Numerical Modelling of Positive Surface Discharges in C-C4F8/CF3I/N2 Gas Mixture under Non-Uniform Field
Abstract
:1. Introduction
2. Model Description
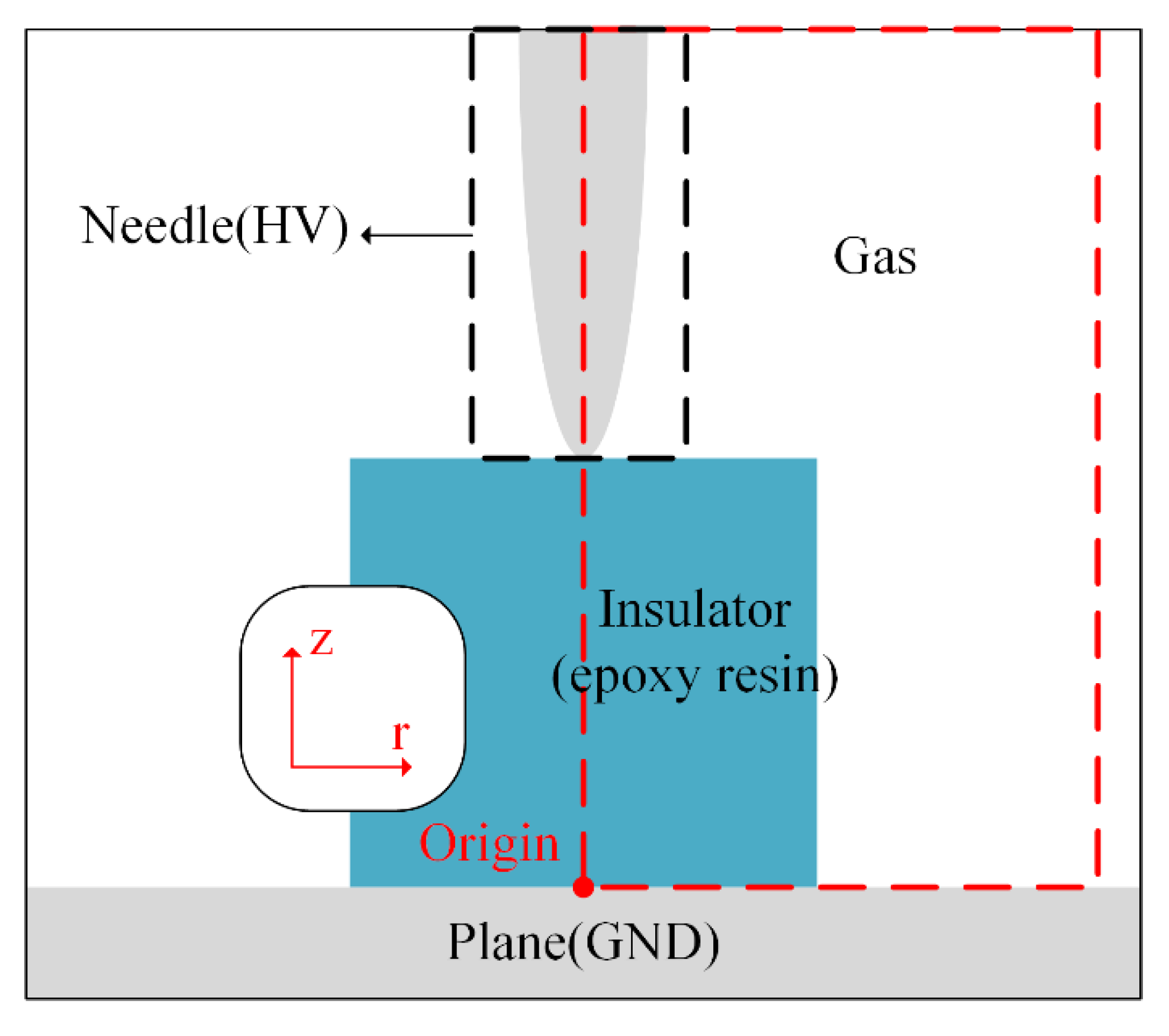
2.1. Physical Model
2.2. Numerical Simulation Model
2.3. Boundary and Initial Conditions
2.4. Reactions in Gas Mixture
3. Results and Discussion
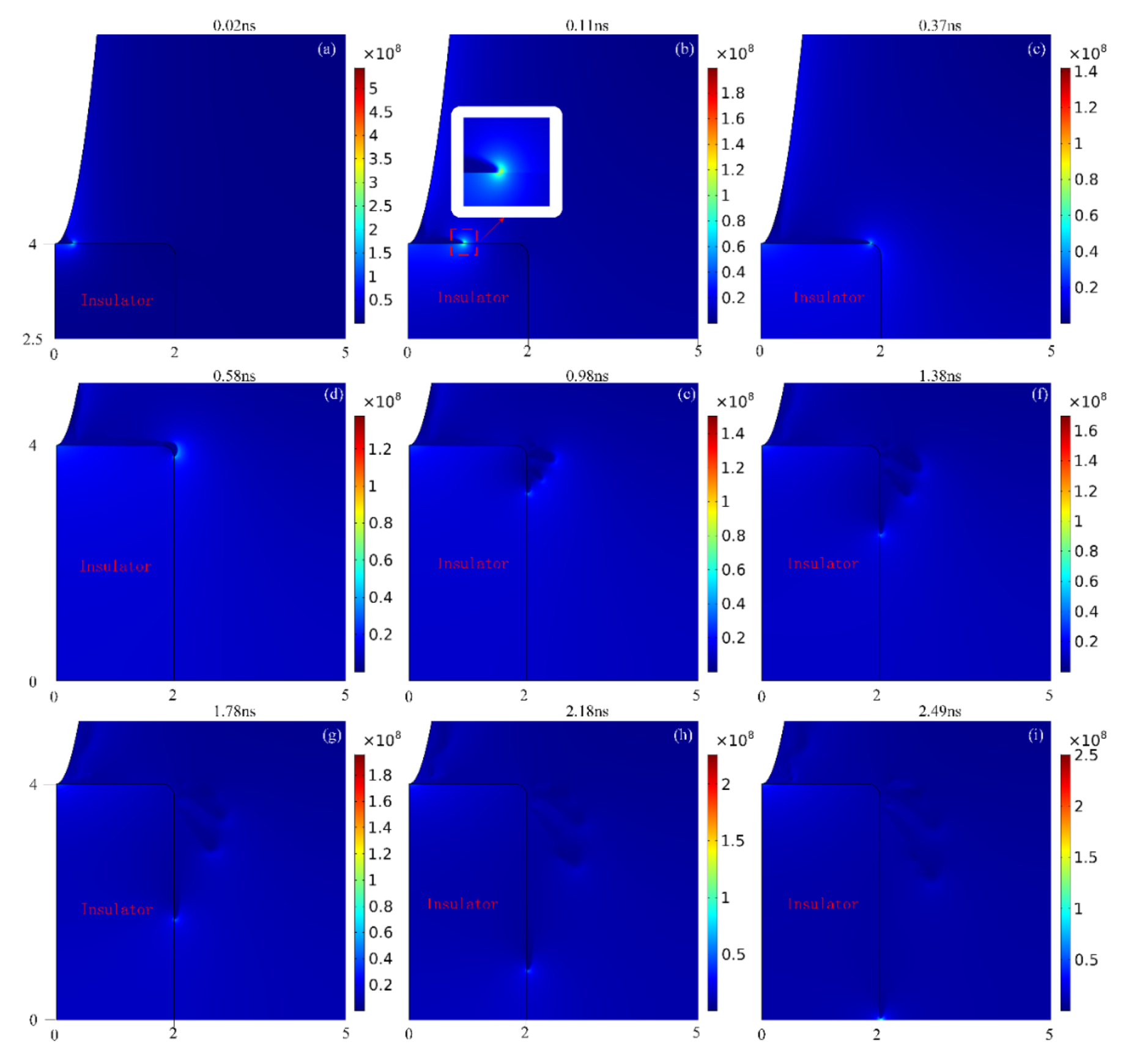
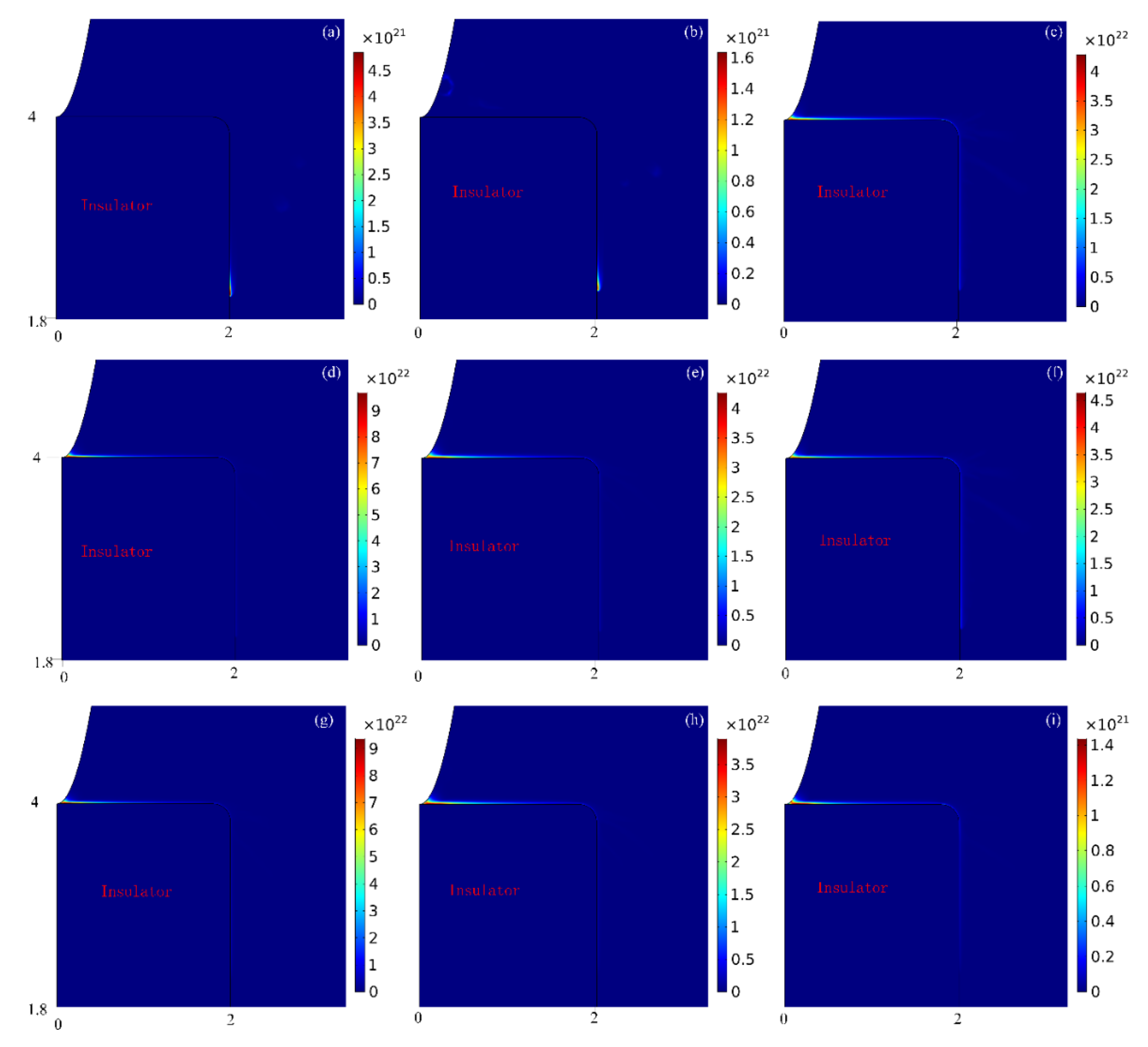
3.1. Streamer Development Process
3.2. Comparison with SF6/N2 and Artificial Air
3.2.1. Comparison of Particle Properties
3.2.2. Comparison of Streamer Characteristics
3.2.3. Comparison of Streamer Branch Characteristics
4. Conclusions
- (1)
- The development process of streamer is as follows: First, the streamer starts from generation and keeps developing along the upper surface of the insulator; then transitions form the upper surface of the insulator to the right surface, and at the meanwhile, the streamer branch appears; later, the streamer along the surface keeps accelerating towards the plane electrode, while the streamer branches become weaker and weaker. Finally, the moment of contact between the streamer and the plane electrode, the discharge in the area near the plane electrode is intensified, and after that the discharge on the insulator surface is also intensified.
- (2)
- In terms of particle properties, the differences in model A and B are not very different, while in model C, the electron concentration is much larger and more dispersed than the first two models, and its negative ion concentration is much smaller than that of the first two.
- (3)
- For the streamer properties, the streamer in model A develops faster than in model B, but the streamer development speed of both is much slower than that of model C. This is due to the SF6, CF3I and c-C4F8 molecules in model A and B have strong attachment, adsorbing a large number of electrons, and high electron density is also an important reason for the accelerated development of streamers. In addition, the streamer channels in models A and B are less obvious than those in model C, and the streamer channels in models A and B can almost only be seen near the streamer head.
- (4)
- Finally, in terms of streamer branches, model C is more prone to streamer branches, because many short streamer branches occur when the main streamer develops on the right surface of the insulator, whereas this phenomenon is not observed in models A and B.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Xiao, D. The effective ionization coefficients and electron drift velocities in gas mixtures of CF3I with N2 and CO2 obtained from Boltzmann equation analysis. Chin. Phys. B 2013, 22, 035101. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Wu, J.; Jia, S. Analysis of the insulation characteristics of CF3I mixtures with CF4, CO2, N2, O2 and air. J. Phys. D Appl. Phys. 2013, 46, 345203. [Google Scholar] [CrossRef]
- Toyota, H.; Matsuoka, S.; Hidaka, K. Measurement of sparkover voltage and time lag characteristics in CF3I-N2 and CF3I-air gas mixtures by using steep-front square voltage. IEEJ Trans. Fundam. Mater. 2005, 125, 409–414. [Google Scholar] [CrossRef]
- Yokomizu, Y.; Ochiai, R.; Matsumura, T. Electrical and thermal conductivities of high-temperature CO2-CF3I mixture and transient conductance of residual arc during its extinction process. J. Phys. D Appl. Phys. 2009, 42, 215204. [Google Scholar] [CrossRef]
- Katagiri, H.; Kasuya, H.; Mizoguchi, H.; Yanabu, S. Investigation of the performance of CF3I gas as a possible substitute for SF6. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1424–1429. [Google Scholar] [CrossRef]
- Zhong, R.; Zhao, S.; Xiao, D.; Wang, H.; Jiang, X.; Yu, Z.; Deng, Y. Investigation of the performance of CF3I/c-C4F8/N2 and CF3I/c-C4F8/CO2 gas mixtures from electron transport parameters. Plasma. Sci. Technol. 2020, 22, 055402. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, Y.; Zhang, Z.; Xiao, D. Analysis of the insulation characteristics of c-C4F8/CO2 gas mixtures by the Monte Carlo method. J. Phys. D Appl. Phys. 2008, 41, 015206. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, D. Analysis of the insulation characteristics of c-C4F8 and N2 gas mixtures by Boltzmann equation method. Eur. Phys. J. Appl. Phys. 2012, 57, 20801. [Google Scholar] [CrossRef]
- Zhong, R.; Zhao, S.; Zhao, X.; Xue, P.; Jiao, J.; Xiao, D. Insulation characteristics of CF3I/c-C4F8/N2 gas mixtures in slightly non-uniform electric field. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1371–1379. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Han, Y.; Cressault, Y. Experimental studies on power frequency breakdown voltage of CF3I/N2 mixed gas under different electric fields. Appl. Phys. Lett. 2016, 108, 092901. [Google Scholar] [CrossRef]
- Zang, Y.; Qian, Y.; Wang, H.; Xu, A.; Sheng, G.; Jiang, X. Method of GIL discharge localization based on natural neighbor interpolation and ECOC-MLP-SVM using optical simulation technology. High Volt. 2021, 6, 514–524. [Google Scholar] [CrossRef]
- Meyer, H.; Mauseth, F.; Marskar, R.; Pedersen, A.; Blaszczyk, A. Streamer and surface charge dynamics in non-uniform air gaps with a dielectric barrier. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1163–1171. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Li, W.; Sun, G.; Xue, J.; Wang, C.; Zhang, G. 3D printing fabrication of conductivity non-uniform insulator for surface flashover mitigation. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1172–1180. [Google Scholar] [CrossRef]
- Li, C.; Lin, C.; Chen, G.; Tu, Y.; Zhou, Y.; Li, Q.; Zhang, B.; He, J. Field-dependent charging phenomenon of HVDC spacers based on dominant charge behaviors. Appl. Phys. Lett. 2019, 114, 202904. [Google Scholar] [CrossRef]
- Dickinson, E.; Ekstrom, H.; Fontes, E. COMSOL Multiphysics®: Finite element software for electrochemical analysis. A mini-review. Electrochem. Commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Peng, B.; Jiang, N.; Yao, X.; Ruan, Y.; Wang, D.; Shang, K.; Lu, N.; Namihira, T.; Li, J.; Wu, Y. Experimental and numerical studies of primary and secondary streamers in a pulsed surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2019, 52, 325202. [Google Scholar] [CrossRef]
- Sima, W.; Shi, J.; Yang, Q. Surface discharge simulation in SF6 and N2 mixtures with a plasmachemical model. Surf. Rev. Lett. 2014, 21, 1450010. [Google Scholar] [CrossRef]
- Li, X.; Sun, A.; Teunissen, J. A computational study of negative surface discharges: Characteristics of surface streamers and surface charges. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1178–1186. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The synergistic effects of the micro-BN and nano-Al2O3 in micro-nano composites on enhancing the thermal conductivity for insulating epoxy resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Luo, B.; He, H.; Cheng, C.; Xia, S.; Chen, W. Numerical simulation of the positive streamer propagation and chemical reactions in SF6/N2 mixtures under non-uniform field. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 782–790. [Google Scholar] [CrossRef]
- Dhali, S.; Pal, K. Numerical simulation of streamers in SF6. J. Appl. Phys. 1988, 63, 1355–1362. [Google Scholar] [CrossRef]
- Kulikovsky, A. Two-dimensional simulation of the positive streamer in N2 between parallel-plate electrodes. J. Phys. D Appl. Phys. 1995, 28, 2483–2493. [Google Scholar] [CrossRef]
- Dordizadeh, P.; Adamiak, K.; Castle, G. Numerical investigation of the formation of Trichel pulses in a needle-plane geometry. J. Phys. D Appl. Phys. 2015, 48, 415203. [Google Scholar] [CrossRef]
- Tran, T.; Golosnoy, I.; Lewin, P.; Georghiou, G. Numerical modelling of negative discharges in air with experimental validation. J. Phys. D Appl. Phys. 2011, 44, 015203. [Google Scholar] [CrossRef] [Green Version]
- Hagelaar, G.; Hoog, F.; Kroesen, G. Boundary condition in fluid models in gas discharges. Phys. Rev. E 2000, 62, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Akaram, S.; Castellon, J.; Agnel, S. Progress in gas/solid interface charging phenomena. Coatings 2020, 10, 1184. [Google Scholar] [CrossRef]
- Christophorou, L.; Olthoff, J. Electron interactions with CF3I. J. Phys. Chem. Ref. Data 1999, 29, 553–569. [Google Scholar] [CrossRef]
- Itoh, H.; Mlyachi, T.; Kawaguchi, M.; Nakao, Y.; Tagashira, H. Electron transport coefficients in SF6 and c-C4F8 mixtures. J. Phys. D Appl. Phys. 2000, 24, 277–282. [Google Scholar] [CrossRef]
- Christophorou, L.; Olthoff, J. Electron interactions with c-C4F8. J. Phys. Chem. Ref. Data 2001, 30, 449–473. [Google Scholar] [CrossRef]
- Hua, W.; Fukagata, K. Near-surface electron transport and its influence on the discharge structure of nanosecond-pulsed dielectric-barrier-discharge under different electrode polarities. Phys. Plasmas 2019, 26, 013514. [Google Scholar] [CrossRef]
- Yu, R.; Gao, B.; Hu, G.; Zhou, P.; Wu, J. Development of white mark on pressboard surface and change of discharge characteristic parameters in needle-plane model. High Volt. Eng. 2020, 46, 240–249. [Google Scholar]







| Seq | Reactions | Type |
|---|---|---|
| R1 | Attachment | |
| R2 | Attachment | |
| R3 | Attachment | |
| R4 | Attachment | |
| R5 | Attachment | |
| R6 | Elastic | |
| R7 | Ionization | |
| R8 | Ionization | |
| R9 | Ionization | |
| R10 | Ionization | |
| R11 | Ionization | |
| R12 | Ionization | |
| R13 | Attachment | |
| R14 | Attachment | |
| R15 | Attachment | |
| R16 | Elastic | |
| R17 | Ionization | |
| R18 | Ionization | |
| R19 | Ionization | |
| R20 | Ionization | |
| R21 | Elastic | |
| R22 | Ionization | |
| R23 | Attachment | |
| R24 | Attachment | |
| R25 | Attachment | |
| R26 | Attachment | |
| R27 | Attachment | |
| R28 | Attachment | |
| R29 | Attachment | |
| R30 | Elastic | |
| R31 | Ionization | |
| R32 | Attachment | |
| R33 | Attachment | |
| R34 | Elastic | |
| R35 | Ionization | |
| R36-37 | Excitation | |
| R38-43 | Excitation | |
| R44-66 | Excitation | |
| R67-70 | Excitation | |
| R71-83 | Excitation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Qian, Y.; Zang, Y.; Li, Z.; Zhou, X. Numerical Modelling of Positive Surface Discharges in C-C4F8/CF3I/N2 Gas Mixture under Non-Uniform Field. Energies 2021, 14, 8299. https://doi.org/10.3390/en14248299
Fan B, Qian Y, Zang Y, Li Z, Zhou X. Numerical Modelling of Positive Surface Discharges in C-C4F8/CF3I/N2 Gas Mixture under Non-Uniform Field. Energies. 2021; 14(24):8299. https://doi.org/10.3390/en14248299
Chicago/Turabian StyleFan, Binhai, Yong Qian, Yiming Zang, Ze Li, and Xiaoli Zhou. 2021. "Numerical Modelling of Positive Surface Discharges in C-C4F8/CF3I/N2 Gas Mixture under Non-Uniform Field" Energies 14, no. 24: 8299. https://doi.org/10.3390/en14248299
APA StyleFan, B., Qian, Y., Zang, Y., Li, Z., & Zhou, X. (2021). Numerical Modelling of Positive Surface Discharges in C-C4F8/CF3I/N2 Gas Mixture under Non-Uniform Field. Energies, 14(24), 8299. https://doi.org/10.3390/en14248299






