffusion layer of PEM fuel cells. J. Memb. Sci. 2005, 261, 98–106. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Garofalo, M.L.; Lu, Z. Water Management in A PEMFC: Water Transport Mechanism and Material Degradation in Gas Diffusion Layers. Fuel Cells 2011, 11, 814–823. [Google Scholar] [CrossRef]
- Ha, T.; Cho, J.; Park, J.; Min, K.; Kim, H.-S.; Lee, E.; Jyoung, J.-Y. Experimental study of the effect of dissolution on the gas diffusion layer in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 12427–12435. [Google Scholar] [CrossRef]
- Burheim, O.S.; Ellila, G.; Fairweather, J.D.; Labouriau, A.; Kjelstrup, S.; Pharoah, J.G. Ageing and thermal conductivity of Porous Transport Layers used for PEM Fuel Cells. J. Power Sources 2013, 221, 356–365. [Google Scholar] [CrossRef]
- Yang, P.; Wu, X.; Xie, Z.; Wang, P.; Liu, C.; Huang, Q. Durability improving and corrosion-Resistance mechanism of graphene oxide modified ultra-Thin carbon paper used in PEM fuel cell. Corros. Sci. 2018, 130, 95–102. [Google Scholar] [CrossRef]
- Fishman, Z.; Bazylak, A. Heterogeneous Through-Plane Porosity Distributions for Treated PEMFC GDLs I. PTFE Effect. J. Electrochem. Soc. 2011, 158, B841. [Google Scholar] [CrossRef]
- Ismail, M.S.; Damjanovic, T.; Ingham, D.B.; Pourkashanian, M.; Westwood, A. Effect of polytetrafluoroethylene-Treatment and microporous layer-Coating on the electrical conductivity of gas diffusion layers used in proton exchange membrane fuel cells. J. Power Sources 2010, 195, 2700–2708. [Google Scholar] [CrossRef]
- Ismail, M.S.; Ingham, D.B.; Ma, L.; Pourkashanian, M. The contact resistance between gas diffusion layers and bipolar plates as they are assembled in proton exchange membrane fuel cells. Renew. Energy 2013, 52, 40–45. [Google Scholar] [CrossRef]
- El-kharouf, A.; Mason, T.J.; Brett, D.J.L.; Pollet, B.G. Ex-situ characterisation of gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2012, 218, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Sadeghifar, H. In-Plane and through-Plane electrical conductivities and contact resistances of a Mercedes-Benz catalyst-Coated membrane, gas diffusion and micro-porous layers and a Ballard graphite bipolar plate: Impact of humidity, compressive load and polytetrafluoroe. Energy Convers. Manag. 2017, 154, 191–202. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, P.; Wang, P.; Xie, Z.; Wu, X. Titanium nitride (TiN)–Polytetrafluoroethylene (PTFE)-Modified carbon paper used in PEM fuel cells: Characterization and corrosion-Resistant mechanism. Appl. Phys. A 2020, 126, 37. [Google Scholar] [CrossRef]
- Burheim, O.S.; Pharoah, J.G. A review of the curious case of heat transport in polymer electrolyte fuel cells and the need for more characterisation. Curr. Opin. Electrochem. 2017, 5, 36–42. [Google Scholar] [CrossRef]
- Blumm, J.; Lindemann, A.; Meyer, M.; Strasser, C. Characterization of PTFE Using Advanced Thermal Analysis Techniques. Int. J. Thermophys. 2010, 31, 1919–1927. [Google Scholar] [CrossRef]
- Sadeghi, E.; Djilali, N.; Bahrami, M. Effective thermal conductivity and thermal contact resistance of gas diffusion layers in proton exchange membrane fuel cells. Part 2: Hysteresis effect under cyclic compressive load. J. Power Sources 2010, 195, 8104–8109. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Effect of Polytetrafluoroethylene (PTFE) and micro porous layer (MPL) on thermal conductivity of fuel cell gas diffusion layers: Modeling and experiments. J. Power Sources 2014, 248, 632–641. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Counter-Intuitive reduction of thermal contact resistance with porosity: A case study of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 6833–6841. [Google Scholar] [CrossRef]
- Burheim, O.S.; Crymble, G.A.; Bock, R.; Hussain, N.; Pasupathi, S.; du Plessis, A.; le Roux, S.; Seland, F.; Su, H.; Pollet, B.G. Thermal conductivity in the three layered regions of micro porous layer coated porous transport layers for the PEM fuel cell. Int. J. Hydrogen Energy 2015, 40, 16775–16785. [Google Scholar] [CrossRef] [Green Version]
- Khandelwal, M.; Mench, M.M. Direct measurement of through-plane thermal conductivity and contact resistance in fuel cell materials. J. Power Sources 2006, 161, 1106–1115. [Google Scholar] [CrossRef]
- Zamel, N.; Litovsky, E.; Li, X.; Kleiman, J. Measurement of the through-plane thermal conductivity of carbon paper diffusion media for the temperature range from −50 to +120 °C. Int. J. Hydrogen Energy 2011, 36, 12618–12625. [Google Scholar] [CrossRef]
- Zamel, N.; Litovsky, E.; Shakhshir, S.; Li, X.; Kleiman, J. Measurement of in-plane thermal conductivity of carbon paper diffusion media in the temperature range of −20 °C to +120 °C. Appl. Energy 2011, 88, 3042–3050. [Google Scholar] [CrossRef]
- Sadeghi, E.; Djilali, N.; Bahrami, M. A novel approach to determine the in-plane thermal conductivity of gas diffusion layers in proton exchange membrane fuel cells. J. Power Sources 2011, 196, 3565–3571. [Google Scholar] [CrossRef]
- Alhazmi, N.; Ismail, M.S.; Ingham, D.B.; Hughes, K.J.; Ma, L.; Pourkashanian, M. The in-Plane thermal conductivity and the contact resistance of the components of the membrane electrode assembly in proton exchange membrane fuel cells. J. Power Sources 2013, 241, 136–145. [Google Scholar] [CrossRef]
- Alhazmi, N.; Ingham, D.B.; Ismail, M.S.; Hughes, K.; Ma, L.; Pourkashanian, M. The through-plane thermal conductivity and the contact resistance of the components of the membrane electrode assembly and gas diffusion layer in proton exchange membrane fuel cells. J. Power Sources 2014, 270, 59–67. [Google Scholar] [CrossRef]
- Teertstra, P.; Karimi, G.; Li, X. Measurement of in-plane effective thermal conductivity in PEM fuel cell diffusion media. Electrochim. Acta 2011, 56, 1670–1675. [Google Scholar] [CrossRef]
- Ramousse, J.; Didierjean, S.; Lottin, O.; Maillet, D. Estimation of the effective thermal conductivity of carbon felts used as PEMFC Gas Diffusion Layers. Int. J. Therm. Sci. 2008, 47, 1–6. [Google Scholar] [CrossRef]
- Karimi, G.; Li, X.; Teertstra, P. Measurement of through-Plane effective thermal conductivity and contact resistance in PEM fuel cell diffusion media. Electrochim. Acta 2010, 55, 1619–1625. [Google Scholar] [CrossRef]
- Hamour, M.; Garnier, J.P.; Grandidier, J.C.; Ouibrahim, A.; Martemianov, S. Thermal-Conductivity Characterization of Gas Diffusion Layer in Proton Exchange Membrane Fuel Cells and Electrolyzers Under Mechanical Loading. Int. J. Thermophys. 2011, 32, 1025–1037. [Google Scholar] [CrossRef]
- Lee, C.; Mérida, W. Gas diffusion layer durability under steady-State and freezing conditions. J. Power Sources 2007, 164, 141–153. [Google Scholar] [CrossRef]
- Cho, J.; Ha, T.; Park, J.; Kim, H.-S.; Min, K.; Lee, E.; Jyoung, J.-Y. Analysis of transient response of a unit proton-Exchange membrane fuel cell with a degraded gas diffusion layer. Int. J. Hydrogen Energy 2011, 36, 6090–6098. [Google Scholar] [CrossRef]
- John Felix Kumar, R.; Radhakrishnan, V.; Haridoss, P. Enhanced mechanical and electrochemical durability of multistage PTFE treated gas diffusion layers for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2012, 37, 10830–10835. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, H.; Ma, H.; Zhong, H. Electrochemical durability of gas diffusion layer under simulated proton exchange membrane fuel cell conditions. Int. J. Hydrogen Energy 2009, 34, 8185–8192. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.; Oh, H.; Min, K.; Lee, E.; Jyoung, J.-Y. Analysis of the transient response and durability characteristics of a proton exchange membrane fuel cell with different micro-Porous layer penetration thicknesses. Appl. Energy 2013, 111, 300–309. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, P.; Gao, P.; Wu, X.; Xie, Z. Enhancement in corrosion resistance and electrical conductivity of hydrophobic-Treated CP by PTFE emulsion containing TiN NPs. Micro Nano Lett. 2019, 14, 1087–1091. [Google Scholar] [CrossRef]
- Poornesh, K.K.; Cho, C.D.; Lee, G.B.; Tak, Y.S. Gradation of mechanical properties in gas-Diffusion electrode. Part 2: Heterogeneous carbon fiber and damage evolution in cell layers. J. Power Sources 2010, 195, 2718–2730. [Google Scholar] [CrossRef]
- Millichamp, J.; Mason, T.J.; Neville, T.P.; Rajalakshmi, N.; Jervis, R.; Shearing, P.R.; Brett, D.J.L. Mechanisms and effects of mechanical compression and dimensional change in polymer electrolyte fuel cells–A review. J. Power Sources 2015, 284, 305–320. [Google Scholar] [CrossRef]
- Destyorini, F.; Indriyati; Indayaningsih, N.; Prihandoko, B.; Syahrial, A.Z. Properties of carbon composite paper derived from coconut coir as a function of polytetrafluoroethylene content. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012054. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.S.; Hassanpour, A.; Ingham, D.B.; Ma, L.; Pourkashanian, M. On the Compressibility of Gas Diffusion Layers in Proton Exchange Membrane Fuel Cells. Fuel Cells 2012, 12, 391–397. [Google Scholar] [CrossRef]
- Lim, C.; Wang, C.Y. Effects of hydrophobic polymer content in GDL on power performance of a PEM fuel cell. Electrochim. Acta 2004, 49, 4149–4156. [Google Scholar] [CrossRef]
- Song, J.M.; Uchida, H.; Watanabe, M. Effect of Wet-Proofing Treatment of Carbon Backing Layer in Gas Diffusion Electrodes on the PEFC Performance. Electrochemistry 2005, 73, 189–193. [Google Scholar] [CrossRef]
- Yan, W.; Hsueh, C.; Soong, C.; Chen, F.; Cheng, C.; Mei, S. Effects of fabrication processes and material parameters of GDL on cell performance of PEM fuel cell. Int. J. Hydrogen Energy 2007, 32, 4452–4458. [Google Scholar] [CrossRef]
- Hung, C.-J.; Liu, C.-H.; Ko, T.-H.; Chen, W.-H.; Cheng, S.-H.; Chen, W.-S.; Yu, A.; Kannan, A.M. Effect of diffusion layers fabricated with different fiber diameters on the performance of low temperature proton exchange membrane fuel cells. J. Power Sources 2013, 221, 134–140. [Google Scholar] [CrossRef]
- Park, S.B.; Park, Y. Fabrication of gas diffusion layer (GDL) containing microporous layer using flourinated ethylene prophylene (FEP) for proton exchange membrane fuel cell (PEMFC). Int. J. Precis. Eng. Manuf. 2012, 13, 1145–1151. [Google Scholar] [CrossRef]
- Öztürk, A.; Fıçıcılar, B.; Eroğlu, İ.; Bayrakçeken Yurtcan, A. Facilitation of water management in low Pt loaded PEM fuel cell by creating hydrophobic microporous layer with PTFE, FEP and PDMS polymers: Effect of polymer and carbon amounts. Int. J. Hydrogen Energy 2017, 42, 21226–21249. [Google Scholar] [CrossRef]
- Hasanpour, S.; Ahadi, M.; Bahrami, M.; Djilali, N.; Akbari, M. Woven gas diffusion layers for polymer electrolyte membrane fuel cells: Liquid water transport and conductivity trade-Offs. J. Power Sources 2018, 403, 192–198. [Google Scholar] [CrossRef]
- Latorrata, S.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Development of an optimal gas diffusion medium for polymer electrolyte membrane fuel cells and assessment of its degradation mechanisms. Int. J. Hydrogen Energy 2015, 40, 14596–14608. [Google Scholar] [CrossRef]
- Latorrata, S.; Cristiani, C.; Dotelli, G. Performance Evaluation and Durability Enhancement of FEP-Based Gas Diffusion Media for PEM Fuel Cells. Energies 2017, 10, 2063. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, S.; Lavielle, N.; Hatton, B.D.; Bazylak, A. Novel electrospun gas diffusion layers for polymer electrolyte membrane fuel cells: Part I. Fabrication, morphological characterization, and in situ performance. J. Power Sources 2017, 352, 272–280. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Costa, C.; Ong, A.L. Microporous layers based on poly(vinylidene fluoride) and sulfonated poly(vinylidene fluoride). Int. J. Hydrogen Energy 2015, 40, 14690–14698. [Google Scholar] [CrossRef]
- Li, T.; Lei, Y.; Gu, J.; Yu, T.; Liu, J.; Zou, Z. Application of ployvinylidene fluoride-Hexafluoroprorylene in gas diffusion layer of proton exchange membrane fuel cell. Procedia Eng. 2012, 27, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Jao, T.-C.; Pasupathi, S.; Bladergroen, B.J.; Linkov, V.; Pollet, B.G. A novel dual catalyst layer structured gas diffusion electrode for enhanced performance of high temperature proton exchange membrane fuel cell. J. Power Sources 2014, 246, 63–67. [Google Scholar] [CrossRef]
- Su, H.; Pasupathi, S.; Bladergroen, B.; Linkov, V.; Pollet, B.G. Optimization of gas diffusion electrode for polybenzimidazole-Based high temperature proton exchange membrane fuel cell: Evaluation of polymer binders in catalyst layer. Int. J. Hydrogen Energy 2013, 38, 11370–11378. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.-J.; Chen, Y.-S. Experimental Study on the Effect of Hydrogen Sulfide on High-Temperature Proton Exchange Membrane Fuel Cells by Using Electrochemical Impedance Spectroscopy. Catalysts 2018, 8, 441. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-L.; Wu, T.-J.; Lin, Y.-T.; Wu, H.-C. Effect of polyvinylidene difluoride in the catalyst layer on high-Temperature PEMFCs. Int. J. Hydrogen Energy 2015, 40, 9400–9409. [Google Scholar] [CrossRef]
- Balzarotti, R.; Latorrata, S.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Development and Characterization of Non-Conventional Micro-Porous Layers for PEM Fuel Cells. Energies 2015, 8, 7070–7083. [Google Scholar] [CrossRef] [Green Version]
- Latorrata, S.; Sansotera, M.; Gola, M.; Stampino, P.G.; Navarrini, W.; Dotelli, G. Innovative Perfluoropolyether-Functionalized Gas Diffusion Layers with Enhanced Performance in Polymer Electrolyte Membrane Fuel Cells. Fuel Cells 2020, 20, 166–175. [Google Scholar] [CrossRef]
- Balzarotti, R.; Latorrata, S.; Mariani, M.; Gallo Stampino, P.; Dotelli, G. Optimization of Perfluoropolyether-Based Gas Diffusion Media Preparation for PEM Fuel Cells. Energies 2020, 13, 1831. [Google Scholar] [CrossRef] [Green Version]
- Gola, M.; Sansotera, M.; Navarrini, W.; Bianchi, C.L.; Gallo Stampino, P.; Latorrata, S.; Dotelli, G. Perfluoropolyether-Functionalized gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2014, 258, 351–355. [Google Scholar] [CrossRef]
- Sansotera, M.; Navarrini, W.; Gola, M.; Dotelli, G.; Stampino, P.G.; Bianchi, C.L. Conductivity and superhydrophobic effect on PFPE-modified porous carbonaceous materials. Int. J. Hydrogen Energy 2012, 37, 6277–6284. [Google Scholar] [CrossRef]
- Latorrata, S.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Novel superhydrophobic microporous layers for enhanced performance and efficient water management in PEM fuel cells. Int. J. Hydrogen Energy 2014, 39, 5350–5357. [Google Scholar] [CrossRef]



| Material | PTFE Loading wt.% | Temperature °C | In-Plane Thermal Conductivity W m−1 K−1 | Ref. |
|---|---|---|---|---|
| Carbon Paper | 5 | 65–70 | 17.39 | [79] |
| 10 | 65–70 | 17.33 | ||
| 20 | 65–70 | 17.58 | ||
| 30 | 65–70 | 17.81 | ||
| Carbon Paper | 0 | 70 | 12.5 ± 0.9 | [78] |
| 5 | 70 | 10.6 ± 0.7 | ||
| 20 | 70 | ~11.0 | ||
| 50 | 70 | ~11.7 | ||
| Carbon Paper | 0 | 110 | 13.3 | [82] |
| 6 | 110 | 12.0 | ||
| 11 | 110 | 9.73 | ||
| 19 | 110 | 10.5 | ||
| 30 | 110 | 15.1 | ||
| Carbon Paper | 0 | 65 | 11.8 | [80] |
| 5 | 65 | 11.6 | ||
| 10 | 65 | 12.9 | ||
| 20 | 65 | 14.1 | ||
| 30 | 65 | 14.8 |
| Material | PTFE Loading wt.% | Compression MPa | Temperature °C | Through-Plane Thermal Conductivity W m−1 K−1 | Ref. |
|---|---|---|---|---|---|
| Carbon Paper | 0 | 2 | 56 | 0.48 ± 0.09 | [76] |
| 5 | 2 | 58 | 0.31 ± 0.06 | ||
| 20 | 2 | 58 | 0.22 ± 0.04 | ||
| Carbon Paper | 5 | ns 1 | 20 | 1.008 | [83] |
| Carbon Paper | 12 | 1.38 | 70 | 0.55 | [84] |
| 19 | 1.38 | 70 | 0.56 | ||
| 29 | 1.38 | 70 | 0.62 | ||
| Carbon Paper | 5 | 0.5 | ns | 1.6 | [72] |
| Carbon Paper | 30 | 2 | ns | 1.4 | [85] |
| Carbon Paper | 0 | 1.6 | 50 | 1.6 | [77] |
| 60 | 1.6 | 50 | 1.3 | ||
| Carbon Paper | 0 | 1.4 | 60 | 0.58 | [73] |
| 5 | 1.4 | 60 | 0.55 | ||
| 20 | 1.4 | 60 | 0.50 | ||
| Carbon Paper | 0 | 2 | 35 | 0.55 | [81] |
| 5 | 2 | 35 | 0.34 | ||
| 10 | 2 | 35 | 0.29 | ||
| 20 | 2 | 35 | 0.32 | ||
| 30 | 2 | 35 | 0.33 |
| GDM | Global Cell Efficiency % | |||
|---|---|---|---|---|
| 0 h | 1000 h c.c. | 1000 h ASTc | 1000 h ASTm | |
| FEP-3 | 41.30 | 38.00 | 37.23 | 27.27 |
| FEP-6 | 42.51 | 39.50 | 38.08 | 29.33 |
| FEP-9 | 42.51 | 42.00 | 41.66 | 35.59 |
| FEP-12 | 43.72 | 43.70 | 42.94 | 41.66 |
| GDM | Maximum Power Density mW cm−2 | |
|---|---|---|
| RH 80–100% | RH 80–60% | |
| PFPE | 447 | 476 |
| FEP | 519 | 481 |
| PFA | 456 | 421 |
| PTFE | 422 | 417 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, M.; Basso Peressut, A.; Latorrata, S.; Balzarotti, R.; Sansotera, M.; Dotelli, G. The Role of Fluorinated Polymers in the Water Management of Proton Exchange Membrane Fuel Cells: A Review. Energies 2021, 14, 8387. https://doi.org/10.3390/en14248387
Mariani M, Basso Peressut A, Latorrata S, Balzarotti R, Sansotera M, Dotelli G. The Role of Fluorinated Polymers in the Water Management of Proton Exchange Membrane Fuel Cells: A Review. Energies. 2021; 14(24):8387. https://doi.org/10.3390/en14248387
Chicago/Turabian StyleMariani, Marco, Andrea Basso Peressut, Saverio Latorrata, Riccardo Balzarotti, Maurizio Sansotera, and Giovanni Dotelli. 2021. "The Role of Fluorinated Polymers in the Water Management of Proton Exchange Membrane Fuel Cells: A Review" Energies 14, no. 24: 8387. https://doi.org/10.3390/en14248387
APA StyleMariani, M., Basso Peressut, A., Latorrata, S., Balzarotti, R., Sansotera, M., & Dotelli, G. (2021). The Role of Fluorinated Polymers in the Water Management of Proton Exchange Membrane Fuel Cells: A Review. Energies, 14(24), 8387. https://doi.org/10.3390/en14248387











