Performance of Passive and Active Balancing Systems of Lithium Batteries in Onerous Mine Environment
Abstract
:1. Introduction
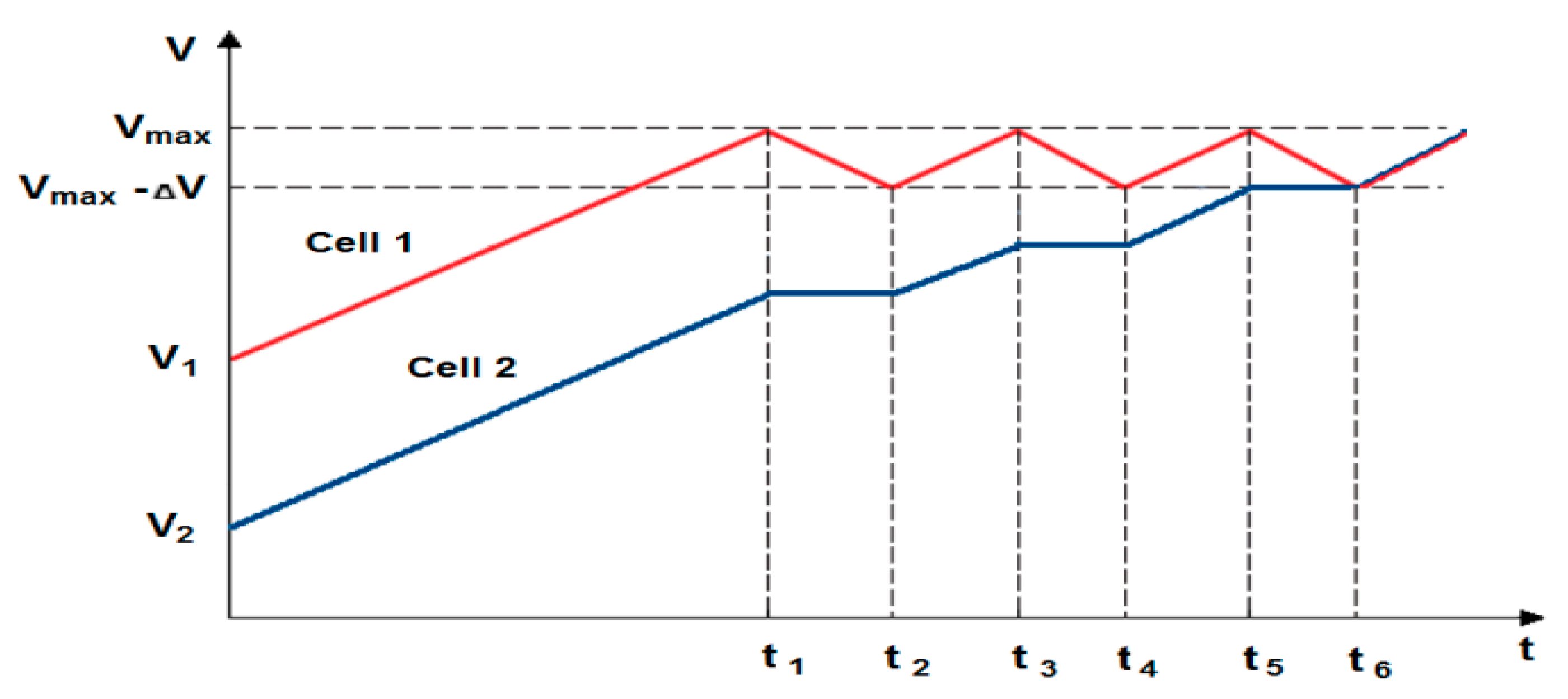
2. Balancing (Equalization) Cells Solutions
- -
- balancing by parametric selection of cells,
- -
- passive balancing,
- -
- active balancing [26].
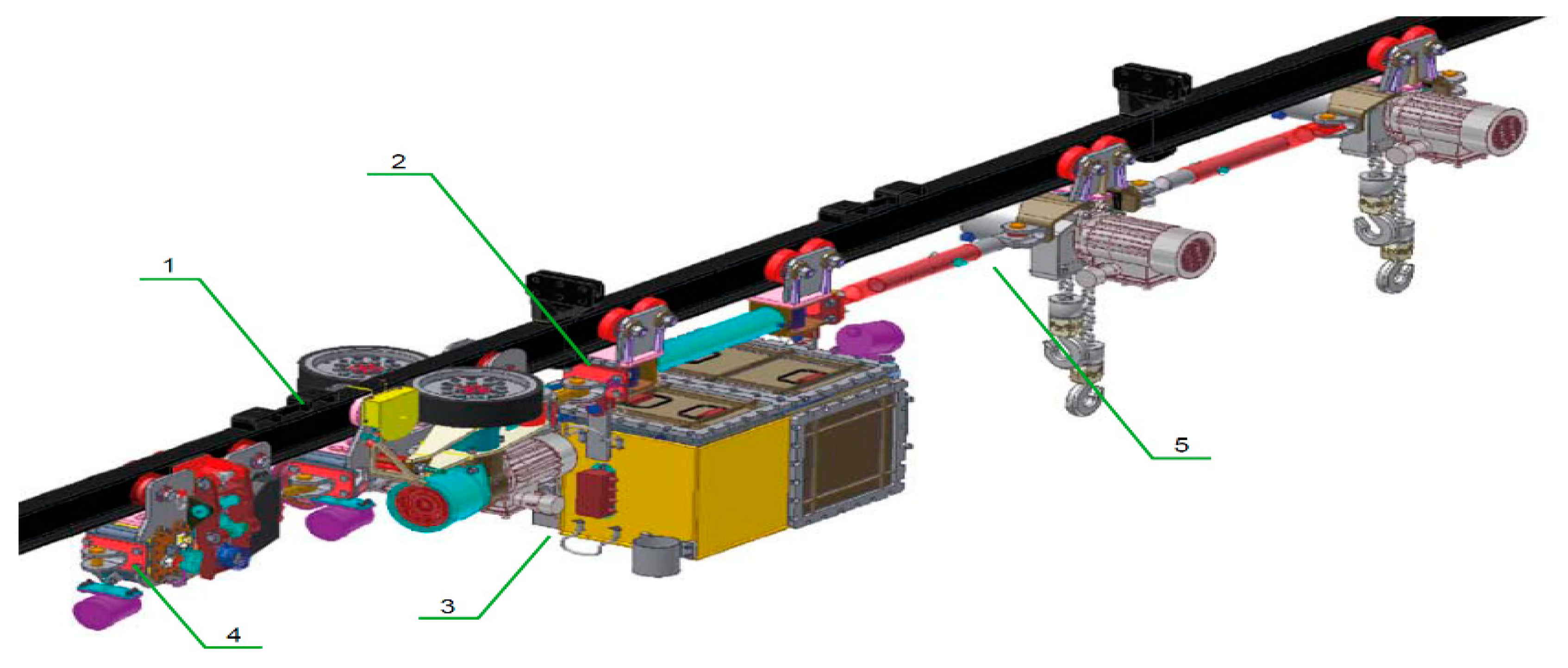
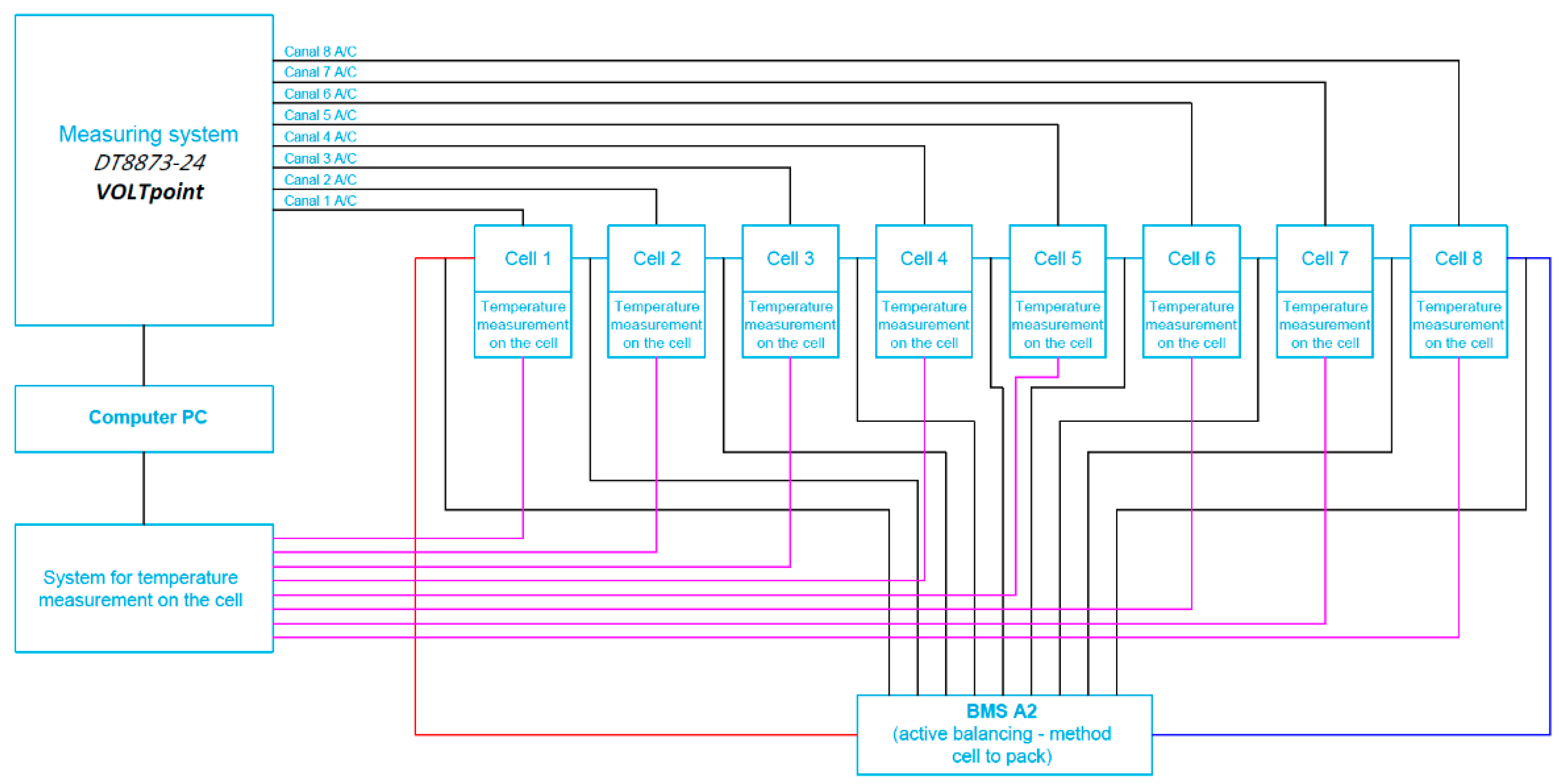
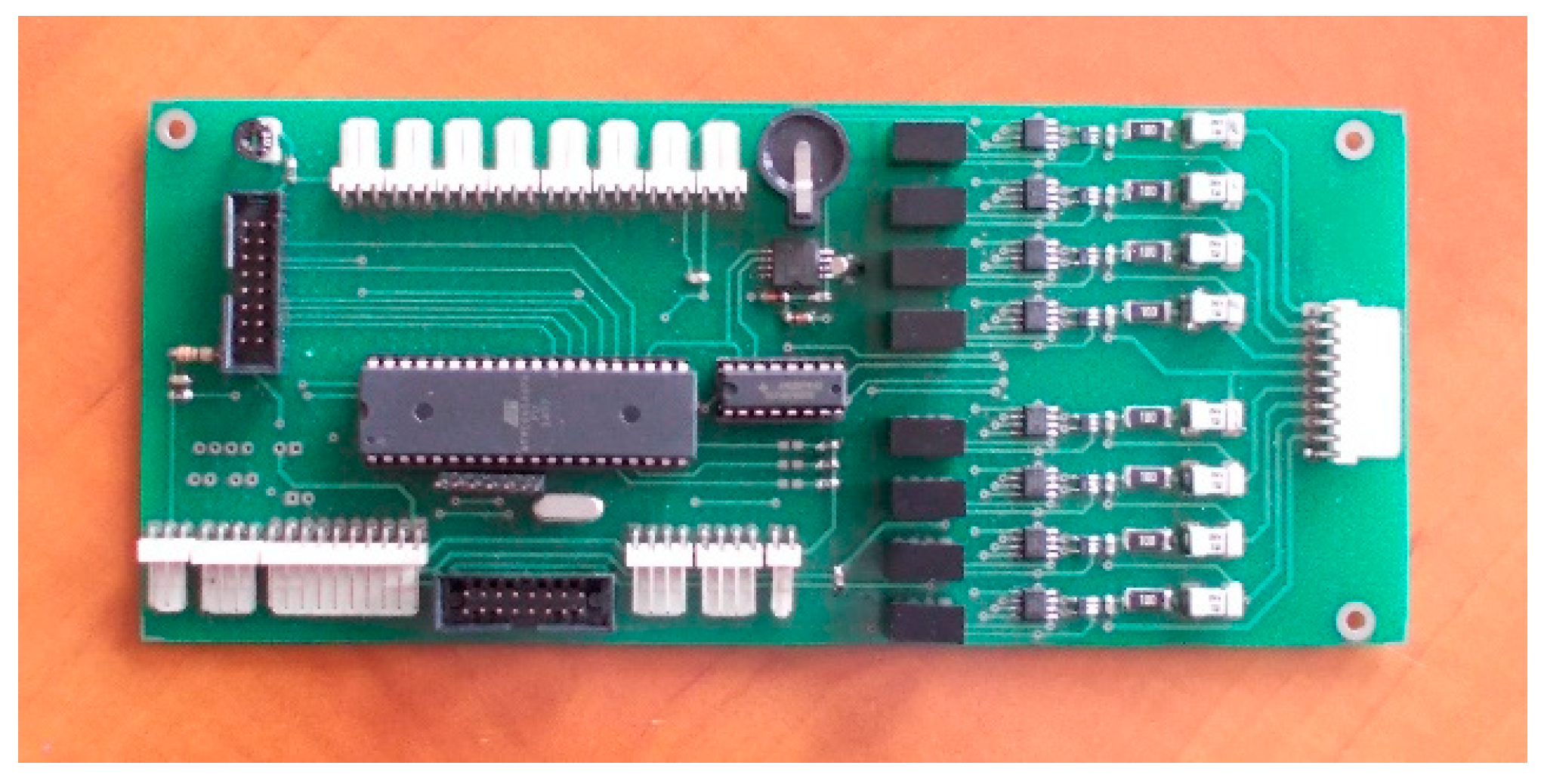
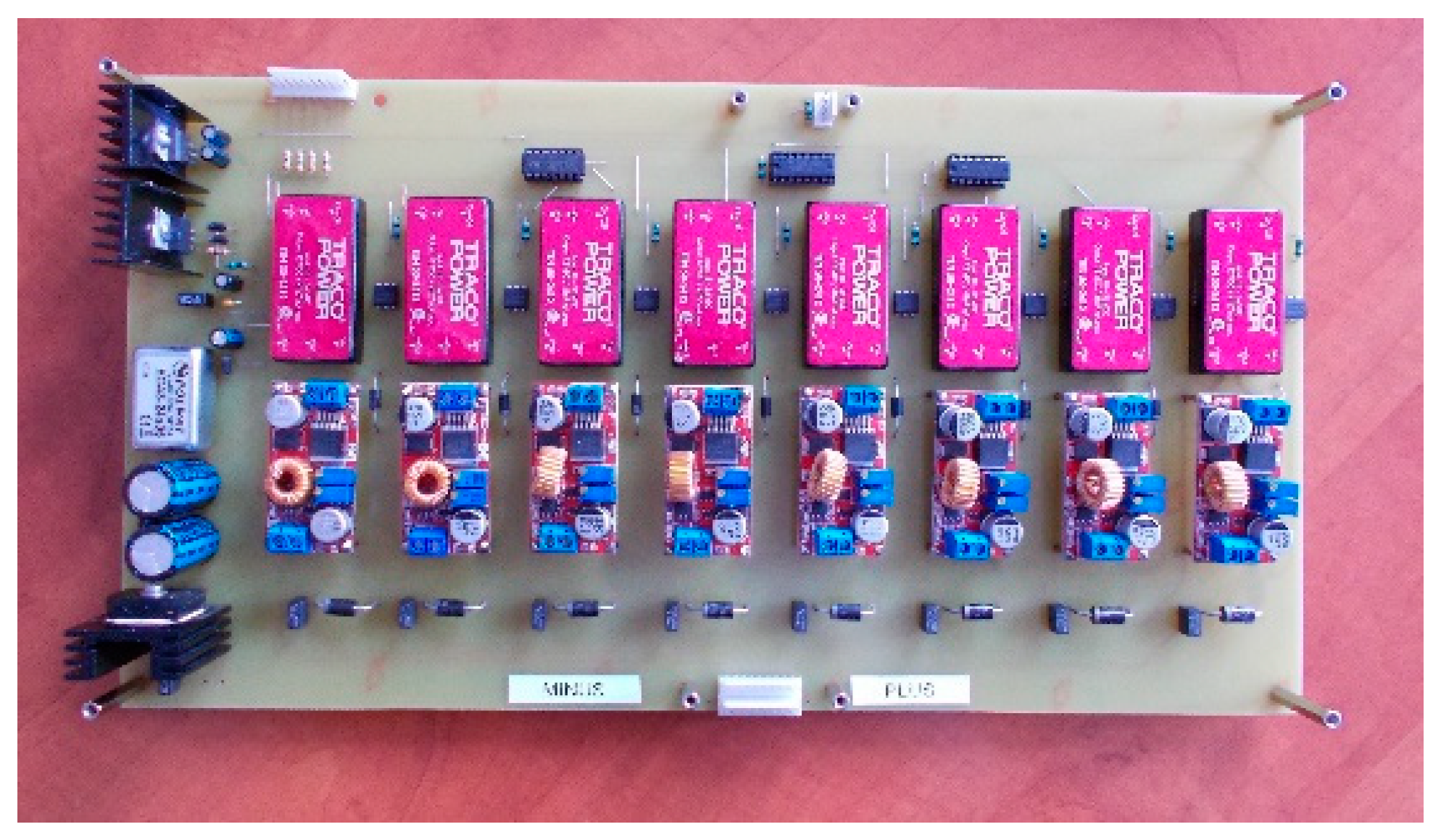
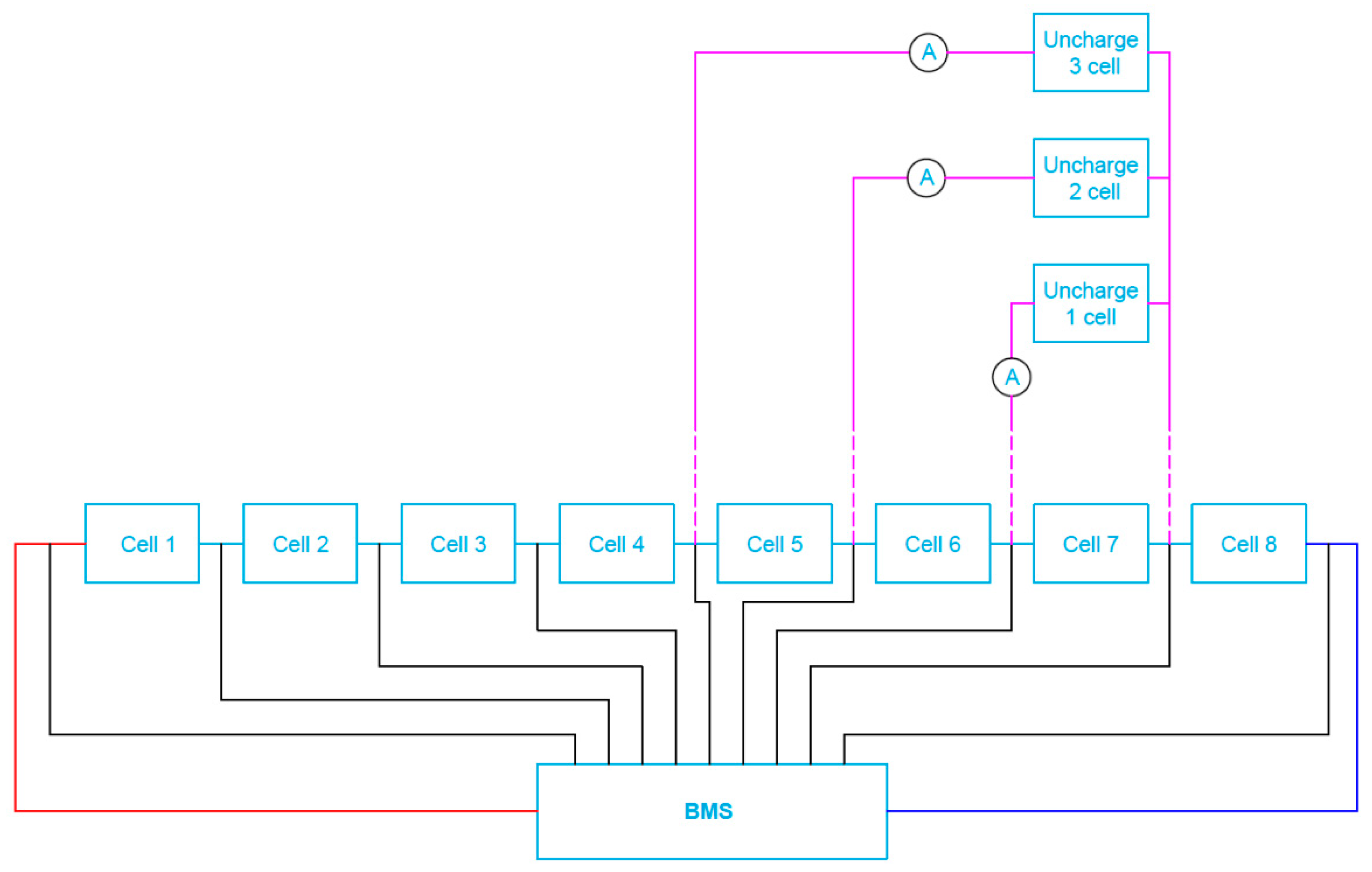
3. Structure of Developed Active BMS for Mine Suspended Vehicles
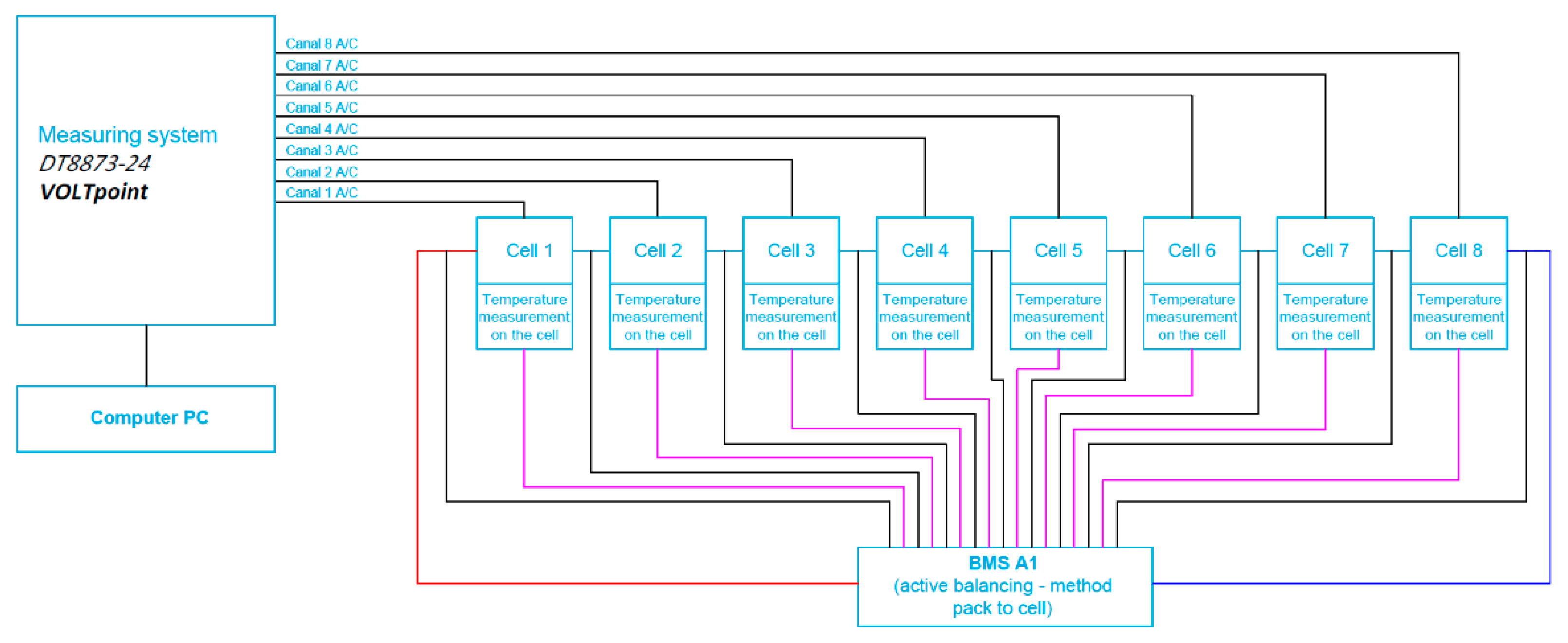
4. The Scope and Way of Testing
- ▪
- Voltage: 3.2 V,
- ▪
- Capacity: 10 Ah,
- ▪
- Internal resistance: <6 mOhm,
- ▪
- Charging voltage: 3.65 V ± 0.05 V,
- ▪
- Energy density: 105 Wh/kg,
- ▪
- Technology: lithium-iron-phosphate (LiFePO4),
- ▪
- Maximum discharge voltage: 2.5 V–2.0 V,
- ▪
- Range of operational temperatures:
- ▪
- Charging: 0 ÷ 45 °C,
- ▪
- Discharging: −20 ÷ 65 °C,
- ▪
- Life: over 2000 cycles (80% of capacity when loading with 10A current).
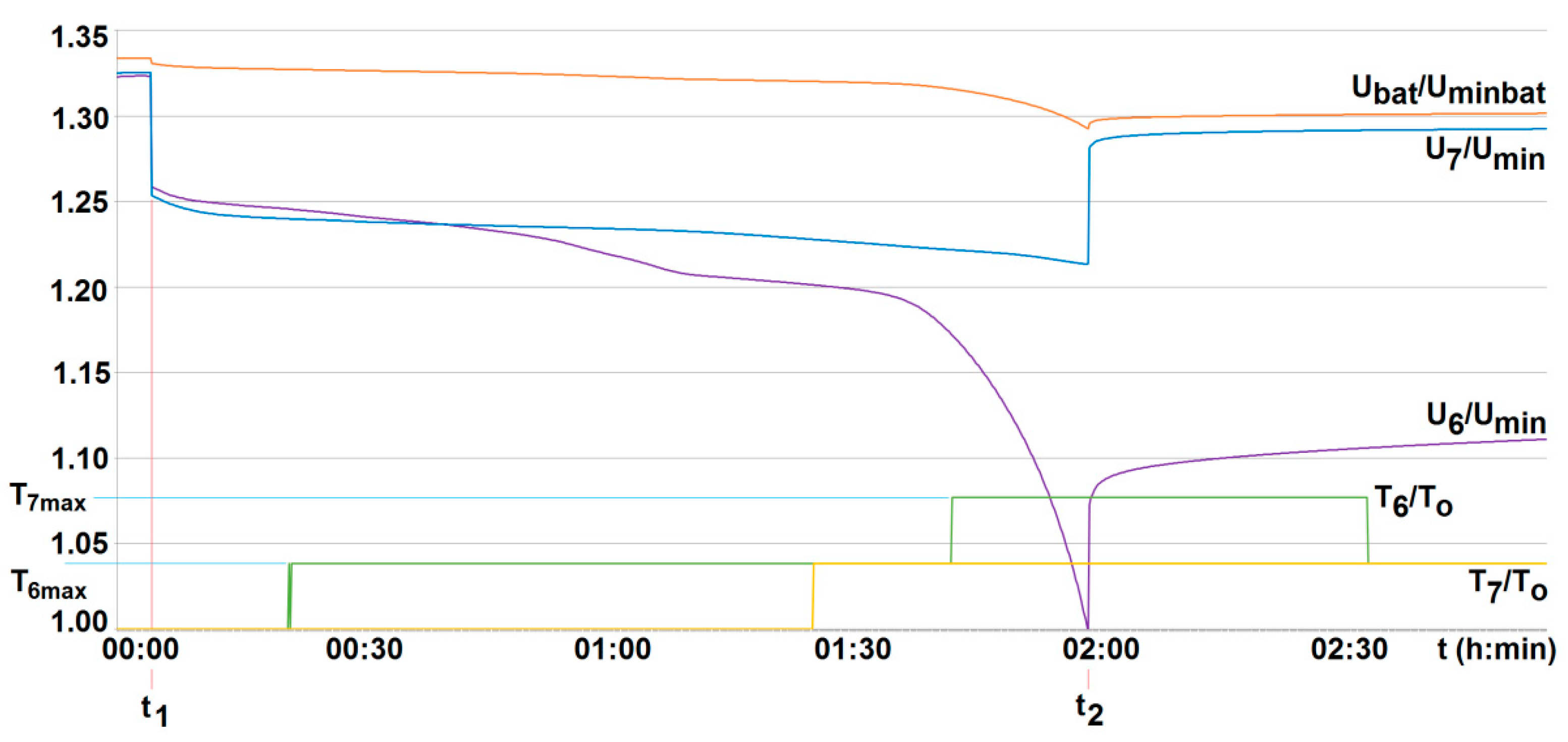
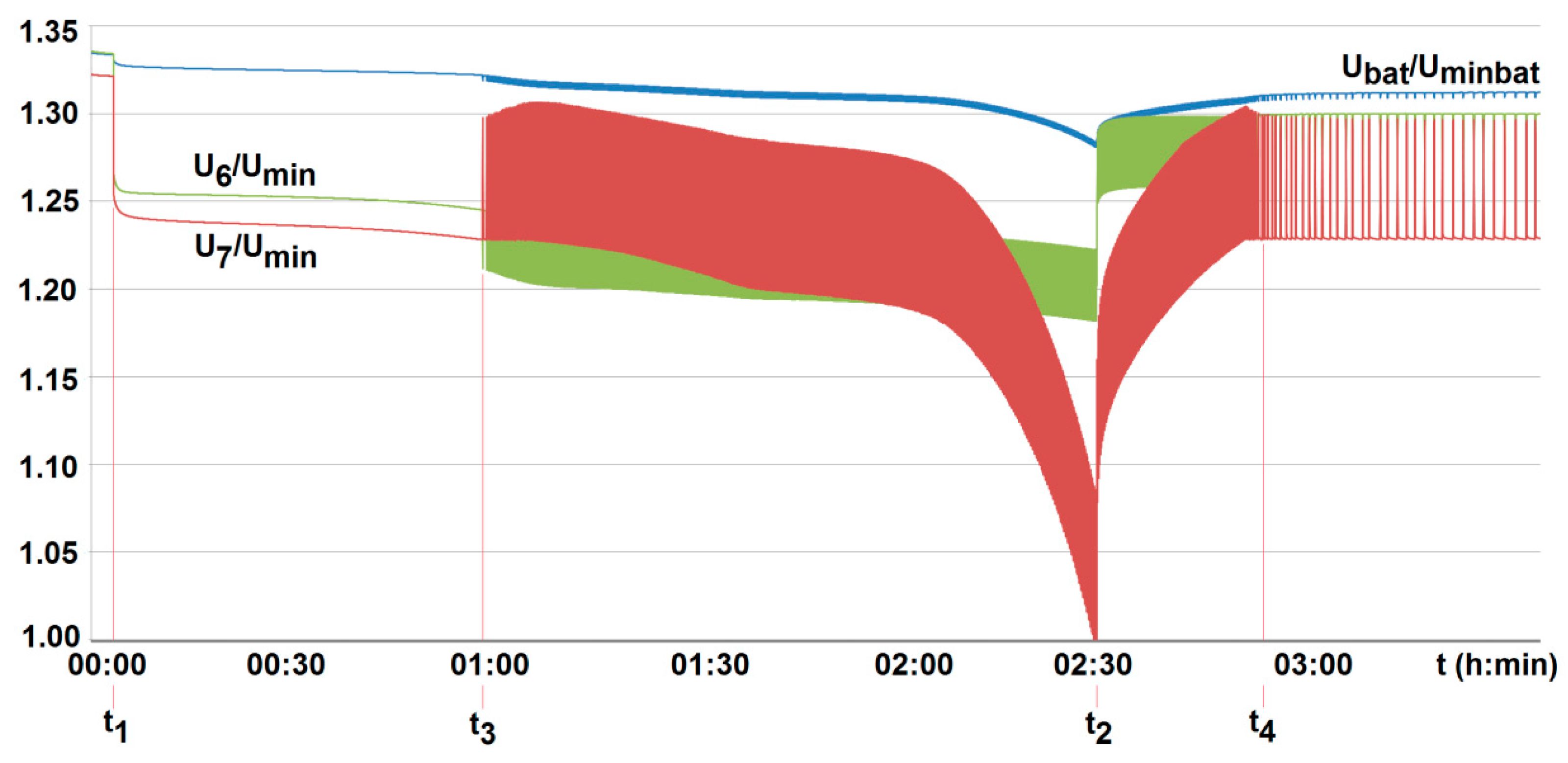
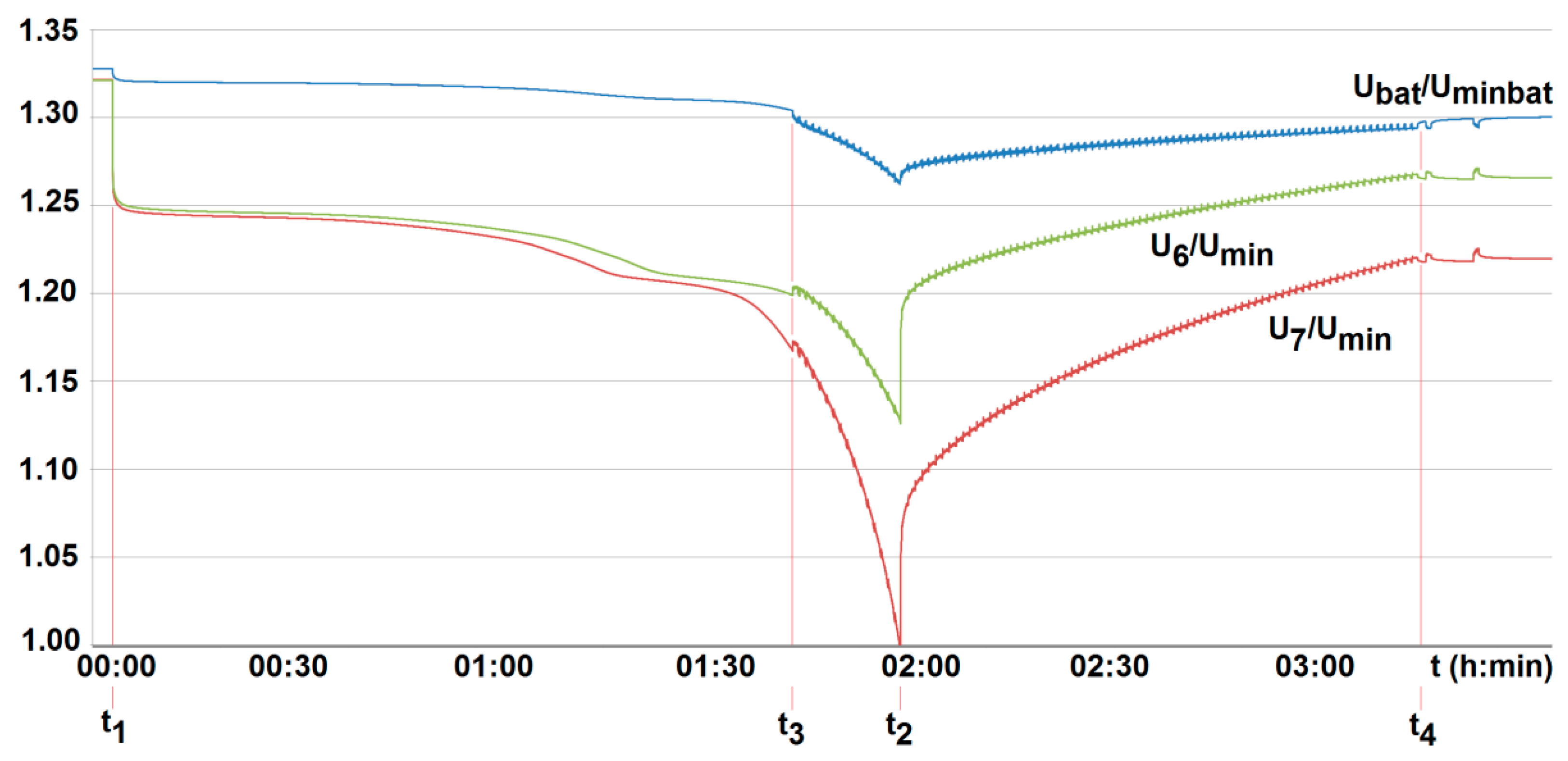
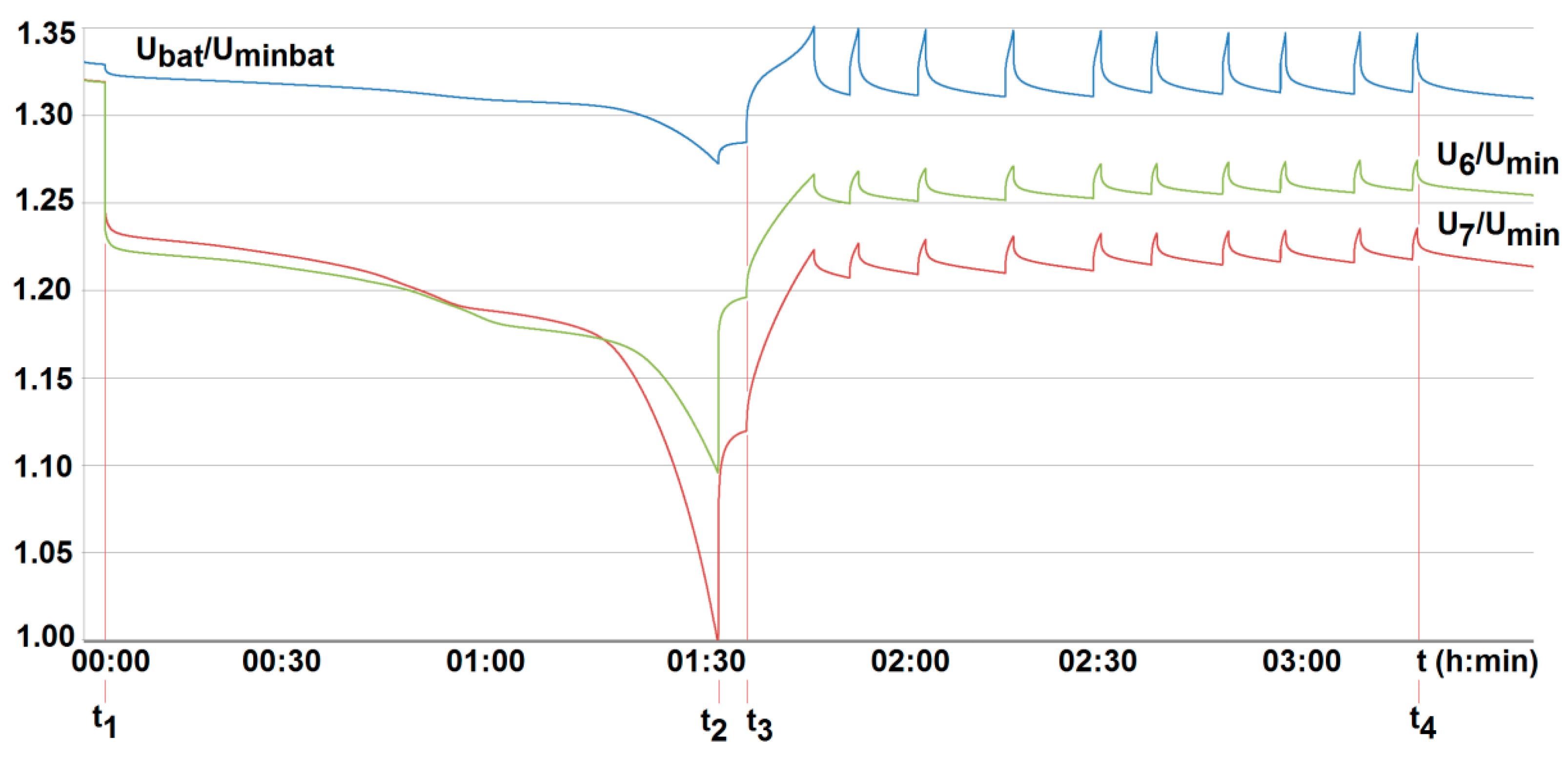
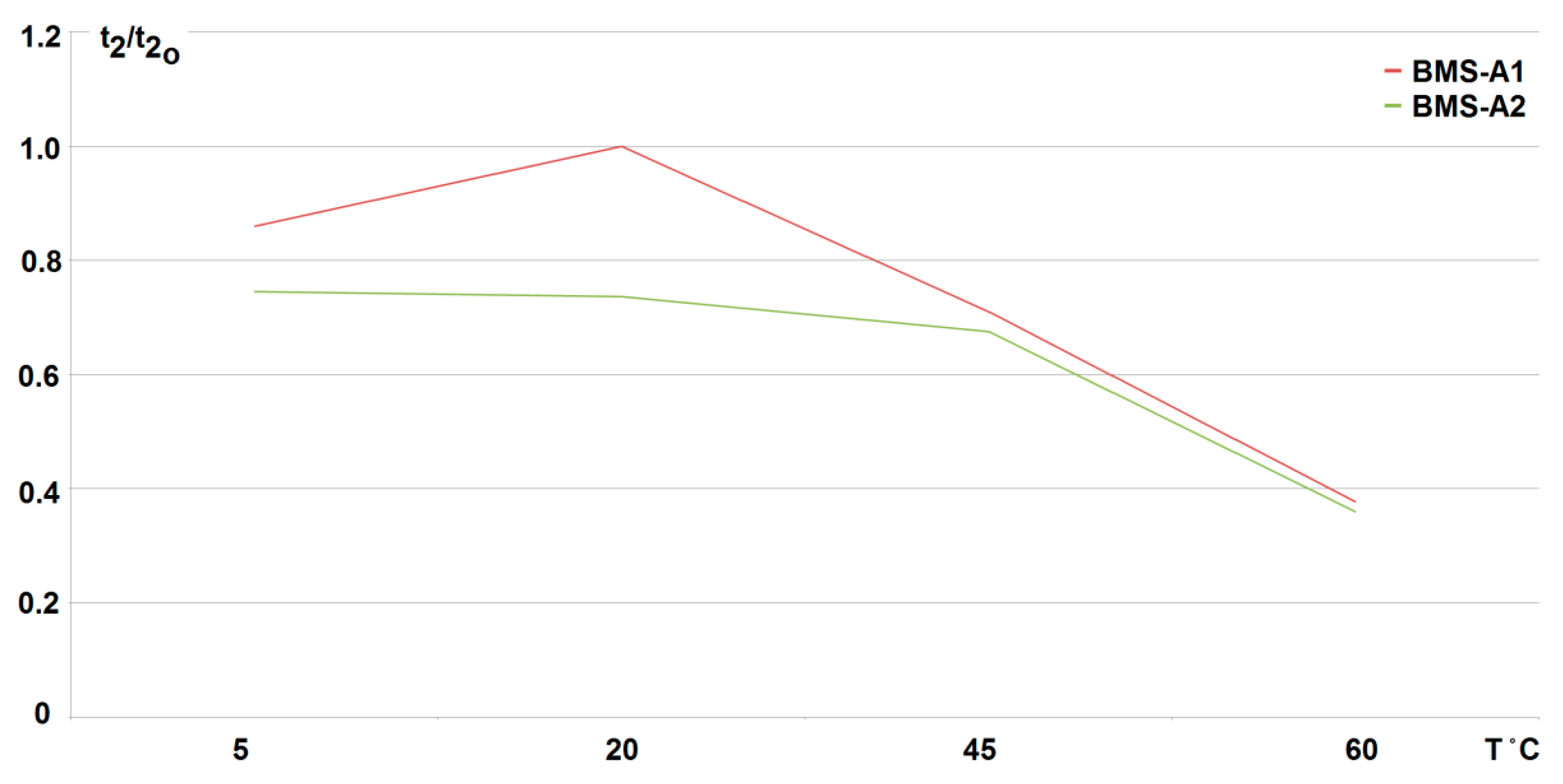
5. Results and Discussion
6. Conclusions
- -
- To ensure long-term, reliable, and safe operation of lithium-iron-phosphate cells under onerous mining environments, the on-line control of their parameters under work needs to be performed. This applies, of course, firstly to both temperature and voltage. This is due to limited cooling conditions and high thermal inertia of cells. As a result, their temperature may reach significant and unacceptable values, not only during deep discharge. This may occur for example, during short lasting but frequently and randomly repeated in time (manufacturer-permitted) pulse discharge currents.
- -
- The conducted tests have also shown that particular cells of the same type behave differently, even when loaded with the same current value. They display different values of temperature and time of voltage(power) fading. Moreover, the difference in parameters of individual cells may change during operation. For this reason, their balancing is also needed. Therefore, the lithium-iron-phosphate cell may be used to power selected suspended battery vehicles, providing BMS is applied.
- -
- There are many different types of BMS systems available on the market, especially passive solutions intended mainly for electric vehicles. However, their use in mining conditions is a problem, due to the undesirable effect of heat dissipation on the equalizing resistors. Therefore, the value of balancing currents must be limited. This results in an unfavorable extension of the cell balancing times, which significantly deteriorates the performance of the device supplied. Therefore, the most advantageous and safest way to power mining equipment is to use active BMS systems appropriately selected and adapted to a particular application.
- -
- The obtained results of tests carried out for two active BMS, developed by the authors and intended to power the suspended mining vehicle, show that the battery-to-cell method is the most useful. For example, the duration of the voltage fad (i.e., the respective lifetime) increased by more than 70% compared to operation without BMS, for 25% of loaded cells. As the number of loaded (discharged) cells increases (provided it is no more than 50%), this effect becomes more visible. It should be noted that although the influence of the ambient temperature significantly shortens the discharge time, it does not cause an excessive increase in the cell temperature. As a result of investigations, the usefulness of the developed active BMS in practice was determined, enabling the extension of the lithium-iron-phosphate battery life for a single recharge under the onerous mine environment, which is a novelty.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radchenko, D.; Bondarenko, A. Mining engineering system as an energy asset in industry 4.0. In Proceedings of the Rudenko International Conference Methodological Problems in Reliability Study of Large Energy Systems (RSES 2018), Funchal, Madeira Island, Portugal, 24–26 October 2018; EDP Sciences: Les Ulis, France, 2018; Volume 58, p. 01009. [Google Scholar]
- Kałuża, E.; Piksa, P.; Setlak, R. Wyznaczanie parametrów energetycznych kwasowych ogniw trakcyjnych stosowanych w lokomotywach dołowych. Wiadomości Elektrotechniczne 2005, 12, 47–49. [Google Scholar]
- Pemberton, J.C. Underground transportation of men and materials in Australian coal mines. Colliery Guard. 1982, 230, S12–S14. [Google Scholar]
- Świątek, J. Ewolucja technologii akumulatorów kwasowo-ołowiowych. Energetyka 2000, 4, 154–158. [Google Scholar]
- Ma, L.; Chen, Q. Problems and research on underground charging safety of power battery for coal mine robot. In Proceedings of the 3rd International Conference on Green Energy and Sustainable Development, Shenyang, China, 14–15 November 2020; IOP Publishing: Bristol, UK, 2020; Volume 651, p. 032100. [Google Scholar]
- Polnik, B. Jakość energii elektrycznej napędów górniczych lokomotyw akumulatorowych w aspekcie emisji gazu elektrolitycznego. Masz. Elektr. Zesz. Probl. 2015, 106, 73–78. [Google Scholar]
- Reyes, M.A.; Novak, T. Injury surveillance and safety considerations for large-format lead-acid batteries used in mining applications. IEEE Trans. Ind. Appl. 2015, 52, 1925–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsukov, Y. Battery Cell Balancing: What to Balance and How; Texas Instruments, Inc.: Dallas, TX, USA, 2005. [Google Scholar]
- Semykina, I.; Zavyalov, V.; Dubkov, E.; Veliliaev, A.H. On the possibility of wireless battery charging in a gaseous-and-dusty mine. In Proceedings of the 10th Anniversary Russian-Chinese Symposium Clean Coal Technologies: Mining, Processing, Safety, and Ecology, Kemerovo, Russia, 19–21 October 2021; EDP Sciences: Les Ulis, France, 2021; Volume 303, p. 01032. [Google Scholar]
- Cao, J.; Schofield, N.; Emadi, A. Battery Balancing Methods: A Comprehensive Review. In Proceedings of the IEEE Vehicle Power and Propulsion Conference, VPPC, Harbin, China, 3–5 September 2008. [Google Scholar]
- Kurpiel, W.; Polnik, B.; Miedziński, B. System nadzorujący pracę baterii akumulatorów (BMS) w celu zwiększenia bezpieczeństwa ich funkcjonowania i żywotności stosowanych ogniw. Mech. I Autom. Górnictwa 2014, 5, 47–49. [Google Scholar]
- Kurpiel, W.; Polnik, B.; Miedziński, B. Właściwości eksploatacyjne ogniw litowych. Elektro Info 2018, 10, 44–48. [Google Scholar]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process. Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Moore, S.W.; Schneider, P.J. A Review of Cell Equalization Methods for Lithium Ion and Lithium Polymer Battery Systems; Technical Paper 2001-01-0959; SAE International: Warrendale, PA, USA, 2001; p. 7. [Google Scholar]
- Wen, S. Cell Balancing Buys Extra Run Time and Battery Life; Texas Instruments, Inc.: Dallas, TX, USA, 2009. [Google Scholar]
- Ziegler, A.; Oeser, D.; Arndt, B.; Ackva, A. Comparison of Active and Passive Balancing by a Long Term Test Including a Post-Mortem Analysis of all Single Battery Cells. In Proceedings of the 2018 International IEEE Conference and Workshop in Óbuda on Electrical and Power Engineering (CANDO-EPE), Budapest, Hungary, 20–21 November 2018; pp. 000015–000020. [Google Scholar]
- Xiong, R.; Li, L.; Tian, J. Towards a smarter battery management system: A critical review on battery state of health monitoring methods. J. Power Sources 2018, 405, 18–29. [Google Scholar] [CrossRef]
- Xiong, R.; Ma, S.; Li, H.; Sun, F.; Li, J. Toward a safer battery management system: A critical review on diagnosis and prognosis of battery short circuit. Iscience 2020, 23, 101010. [Google Scholar] [CrossRef]
- Lipu, M.H.; Hannan, M.A.; Karim, T.F.; Hussain, A.; Saad, M.H.; Ayob, A.; Miah, M.S.; Mahlia, T.I. Intelligent algorithms and control strategies for battery management system in electric vehicles: Progress, challenges and future outlook. J. Clean. Prod. 2021, 292, 126044. [Google Scholar] [CrossRef]
- Carkhuff, B.G.; Demirev, P.A.; Srinivasan, R. Impedance-based battery management system for safety monitoring of lithium-ion batteries. IEEE Trans. Ind. Electron. 2018, 65, 6497–6504. [Google Scholar] [CrossRef]
- Frost, D.F.; Howey, D.A. Completely decentralized active balancing battery management system. IEEE Trans. Power Electron. 2017, 33, 729–738. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Mi, C.; Chen, Z.; Cao, B. SOC Based Battery Cell Balancing with a Novel Topology and Reduced Component Count. Energies 2013, 6, 2726–2740. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, J.M.; Yi, J.; Won, C.Y. Battery Management System Algorithm for Energy Storage Systems Considering Battery Efficiency. Electronics 2021, 10, 1859. [Google Scholar] [CrossRef]
- Perişoară, L.A.; Guran, I.C.; Costache, D.C. A passive battery management system for fast balancing of four LiFePO4 cells. In Proceedings of the 2018 IEEE 24th International Symposium for Design and Technology in Electronic Packaging (SIITME), Iasi, Romania, 25–28 October 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Daowd, M.; Omar, N.; Van Den Bossche, P.; Van Mierlo, J. Passive and Active Battery Balancing comparison based on MATLAB Simulation. In Proceedings of the 7th IEEE Vehicle Power and Propulsion Conference, VPPC’11, Chicago, IL, USA, 6–9 September 2011. [Google Scholar]
- Lukasiewycz, M.; Kauer, M.; Steinhorst, S. Synthesis of Active Cell Balancing Architectures for Battery Packs. IEEE Trans. Comput. Aided Des. Integr. Circuits Syst. 2016, 35, 1876–1889. [Google Scholar] [CrossRef]
- EN 60079-1:2014/AC:2018-09. Explosive Atmospheres—Part 1: Equipment Protection by Flameproof Enclosures “d”. Available online: https://standards.iteh.ai/catalog/standards/clc/e87e6b2c-cf55-4187-aeb3-eb0490b0bdfe/en-60079-1-2014 (accessed on 17 August 2021).
- Diao, W.; Xue, N.; Bhattacharjee, V.; Jiang, J.; Karabasoglu, O.; Pecht, M. Active battery cell equalization based on residual available energy maximization. Appl. Energy 2018, 210, 690–698. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; He, Y.; Zeng, G. Active vehicle battery equalization scheme in the condition of constant-voltage/current charging and discharging. IEEE Trans. Veh. Technol. 2017, 12, 47–49. [Google Scholar]
- Shah, S.; Murali, M.; Gandhi, P. A Practical Approach of Active Cell Balancing in a Battery Management System. In Proceedings of the IEEE International Conference on Power Electronics, Drives and Energy Systems (PEDES), Chennai, India, 18–21 December 2018; pp. 1–6. [Google Scholar]
- Jura, J.; Bartoszek, S. Układ aktywnego balansowania baterii ogniw litowych przeznaczony do górniczych maszyn mobilnych. Masz. Górnicze 2019, 1, 52–65. [Google Scholar]
- Documentation of Battery LiFePO4 3.2 V. Available online: https://www.bto.pl/produkt/31801/headway-lfp38120(s)-10000mah-lifepo4 (accessed on 10 August 2021).
- Taylor, R.J. Introduction to Error Analysis, the Study of Uncertainties in Physical Measurements, 2nd ed.; University Science Books: Sausalito, CA, USA, 1997. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpiel, W.; Deja, P.; Polnik, B.; Skóra, M.; Miedziński, B.; Habrych, M.; Debita, G.; Zamłyńska, M.; Falkowski-Gilski, P. Performance of Passive and Active Balancing Systems of Lithium Batteries in Onerous Mine Environment. Energies 2021, 14, 7624. https://doi.org/10.3390/en14227624
Kurpiel W, Deja P, Polnik B, Skóra M, Miedziński B, Habrych M, Debita G, Zamłyńska M, Falkowski-Gilski P. Performance of Passive and Active Balancing Systems of Lithium Batteries in Onerous Mine Environment. Energies. 2021; 14(22):7624. https://doi.org/10.3390/en14227624
Chicago/Turabian StyleKurpiel, Wojciech, Przemysław Deja, Bartosz Polnik, Marcin Skóra, Bogdan Miedziński, Marcin Habrych, Grzegorz Debita, Monika Zamłyńska, and Przemysław Falkowski-Gilski. 2021. "Performance of Passive and Active Balancing Systems of Lithium Batteries in Onerous Mine Environment" Energies 14, no. 22: 7624. https://doi.org/10.3390/en14227624
APA StyleKurpiel, W., Deja, P., Polnik, B., Skóra, M., Miedziński, B., Habrych, M., Debita, G., Zamłyńska, M., & Falkowski-Gilski, P. (2021). Performance of Passive and Active Balancing Systems of Lithium Batteries in Onerous Mine Environment. Energies, 14(22), 7624. https://doi.org/10.3390/en14227624







