Clarifying the Effect of Clay Minerals on Methane Adsorption Capacity of Marine Shales in Sichuan Basin, China
Abstract
:1. Introduction
2. Samples and Experiments
2.1. Samples
2.2. Mineral Analysis
2.3. TOC Experiments
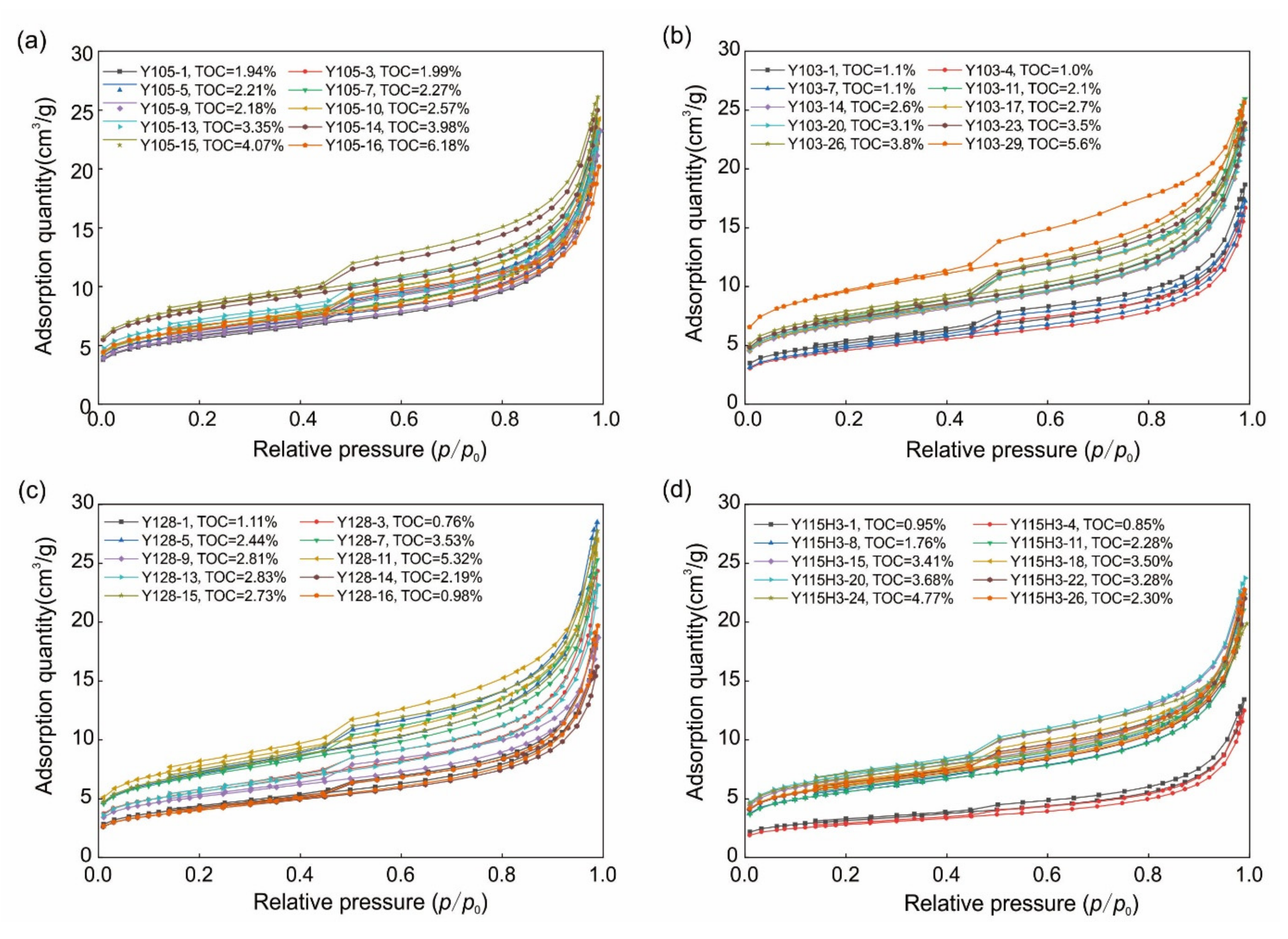
2.4. Low-Temperature N2 Adsorption Experiments
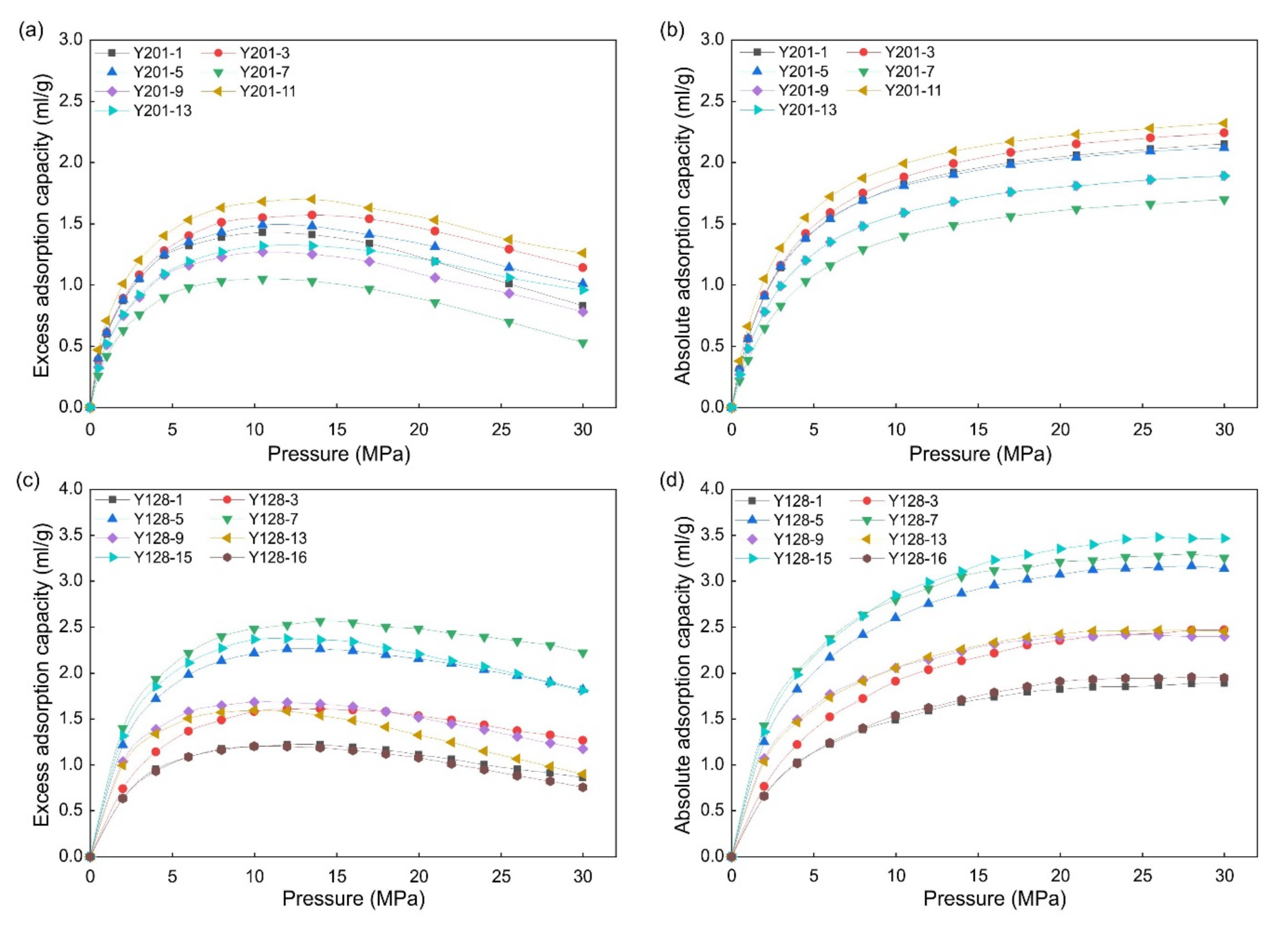
2.5. Methane Adsorption Experiments
3. Results
3.1. TOC and Clay Mineral Composition
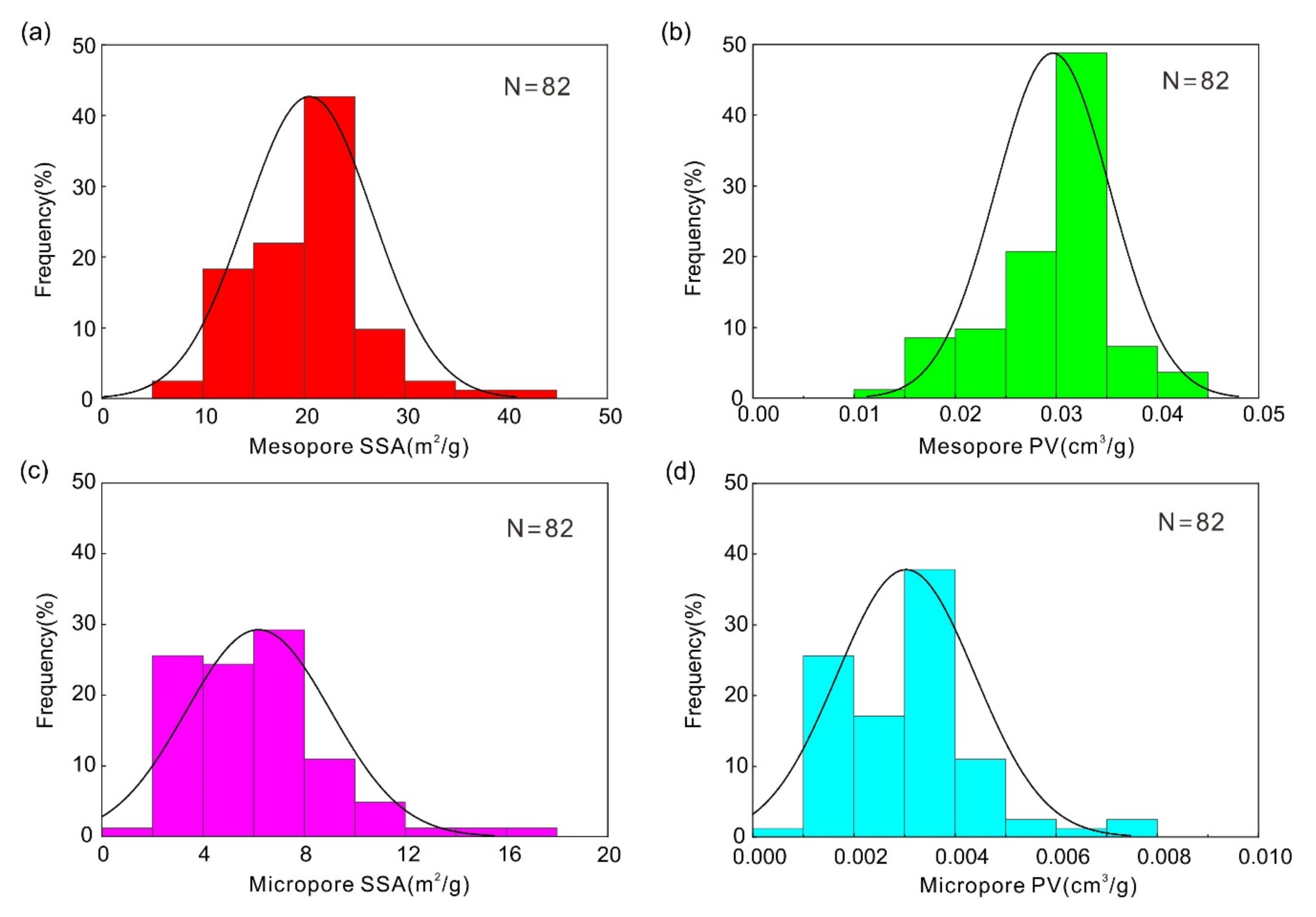
3.2. SSA and PV Characteristics
3.3. Methane Adsorption Capacity
4. Discussion
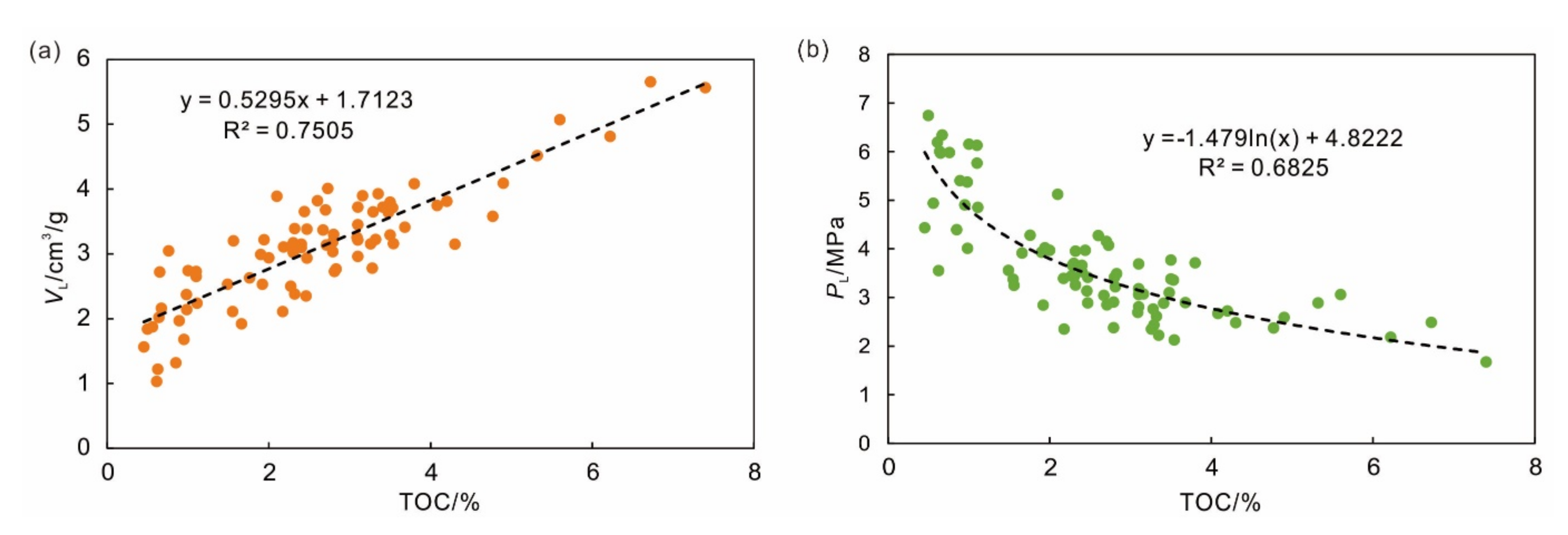
4.1. Effect of TOC on Adsorption Capacity
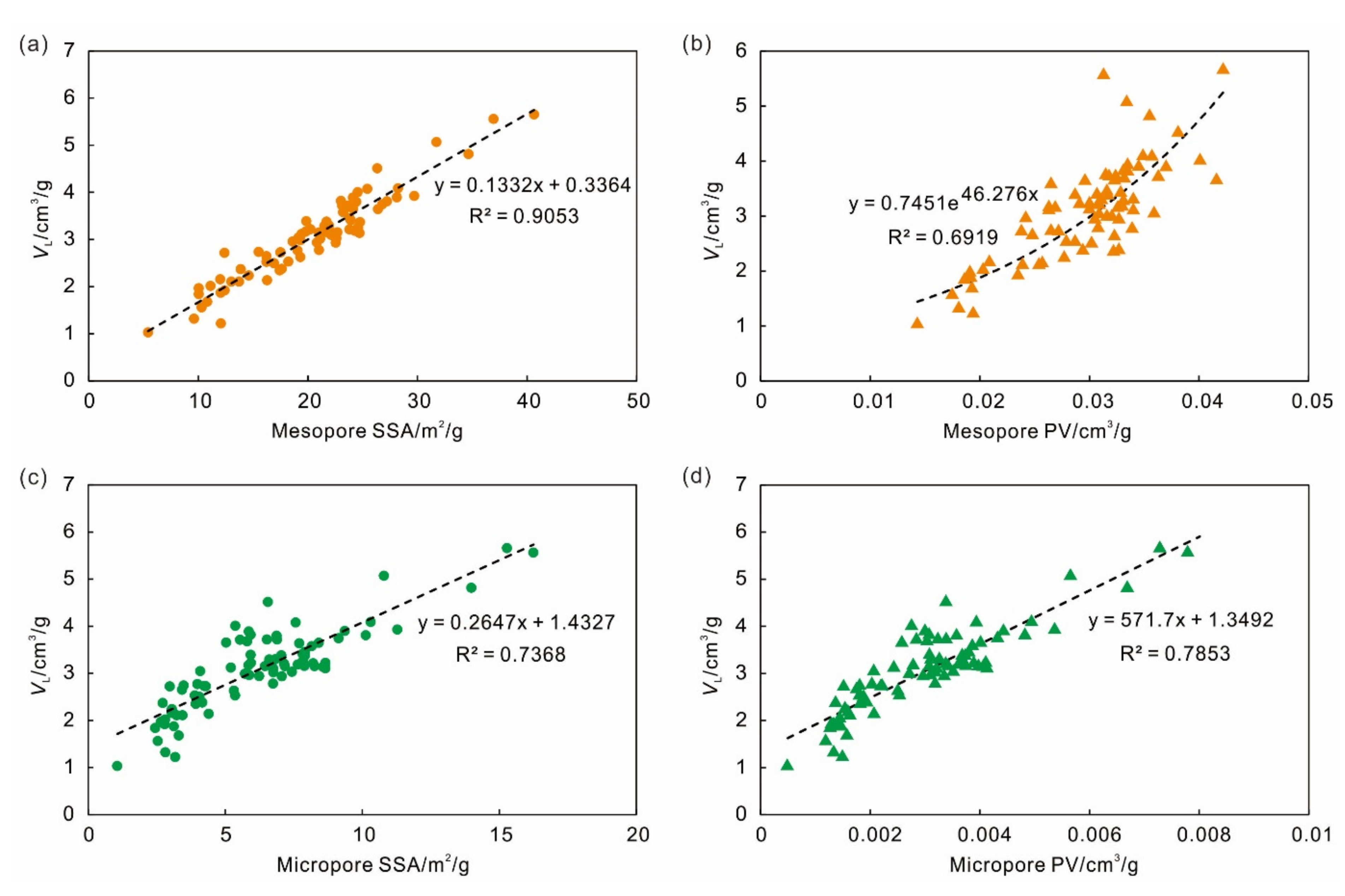
4.2. Effect of Pore Characteristics on Adsorption Capacity
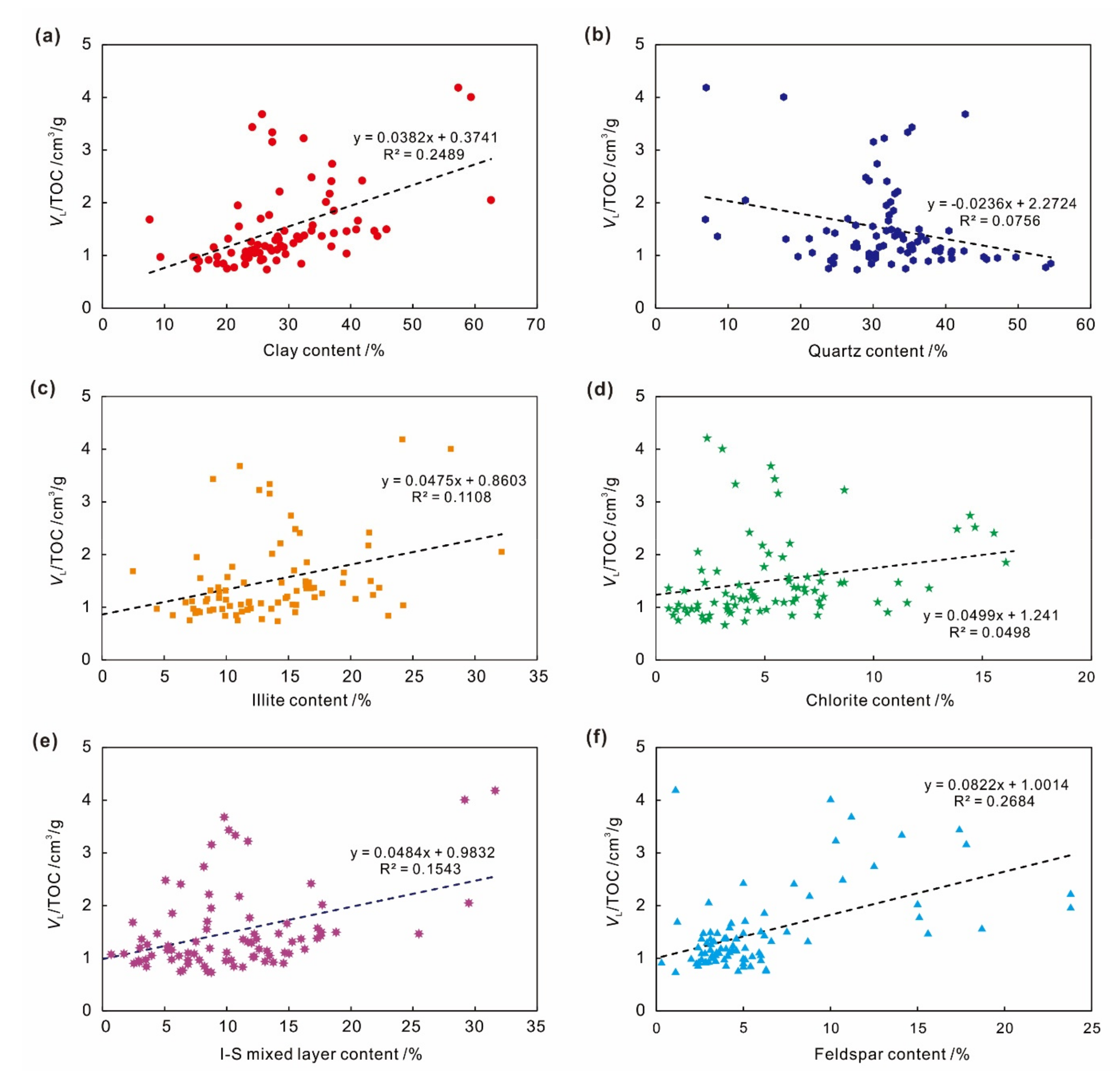
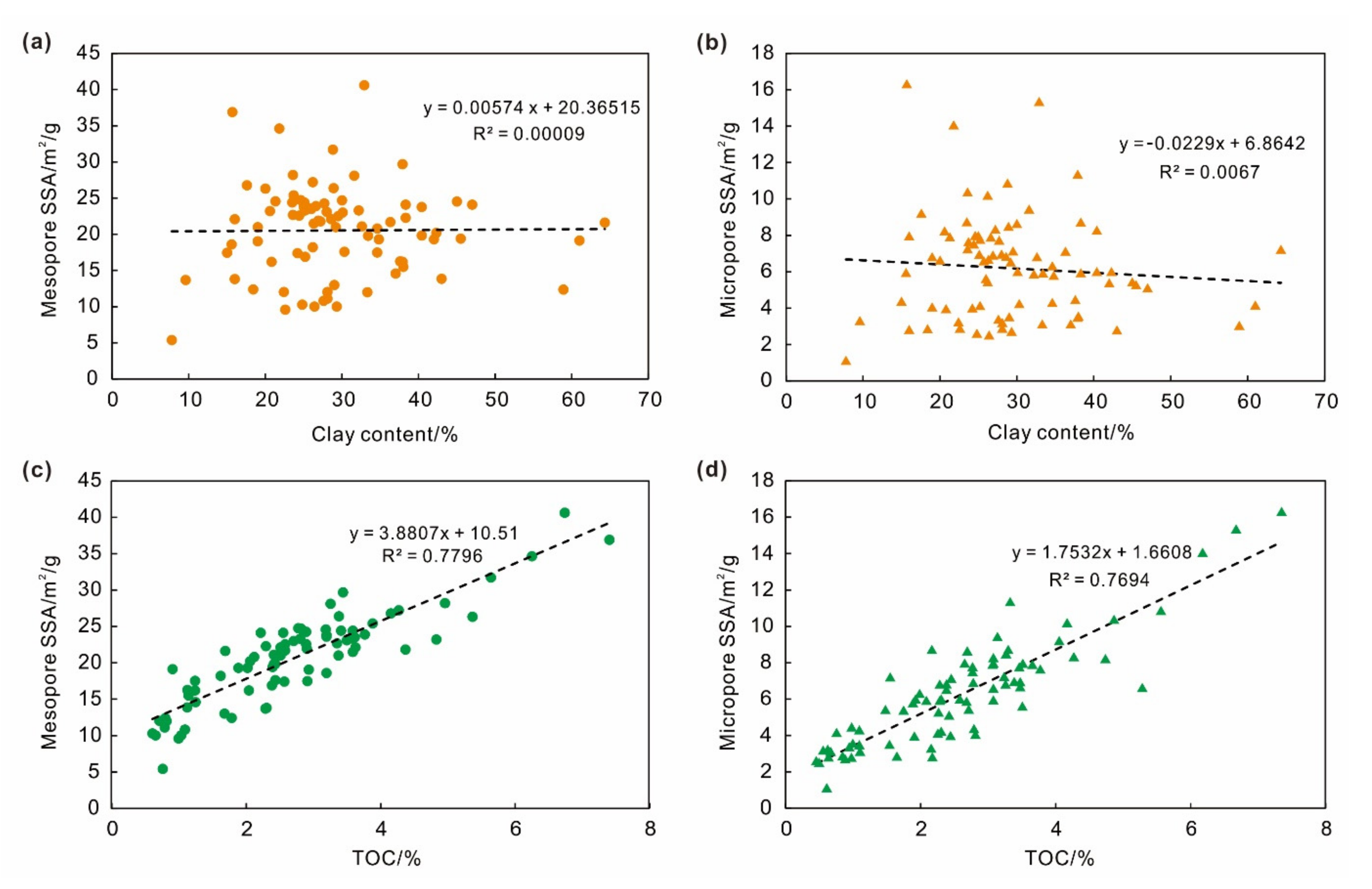
4.3. Effect of Mineral Composition on Adsorption Capacity
4.4. Competitive Adsorption between OM and Clay Minerals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.; Xie, J. The progress and prospects of shale gas exploration and development in southern Sichuan Basin, SW China. Pet. Explor. Dev. 2018, 45, 172–182. [Google Scholar] [CrossRef]
- Ma, Y.; Cai, X.; Zhao, P. China’s shale gas exploration and development: Understanding and practice. Pet. Explor. Dev. 2018, 45, 589–603. [Google Scholar] [CrossRef]
- Zou, C.; Zhu, R.; Chen, Z.-Q.; Ogg, J.G.; Wu, S.; Dong, D.; Qiu, Z.; Wang, Y.; Wang, L.; Lin, S.; et al. Organic-matter-rich shales of China. Earth-Sci. Rev. 2019, 189, 51–78. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Cong, L.; Wang, H.; Shi, Z.; Wu, J.; Pan, S. Development progress, potential and prospect of shale gas in China. Nat. Gas Ind. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- Feng, Z.; Hao, F.; Zhou, S.; Wu, W.; Tian, J.; Xie, C.; Cai, Y. Pore Characteristics and Methane Adsorption Capacity of Different Lithofacies of the Wufeng Formation–Longmaxi Formation Shales, Southern Sichuan Basin. Energy Fuels 2020, 34, 8046–8062. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Liu, D.; Jiao, P.; Liu, H. Progress and prospect of key experimental technologies for shale gas geological evaluation. Nat. Gas. Ind. 2020, 40, 1–17. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Zhou, S.; Feng, Z.; Liu, H.; Guo, W. Insights into NMR response characteristics of shales and its application in shale gas reservoir evaluation. J. Nat. Gas Sci. Eng. 2020, 84, 103674. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. Characterizing the shale gas resource potential of Devonian–Mississippian strata in the Western Canada sedimentary basin: Application of an integrated formation evaluation. AAPG Bull. 2008, 92, 87–125. [Google Scholar] [CrossRef]
- Ambrose, R.J.; Hartman, R.C.; Campos, M.D.; Akkutlu, I.Y.; Sondergeld, C.H. Shale Gas-in-Place Calculations Part I: New Pore-Scale Considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Luxbacher, K.; Pitcher, E. Adsorption Models for Methane in Shales: Review, Comparison, and Application. Energy Fuels 2017, 31, 10787–10801. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wu, K.; Wang, K.; Luo, J.; Feng, D.; Qu, S.; Li, X. A multi-site model to determine supercritical methane adsorption in energetically heterogeneous shales. Chem. Eng. J. 2018, 349, 438–455. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Experimental Investigation and Mathematical Modeling of Gas Diffusivity by Carbon Dioxide and Methane Kinetic Adsorption. Ind. Eng. Chem. 2019, 58, 12392–12400. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, Y.; Soliman, M.Y.; Shen, Y. Experimental measurement and analytical estimation of methane absorption in shale kerogen. Fuel 2019, 240, 192–205. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Frère, M.; De Weireld, G. High-pressure adsorption measurements. A comparative study of the volumetric and gravimetric methods. Meas. Sci. Technol. 2004, 15, 848–858. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-Pressure Methane Sorption Isotherms of Black Shales from The Netherlands. Energy Fuels 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.; Robinson, R.L.; Gasem, K.A. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S.; Duffy, G. Application of a Modified Dubinin−Radushkevich Equation to Adsorption of Gases by Coals under Supercritical Conditions. Energy Fuels 2007, 21, 992–997. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Stadie, N.; Yu, L.; Hall, M. A dual-site Langmuir equation for accurate estimation of high pressure deep shale gas resources. Fuel 2016, 185, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Li, J. Development of adsorption ratio equation and state equation of liquid and their geological significance. Capillarity 2021, 4, 63–65. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. Impact of mass balance calculations on adsorption capacities in microporous shale gas reservoirs. Fuel 2007, 86, 2696–2706. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Tian, H.; Li, T.; Zhang, T.; Xiao, X. Characterization of methane adsorption on overmature Lower Silurian–Upper Ordovician shales in Sichuan Basin, southwest China: Experimental results and geological implications. Int. J. Coal Geol. 2016, 156, 36–49. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J. Selective adsorption of CO2/CH4 mixture on clay-rich shale using molecular simulations. J. CO2 Util. 2020, 39, 101143. [Google Scholar] [CrossRef]
- Zhu, H.; Ju, Y.; Huang, C.; Chen, F.; Chen, B.; Yu, K. Microcosmic gas adsorption mechanism on clay-organic nanocomposites in a marine shale. Energy 2020, 197, 117256. [Google Scholar] [CrossRef]
- Li, A.; Han, W.; Fang, Q.; Memon, A.; Ma, M. Experimental investigation of methane adsorption and desorption in water-bearing shale. Capillarity 2020, 3, 45–55. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Huang, W.-L. Selective adsorption of hydrocarbon gases on clays and organic matter. Org. Geochem. 2004, 35, 413–423. [Google Scholar] [CrossRef]
- Lu, X.; Li, F.; Watson, A.T. Adsorption measurements in Devonian shales. Fuel 1995, 74, 599–603. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Wang, W.; Zhang, Y.; Yin, J.; Lu, S.; Chen, F.; Meng, Y.; Zhang, X.; Chen, X.; et al. Key factors controlling the gas adsorption capacity of shale: A study based on parallel experiments. Appl. Geochem. 2015, 58, 88–96. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, W.; Lu, L.; Li, M.; Chen, P.; Ji, X. Analysis of influence factors of methane adsorption capacity of the Lower Silurian shale. Pet. Sci. Technol. 2018, 36, 2112–2118. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, S.; Xing, X.; Zhang, W.; Wang, S. Advances and challenges in shale oil development: A critical review. Adv. Geo-Energy Res. 2020, 4, 406–418. [Google Scholar] [CrossRef]
- Luo, P.; Zhong, N.; Khan, I.; Wang, X.; Wang, H.; Luo, Q.; Guo, Z. Effects of pore structure and wettability on methane adsorption capacity of mud rock: Insights from mixture of organic matter and clay minerals. Fuel 2019, 251, 551–561. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Cancino, O.P.O.; Peredo-Mancilla, D.; Pozo, M.; Pérez, E.; Bessieres, D. Effect of Organic Matter and Thermal Maturity on Methane Adsorption Capacity on Shales from the Middle Magdalena Valley Basin in Colombia. Energy Fuels 2017, 31, 11698–11709. [Google Scholar] [CrossRef]
- Shabani, M.; Moallemi, S.A.; Krooss, B.M.; Amann-Hildenbrand, A.; Zamani-Pozveh, Z.; Ghalavand, H.; Littke, R. Methane sorption and storage characteristics of organic-rich carbonaceous rocks, Lurestan province, southwest Iran. Int. J. Coal Geol. 2018, 186, 51–64. [Google Scholar] [CrossRef]
- Jeon, P.R.; Choi, J.; Yun, T.S.; Lee, C.-H. Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 2014, 255, 705–715. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, X.; Li, Y.; Wu, K.; Shi, J.; Yang, L.; Feng, D.; Zhang, T.; Yu, P. Effect of water distribution on methane adsorption capacity in shale clay. Int. J. Coal Geol. 2016, 159, 135–154. [Google Scholar] [CrossRef]
- Shen, W.; Li, X.; Ma, T.; Cai, J.; Lu, X.; Zhou, S. High-pressure methane adsorption behavior on deep shales: Experiments and modeling. Phys. Fluids 2021, 33, 063103. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, L.; Pang, Z.; Liang, P.; Guo, W.; Zhou, S.; Lu, B. Reservoir heterogeneity of the Longmaxi Formation and its significance for shale gas enrichment. Energy Sci. Eng. 2020, 8, 4229–4249. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Z.; Liu, K.; Tan, J.; Gao, F.; Wang, P. Pore structure characterization for organic-rich Lower Silurian shale in the Upper Yangtze Platform, South China: A possible mechanism for pore development. J. Nat. Gas Sci. Eng. 2017, 46, 1–15. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, H.; Ning, Y.; Guo, W.; Zhang, Q. Experimental study of supercritical methane adsorption in Longmaxi shale: Insights into the density of adsorbed methane. Fuel 2018, 211, 140–148. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Mikhail, R.; Brunauer, S.; Bodor, E. Investigations of a complete pore structure analysis-I: Analysis of micropores. J. Colloid Interface Sci. 1968, 26, 45–53. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, H.; Chen, H.; Wang, H.; Guo, W.; Liu, D.; Zhang, Q.; Wu, J.; Shen, W. A comparative study of the nanopore structure characteristics of coals and Longmaxi shales in China. Energy Sci. Eng. 2019, 7, 2768–2781. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, D.; Wang, H.; Li, X. A modified BET equation to investigate supercritical methane adsorption mechanisms in shale. Mar. Pet. Geol. 2019, 105, 284–292. [Google Scholar] [CrossRef]
- Chai, D.; Yang, G.; Fan, Z.; Li, X. Gas transport in shale matrix coupling multilayer adsorption and pore confinement effect. Chem. Eng. J. 2019, 370, 1534–1549. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, Z.; Li, X.; Yang, Y.; Zhao, C.; Connell, L.D.; Li, S.; He, J. Experimental study and modelling of methane adsorption and diffusion in shale. Fuel 2014, 117, 509–519. [Google Scholar] [CrossRef]
- Wood, D.A. Techniques used to calculate shale fractal dimensions involve uncertainties and imprecisions that require more careful consideration. Adv. Geo-Energy Res. 2021, 5, 153–165. [Google Scholar] [CrossRef]
- Gai, H.; Li, T.; Wang, X.; Tian, H.; Xiao, X.; Zhou, Q. Methane adsorption characteristics of overmature Lower Cambrian shales of deepwater shelf facies in Southwest China. Mar. Pet. Geol. 2020, 120, 104565. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Chen, S.; Tao, S.; Tang, D.; Xu, H.; Li, S.; Zhao, J.; Jiang, Q.; Yang, H. Pore Structure Characterization of Different Rank Coals Using N2 and CO2 Adsorption and Its Effect on CH4 Adsorption Capacity: A Case in Panguan Syncline, Western Guizhou, China. Energy Fuels 2017, 31, 6034–6044. [Google Scholar] [CrossRef]
- Suárez-Ruiz, I.; Juliao, T.; Suárez-García, F.; Marquez, R.; Ruiz, B. Porosity development and the influence of pore size on the CH4 adsorption capacity of a shale oil reservoir (Upper Cretaceous) from Colombia. Role of solid bitumen. Int. J. Coal Geol. 2016, 159, 1–17. [Google Scholar] [CrossRef]
- Li, J.; Wu, K.; Chen, Z.; Wang, W.; Yang, B.; Wang, K.; Luo, J.; Yu, R. Effects of energetic heterogeneity on gas adsorption and gas storage in geologic shale systems. Appl. Energy 2019, 251, 113368. [Google Scholar] [CrossRef]
- Gao, Z.; Xiong, S. Methane Adsorption Capacity Reduction Process of Water-Bearing Shale Samples and Its Influencing Factors: One Example of Silurian Longmaxi Formation Shale from the Southern Sichuan Basin in China. J. Earth Sci. 2021, 32, 946–959. [Google Scholar] [CrossRef]
- Li, B.; Mehmani, A.; Chen, J.; Georgi, D.T.; Jin, G. The Condition of Capillary Condensation and Its Effects on Adsorption Isotherms of Unconventional Gas Condensate Reservoirs. proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September 2013. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Ren, C.; Zhang, Y.; Wang, B. An adsorption model for evaluating methane adsorption capacity in shale under various pressures and moisture. J. Nat. Gas Sci. Eng. 2020, 81, 103426. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Gao, Z.; Zhang, Y.; Wang, B. Adsorption behavior, including the thermodynamic characteristics of wet shales under different temperatures and pressures. Chem. Eng. Sci. 2021, 230, 116228. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, R.; Xie, Q.; You, L.; Liu, K.; Saeedi, A. Investigation of moisture effect on methane adsorption capacity of shale samples. Fuel 2018, 232, 323–332. [Google Scholar] [CrossRef]
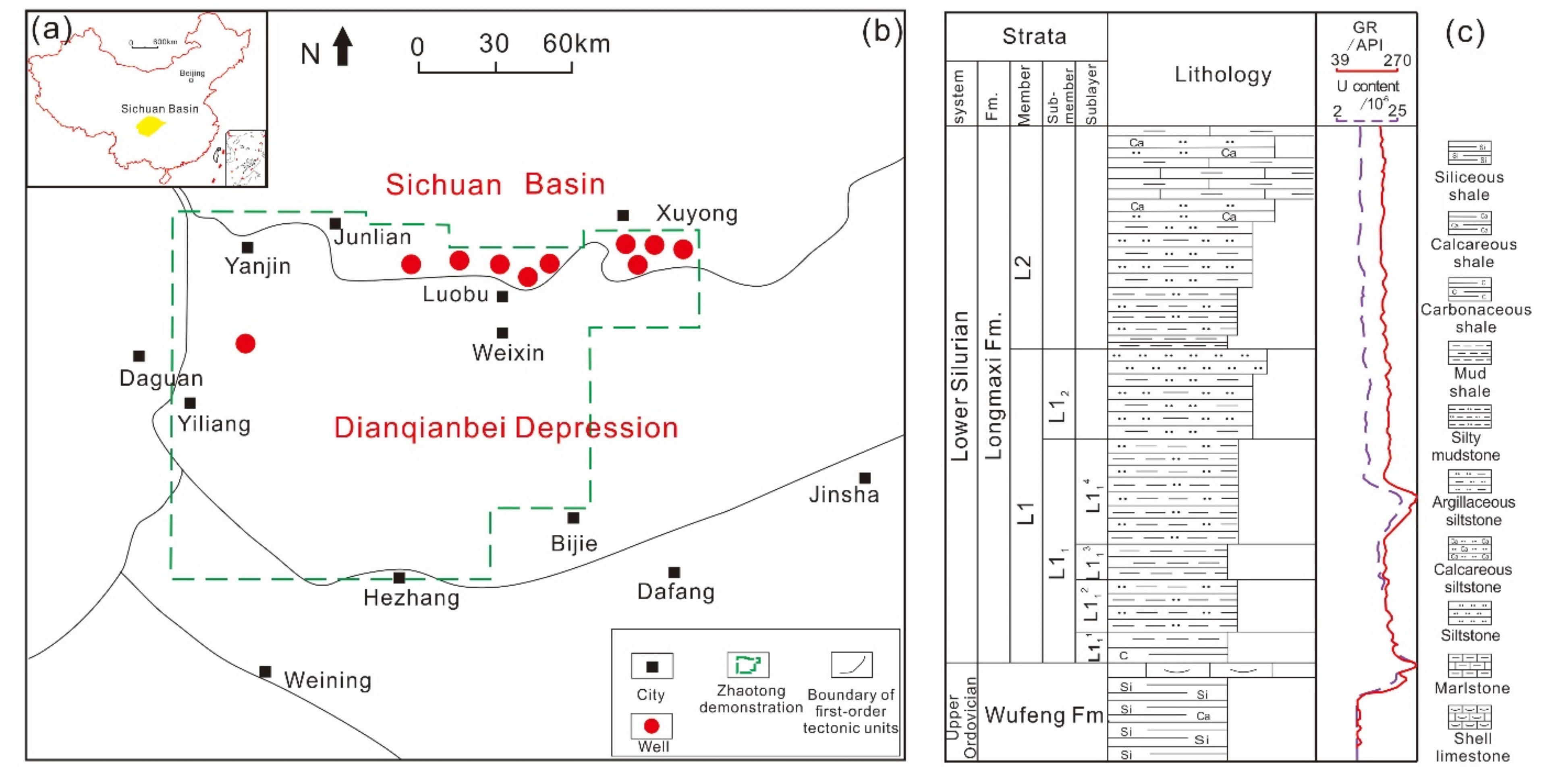



| No. | Well | Number of Samples | Depth (m) | TOC (%) | Quartz Content (%) | Clay Content (%) |
|---|---|---|---|---|---|---|
| 1 | Y105 | 10 | 1654.40–1691.99 | 1.90–4.90 (3.03) | 28.1–38.0 (33.7) | 23.6–34.8 (29.0) |
| 2 | Y103 | 10 | 1027.00–1086.96 | 1.00–5.60 (2.66) | 24.6–41.6 (34.7) | 23.7–38.3 (31.4) |
| 3 | Y201 | 8 | 2734.55–2776.33 | 0.61–2.46 (1.87) | 7.0–34.4 (23.4) | 7.8–30.3 (20.7) |
| 4 | Y128 | 10 | 2235.66–2267.03 | 0.76–5.32 (2.47) | 18.0–37.0 26.8) | 15.0–61.0 (32.9) |
| 5 | Y115H3 | 10 | 2989.42–3039.78 | 0.85–4.77 (2.68) | 18.3–55.5 (36.4) | 19.0–45.5 (30.9) |
| 6 | Y112H12 | 10 | 2125.71–2186.67 | 0.50–3.48 (1.67) | 7.1–48.0 (33.7) | 15.6–58.9 (33.6) |
| 7 | Y151 | 9 | 1704.16–1761.95 | 0.56–6.22 (2.63) | 27.0–54.8 (37.1) | 21.8–40.4 (29.1) |
| 8 | Y107 | 5 | 1233.43–1255.51 | 1.56–3.54 (2.89) | 12.6–50.6 (36.2) | 16.0–64.3 (32.1) |
| 9 | Y137 | 5 | 1014.50–1035.66 | 2.67–6.72 (3.94) | 28.2–40.1 (34.2) | 17.6–32.9 (25.6) |
| 10 | Y138 | 5 | 1937.57–1987.98 | 0.46–7.40 (2.80) | 24.3–36.0 (29.2) | 15.7–38.3 (27.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhou, S.; Zhang, J.; Feng, Z.; Jiao, P.; Zhang, L.; Zhang, Q. Clarifying the Effect of Clay Minerals on Methane Adsorption Capacity of Marine Shales in Sichuan Basin, China. Energies 2021, 14, 6836. https://doi.org/10.3390/en14206836
Wang H, Zhou S, Zhang J, Feng Z, Jiao P, Zhang L, Zhang Q. Clarifying the Effect of Clay Minerals on Methane Adsorption Capacity of Marine Shales in Sichuan Basin, China. Energies. 2021; 14(20):6836. https://doi.org/10.3390/en14206836
Chicago/Turabian StyleWang, Hongyan, Shangwen Zhou, Jiehui Zhang, Ziqi Feng, Pengfei Jiao, Leifu Zhang, and Qin Zhang. 2021. "Clarifying the Effect of Clay Minerals on Methane Adsorption Capacity of Marine Shales in Sichuan Basin, China" Energies 14, no. 20: 6836. https://doi.org/10.3390/en14206836
APA StyleWang, H., Zhou, S., Zhang, J., Feng, Z., Jiao, P., Zhang, L., & Zhang, Q. (2021). Clarifying the Effect of Clay Minerals on Methane Adsorption Capacity of Marine Shales in Sichuan Basin, China. Energies, 14(20), 6836. https://doi.org/10.3390/en14206836








