Kinetic and Thermodynamic Analysis of High-Pressure CO2 Capture Using Ethylenediamine: Experimental Study and Modeling
Abstract
:1. Introduction
2. Methods
2.1. Materials
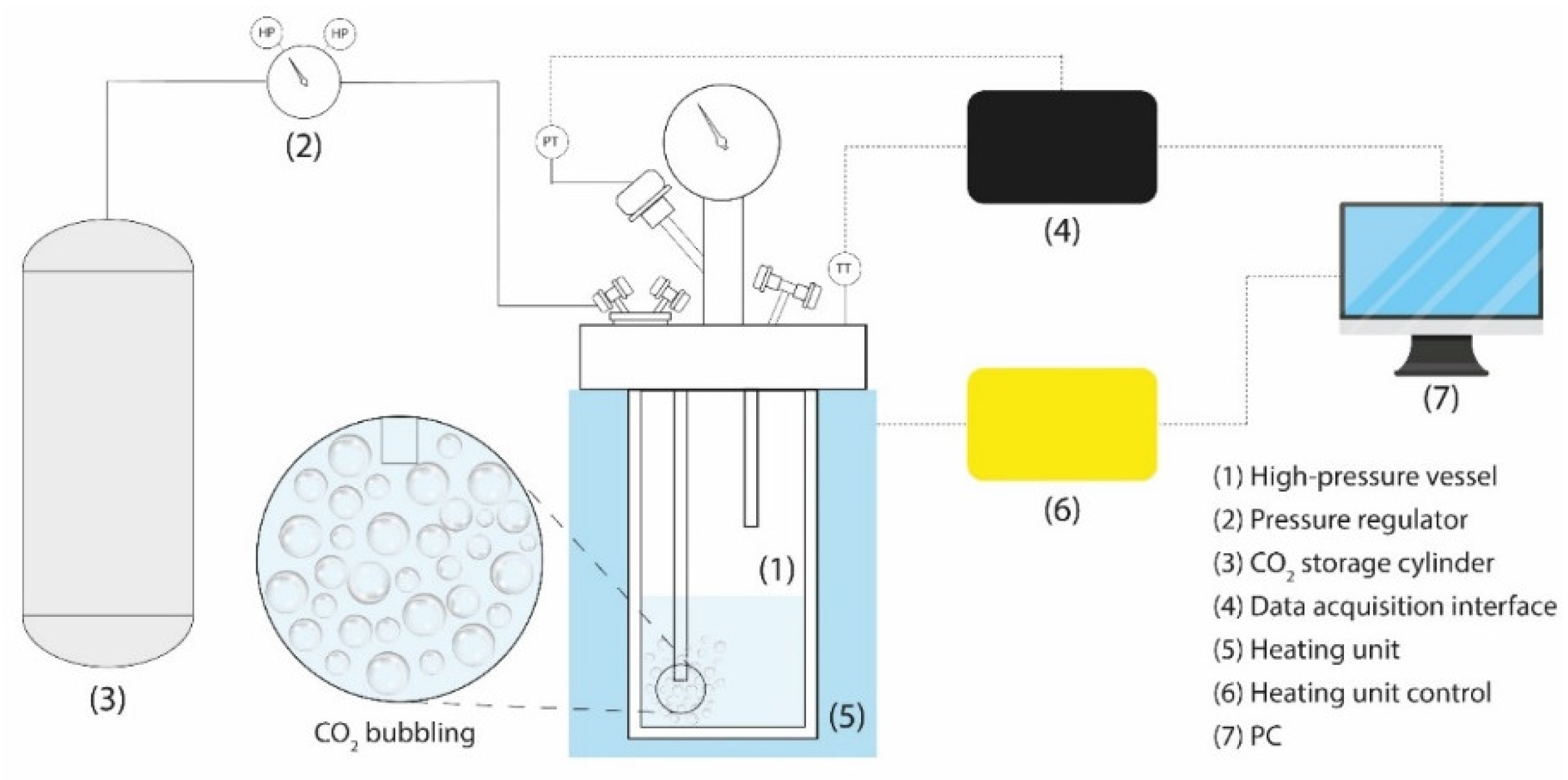
2.2. Experimental Setup
2.3. CO2 Solubility Testing Procedure
2.4. CO2 Capture Testing Procedure Using EDA
2.5. Data Processing
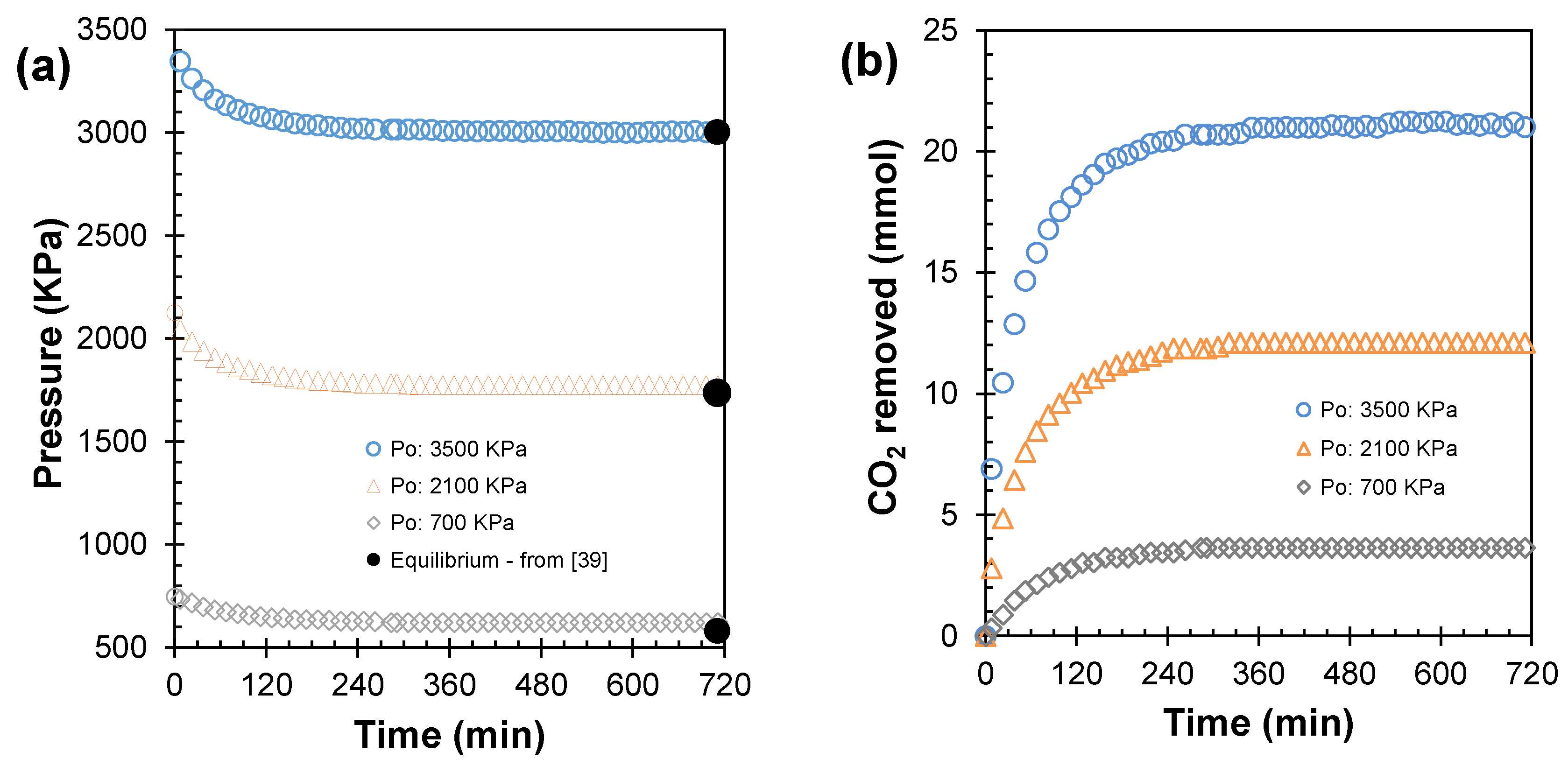
- The quantity of CO2 removed refers to the gaseous phase moles transferred to the liquid phase, with respect to the initial amount of gas loaded into the high-pressure vessel. This quantity was calculated using Equation (5):where is the CO2 mole number in the gas phase, is the vessel pressure, is the gas phase volume, is the compressibility factor calculated by the Peng–Robinson equation of state [36], and is the vessel temperature. Moreover, and are the initial time and an instantaneous time of the experiment, respectively.
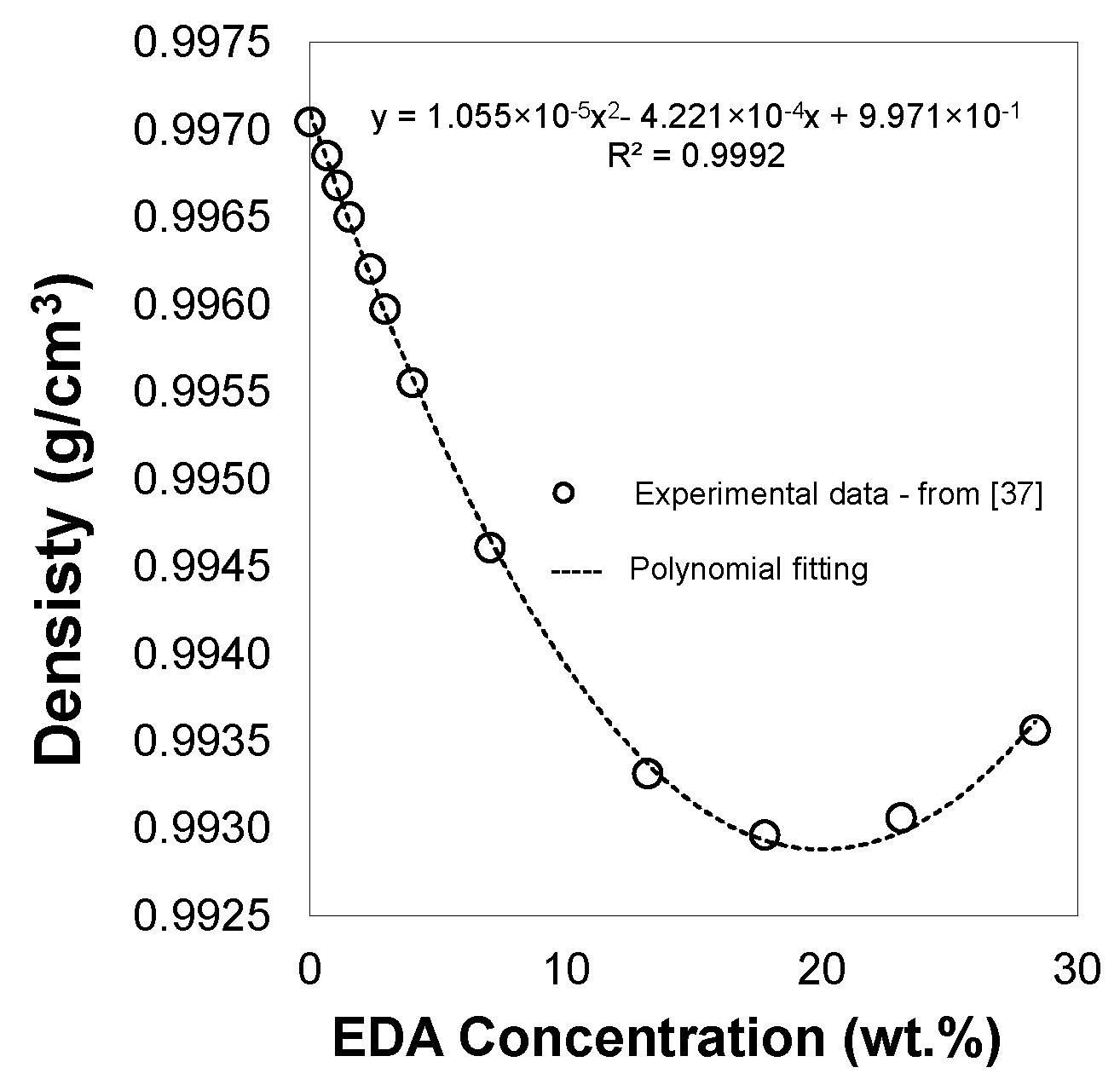
- The CO2 loading refers to the amount of CO2 removed from the gas phase for each liquid (amine + water) mole initially introduced into the high-pressure vessel. The CO2 loading was calculated using Equation (6):where is the initial number of moles in the liquid phase. Appendix A shows the number of moles in each solution for the different amine concentrations used in this study. For this calculation, the experimental density data of aqueous EDA solutions at 303 K reported by Egorov et al. [37] were used.
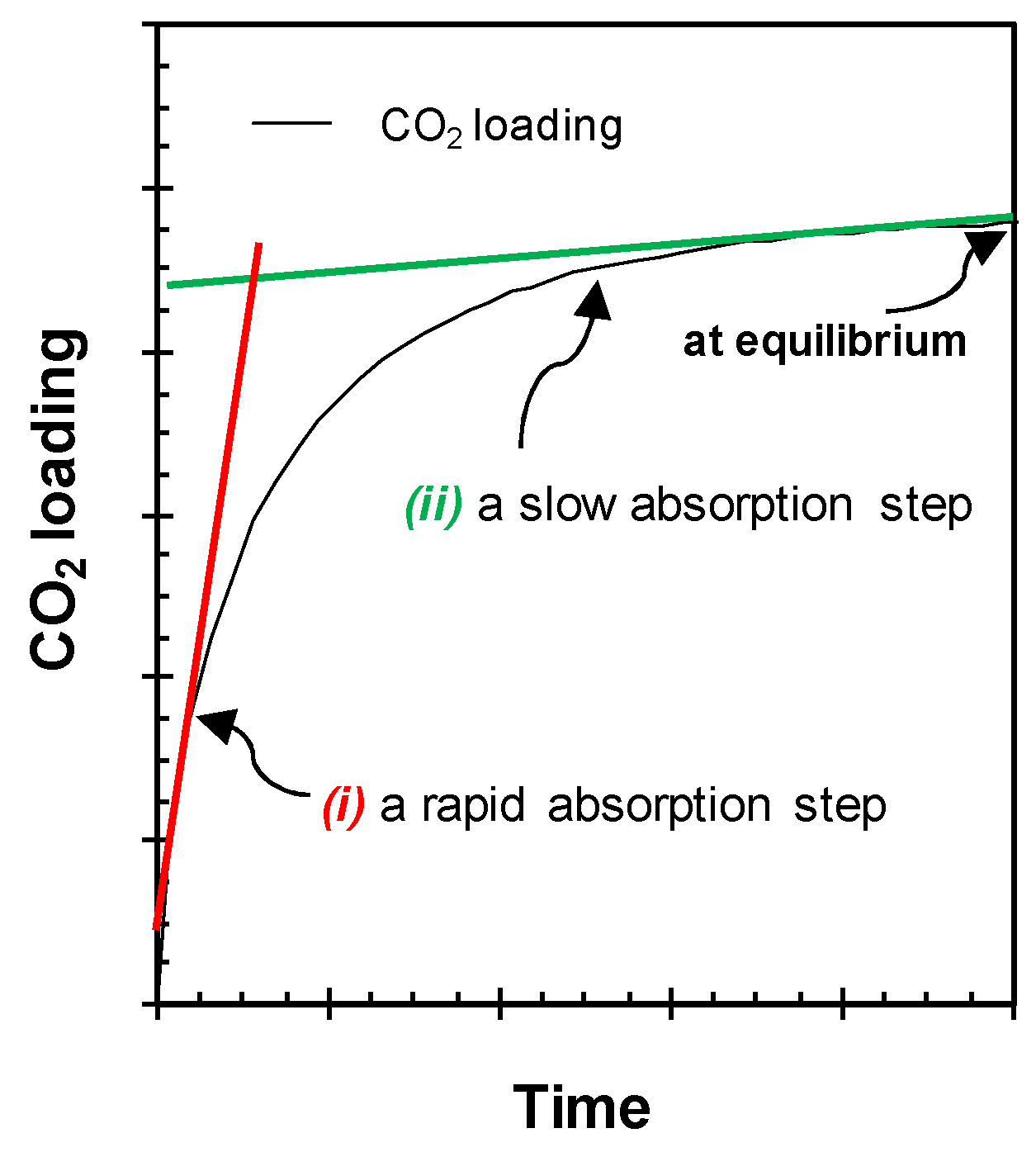
- , , and refer to the time required to reach 25%, 50%, and 90%, respectively, of the total amount of CO2 removed from the gas phase at the end of each experiment.
- refers to the CO2 capture rate. This was calculated directly on the curve of the removed amount of CO2 from the gas as a function of time, and corresponds to the maximum value of gas consumption in the experiments’ first instants. This value was obtained numerically using the initial slope method [38].
3. Results and Discussion
3.1. CO2 Solubility Testing
3.2. CO2 Capture Testing Using EDA
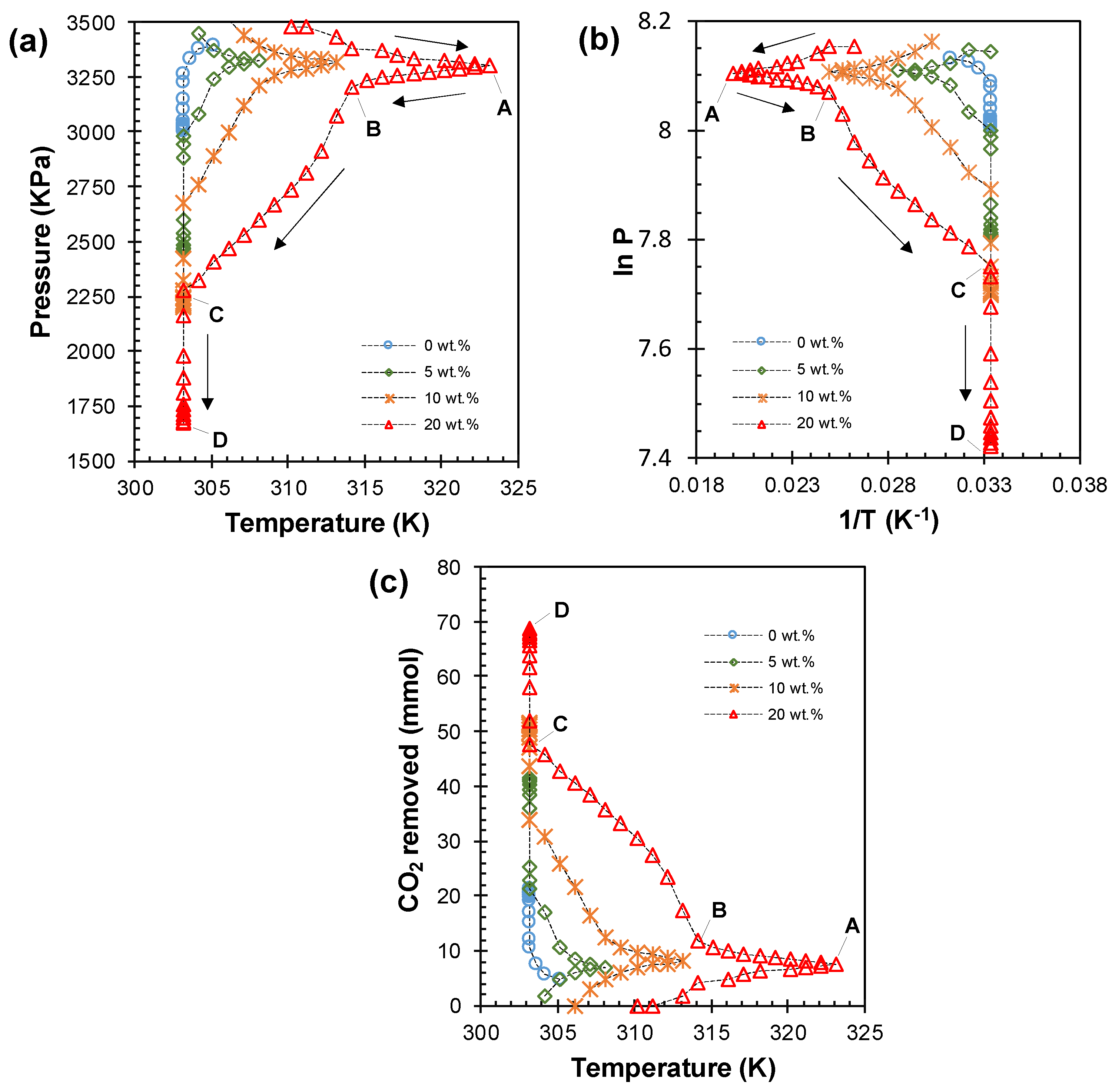
3.3. CO2 Capture Using EDA: Reaction Exothermicity Effects
3.4. CO2 Capture Using EDA: Kinetic and Thermodynamic Analysis
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Mole Number in EDA Aqueous Solutions
| EDA Conc. (wt.%) | Density (g/cm3) | (mole) | (mole) | (mole) |
|---|---|---|---|---|
| 0 | 0.997 | - | 1.660 | 1.660 |
| 5 | 0.995 | 0.025 | 1.574 | 1.599 |
| 10 | 0.994 | 0.050 | 1.490 | 1.540 |
| 20 | 0.993 | 0.099 | 1.323 | 1.422 |
References
- International Energy Agency. Global Energy Review 2020; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/global-energy-review-2020 (accessed on 1 September 2021).
- Center for Climate and Energy Solutions. Global Emissions; C2ES: Arlington, TX, USA, 2020; Available online: https://www.c2es.org/content/international-emissions (accessed on 1 September 2021).
- United Nations Framework Convention on Climate Change. The Paris Agreement. Adopted 12 December 2015. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 1 September 2021).
- Intergovernmental Panel on Climate Change. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005; p. 431. ISBN 978-0-521-86336-0. [Google Scholar]
- Liang, Z.; Fu, K.; Idem, R.; Tontiwachwuthikul, P. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents. Chin. J. Chem. Eng. 2016, 24, 278–288. [Google Scholar] [CrossRef]
- Kohl, A.; Nielsen, R. Chapter 2-alkanolamines for hydrogen sulfide and carbon dioxide removal. In Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 40–186. [Google Scholar] [CrossRef]
- Nwaoha, C.; Beaulieu, M.; Tontiwachwuthikul, P.; Gibson, M. Techno-economic analysis of CO2 capture from a 1.2 million MTPA cement plant using AMP-PZ-MEA blend. Int. J. Greenh. Gas Control 2018, 78, 400–412. [Google Scholar] [CrossRef]
- Rochelle, G. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Feron, P.; Cousins, A.; Jiang, K.; Zhai, R.; Garcia, M. An update of the benchmark post-combustion CO2-capture technology. Fuel 2020, 273, 117776. [Google Scholar] [CrossRef]
- Adu, E.; Zhang, Y.D.; Liu, D.; Tontiwachwuthikul, P. Parametric process design and economic analysis of post-combustion CO2 capture and compression for coal- and natural gas-fired power plants. Energies 2020, 13, 2519. [Google Scholar] [CrossRef]
- Garba, Z.; Galadima, A. Carbon capture and storage (CCS) technology: Challenges to implementation. In Encyclopedia of Renewable and Sustainable Materials, 1st ed.; Hashmi, S., Chudhury, I., Eds.; Elsevier: New York, NY, USA, 2020; Volume 3, pp. 291–299. [Google Scholar] [CrossRef]
- Ishaq, H.; Ali, U.; Sher, F.; Anus, M.; Imran, M. Process analysis of improved process modifications for ammonia-based post-combustion CO2 capture. J. Environ. Chem. Eng. 2021, 9, 104928. [Google Scholar] [CrossRef]
- Nwaoha, C.; Tontiwachwuthikul, P.; Benamor, A. CO2 capture from lime kiln using AMP-DA2MP amine solvent blend: A pilot plant study. J. Environ. Chem. Eng. 2018, 6, 7102–7110. [Google Scholar] [CrossRef]
- Raznahan, M.; Riahi, S.; Mousavi, S. A simple, robust and efficient structural model to predict CO2 absorption for different amine solutions: Concern to design new amine compounds. J. Environ. Chem. Eng. 2020, 8, 104572. [Google Scholar] [CrossRef]
- Borhani, T.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Ume, C.; Ozturk, M.; Alper, E. Kinetics of CO2 absorption by a blended aqueous amine solution. Chem. Eng. Technol. 2012, 35, 464–468. [Google Scholar] [CrossRef]
- Lestari, I.; Rahayu, D.; Nurani, D.; Krisnandi, Y.; Budianto, E. Ethylenediamine-derived imidazoline synthesis using MAOS (Microwave Assisted Organic Synthesis) method. AIP Conf. Proc. 2019, 2168, 020066. [Google Scholar] [CrossRef]
- Li, J.; Henni, A.; Tontiwachwuthikul, P. Reaction kinetics of CO2 in aqueous ethylenediamine, ethyl ethanolamine, and diethyl monoethanolamine solutions in the temperature range of 298-313 K, using the stopped-flow technique. Ind. Eng. Chem. Res. 2007, 46, 4426–4434. [Google Scholar] [CrossRef]
- Salvi, A.; Vaidya, P.; Kenig, E. Kinetics of carbon dioxide removal by ethylenediamine and diethylenetriamine in aqueous solutions. Can. J. Chem. Eng. 2014, 92, 2021–2028. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, X.; Nguyen, T.; Voice, A.; Rochelle, G. Aqueous ethylenediamine for CO2 capture. ChemSusChem 2010, 3, 913–918. [Google Scholar] [CrossRef]
- Hafizi, A.; Mokari, M.; Khalifeh, R.; Farsi, M.; Raphimpour, M. Improving the CO2 solubility in aqueous mixture of MDEA and different polyamine promoters: The effects of primary and secondary functional groups. J. Mol. Liq. 2020, 297, 111803. [Google Scholar] [CrossRef]
- Kumar, S.; Padhan, R.; Mondal, M. Equilibrium solubility measurement and modeling of CO2 absorption in aqueous blend of 2-(diethyl amino) ethanol and ethylenediamine. J. Chem. Eng. Data 2020, 65, 523–531. [Google Scholar] [CrossRef]
- Nakhjiri, A.; Heydarinasab, A. Computational simulation and theoretical modeling of CO2 separation using EDA, PZEA and PS absorbents inside the hollow fiber membrane contactor. J. Ind. Eng. Chem. 2019, 78, 106–115. [Google Scholar] [CrossRef]
- Ciftja, A.; Hartono, A.; Svendsen, H. Carbamate formation in aqueous—diamine—CO2 systems. Energy Procedia 2013, 37, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.; Svendsen, H. Computational chemistry study of reactions, equilibrium and kinetics of chemical CO2 absorption. Int. J. Greenh. Gas Control 2007, 1, 151–157. [Google Scholar] [CrossRef]
- Thompson, J.; Richburg, H.; Liu, K. Thermal degradation pathways of aqueous diamine CO2 capture solvents. Energy Procedia 2017, 114, 2030–2038. [Google Scholar] [CrossRef]
- Hikita, H.; Asai, S.; Ishikawa, H.; Honda, M. The kinetics of reactions of carbon dioxide with monoisopropanolamine, diglycolamine and ethylenediamine by a rapid mixing method. Chem. Eng. J. 1977, 14, 27–30. [Google Scholar] [CrossRef]
- Jensen, A.; Christensen, R. Studies on carbamates. XI. The carbamate of ethylenediamine. Acta Chem. Scand. 1955, 9, 486–492. [Google Scholar] [CrossRef]
- Gaines, G. The structure of (ammonioethyl)carbamate in solution. J. Org. Chem. 1985, 50, 410–411. [Google Scholar] [CrossRef]
- Lee, B.; Lee, K.; Lim, B.; Cho, J.; Nam, W.; Hur, N. Direct synthesis of imines via solid state reactions of carbamates with aldehydes. Adv. Synth. Catal. 2013, 355, 389–394. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng-Gang, F.; You-Ting, W.; Yuan-Tao, W.; Ai-Min, L.; Zhi-Bing, Z. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem. Eng. J. 2010, 160, 691–697. [Google Scholar] [CrossRef]
- Khan, S.; Hailegiorgis, S.; Man, Z.; Garg, S.; Shariff, A.; Farrukh, S.; Ayoub, M.; Ghaedi, H. High-pressure absorption study of CO2 in aqueous N-methyldiethanolamine (MDEA) and MDEA-piperazine (PZ)-1-butyl-3-methylimidazolium trifluoromethanesulfonate [bmim][OTf] hybrid solvents. J. Mol. Liq. 2018, 249, 1236–1244. [Google Scholar] [CrossRef] [Green Version]
- Kidnay, A.; Parrish, W.; McCartney, G. Fundamentals of Natural Gas Processing, 3rd ed.; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 217–248. ISBN 978-1-138-61279-2. [Google Scholar]
- Abro, M.; Yu, L.; Yu, G.; Chen, X.; Qazi, A. Experimental investigation of hydrodynamic parameters and bubble characteristics in CO2 absorption column using pure ionic liquid and binary mixtures: Effect of porous sparger and operating conditions. Chem. Eng. Sci. 2021, 229, 116041. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y.; Jin, F. Effect of CO2 bubbling into aqueous solutions used for electrochemical reduction of CO2 for energy conversion and storage. J. Phys. Chem. C 2015, 119, 55–61. [Google Scholar] [CrossRef]
- Peng, D.; Robinson, D. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Egorov, G.; Makarov, D.; Kolker, A. Volume properties of liquid mixture of {water (1) + ethylenediamine (2)} over the temperature range from 274.15 to 333.15 K at atmospheric pressure. Thermochim. Acta 2016, 639, 148–159. [Google Scholar] [CrossRef]
- Fogler, S. Elements of Chemical Reaction Engineering, 3rd ed.; Prentice-Hall India: New Delhi, India, 2004; pp. 223–281. ISBN 81-203-2234-7. [Google Scholar]
- Ricaurte, M.; Torré, J.-P.; Asbai, A.; Broseta, D.; Dicharry, C. Experimental data, modeling, and correlation of carbon dioxide solubility in aqueous solutions containing low concentrations of clathrate hydrate promoters: Application to CO2-CH4 gas mixtures. Ind. Eng. Chem. Res. 2012, 51, 3157–3169. [Google Scholar] [CrossRef] [Green Version]
- Farajzadeh, R.; Barati, A.; Delil, H.; Bruining, J.; Zitha, P. Mass transfer of CO2 into water and surfactant solutions. Pet. Sci. Technol. 2007, 25, 1493–1511. [Google Scholar] [CrossRef]
- Diamond, L.; Akinfiev, N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Ph. Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Hemmati, A.; Farahzad, R.; Surendar, A.; Aminahmadi, B. Validation of mass transfer and liquid holdup correlations for CO2 absorption process with methyldiethanolamine solvent and piperazine as an activator. Process Saf. Environ. Prot. 2019, 126, 214–222. [Google Scholar] [CrossRef]
- Bourne, J.; von Stockar, U.; Coggan, G. Gas absorption with heat effects. Experimental results. Ind. Eng. Chem. Process. Des. Dev. 1974, 13, 124–132. [Google Scholar] [CrossRef]
- Green, D.; Perry, R. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2008; pp. 14-1–14-129. ISBN 0-07-159313-6. [Google Scholar]
- Hikita, H.; Ishikawa, H.; Murakami, T.; Ishii, T. Densities, viscosities and amine diffusivities of aqueous MIPA, DIPA, DGA and EDA solutions. J. Chem. Eng. Jpn. 1981, 14, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Lui, Y.; Wang, F. Detailed experimental study on the performance of monoethanolamine, diethanolamine, and diethylenetriamine at absorption/regeneration conditions. J. Clean. Prod. 2016, 125, 296–308. [Google Scholar] [CrossRef]
- Bui, M.; Tait, P.; Lucquiaud, M.; Mac Dowell, N. Dynamic operation and modelling of amine-based CO2 capture at pilot scale. Int. J. Greenh. Gas Control 2018, 79, 134–153. [Google Scholar] [CrossRef]
- Rabensteiner, M.; Kinger, G.; Koller, M.; Gronald, G.; Hochenauer, C. Pilot plant study of ethylenediamine as a solvent for post combustion carbon dioxide capture and comparison to monoethanolamine. Int. J. Greenh. Gas Control 2014, 27, 1–14. [Google Scholar] [CrossRef]
- Stec, M.; Tatarczuk, A.; Więcław-Solny, L.; Krótki, A.; Ściążko, M.; Tokarski, S. Pilot plant results for advanced CO2 capture process using amine scrubbing at the Jaworzno II Power Plant in Poland. Fuel 2015, 151, 50–56. [Google Scholar] [CrossRef]
- Hemmati, A.; Rashidi, H. Mass transfer investigation and operational sensitivity analysis of amine-based industrial CO2 capture plant. Chin. J. Chem. Eng. 2019, 27, 534–543. [Google Scholar] [CrossRef]
- Li, F.; Hemmati, A.; Rashidi, H. Industrial CO2 absorption into methyldiethanolamine/piperazine in place of monoethanolamine in the absorption column. Process Saf. Environ. Prot. 2020, 142, 83–91. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.; Gallego, L.; Portillo, E.; Navarrete, B. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Hu, L.; Liu, N.; Zhou, J.; Cen, K. Regulating crystal structures of EDA-carbamates in solid-liquid phase changing CO2 capture solutions. Fuel 2019, 252, 47–54. [Google Scholar] [CrossRef]
- Momeni, M.; Riahi, S. An investigation into the relationship between molecular structure and rich/lean loading of linear amine-based CO2 absorbents. Int. J. Greenh. Gas Control 2015, 61, 157–164. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Mishra, A.; Kumar, A. Amine tethered pore-expanded MCM-41 for CO2 capture: Experimental, isotherm and kinetic modeling studies. Chem. Eng. J. 2016, 303, 89–99. [Google Scholar] [CrossRef]
- Da Silva, E.; Svendsen, H. Comment on “Reaction kinetics of CO2 in aqueous ethylenediamine, ethyl ethanolamine, and diethyl monoethanolamine solutions in the temperature range of 298-313 K, using the stopped-flow technique”. Ind. Eng. Chem. Res. 2008, 47, 990. [Google Scholar] [CrossRef]
- Wai, S.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic capacity, heat duty, and absorption kinetic modeling of AMP-DETA blend for post-combustion CO2 capture. Sep. Purif. Technol. 2018, 94, 89–95. [Google Scholar] [CrossRef]
- Muchan, P.; Narku-Tetteh, J.; Saiwan, C.; Idem, R.; Supap, T. Effect of number of amine groups in aqueous polyamine solution on carbon dioxide (CO2) capture activities. Sep. Purif. Technol. 2017, 184, 128–134. [Google Scholar] [CrossRef]
- Singh, P. Amine Base Solvent for CO2 Absorption “from Molecular Structure to Process”. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2011. [Google Scholar] [CrossRef] [Green Version]
- Bernhardsen, I.; Knuutila, H. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Gomes, J.; Santos, S.; Bordado, J. Choosing amine-based absorbents for CO2 capture. Environ. Technol. 2015, 36, 19–25. [Google Scholar] [CrossRef] [PubMed]


| Kinetic Data | Thermodynamic Data | |||||||
|---|---|---|---|---|---|---|---|---|
(KPa) | (mmol/min) | (min) | (min) | (min) | (KPa) | CO2 Removed (mmol) | CO2 Loading (*) (mmol CO2/mol H2O) | CO2 Solubility (**) (mmol CO2/mol H2O) |
| 700 | 0.034 | 24.52 | 45.04 | 192.55 | 623 | 3.64 | 2.20 | 2.39 |
| 2100 | 0.189 | 8.87 | 32.43 | 167.90 | 1774 | 12.10 | 7.30 | 7.57 |
| 3500 | 0.654 | 2.20 | 23.31 | 145.30 | 3007 | 21.25 | 12.82 | 12.89 |
| Kinetic Data | Thermodynamic Data | ||||||
|---|---|---|---|---|---|---|---|
| EDA Conc. (wt.%) | (mmol/min) | (min) | (min) | (min) | (KPa) | CO2 Removed (mmol) | CO2 Loading (*) (mmol CO2 /mol Liquid) |
| 0 | 0.654 | 2.20 | 23.31 | 145.30 | 3007 | 21.25 | 12.82 |
| 5 | 0.974 | 4.35 | 24.23 | 155.11 | 2450 | 41.63 | 26.03 |
| 10 | 1.040 | 5.28 | 27.28 | 172.16 | 2195 | 51.81 | 33.66 |
| 20 | 0.859 | 11.55 | 44.56 | 255.91 | 1640 | 69.72 | 49.03 |
| EDA Conc. (wt.%) | Kinetic Data | ||||
|---|---|---|---|---|---|
| AAD (%) | |||||
| 0 | 8.158 | 12.764 | 7.083 | 226.615 | 0.586 |
| 5 | 7.687 | 7.380 | 13.737 | 29.199 | 0.191 |
| 10 | 10.657 | 6.776 | 17.311 | 27.009 | 0.196 |
| 20 | 18.088 | 4.821 | 25.356 | 20.363 | 0.321 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarroel, J.A.; Palma-Cando, A.; Viloria, A.; Ricaurte, M. Kinetic and Thermodynamic Analysis of High-Pressure CO2 Capture Using Ethylenediamine: Experimental Study and Modeling. Energies 2021, 14, 6822. https://doi.org/10.3390/en14206822
Villarroel JA, Palma-Cando A, Viloria A, Ricaurte M. Kinetic and Thermodynamic Analysis of High-Pressure CO2 Capture Using Ethylenediamine: Experimental Study and Modeling. Energies. 2021; 14(20):6822. https://doi.org/10.3390/en14206822
Chicago/Turabian StyleVillarroel, Josselyne A., Alex Palma-Cando, Alfredo Viloria, and Marvin Ricaurte. 2021. "Kinetic and Thermodynamic Analysis of High-Pressure CO2 Capture Using Ethylenediamine: Experimental Study and Modeling" Energies 14, no. 20: 6822. https://doi.org/10.3390/en14206822
APA StyleVillarroel, J. A., Palma-Cando, A., Viloria, A., & Ricaurte, M. (2021). Kinetic and Thermodynamic Analysis of High-Pressure CO2 Capture Using Ethylenediamine: Experimental Study and Modeling. Energies, 14(20), 6822. https://doi.org/10.3390/en14206822






