Experimental Investigation of the Ash Deposition Characteristics of Biomass Pretreated by Ash Removal during Co-Combustion with Sub-Bituminous Coal
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation and Analysis
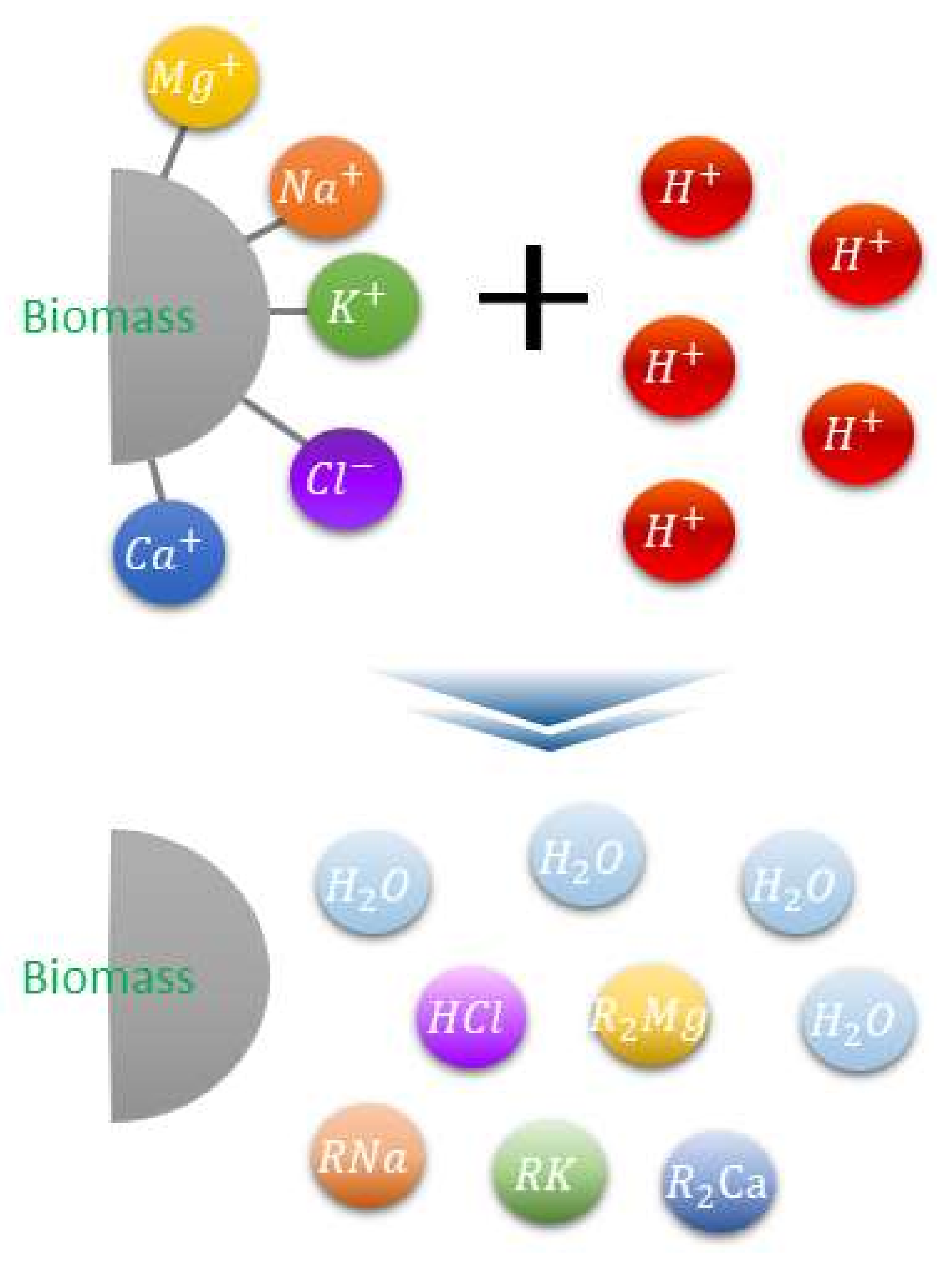
2.2. Ash Removal
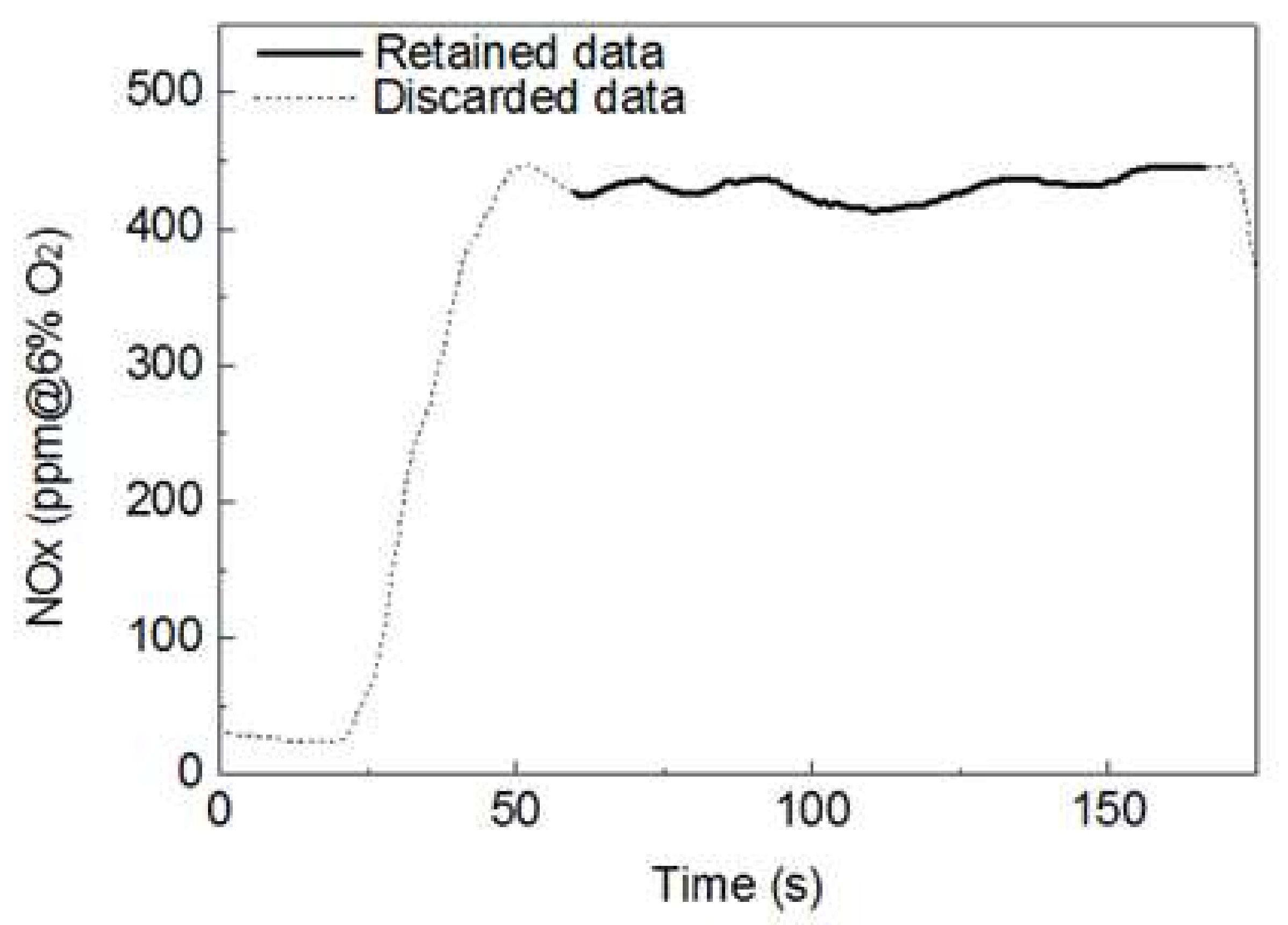
2.3. Gas Emission and Unburned Carbon Analysis
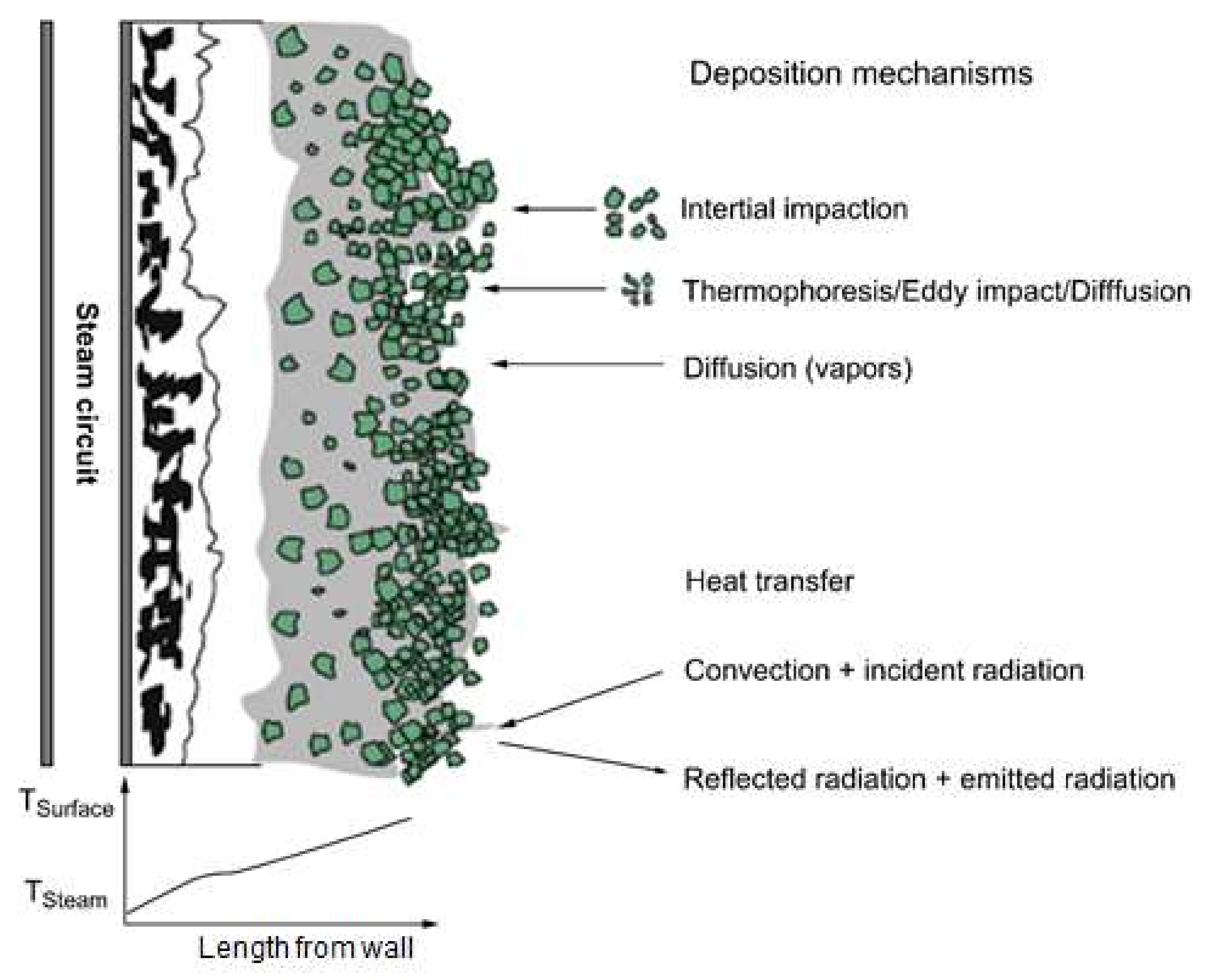
2.4. Ash Deposition Rate Experiment
3. Results and Discussion
3.1. Ash Removal Associated with the Pretreatment
3.2. Gases and UBC Associated with the ALB Co-Combustion
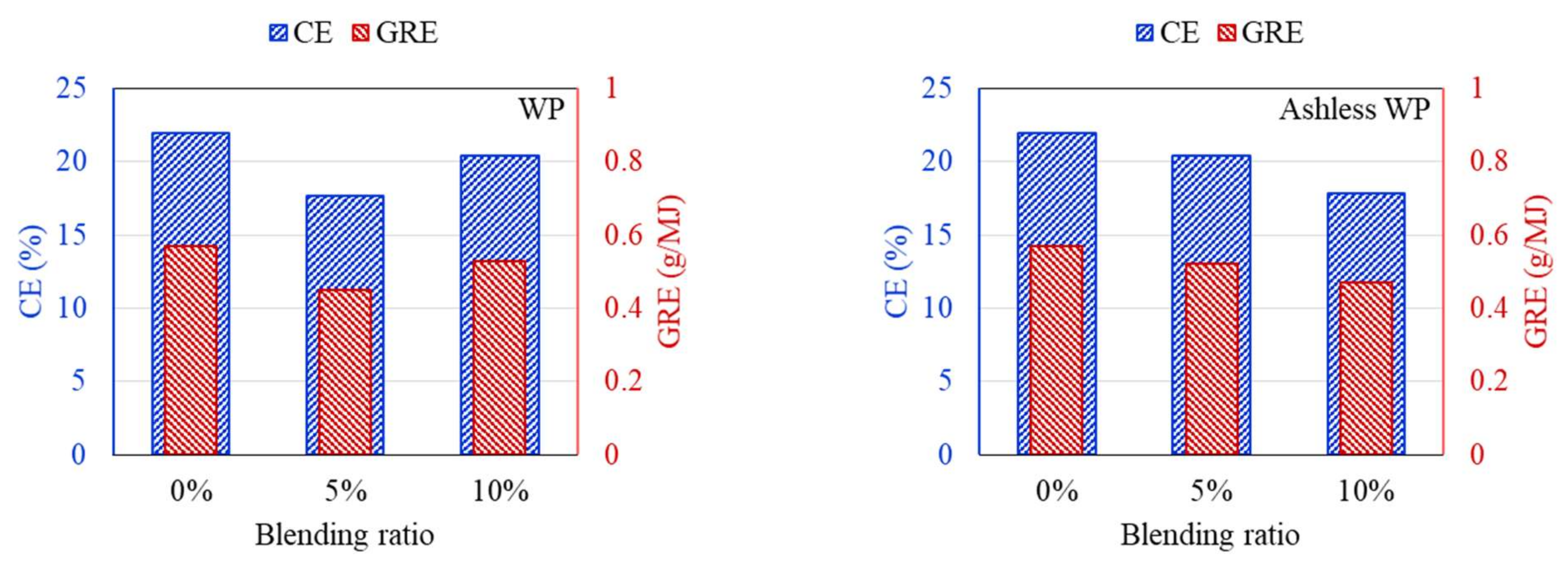
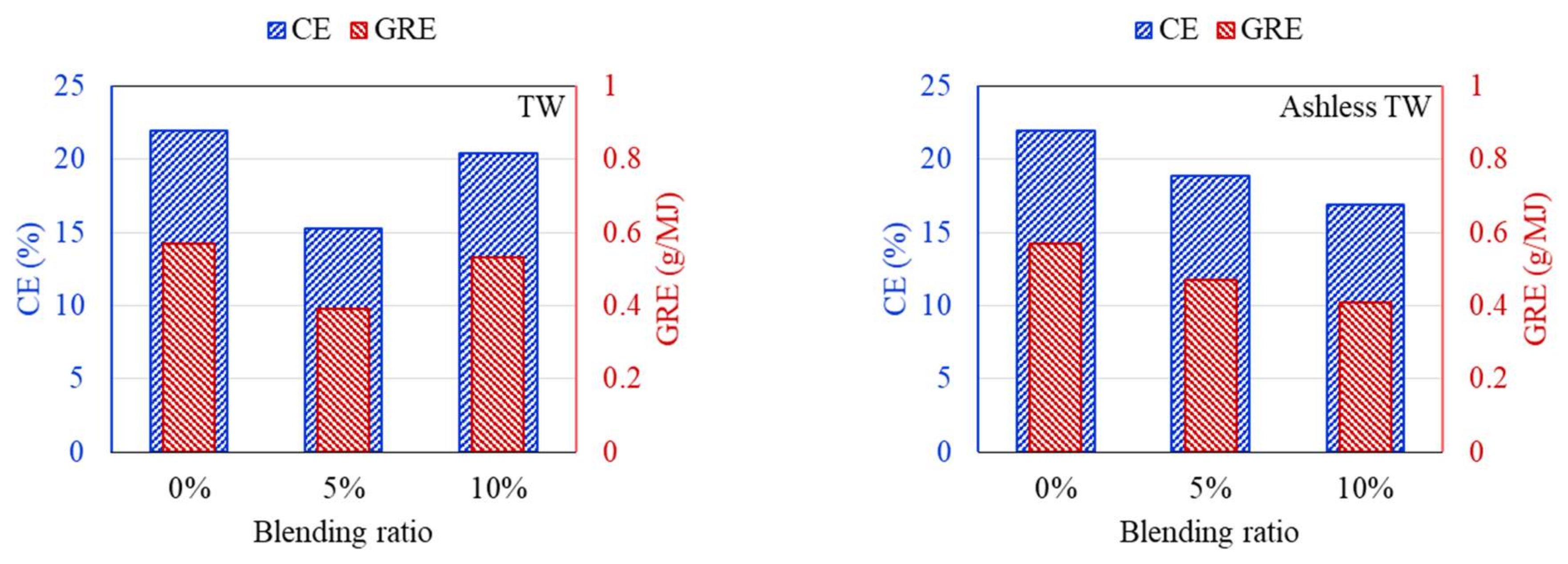
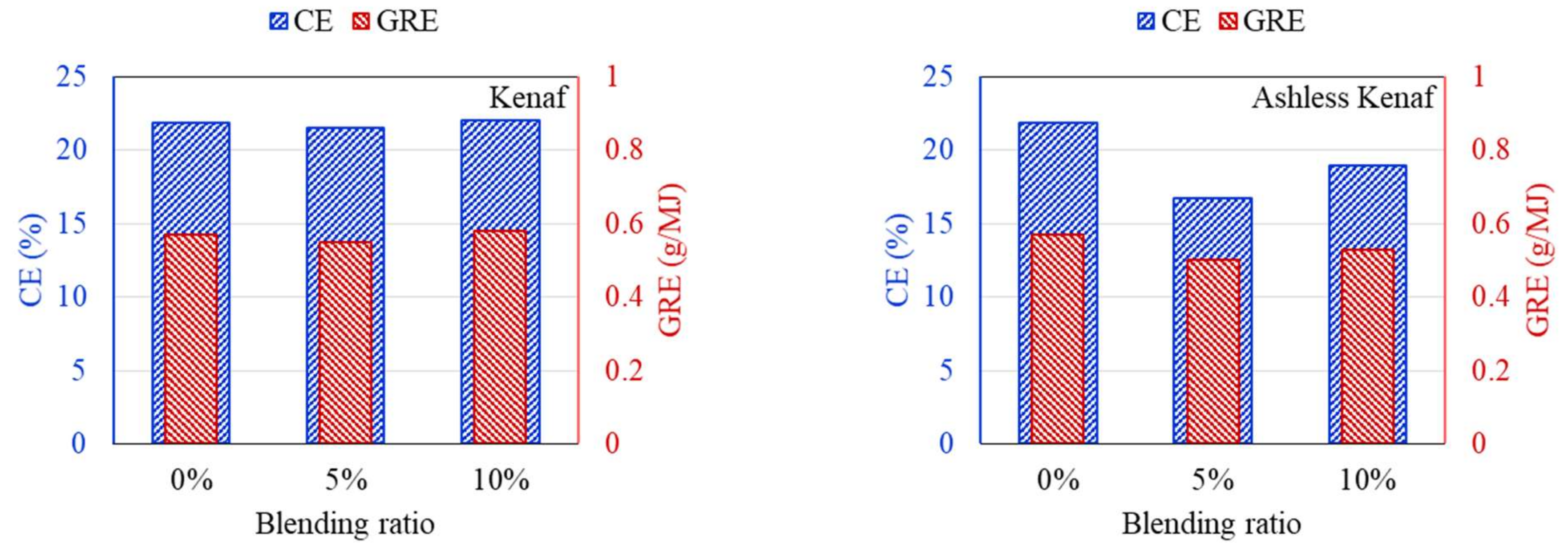
3.3. Ash Deposition Rate after the ALB Cofiring
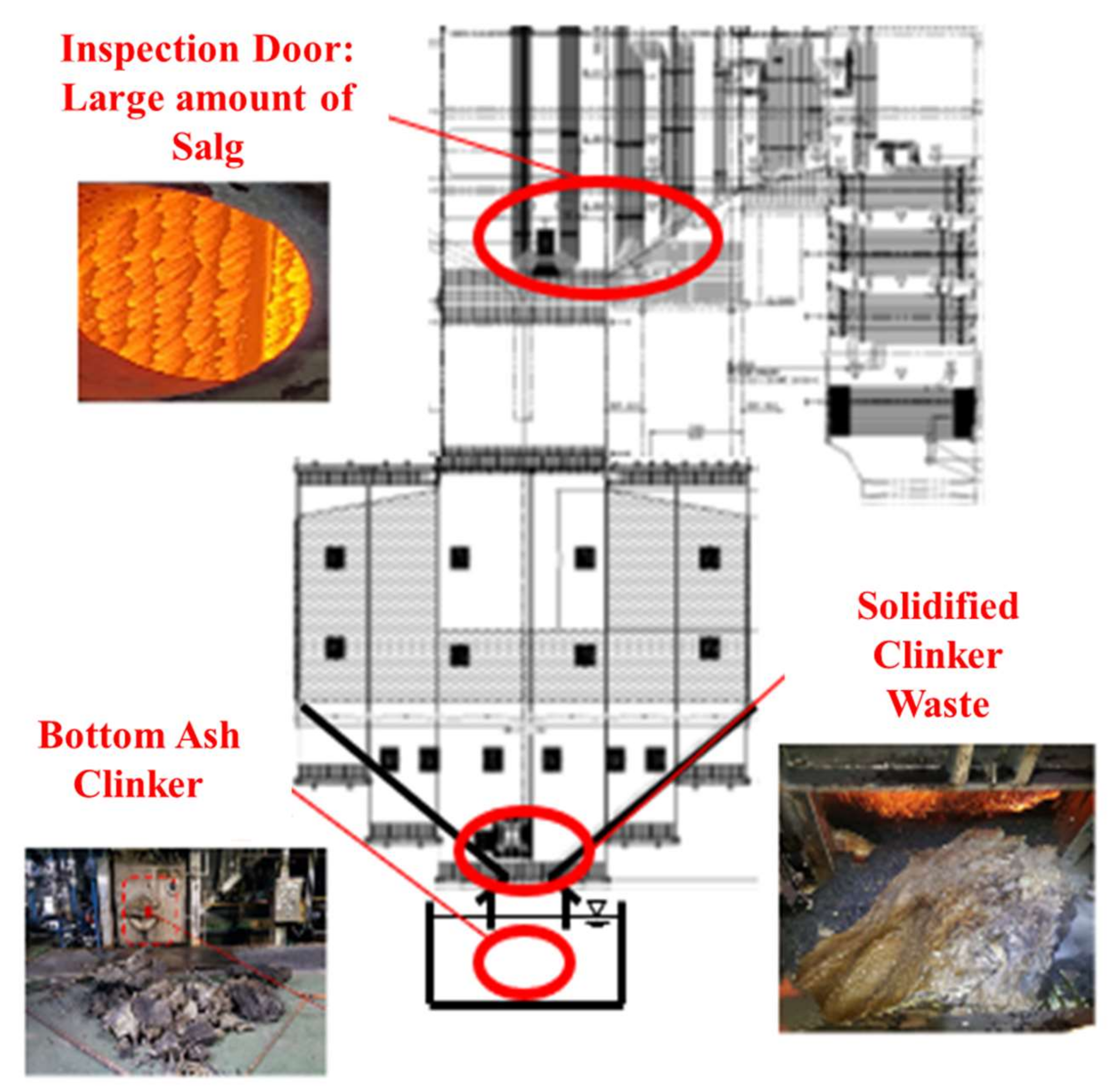
3.4. Effect of Ash Removal Pretreatment on the Boiler
4. Conclusions and Summary
- (1)
- XRF and ion chromatography revealed that ash removal pretreatment decreased the ash content produced by the three types of biomass, and the abundance of Na, Mg, K, and Cl, which are associated with clinker generation, decreased by > 80%.
- (2)
- DTF and gas analysis revealed that after mixing with 5% and 10% biomass, the NOx and UBC decreased, while SOx slightly increased. However, in most cases, the SOx values ranged between 30–50 ppm, which are lower than those for bituminous and sub-bituminous coal. Moreover, the NOx and SOx values for all samples decreased slightly, however, converged toward an error of approximately 10 ppm, while the UBC showed no trend.
- (3)
- Ash deposition in the DTF, CE, and GRE was analyzed. Raw biomass exhibited a V-shaped variation due to the presence of alkali contents in raw biomass. With ash removal pretreatment, these effects disappeared as CE and GRE gradually decreased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | Ashless biomass, biomass from the ash removal process |
| DTF | Drop Tube Furnace |
| WP | Wood Pellet |
| TW | Thinned Wood |
| UBC | Unburned Carbon |
| CE | Capture Efficiency |
| GRE | Energy Based Growth Rate |
References
- WPAC Drax Biomass. 2016. Available online: https://www.pellet.org/wpac-agm/images/2016/Drax-Biomass---WPAC-2016---For-Sharing.pdf (accessed on 5 June 2020).
- Data Basis Made with Natural Earth, AMI/LK/MIO. 2017. Available online: https://www.ufop.de/ (accessed on 20 June 2019).
- Islam, A.S.; Ahiduzzaman, M. Green electricity from rice husk. In A model for Bangladesh, Thermal Power Plants-Advanced Applications; Rasul, M., Ed.; IntechOpen: London, UK, 2013; pp. 127–144. [Google Scholar]
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity evaluation and emission characteristics of poultry litter co-combustion process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef] [Green Version]
- Papilo, P.; Kusumanto, I.; Kunaifi, K. Assessment of agricultural biomass potential to electricity generation in Riau Province. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012006. [Google Scholar] [CrossRef] [Green Version]
- Bryers, R.W. USA Fireside Slagging, Fouling, and High-Temperature Corrosion of Heat-Transfer Surface Due to Impurities in Steam-Raising Fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal properties and combustion-related problems prediction of agricultural crop residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Song, G.; Namkung, H.; Park, S.; Kim, J.; Choi, J.; Jeon, C.; Choi, Y. Characterization of PM2.5 and Gaseous emissions during combustion of ultra-clean biomass via dual-stage treatment. Atmos. Environ. 2018, 193, 168–176. [Google Scholar] [CrossRef]
- ASTM D3173/D3173M-17a. Standard Test Method for Moisture in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM D3175-20. Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM D3172-13. Standard Test Method for Fixed Carbon in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM D3174-12(2018)e1. Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM D5373-16. Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM D5865-13. Standard Test Method for Gross Calorific Value of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Peng, B.; Wu, D.; Lai, J.; Xiao, H.; Li, P. Simultaneous determination of halogens (F, Cl, Br, and I) in coal using pyrohydrolysis combined with ion chromatography. Fuel 2012, 94, 629–631. [Google Scholar] [CrossRef]
- Ballantyne, T.R.; Ashman, P.J.; Mullinger, P.J. A new method for determining the conversion of low-ash coals using synthetic ash as a tracer. Fuel 2005, 84, 1980–1985. [Google Scholar] [CrossRef]
- Lourival, J.; Mendes, N.; Bazzo, E. Characterization and growth modeling of ash deposits in coal fired boilers. Powder Technol. 2012, 217, 61–68. [Google Scholar]
- Standards Association of Australia Coal and Coke Analysis and Testing, Part 15: Higher Rank Coal Ash and Coke Ash Fusibility, 3rd ed.; Standards Australia: Homebush, NSW, Australia, 1995.
- Rushdi, A.; Sharma, A.; Gupta, R. An experimental study of the effect of coal blending on ash deposition. Fuel 2004, 83, 495–506. [Google Scholar] [CrossRef]
- Jeong, T.; Sh, L.; Kim, J.; Lee, B.; Jeon, C. Experimental investigation of ash deposit behavior during co-combustion of bituminous coal with wood pellets and empty fruit bunches. Energies 2019, 12, 2087. [Google Scholar] [CrossRef] [Green Version]
- An, K.J.; Lee, B.H.; Kim, S.I.; Jeon, C.H. Study on slagging characteristic of hybrid coals using the thermo-mechanical analysis. In Proceedings of the Yeongheung Spring Conference on K.S.M.E., Yoengheung, Korea, 31 May 2013. [Google Scholar]
- Blanchard, R. Measurements and Modeling of Coal Ash Deposition in an Entrained Flow Reactor. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2008. [Google Scholar]
- Pudasainee, D.; Kim, J.H.; Lee, S.-H.; Park, J.-M.; Jang, H.-N.; Song, G.-J.; Seo, Y.-C. Hazardous air pollutants emission from coal and oil-fired power plants. Asia Pac. J. Chem. Eng. 2010, 5, 299–303. [Google Scholar] [CrossRef]
- Li, S.; Xu, T.; Sun, P.; Zhou, Q.; Tan, H.; Hui, S. NOx and SOx emissions of a high sulfur self-retention coal during air-staged combustion. Fuel 2008, 87, 723–731. [Google Scholar] [CrossRef]
- Jiang, Y.; Jeon, C. Comparative study on combustion and emission characteristics of torrefied and ashless biomass with coal through the 500 MW tangentially fired boiler simulation. Energy Fuels 2021, 35, 561–574. [Google Scholar] [CrossRef]
- Abreu, P.; Casaca, C.; Costa, M. Ash deposition during the co-firing of bituminous coal with pine sawdust and olive stones in a laboratory furnace. Fuel 2010, 89, 4040–4048. [Google Scholar] [CrossRef]
- Kupka, T.; Mancini, M.; Irmer, M.; Weber, R. Investigation of ash deposit formation during co-firing of coal with sewage sludge, saw-dust and refuse derived fuel. Fuel 2008, 87, 2824–2837. [Google Scholar] [CrossRef]
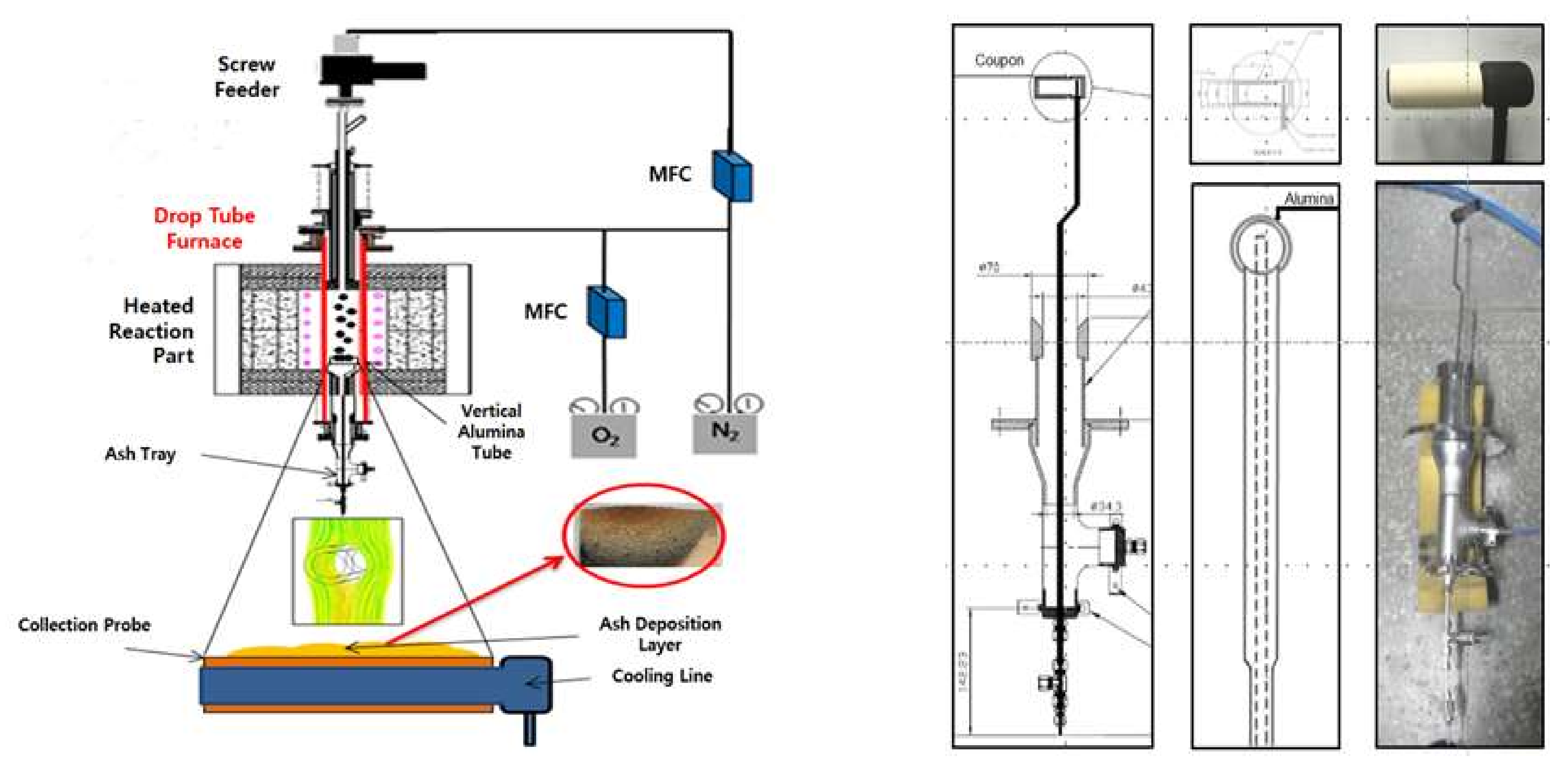
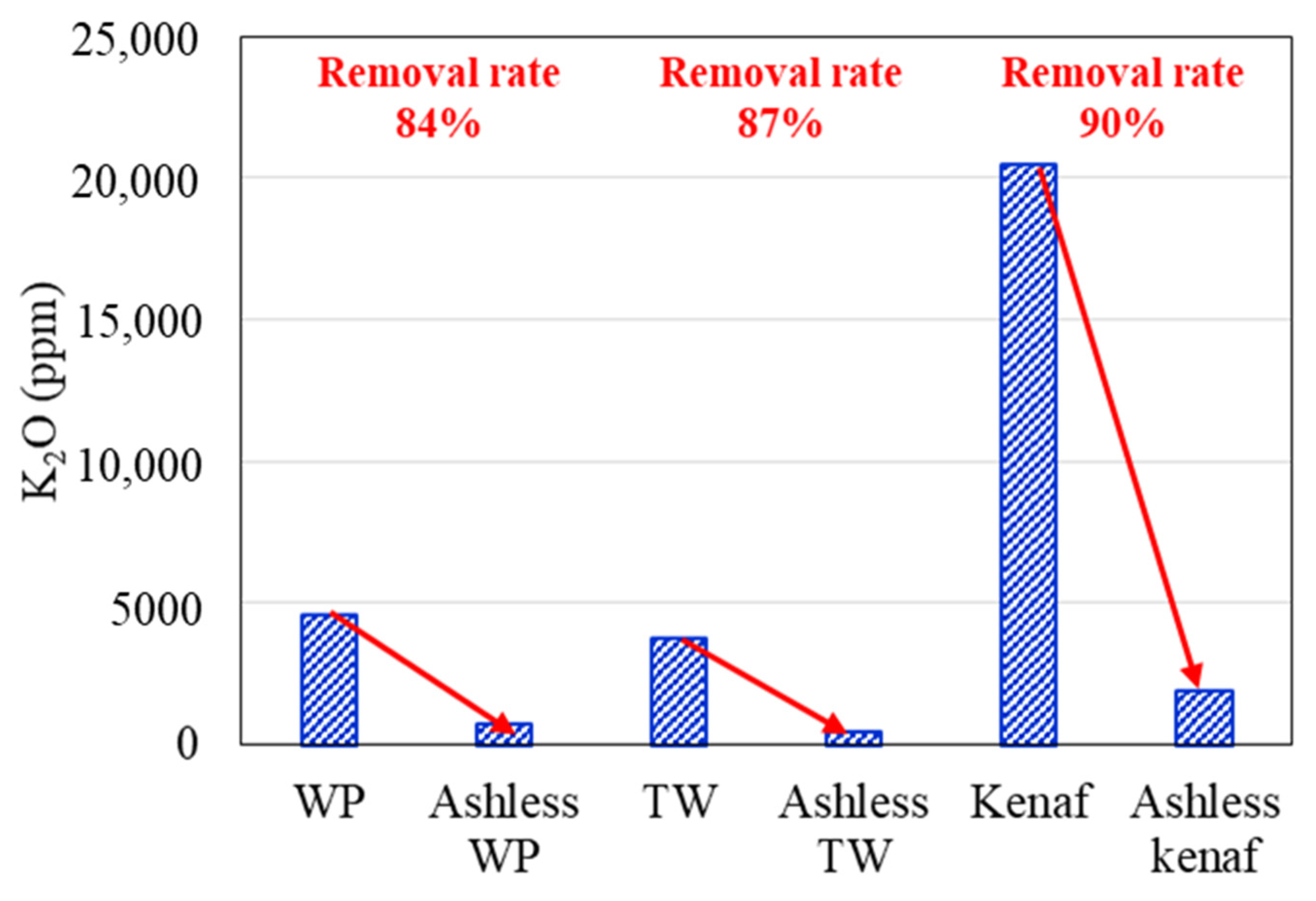
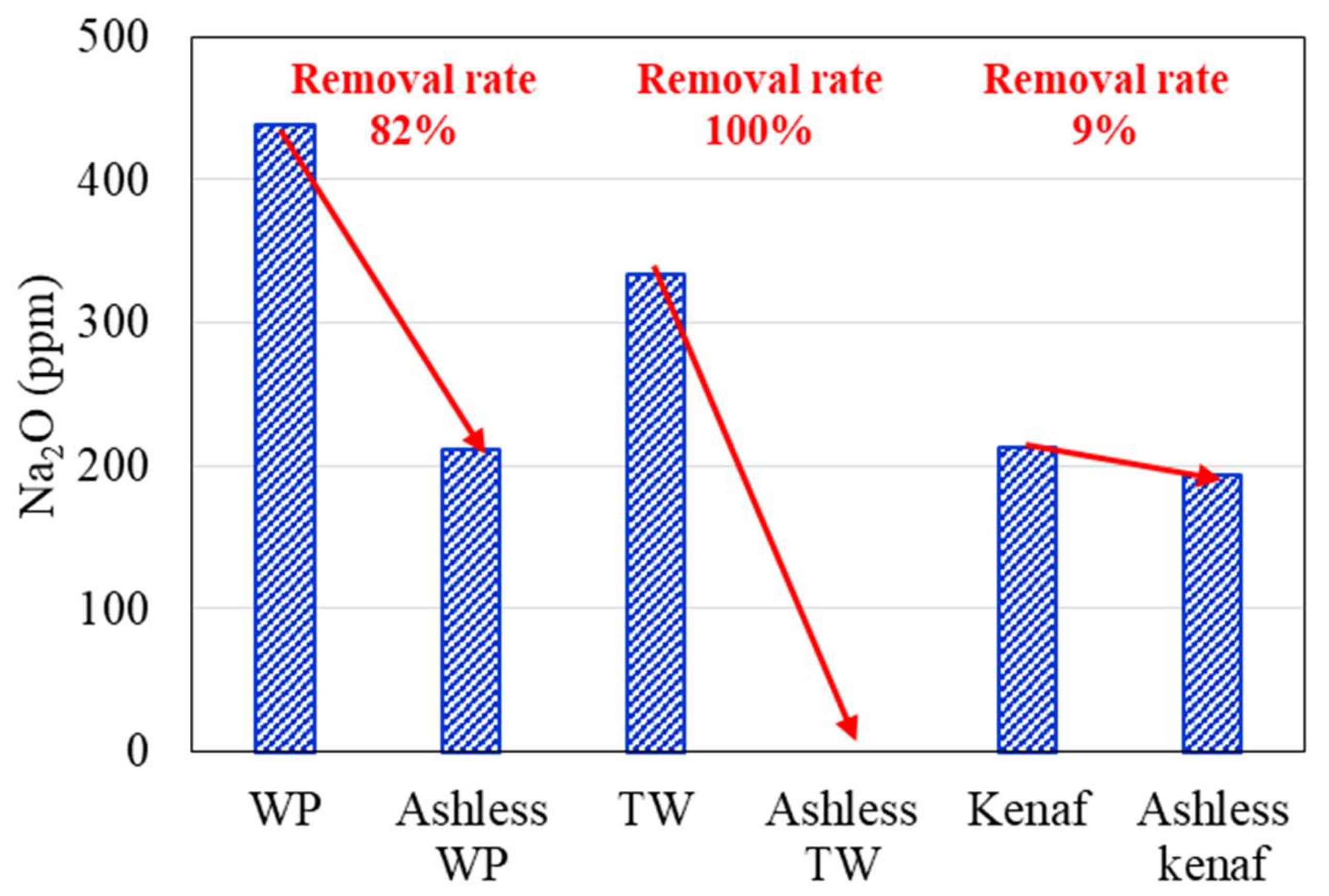
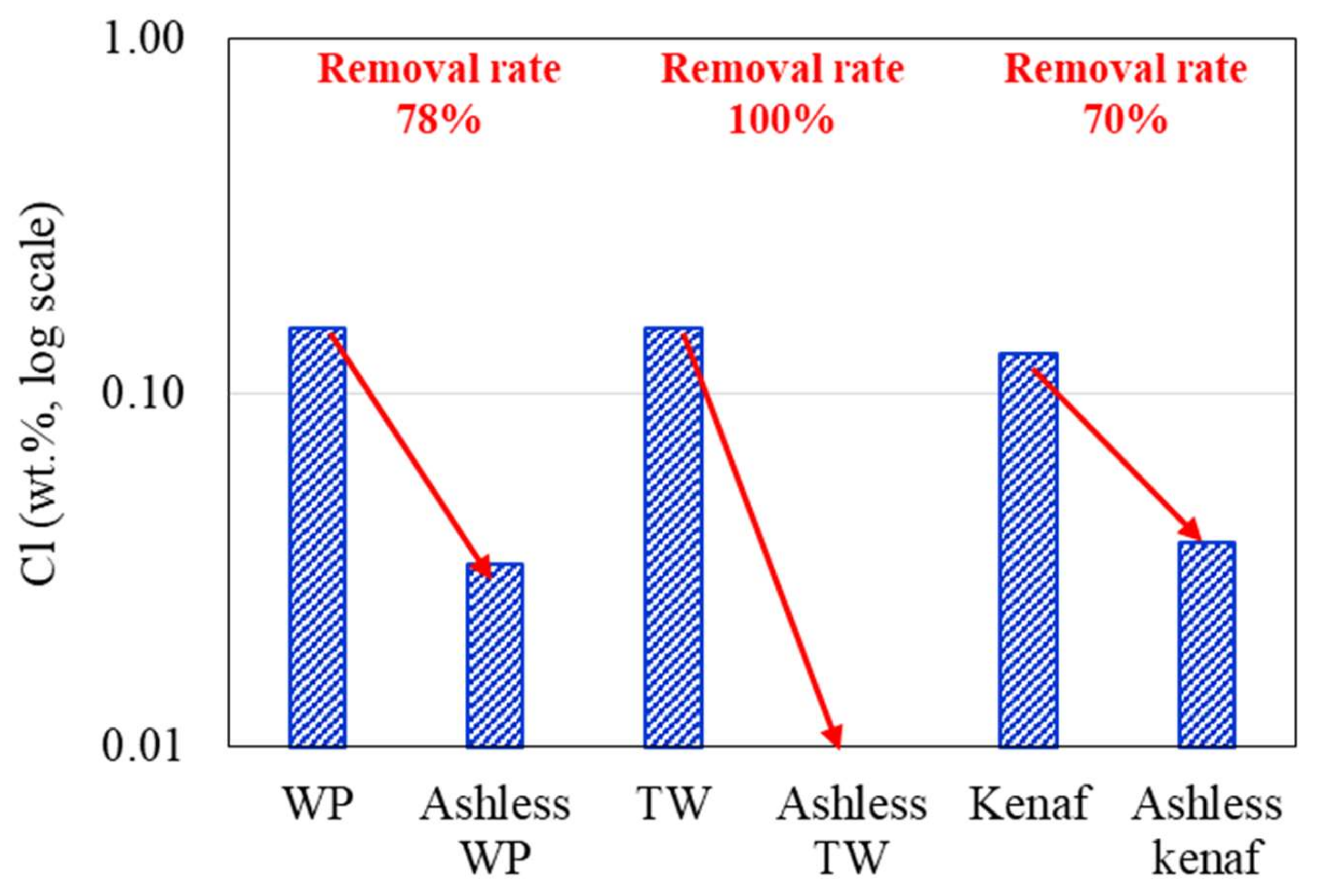
| Sample | Coal A | WP | Ashless WP | TW | Ashless TW | Kenaf | Ashless Kenaf |
|---|---|---|---|---|---|---|---|
| Proximate analysis (ad) | |||||||
| Inherent moisture (wt.%) | 3.31 | 6.60 | 5.82 | 3.28 | 3.30 | 3.38 | 3.08 |
| Ash (wt.%) | 15.30 | 3.46 | 1.55 | 4.52 | 1.57 | 6.08 | 1.94 |
| Volatile matter (wt.%) | 33.17 | 69.90 | 74.46 | 71.69 | 74.17 | 72.19 | 76.65 |
| Fixed carbon (wt.%) | 48.23 | 20.04 | 18.18 | 20.52 | 20.96 | 18.37 | 18.33 |
| Ultimate analysis (DAF) | |||||||
| C (wt.%) | 84.63 | 43.03 | 49.30 | 48.68 | 48.95 | 43.55 | 47.30 |
| H (wt.%) | 5.37 | 5.38 | 5.69 | 6.18 | 6.07 | 5.78 | 6.29 |
| O (wt.%) | 7.48 | 50.54 | 44.70 | 44.41 | 46.90 | 48.31 | 50.11 |
| N (wt.%) | 1.76 | 2.88 | 0.30 | 2.59 | 2.52 | 2.86 | 2.86 |
| S (wt.%) | 0.76 | 0.70 | 0.01 | 0.00 | 0.00 | 0.05 | 0.00 |
| LHV (kcal/kg) | 5593 | 4177 | 3864 | 4566 | 4554 | 4129 | 4411 |
| Sample (wt.%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Others | Cl− |
|---|---|---|---|---|---|---|---|---|---|
| Coal A | 65.45 | 23.97 | 4.87 | 1.07 | 0.7 | 0.27 | 1.24 | 2.44 | N.D |
| WP | 24.62 | 3.87 | 3.04 | 24.92 | 5.52 | 1.27 | 13.25 | 23.52 | 0.13 |
| Ashless WP | 32.69 | 4.97 | 3.86 | 27.75 | 2.72 | 1.36 | 4.85 | 21.8 | 0.04 |
| TW | 46.55 | 10.60 | 3.66 | 19.48 | 3.45 | 0.74 | 8.36 | 7.16 | 0.03 |
| Ashless TW | 72.56 | 9.37 | 0.70 | 11.23 | 0.00 | 0.00 | 3.06 | 3.08 | 0.01 |
| Kenaf | 6.71 | 2.08 | 0.82 | 13.75 | 8.94 | 0.35 | 33.67 | 33.68 | 3.85 |
| Ashless kenaf | 20.71 | 4.79 | 4.75 | 23.97 | 6.37 | 1.00 | 9.73 | 29.06 | 0.03 |
| Sample | NOx (ppm) | SOx (ppm) | UBC (%) |
|---|---|---|---|
| Coal A | 102.85 | 27.72 | 40.43 |
| Coal A + WP (5%) | 87.14 | 40.51 | 28.18 |
| Coal A + WP (10%) | 95.64 | 38.27 | 37.53 |
| Coal A + Ashless WP (5%) | 96.34 | 39.32 | 20.04 |
| Coal A + Ashless WP (10%) | 93.73 | 42.25 | 15.45 |
| Coal A + TW (5%) | 84.15 | 30.64 | 27.92 |
| Coal A + TW (10%) | 84.89 | 35.74 | 27.24 |
| Coal A + Ashless TW (5%) | 91.03 | 44.81 | 36.16 |
| Coal A + Ashless TW (10%) | 93.17 | 39.54 | 33.32 |
| Coal A + Kenaf (5%) | 88.87 | 52.23 | 27.63 |
| Coal A + Kenaf (10%) | 82.69 | 40.22 | 25.50 |
| Coal A + Ashless Kenaf (5%) | 84.75 | 39.21 | 39.18 |
| Coal A + Ashless Kenaf (10%) | 90.86 | 47.44 | 38.51 |
| Sample | Total Feed (g) | Deposited Mass (g) |
|---|---|---|
| Coal A | 10 | 0.134 |
| Coal A + WP (5%) | 10 | 0.104 |
| Coal A + WP (10%) | 10 | 0.125 |
| Coal A + Ashless WP (5%) | 10 | 0.119 |
| Coal A + Ashless WP (10%) | 10 | 0.109 |
| Coal A + TW (5%) | 10 | 0.090 |
| Coal A + TW (10%) | 10 | 0.125 |
| Coal A + Ashless TW (5%) | 10 | 0.110 |
| Coal A + Ashless TW (10%) | 10 | 0.099 |
| Coal A + Kenaf (5%) | 10 | 0.128 |
| Coal A + Kenaf (10%) | 10 | 0.135 |
| Coal A + Ashless Kenaf (5%) | 10 | 0.098 |
| Coal A + Ashless Kenaf (10%) | 10 | 0.116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-G.; Ku, M.-J.; Kim, K.-H.; Kim, J.-S.; Kim, S.-M.; Jeon, C.-H. Experimental Investigation of the Ash Deposition Characteristics of Biomass Pretreated by Ash Removal during Co-Combustion with Sub-Bituminous Coal. Energies 2021, 14, 7391. https://doi.org/10.3390/en14217391
Lee D-G, Ku M-J, Kim K-H, Kim J-S, Kim S-M, Jeon C-H. Experimental Investigation of the Ash Deposition Characteristics of Biomass Pretreated by Ash Removal during Co-Combustion with Sub-Bituminous Coal. Energies. 2021; 14(21):7391. https://doi.org/10.3390/en14217391
Chicago/Turabian StyleLee, Dae-Gyun, Min-Jong Ku, Kyeong-Ho Kim, Jae-Sung Kim, Seung-Mo Kim, and Chung-Hwan Jeon. 2021. "Experimental Investigation of the Ash Deposition Characteristics of Biomass Pretreated by Ash Removal during Co-Combustion with Sub-Bituminous Coal" Energies 14, no. 21: 7391. https://doi.org/10.3390/en14217391
APA StyleLee, D.-G., Ku, M.-J., Kim, K.-H., Kim, J.-S., Kim, S.-M., & Jeon, C.-H. (2021). Experimental Investigation of the Ash Deposition Characteristics of Biomass Pretreated by Ash Removal during Co-Combustion with Sub-Bituminous Coal. Energies, 14(21), 7391. https://doi.org/10.3390/en14217391







