Analysis on the Improvement of Thermal Performance of Phase Change Material Ba (OH)2·8H2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Equipment
2.2. Experimental Method
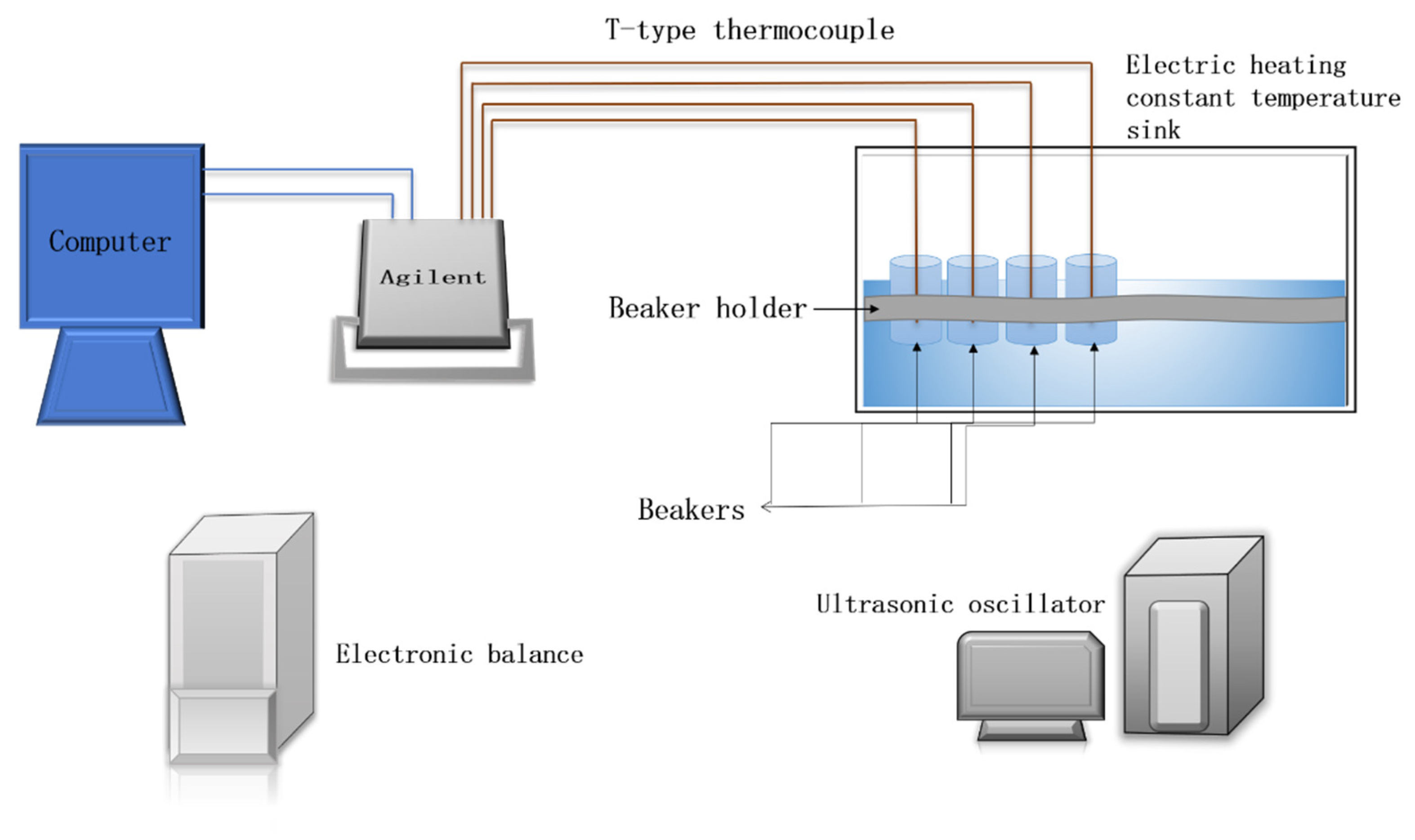
2.3. Phase Change Material Experiment Platform Construction
3. Experimental Results and Discussions
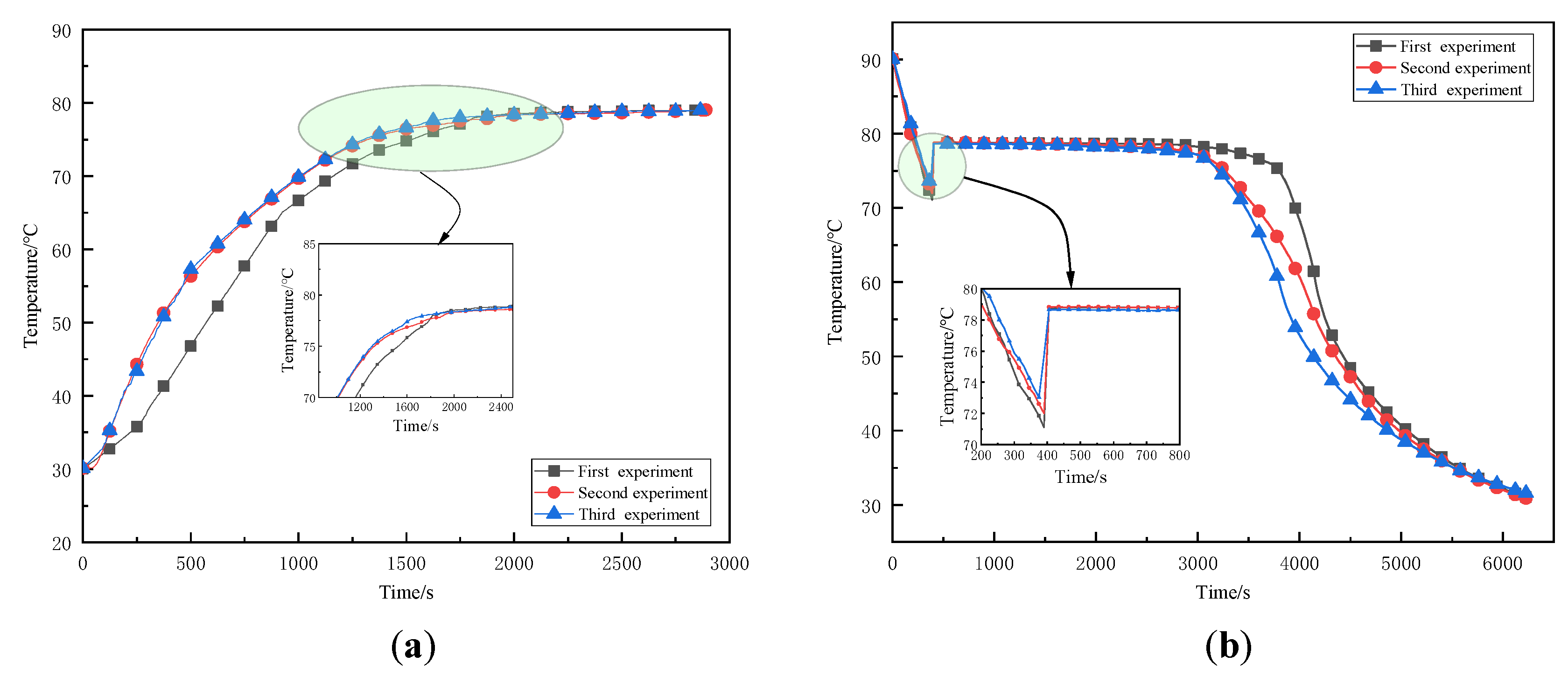
3.1. Test Results and Analysis of Substrate Supercooling
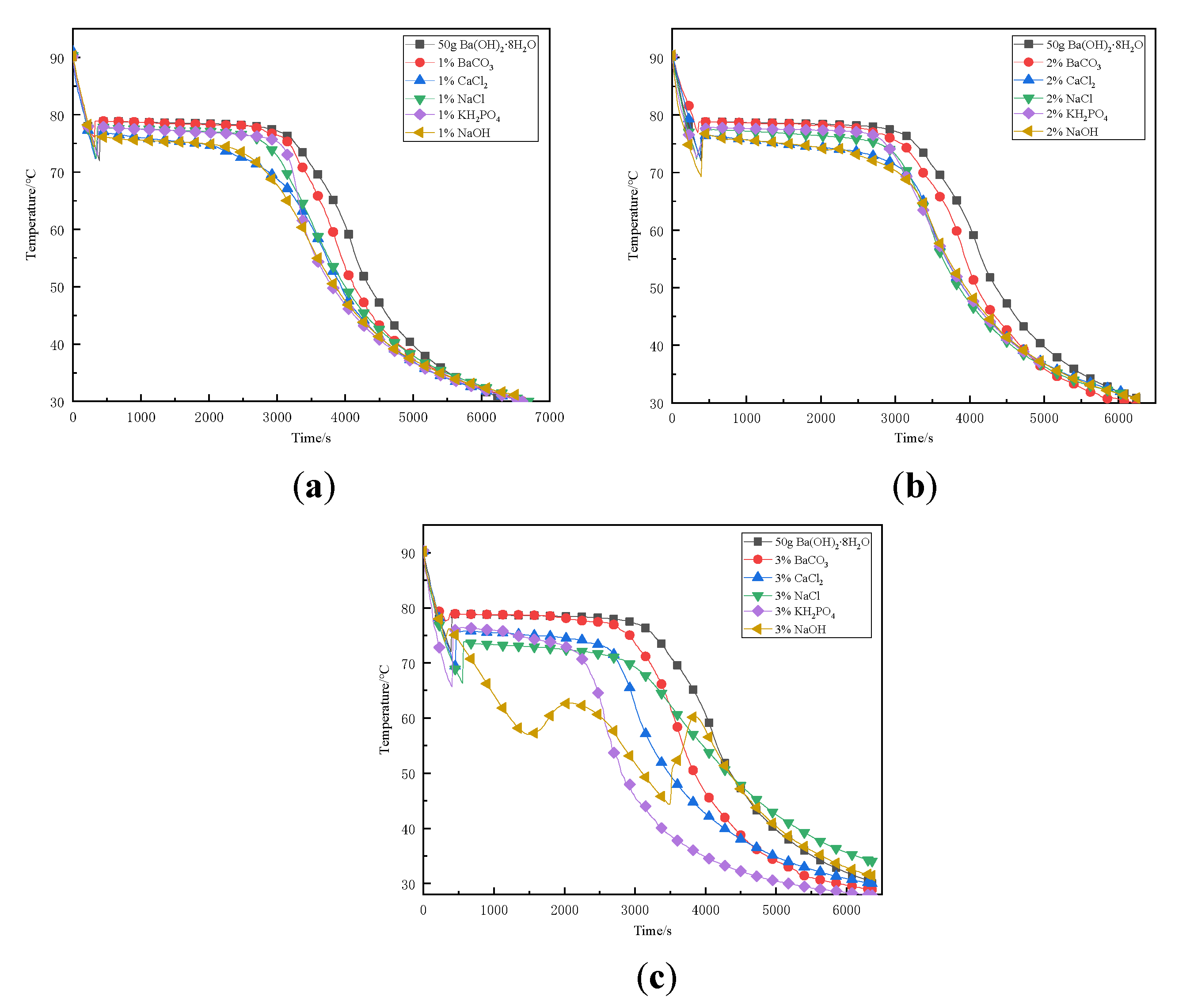
3.2. Analysis of the Influence of Different Types of Nucleating Agents on Supercooling Degree of Ba(OH)2·8H2O
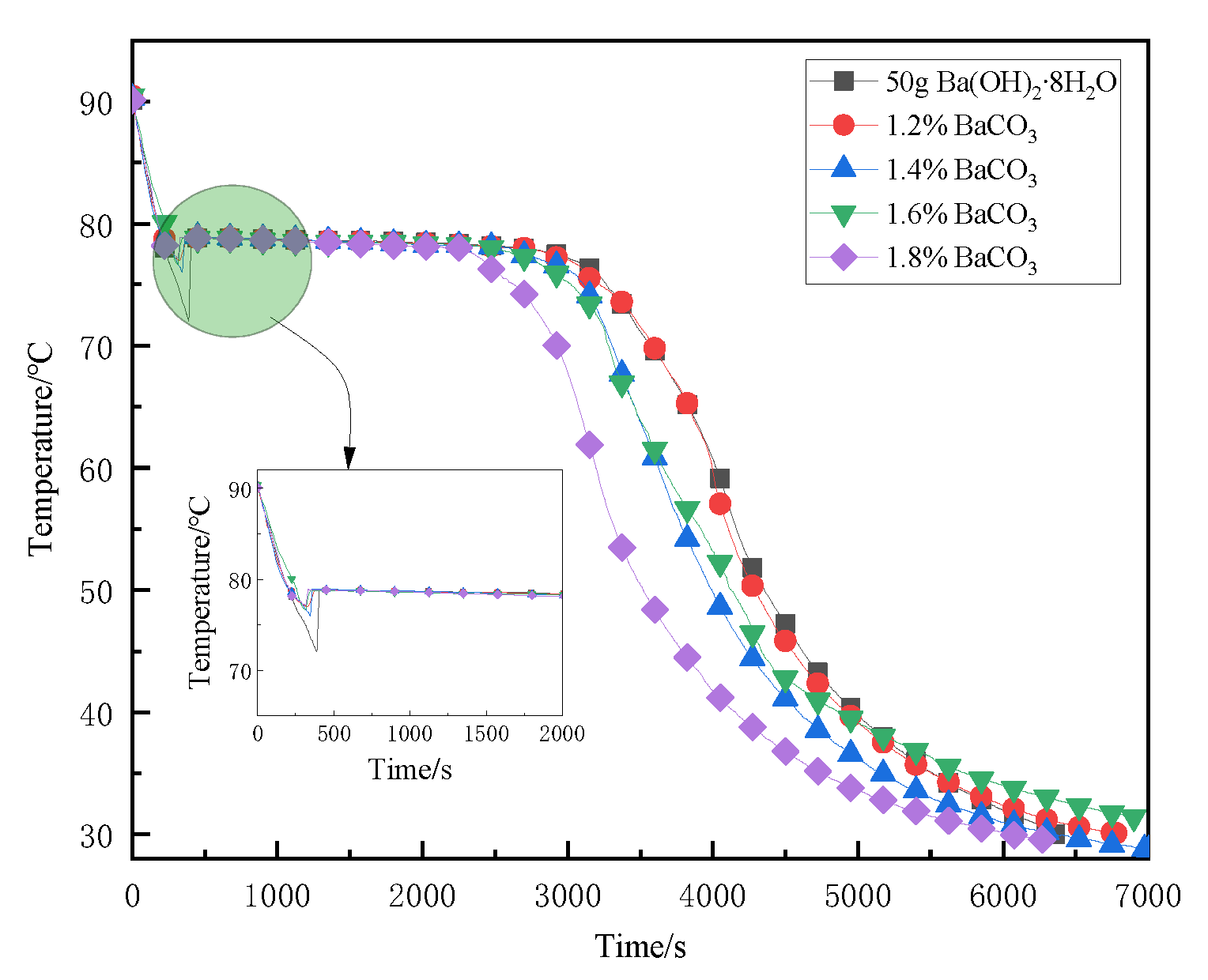
3.3. Adding Different Mass Fractions of Nucleating Agents in the Supercooling Test
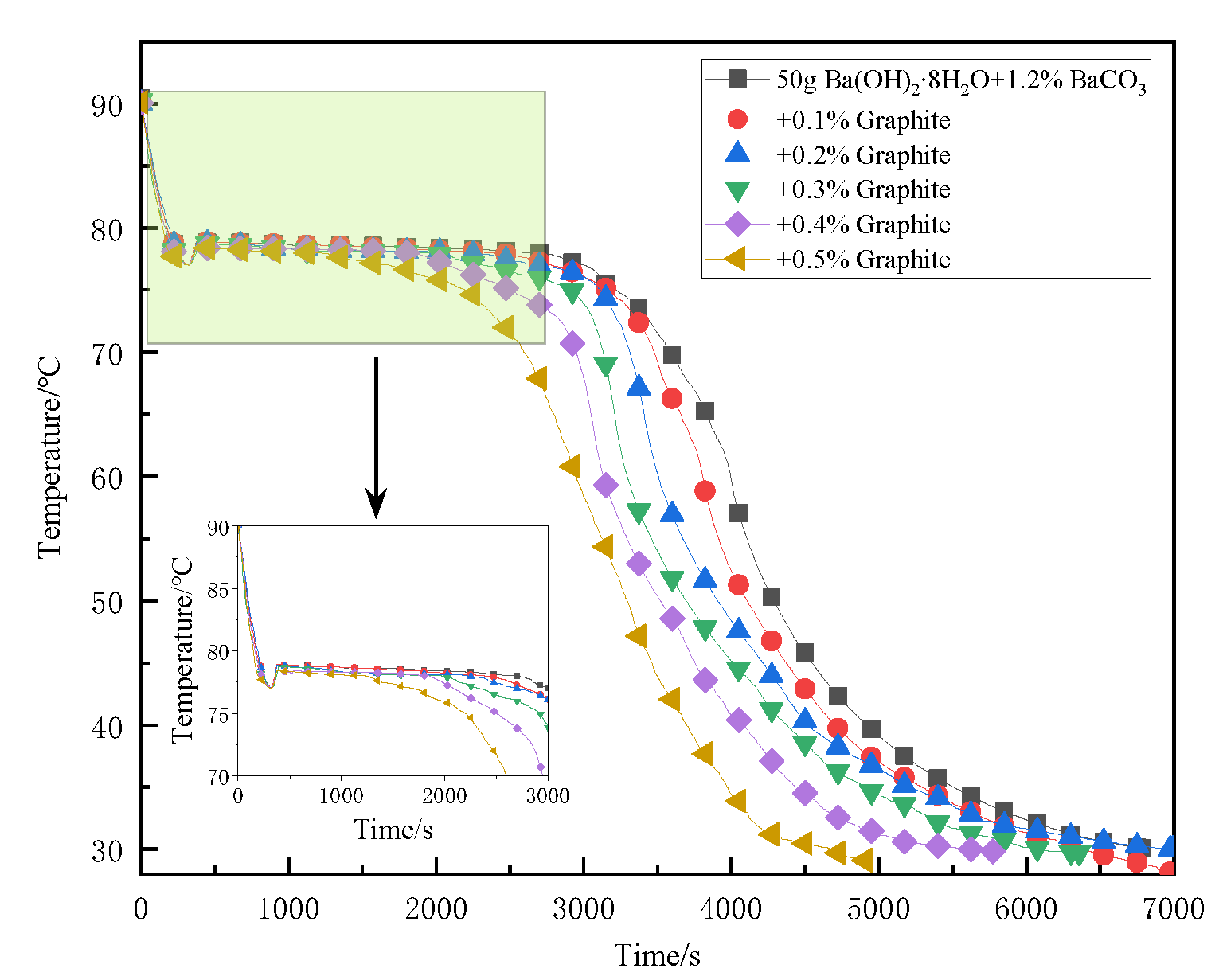
3.4. Add Graphite Powder with Different Mass Fractions for Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, S.; Luo, Y. Multiscale Modeling of Lignocellulosic Biomass Thermochemical Conversion Technology: An Overview on the State-of-the-Art. Energy Fuels 2020, 34, 11867–11886. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Zhang, G.; Xiong, B. Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bauer, N.; Mouratiadou, I.; Luderer, G.; Baumstark, L.; Brecha, R.J.; Edenhofer, O.; Kriegler, E. Global fossil energy markets and climate change mitigation–an analysis with REMIND. Clim. Chang. 2016, 136, 69–82. [Google Scholar] [CrossRef]
- Nol, J.A.; Kahwaji, S.; Desgrosseilliers, L.; Groulx, D.; White, M.A. Phase Change Materials. Storing Energy 2016, 2, 249–272. [Google Scholar]
- Li, C.; Gou, F.; Yu, R. Application of Phase Change Heat Storage Technology in Vehicle Engineering Field. IOP Conf. Ser. Earth Environ. Sci. 2020, 615, 12052. [Google Scholar] [CrossRef]
- Ren, Q.; He, Y.-L.; Su, K.-Z.; Chan, C.L. Investigation of the effect of metal foam characteristics on the PCM melting performance in a latent heat thermal energy storage unit by pore-scale lattice Boltzmann modeling. Numer. Heat Transf. Part A Appl. 2017, 72, 745–764. [Google Scholar] [CrossRef]
- Irsyad, M. Harmen Heat transfer characteristics of coconut oil as phase change material to room cooling application. IOP Conf. Ser. Earth Environ. Sci. 2017, 60, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Khamooshi, A.; Kani, E.N. Development of a phase-change material for heat storage in gypsum-based building materials. Proc. Inst. Civ. Eng. 2019, 172, 79–88. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, H.; Tripathi, B.P. Synthesis and nanoencapsulation of poly (ethylene glycol)-distearates phase change materials for latent heat storage and release. ACS Appl. Energy Mater. 2020, 3, 5965–5976. [Google Scholar] [CrossRef]
- Mert, H.H. PolyHIPE composite based-form stable phase change material for thermal energy storage. Int. J. Energy Res. 2020, 44, 6583–6594. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Ha Thanh, T.; Lam Quang, V.; Huynh Thanh, D. Determination of the dynamic resistance of the quantum dots solar cells by one I-V curve and electrochemical impedance spectra. Sol. Energy Mater. Sol. Cells 2015, 143, 269–274. [Google Scholar] [CrossRef]
- Petrecca, G. Energy Conversion and Management; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Zhang, Z.; Qin, Z.; Yong, L.I.; Wang, Y. Progress in Supercooling and Suppression Methods of Phase Change Materials. Mater. Rep. 2019, 33, 3613–3619. [Google Scholar]
- Lu, L.; Zhang, X.; Shao, T.; Zhang, J.; Wang, Q. Study on the Supercooling Degree of Eutectic Hydrated Salt Phase Change Heat Storage Material. Shandong Chem. Ind. 2019, 48, 75–76. [Google Scholar]
- He, M.; Yang, L.; Zhang, Z. Supercooling characteristics of inorganic phase change material CaCl·6HO. CIESC J. 2017, 68, 4016–4024. [Google Scholar]
- Yong, D.; Li, J.; Deng, Y.; Nian, H.; Hua, J. Supercooling Suppression and Thermal Conductivity Enhancement of Na2HPO4·12H2O/Expanded Vermiculite Form-Stable Composite Phase Change Materials with Alumina for Heat Storage. ACS Sustain. Chem. Eng. 2018, 6, 6792–6801. [Google Scholar]
- Li, Z.; Zhong, D.; Zhou, H.; Cai, Q.; Liu, Y.; Liu, L.; Ma, F. Effect of nucleating agent on the thermal storage performance of Ba(OH)2·8H2O as a phase change material. Mater. Res. Express 2019, 6, 095514. [Google Scholar] [CrossRef]
- Sheng, Q.; Xing, Y.; Wang, Z. Influence of nucleating agents on the supercooling of Ba(OH)2·8H2O as phase change material. New Chem. Mater. 2015, 43, 100–102. [Google Scholar]
- Cui, K.; Liu, L.; Sun, M. Study on improving the heat storage property of Ba(OH)2·8H2O with paraffin. Mater. Res. Express 2017, 4, 125502. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Xia, B.; Dong, Z.; Zhang, X. Enhanced Thermal Conductivity of Composite Phase Change Materials based on Carbon Modified Expanded Perlite. Mater. Chem. Phys. 2021, 261, 124226. [Google Scholar] [CrossRef]
- Manting, F.; Xuelai, Z.; Jun, J.I.; Weisan, H.U.A.; Biao, L.I.U.; Xuzhe, W. Progress in hydrated salt based composite phase change materials. Energy Storage Sci. Technol. 2019, 8, 709–717. [Google Scholar] [CrossRef]

| Type | Name | Chemical Formula | Purity Content |
|---|---|---|---|
| Nucleating agent | Barium carbonate | BaCO3 | ≥99.0% |
| Calcium chloride | CaCl2 | ≥96.0% | |
| Sodium chloride | NaCl | ≥99.5% | |
| Potassium Dihydrogen Phosphate | KH2PO4 | ≥99.5% | |
| Sodium hydroxide | NaOH | ≥96.0% | |
| Thermal conductive agent | Graphite | —— | ≥98.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Luo, X.; Cao, S.; Zou, C. Analysis on the Improvement of Thermal Performance of Phase Change Material Ba (OH)2·8H2O. Energies 2021, 14, 7761. https://doi.org/10.3390/en14227761
Lu X, Luo X, Cao S, Zou C. Analysis on the Improvement of Thermal Performance of Phase Change Material Ba (OH)2·8H2O. Energies. 2021; 14(22):7761. https://doi.org/10.3390/en14227761
Chicago/Turabian StyleLu, Xiaohui, Xiaoxue Luo, Shibo Cao, and Changzhen Zou. 2021. "Analysis on the Improvement of Thermal Performance of Phase Change Material Ba (OH)2·8H2O" Energies 14, no. 22: 7761. https://doi.org/10.3390/en14227761
APA StyleLu, X., Luo, X., Cao, S., & Zou, C. (2021). Analysis on the Improvement of Thermal Performance of Phase Change Material Ba (OH)2·8H2O. Energies, 14(22), 7761. https://doi.org/10.3390/en14227761





