Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Scheme
2.2. Experimental Material
2.3. Substrate Pretreatment
2.4. Experimental Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dziennik Ustaw. Available online: https://www.dziennikustaw.gov.pl/M2021000026401.pdf (accessed on 17 November 2021).
- Krajowy Rejestr Wytwórców Biogazu. Available online: https://www.kowr.gov.pl/uploads/pliki/oze/biogaz/rejestr%20wytw%C3%B3rc%C3%B3w%20biogazu%20rolniczego%20z%20dnia%2016.04.2021%20r.pdf (accessed on 17 November 2021).
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L. Giant miscanthus as a substrate for biogas production. J. Ecol. Eng. 2015, 16, 139–142. [Google Scholar]
- Piechota, G. Multi-Step Biogas Quality Improving by Adsorptive Packed Column System as Application to Biomethane Upgrading. J. Environ. Chem. Eng. 2021, 9, 105944. [Google Scholar] [CrossRef]
- Li, P.; Sakuragi, K.; Makino, H. Extraction Techniques in Sustainable Biofuel Production: A Concise Review. Fuel Process. Technol. 2019, 193, 295–303. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave Heating Processing as Alternative of Pretreatment in Second-Generation Biorefinery: An Overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef] [Green Version]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising Evolution of Biofuel Generations. Subject Review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Wang, D.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can Hydrothermal Pretreatment Improve Anaerobic Digestion for Biogas from Lignocellulosic Biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2021, 14, 150. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological Pretreatment of Lignocellulosic Biomass—An Overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Bertilsson, M.; Gorwa-Grauslund, M.F.; Gorsich, S.; Lidén, G. Metabolic Effects of Furaldehydes and Impacts on Biotechnological Processes. Appl. Microbiol. Biotechnol. 2009, 82, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The Impact and Mode of Action of Phenolic Compounds Extracted from Brown Seaweed on Mixed Anaerobic Microbial Cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.Y.; Sandoval, N.R.; Gill, R.T. Cellulosic Hydrolysate Toxicity and Tolerance Mechanisms in Escherichia Coli. Biotechnol. Biofuels 2009, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Cueva, C.; Mingo, S.; Muñoz-González, I.; Bustos, I.; Requena, T.; del Campo, R.; Martín-Álvarez, P.; Bartolomé, B.; Moreno-Arribas, M.V. Antibacterial Activity of Wine Phenolic Compounds and Oenological Extracts against Potential Respiratory Pathogens. Lett. Appl. Microbiol. 2012, 54, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ujor, V.; Wick, M.; Ezeji, T. Identification, Purification and Characterization of Furfural Transforming Enzymes from Clostridium Beijerinckii NCIMB 8052. Anaerobe 2015, 33, 124–131. [Google Scholar] [CrossRef]
- Elalami, D.; Monlau, F.; Carrere, H.; Abdelouahdi, K.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Effect of Coupling Alkaline Pretreatment and Sewage Sludge Co-Digestion on Methane Production and Fertilizer Potential of Digestate. Sci. Total Environ. 2020, 743, 140670. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Production of Hydrogen and Methane from Lignocellulose Waste by Fermentation. A Review of Chemical Pretreatment for Enhancing the Efficiency of the Digestion Process. J. Clean. Prod. 2020, 267, 121721. [Google Scholar] [CrossRef]
- Balat, M. Production of Bioethanol from Lignocellulosic Materials via the Biochemical Pathway: A Review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Du, Z.; Zheng, T.; Wang, P.; Hao, L.; Wang, Y. Fast Microwave-Assisted Preparation of a Low-Cost and Recyclable Carboxyl Modified Lignocellulose-Biomass Jute Fiber for Enhanced Heavy Metal Removal from Water. Bioresour. Technol. 2016, 201, 41–49. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M.; Rusanowska, P. Progress in the Production of Biogas from Virginia Mallow after Alkaline-Heat Pretreatment. Biomass Bioenergy 2019, 126, 174–180. [Google Scholar] [CrossRef]
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short Duration Microwave Assisted Pretreatment Enhances the Enzymatic Saccharification and Fermentable Sugar Yield from Sugarcane Bagasse. Renew. Energy 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Zhu, Z.; Rezende, C.A.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J.; Polikarpov, I.; Gomez, L.D. Efficient Sugar Production from Sugarcane Bagasse by Microwave Assisted Acid and Alkali Pretreatment. Biomass Bioenergy 2016, 93, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid Pretreatment of Lignocellulosic Biomass for Energy Vectors Production: A Review Focused on Operational Conditions and Techno-Economic Assessment for Bioethanol Production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Bichot, A.; Lerosty, M.; Méchin, V.; Bernet, N.; Delgenès, J.P.; García-Bernet, D. Evaluation of Chemical-Free Microwave Pretreatment on Methane Yield of Two Grass Biomass with Contrasted Parietal Content. Energy Convers. Manag. 2021, 229, 113746. [Google Scholar] [CrossRef]
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pretreatment of Lignocellulosic Agricultural Waste for Delignification, Rapid Hydrolysis, and Enhanced Biogas Production: A Review. J. Indian Chem. Soc. 2021, 98, 100147. [Google Scholar] [CrossRef]
- Jackowiak, D.; Bassard, D.; Pauss, A.; Ribeiro, T. Optimisation of a Microwave Pretreatment of Wheat Straw for Methane Production. Bioresour. Technol. 2011, 102, 6750–6756. [Google Scholar] [CrossRef] [PubMed]
- Jackowiak, D.; Frigon, J.C.; Ribeiro, T.; Pauss, A.; Guiot, S. Enhancing Solubilisation and Methane Production Kinetic of Switchgrass by Microwave Pretreatment. Bioresour. Technol. 2011, 102, 3535–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapci, Z. The Effect of Microwave Pretreatment on Biogas Production from Agricultural Straws. Bioresour. Technol. 2013, 128, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, K.J.; Dubis, B.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Productivity and Energy Balance of Maize and Sorghum Grown for Biogas in a Large-Area Farm in Poland: An 11-Year Field Experiment. Ind. Crop. Prod. 2020, 148, 112326. [Google Scholar] [CrossRef]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 2002, 31, 426–428. [Google Scholar] [CrossRef]
- Światek, K.; Lewandowska, M.; Światek, M.; Bednarski, W.; Brzozowski, B. The Improvement of Enzymatic Hydrolysis Efficiency of Rape Straw and Miscanthus Giganteus Polysaccharides. Bioresour. Technol. 2014, 151, 323–331. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Hydrothermal Processing of Lignocellulosic Materials. Holz als roh-und Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Gregg, D.; Saddler, J.N. A Techno-Economic Assessment of the Pretreatment and Fractionation Steps of a Biomass-to-Ethanol Process. Appl. Biochem. Biotechnol. 1996, 57, 711–727. [Google Scholar] [CrossRef]
- Brownell, H.; Yu, E.; Saddler, J. Steam-Explosion Pretreatment of Wood: Effect of Chip Size, Acid, Moisture Content and Pressure Drop. Biotechnol. Bioeng. 1986, 28, 792–801. [Google Scholar] [CrossRef]
- Gabhane, J.; Prince William, S.P.M.; Gadhe, A.; Rath, R.; Vaidya, A.N.; Wate, S. Pretreatment of Banana Agricultural Waste for Bio-Ethanol Production: Individual and Interactive Effects of Acid and Alkali Pretreatments with Autoclaving, Microwave Heating and Ultrasonication. Waste Manag. 2014, 34, 498–503. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, S.M.; Santos, A.M.P.; Rocha, G.J.M.; Souto-Maior, A.M. Diluted Phosphoric Acid Pretreatment for Production of Fermentable Sugars in a Sugarcane-Based Biorefinery. Bioresour. Technol. 2013, 135, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Phutela, U.G. Enhancement of Paddy Straw Digestibility and Biogas Production by Sodium Hydroxide-Microwave Pretreatment. Renew. Energy 2016, 92, 178–184. [Google Scholar] [CrossRef]
- Laghari, S.; Isa, M.; Laghari, A. Delignification of Coconut Husk by Microwave Assisted Chemical Pretreatment. Adv. Environ. Biol. 2015, 9, 1–5. [Google Scholar]
- Gossett, J.M.; Stuckey, D.C.; Owen, W.F.; McCarty, P.L. Heat Treatment and Anaerobic Digestion of Refuse. J. Environ. Eng. Div. 1982, 108, 437–454. [Google Scholar] [CrossRef]
- Díaz, M.J.; Cara, C.; Ruiz, E.; Pérez-Bonilla, M.; Castro, E. Hydrothermal Pre-Treatment and Enzymatic Hydrolysis of Sunflower Stalks. Fuel 2011, 90, 3225–3229. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.-P.; Carrere, H. Predictive Models of Biohydrogen and Biomethane Production Based on the Compositional and Structural Features of Lignocellulosic Materials. Environ. Sci. Technol. 2012, 46, 12217–12225. [Google Scholar] [CrossRef]
- Larsson, S. Ethanol from Lignocellulose-Fermentation Inhibitors, Detoxification and Genetic Engineering of Sacchwomyces Cerevkiae for Enhanced Resistance. Ph.D. Thesis, Lund University, Lund, Sweden, 2000. [Google Scholar]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated Development of Leading Biomass Pretreatment Technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Lee, K.; Mahmood, Q. Bio-Hydrogen Production by Chlorella Vulgaris under Diverse Photoperiods. Bioresour. Technol. 2011, 102, 2101–2104. [Google Scholar] [CrossRef]
- Thomas, H.L.; Arnoult, S.; Brancourt-Hulmel, M.; Carrère, H. Methane Production Variability According to Miscanthus Genotype and Alkaline Pretreatments at High Solid Content. Bioenergy Res. 2019, 12, 325–337. [Google Scholar] [CrossRef]
- Siddhu, M.A.H.; Li, J.; Zhang, J.; Huang, Y.; Wang, W.; Chen, C.; Liu, G. Improve the Anaerobic Biodegradability by Copretreatment of Thermal Alkali and Steam Explosion of Lignocellulosic Waste. BioMed Res. Int. 2016, 2016, 2786598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kainthola, J.; Shariq, M.; Kalamdhad, A.S.; Goud, V.V. Enhanced Methane Potential of Rice Straw with Microwave Assisted Pretreatment and Its Kinetic Analysis. J. Environ. Manag. 2019, 232, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Latrille, E.; da Costa, A.C.; Steyer, J.P.; Carrère, H. Enhancement of Methane Production from Sunflower Oil Cakes by Dilute Acid Pretreatment. Appl. Energy 2013, 102, 1105–1113. [Google Scholar] [CrossRef]
- Dębicki, P.; Styłba, S. Oddziaływania Środowiskowe Pół Elektromagnetycznych: Aspekty Fizyczne, Techniczne i Prawne; Akademia Morska: Szczecin, Poland, 2010. [Google Scholar]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]

| Pretreatment Method | Advantages | Disadvantages |
|---|---|---|
| Mechanical disintegration | Cellulose crystallinity reduction | Energy consumption exceeding the amount of energy produced |
| Steam explosion | Hemicellulose degradation; lignin transformation; high cost-effectiveness | Damage to a part of xylans; incomplete disruption of the lignin–carbohydrate matrix; formation of inhibitors |
| Ammonia fiber explosion | Increasing the specific surface area; removal of lignin and cellulose; lack of inhibitors | No effects in the case of high-lignin substrates |
| Effects of carbon dioxide | Increasing the specific surface area; cost-effectiveness; no inhibitors | No effect on lignin and hemicellulose |
| Acidic | Hydrolysis of hemicellulose to xylose and other sugars; lignin structure modification | High costs; potential corrosion-inducing effect; formation of inhibitors |
| Alkaline | Removal of lignin and hemicellulose; increasing the specific surface area | Time-consuming |
| Biological | Degradation of lignin and hemicellulose; low energy requirement | Very slow course of hydrolysis |
| Parameter | Unit | Value |
|---|---|---|
| Total solids 1 | mg/gTS | 360.0 ± 11.0 |
| Volatile solids 2 | mg/gVS | 326.0 ± 9.1 |
| Cellulose 3 | %TS | 20.1 ± 0.5 |
| Hemicellulose 3 | %TS | 14.6 ± 0.3 |
| Lignin 3 | %TS | 2.6 ± 0.1 |
| Acid Dose (g/gTS) | Hydrolysis Yield (Addition of HCl) (%) | Hydrolysis Yield (Addition of H3PO4) (%) | Hydrolysis Yield (Addition of H2SO4) (%) |
|---|---|---|---|
| Conventional heating | |||
| 0.0 | 0.41 ± 0.00 | 0.35 ± 0.02 | 0.42 ± 0.04 |
| 0.02 | 0.63 ± 0.11 | 0.57 ± 0.04 | 0.48 ± 0.04 |
| 0.05 | 0.86 ± 0.10 | 0.84 ± 0.10 | 0.69 ± 0.02 |
| 0.1 | 1.38 ± 0.14 | 0.82 ± 0.05 | 1.07 ± 0.03 |
| 0.2 | 2.58 ± 0.22 | 1.69 ± 0.09 | 2.89 ± 0.27 |
| 0.4 | 4.75 ± 0.38 | 1.71 ± 0.07 | 5.70 ± 0.27 |
| Microwave heating | |||
| 0.0 | 0.51 ± 0.02 | 0.45 ± 0.02 | 0.51 ± 0.04 |
| 0.02 | 1.45 ± 0.07 | 1.01 ± 0.03 | 0.72 ± 0.06 |
| 0.05 | 2.96 ± 0.25 | 1.78 ± 0.08 | 0.82 ± 0.03 |
| 0.1 | 4.44 ± 0.44 | 2.30 ± 0.17 | 1.64 ± 0.02 |
| 0.2 | 5.95 ± 0.53 | 6.72 ± 0.33 | 8.98 ± 0.19 |
| 0.4 | 10.84 ± 0.66 | 6.91 ± 0.20 | 11.57 ± 0.22 |
| Dose | HCl | H3PO4 | H2SO4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
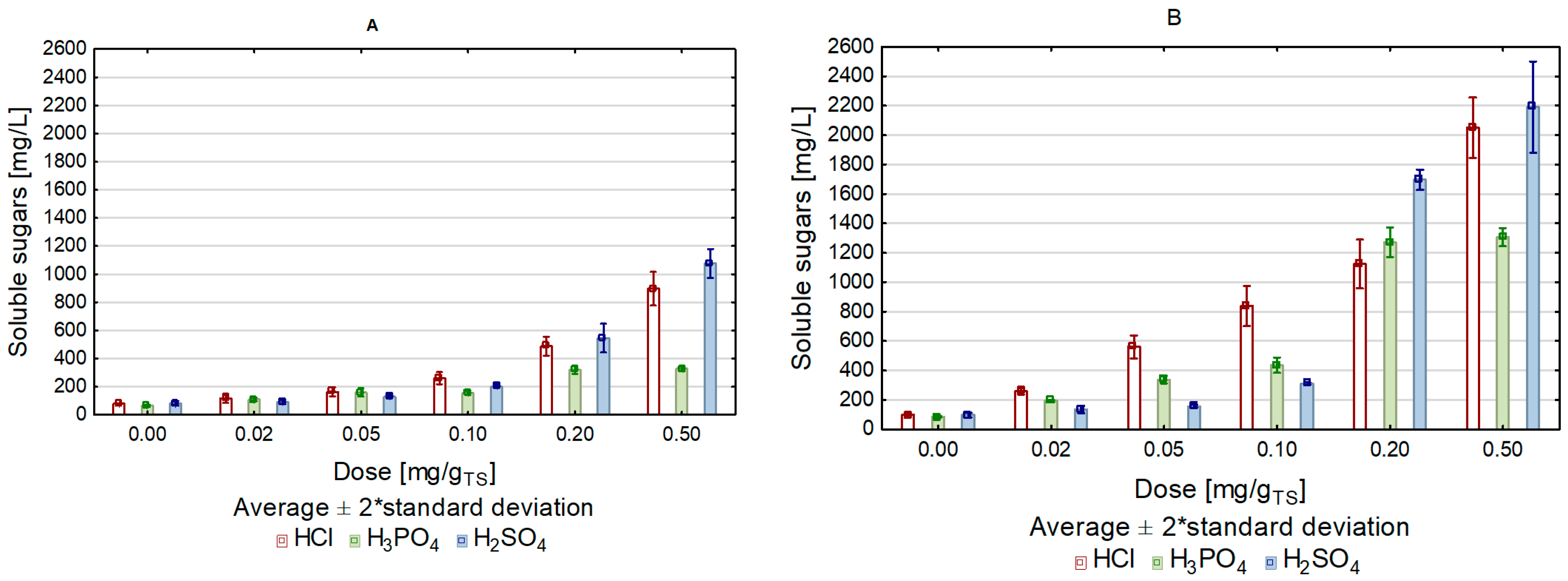
| Soluble Sugars (mg/L) | By-Products Concentration (mg/L) | Σ Soluble Sugars/Σ Furans | Σ Soluble Sugars/Σ Furans and Phenols | Soluble Sugars (mg/L) | By-Products Concentration (mg/L) | Σ Soluble Sugars/Σ Furans | Σ Soluble Sugars/Σ Furans and Phenols | Soluble Sugars (mg/L) | By-Products Concentration (mg/L) | Σ Soluble Sugars/Σ Furans | Σ Soluble Sugars/Σ Furans and Phenols | |||||||
| Furfural | 5-HMF | Phenols | Furfural | 5-HMF | Phenols | Furfural | 5-HMF | Phenols | ||||||||||
| Conventional heating | ||||||||||||||||||
| 0 | 78.7 | 70 | 32 | 23 | 0.77 | 0.63 | 65.3 | 75 | 33 | 21 | 0.60 | 0.51 | 81.2 | 69 | 35 | 20 | 0.78 | 0.65 |
| 0.02 | 118.1 | 98 | 45 | 22 | 0.83 | 0.72 | 109.5 | 101 | 28 | 25 | 0.85 | 0.71 | 92.3 | 98 | 42 | 30 | 0.66 | 0.54 |
| 0.05 | 163.3 | 110 | 48 | 56 | 1.03 | 0.76 | 159.3 | 120 | 48 | 48 | 0.95 | 0.74 | 130.3 | 115 | 57 | 55 | 0.76 | 0.57 |
| 0.1 | 260.7 | 290 | 65 | 89 | 0.73 | 0.59 | 155.7 | 305 | 46 | 65 | 0.44 | 0.37 | 202.4 | 305 | 63 | 78 | 0.55 | 0.45 |
| 0.2 | 487.7 | 330 | 112 | 110 | 1.10 | 0.88 | 318.7 | 535 | 138 | 183 | 0.47 | 0.37 | 545.7 | 358 | 115 | 98 | 1.15 | 0.96 |
| 0.4 | 897.3 | 482 | 185 | 158 | 1.35 | 1.09 | 323.1 | 702 | 215 | 335 | 0.35 | 0.26 | 1076.7 | 498 | 171 | 134 | 1.61 | 1.34 |
| Microwave heating | ||||||||||||||||||
| 0 | 98.2 | 78 | 35 | 28 | 1.24 | 0.70 | 85.7 | 81 | 34 | 26 | 0.75 | 0.61 | 96.3 | 77 | 31 | 24 | 0.89 | 0.73 |
| 0.02 | 275.7 | 118 | 42 | 28 | 1.69 | 1.47 | 192.3 | 105 | 27 | 25 | 1.46 | 1.22 | 135.3 | 98 | 45 | 27 | 0.95 | 0.80 |
| 0.05 | 559.3 | 250 | 56 | 65 | 2.07 | 1.51 | 337.8 | 116 | 46 | 41 | 2.09 | 1.66 | 156.7 | 104 | 59 | 48 | 0.96 | 0.74 |
| 0.1 | 838.0 | 308 | 120 | 89 | 1.47 | 1.62 | 435.2 | 317 | 57 | 67 | 1.16 | 0.99 | 310.2 | 287 | 67 | 81 | 0.88 | 0.71 |
| 0.2 | 1124.3 | 310 | 152 | 125 | 1.12 | 1.92 | 127.0 | 448 | 171 | 191 | 2.05 | 1.57 | 1696.8 | 322 | 128 | 89 | 3.77 | 3.15 |
| 0.4 | 2049.5 | 450 | 190 | 168 | 1.26 | 2.54 | 1306.7 | 698 | 204 | 328 | 1.45 | 1.34 | 2186.7 | 502 | 198 | 155 | 3.12 | 2.56 |
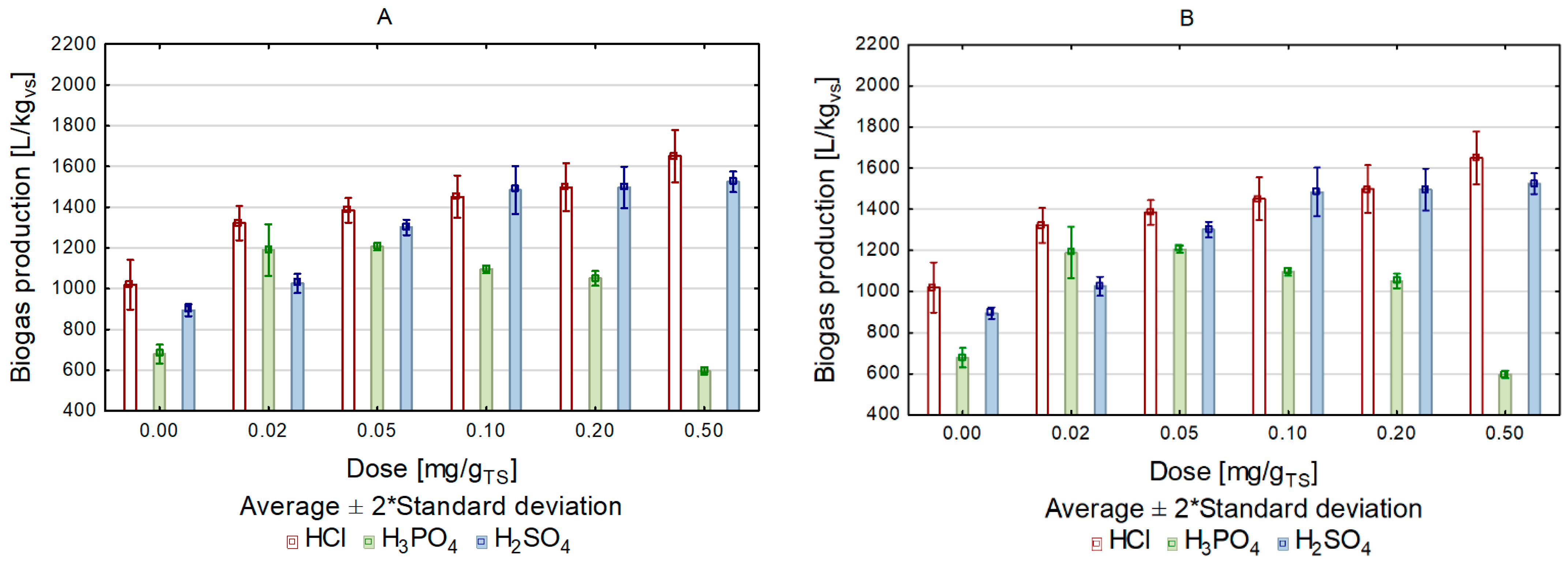
| Acid Dose (g/gvs) | Reaction Rate Constant | Biogas Production Rate | ||||
|---|---|---|---|---|---|---|
| Addition of HCl k (d−1) | Addition of H3PO4 k (d−1) | Addition of H2SO4 k (d−1) | Addition of HCl r (L/d) | Addition of H3PO4 r (L/d) | Addition of H2SO4 r (L/d) | |
| Conventional heating | ||||||
| 0.0 | 0.27 | 0.27 | 0.21 | 0.12 | 0.08 | 0.09 |
| 0.02 | 0.25 | 0.22 | 0.26 | 0.15 | 0.12 | 0.12 |
| 0.05 | 0.23 | 0.2 | 0.23 | 0.14 | 0.11 | 0.14 |
| 0.1 | 0.25 | 0.23 | 0.23 | 0.16 | 0.11 | 0.16 |
| 0.2 | 0.24 | 0.24 | 0.21 | 0.16 | 0.13 | 0.14 |
| 0.4 | 0.2 | 0.27 | 0.2 | 0.15 | 0.08 | 0.14 |
| Microwave heating | ||||||
| 0.0 | 0.27 | 0.27 | 0.29 | 0.14 | 0.09 | 0.13 |
| 0.02 | 0.26 | 0.17 | 0.27 | 0.17 | 0.09 | 0.13 |
| 0.05 | 0.21 | 0.18 | 0.25 | 0.15 | 0.13 | 0.15 |
| 0.1 | 0.22 | 0.17 | 0.21 | 0.16 | 0.98 | 0.18 |
| 0.2 | 0.25 | 0.23 | 0.23 | 0.22 | 0.13 | 0.17 |
| 0.4 | 0.2 | 0.27 | 0.16 | 0.17 | 0.09 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M. Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies 2021, 14, 8018. https://doi.org/10.3390/en14238018
Nowicka A, Zieliński M, Dębowski M, Dudek M. Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies. 2021; 14(23):8018. https://doi.org/10.3390/en14238018
Chicago/Turabian StyleNowicka, Anna, Marcin Zieliński, Marcin Dębowski, and Magda Dudek. 2021. "Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment" Energies 14, no. 23: 8018. https://doi.org/10.3390/en14238018
APA StyleNowicka, A., Zieliński, M., Dębowski, M., & Dudek, M. (2021). Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies, 14(23), 8018. https://doi.org/10.3390/en14238018








