Abstract
Considering the pressing challenges of supply security and climate change, advanced processes to produce electricity and biofuels from biomass have to be developed. Biomass gasification is a very promising technology, but there is a lack of comprehensive reviews, specifically on the technologies for hydrogen chloride hot gas cleanup, which are necessary in order to work at the same temperature and respect the limits of advanced downstream components. In this review, the Cl content of the main biomasses in Europe is given, and data on syngas content and the tolerance of downstream equipment are highlighted. Hot gas cleaning technologies, which have the advantage of improved thermal efficiency are reviewed, analyzing the thermodynamic and primary and secondary methods. This review identifies NaAlO2 and Na2CO3 within 450–550 °C as the most effective sorbents, which are able to reduce the concentration of HCl below 1 ppm. Nevertheless, H2S cannot be simultaneously removed and has to be removed first, because it reduces the HCl adsorption sorbent capacity.
1. Introduction
In order to accomplish the requirement for national energy independence and the reduction of fossil fuel consumption, which bring a consequent mitigation of climate change and greenhouse gas emissions, the application of renewable resources for chemical and energy substitution is nowadays very important. Among the technologies based on renewable resources, biomass-based plants have huge commercial potential, owing to the great biomass availability, the possibility of reaching negative greenhouse gas emissions, and having greater impacts on and cross-fertilization with other sectors; however, efficient and reliable low emission technologies still have to be developed [1,2,3,4,5,6,7,8]. Biomass gasification is one of the most practicable applications within biomass-based power plants, allowing achieving a high-rate production of fuel gas, with small investment costs [9,10,11,12,13,14]. Among the reactors that can be used in biomass gasification processes, fluidized-bed (FB) reactors seem to be the most efficient, since they guarantee high reaction rates and conversion efficiencies, due to very good mixing and gas–solid contact [5,15,16,17,18,19,20]. The resulting fuel gas, which is named syngas, is a mixture of a combustible gases, consisting of hydrogen, methane, carbon monoxide, carbon dioxide, and steam, together with some unwanted by-products; the quantity of which is related to the feedstock, process conditions, and gasifier design [21,22,23,24,25,26]. The syngas can be directly burnt in order to produce heat, only, or it can be used in energy conversion systems to produce electricity and heat or, moreover, it can be used to produce biofuels and biochemicals [27]. Before being used in these kind of applications, raw syngas from the gasifier needs to be cleaned since the organic contaminants (tar) and inorganic contaminants (alkali, sulfur compounds, mainly H2S, and halides, mainly HCl) contained in the syngas are a big risk factor for the lifetime of the plant equipment [28]. Chlorine compounds may cause corrosion of metal equipment, health problems, and environmental issues [29,30,31,32,33,34]. In the gas phase, HCl may react to form other contaminants (e.g., ammonium chloride (NH4Cl) and sodium chloride (NaCl)). These contaminants cause heavy deposition and fouling on the downstream processes [35]. Moreover, chlorides deactivate the catalysts used for chemical synthesis. In order to prevent this kind of damage, the content of HCl that has been demonstrated to not be dangerous for the most syngas applications (such as, gas turbines, fuel cells, ammonia production, methanol synthesis) is less than 1 ppm [32,36,37,38,39,40]. In the literature, there have been few comprehensive reviews on gas cleaning technologies in recent years. Woolcock and Brown produced a full and complete review about hot, warm, and cold technologies for particulate, tar, sulfur, ammonia, and chlorine compounds and alkali removal [41]. In addition, other authors have recapitulated the most common applications of hot gas cleaning for the removal of tar [42,43,44,45], hydrogen sulfide [46,47], and for both hydrogen sulfide and hydrogen chloride [48]. However, there is a lack of a comprehensive review, specifically on technologies for hot gas cleanup for hydrogen chloride, and many researchers did not emphasize removing HCl [49,50,51]. The present review is focused on the investigation of the most recent and effective ways to reduce the hydrogen chloride contained in biomass-derived syngas under 1 ppm by means of hot gas cleaning. The article is organized as follow: the average level of hydrogen chloride in syngas is discussed in Section 2, the hot gas cleaning techniques for HCl removal are in Section 3, the possibility of the simultaneous removal of HCl and H2S is discussed in Section 4, and the risk of exposure to hydrogen chloride is discussed in Section 5.
2. Average Level of Hydrogen Chloride in Biomass-Syngas
Biomass contains a low concentration of halides, especially chloride, and the amount of HCl in the gas produced depends on the initial concentration of chloride in the feedstock. Table 1 reports some of the most abundant biomasses and their HCl concentration.

Table 1.
Chloride content for the most common biomass feedstocks.
A few studies report precise quantities of HCl in syngas. Barisano et al. [58] showed experimentally that the amount of Cl from almond shell biomass that converts into HCl, in a FB gasifier at 850 °C with an equivalent ratio (ER) in the range 0.25–0.28 and steam to biomass (S/B) in the range 0.4–0.5, is 80% of the total. Depending on the biomass feedstock and on the gasification conditions, the produced gas may contain from 40 ppm of HCl to 900 ppm [59]. As previously explained, HCl at such a concentration level corrodes the downstream equipment, for this reason it is necessary to remove it.
Since an FB gasifier is most commonly used, as explained in the Introduction of the present paper, Figure 1 shows the calculated HCl levels obtained by the bubbling fluidized-bed (BFB) steam gasifier in Güssing [60], which worked at 850 °C and 1 bar at an S/B ratio equal to 0.6, with five representative biomasses. Pine side shells, miscanthus, olive prunings, and wood chips are representatives of the category of branches and leaves from forest biomass. Wheat straw and almond Shells are representatives of agricultural biomass. The other biomasses quoted in Table 1 are not reported in Figure 1, because black liquor does not appear to be very promising as a possible feedstock for biomass gasification in a FB reactor, due to its low melting temperature compared to typically used in this type of gasifier. Moreover, from the data about proximate and elemental analysis, sub-coal, municipal solid waste, and digestate appear as difficult feedstocks for application in gasification, due to the high amount of ash produced and high Cl and S contents [61,62]. For the other remaining feedstocks, all of which are woody and herbaceous biomass types, no special issues are foreseen with regard to their use as feedstocks in a process of gasification in a FB reactor.
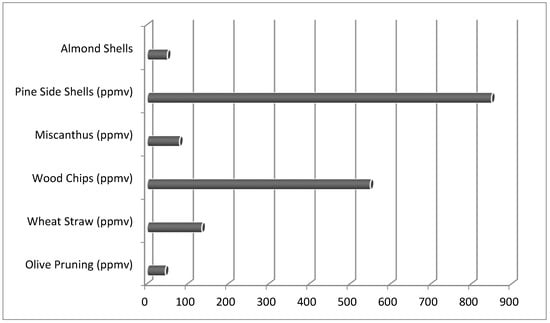
Figure 1.
Predicted HCl level in the “Güssing” gasifier [63].
3. Gas Cleaning Technologies
Gas cleaning can be performed as a low-temperature process (e.g., amine scrubber) or as a mid- to high-temperature cleaning process (e.g., sorbent reactor) [46,64].
Low-temperature clean-up, performed by means of chemical absorbers in the liquid state at low temperature (under 50 °C) in a counter-current with syngas, which is over 800 °C (at its current gasification temperature), has many disadvantages, such as the reduction of the efficiency of the power plant due to the consumption of water and the production of polluted water, which must then be purified [65,66]. For this reason, low-temperature gas cleaning approaches are discouraged.
Mid- (400–600 °C) to high-temperature (600–850 °C) syngas cleaning technologies, which include sorbent technologies, are more suitable for syngas applications, since syngas has a high temperature, and carrying out the process at mid- to high-temperature allows achieving an optimum cycle efficiency and protects downstream equipment and catalysts [67,68].
For making the choice of which sorbent is better to deploy, the following characteristics have to be taken into account [39,69,70,71,72,73]:
- The porosity of the material;
- The ability to work in the presence of high concentrations of H2, CO, CO2, and H2O in the temperature range of 650–950 °C;
- The resistance to deactivation that could be provoked by carbon fouling, sintering, and poisoning (especially sulfur poisoning);
- The ability to regenerate easily;
- The price, which has to be as low as possible;
- Fast adsorption kinetics (kinetic of dechlorination has to be a first-order dependence in HCl);
- High equilibrium constant;
- The resistance to attrition.
3.1. Thermodynamics of HCl Removal
Alkali (Na, K) and alkaline earth (Ca) carbonates are among the most investigated sorbents for HCl removal [40,42]. General sorption reactions (1) and (2) are given below. Since alkaline earth carbonates such as CaCO3 may decompose under the process conditions, depending on the temperature and CO2 partial pressure, reaction (3) of the respective oxide with HCl also needs to be considered.
(Na,K)2CO3 + 2HCl → 2(Na,K)Cl + H2O + CO2
CaCO3 + 2HCl → CaCl2 + H2O + CO2
CaO + 2HCl → CaCl2 + H2O
Figure 2 shows the standard free energy of the formation of the alkali and alkaline earth chlorides, which gives a first indication of the relative suitability of the various sorbent materials. Calculations were performed with FactSageTM version 7.3 (GTT-Technologies, Herzogenrath, Germany), which has be proven to be suitable for the simulation of biomass syngases, including trace gases [74,75,76]. From a thermodynamic point of few, alkali carbonates should achieve much lower HCl concentrations in syngas than alkaline earth carbonates, with potassium performing better than sodium. Furthermore, calcium oxide should perform better than calcium carbonate at lower temperatures, which means that in cases where calcium-based sorbents are used, the CO2 concentration in syngas should be sufficiently low to stabilize the CaO, if possible. A major drawback of alkali carbonates is the relatively high volatility of the formed alkali chlorides, which are an order of magnitude higher than CaCl2, as shown in Figure 3. If the limit for alkali chlorides is set to 1 ppm, sodium and potassium carbonate should be used below 592 °C and 565 °C, respectively, whereas calcium based sorbents can theoretically be used up to 848 °C.
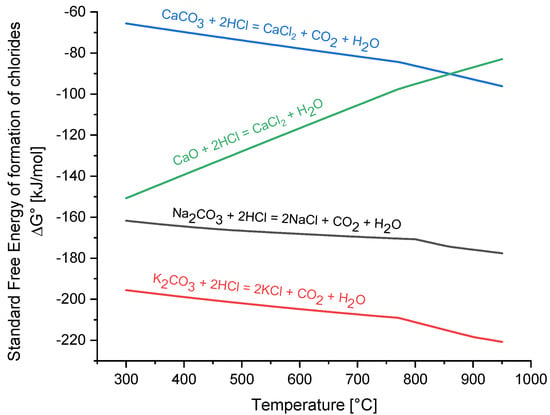
Figure 2.
Standard free energy of the formation of alkali and alkaline earth chlorides.
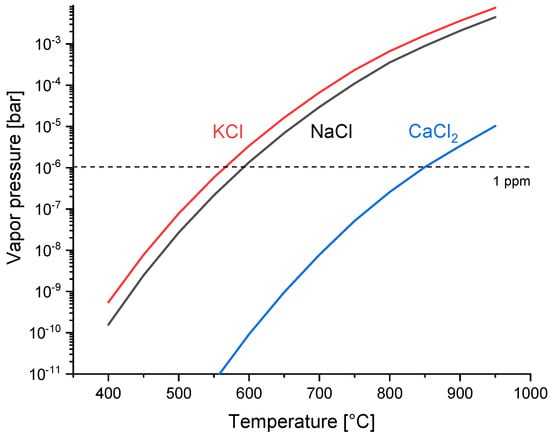
Figure 3.
Vapor pressure of alkali and alkaline earth chlorides. The dashed line marks the concentration of 1 ppmv at 1 bar total pressure.
According to reactions (1)–(3), the achievable HCl level in syngas depends on the syngas composition, i.e., the concentration of H2O and CO2, which in turn depends on the type and ratio of gasification agent. In order to show the magnitude of this influence, unique parameters, such as the H2/C ratio and relative oxygen content of the whole system (ROC) [77] are used in Figure 4 to describe the syngas composition and influence on the HCl concentration achievable with Na2CO3 as a sorbent material. Nevertheless, Nunokawa et al. did not find any effects of steam concentrations of up to 28 vol% on HCl reduction in their investigations [78].
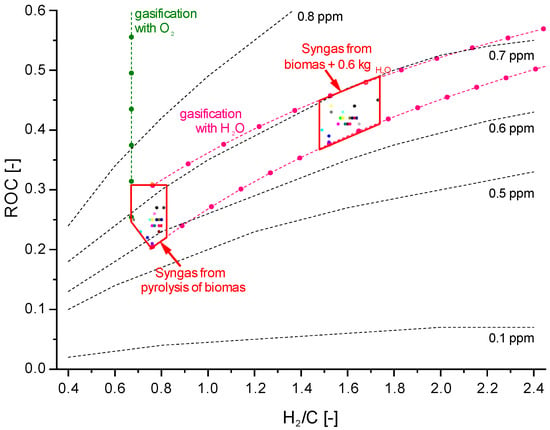
Figure 4.
Achievable HCl concentrations after absorption by Na2CO3 at 500 °C, depending on the syngas composition.
It should be noted, that the very low concentration of HCl in syngas predicted for some biomass feedstocks in Figure 1 is not only caused by their low chlorine content, but also by their high content of available alkalis, forming respective alkali chlorides according to reaction (1). If these alkali chlorides are adsorbed by aluminosilicates, as part of a comprehensive hot gas cleaning concept, the HCl concentration in the syngas will increase correspondingly [62].
3.2. High Temperature Primary Methods for HCl Removal
All the cleaning treatments that happen within a gasifier are called primary methods, and even if they are mostly known for tar removal, they are also effective for an initial reduction of HCl.
The most common catalyst used for contaminant removal is natural dolomite CaMg(CO3)2, which is very cheap [79,80,81]. Pinto et al. [82] found that in the presence of dolomite the highest gas yield and a higher heating value (HHV) of syngas were reached, concluding that dolomite is a highly attractive catalyst. Roche et al. [83] conducted an experiment in a BFB gasifier of dried sludge at 800 °C and at a S/B ratio 1 and demonstrated that the effectiveness of dolomite increases when steam is used as a gasifying agent. In fact, the H2/CO ratio increased from 1.1 to 2.6 when a dolomite and air-steam combination was used instead of dolomite and pure air. Several studies from laboratories and pilot plants [72,84,85,86,87,88,89] have reported calcined dolomite CaO-MgO as the best solution, where both the pore-volume and pore-diameter enhanced the mass transfer.
Mura and Lallai [90] investigated the reaction that happens between calcium oxide and hydrogen chloride, determining that the activation energy of the reaction is 45 kJ mol−1.
Weinell and Jensen [91] analyzed the HCl reaction with lime and limestone at 60–1000 °C. They showed that lime and limestone are able to capture the majority of HCl at 500–600 °C. Then, over 500 °C, the adsorption capacity is reduced by the chemical equilibrium between gas and solid.
Lin et al. [92] investigated the removal of dry HCl using calcined limestone, in a fixed-bed reactor, taking into account the effect of the presence of SO2, CO, and CO2 on the chlorination reactivity of calcined limestone. The study demonstrated that the adsorption of HCl is less affected under various gas atmospheres at 650 °C and the chlorination is faster when CO2 is present. In the temperature range of 750–850 °C, the presence of SO2 or O2 affects the reactivity of calcined limestone toward HCl, causing a reduction of it. In fact, the concurrent sulfidation of chlorides determines the subsequent re-release of HCl to the gas phase; therefore, lowering the achievable level of the chlorination reaction. Instead, the presence of O2 obstructs the conversion of calcined limestone to chlorides, probably through some type of reaction involving dechlorination of the sorbent particles.
Partanen et al. [93] investigated the adsorption of HCl by means of limestone in a fluidized bed reactor. The study revealed that the adsorption of HCl at 850 °C is strongly related to the content of humidity in the syngas; in fact, the presence of steam reduces the achievable conversion at a certain reaction time. The adsorption was higher at temperatures around 650 °C. At 850 °C, the effect of the particle size on the conversion of CaO most likely interfered with the formation of a molten product phase.
Experiments by Gullett et al. [94], Li et al. [95], Petrini et al. [96], and Wang et al. [97], conducted in fixed-bed gasifier, in order to facilitate a rate controlling step, indicated that the limestone and CaO adsorption of HCl, when N2 is used as the reacting gas, is higher at temperature lower than 600 °C. They also showed that the reaction rate and the conversion were influenced by the properties of the sorbent material; smaller particles ensure a better conversion rate, and the reactivity of the sorbent is directly proportional to the particle porosity and the specific surface area.
Weinell et al. [91] investigated the adsorption of HCl using lime and limestone at temperatures up to 1000 °C; they demonstrated that the binding capacity is best at 500–600 °C. They noticed that, at temperatures above 750 °C, a liquid phase of CaCl2 saturated with CaO was produced, but the influence of this liquid phase on the kinetics is not responsible for the reduced adsorption capacity of CaO at higher temperature; the cause is the chemical equilibrium between the gas and solid. Therefore, only a low adsorption is possible at the common gasification temperatures (700–900 °C). This is the reason why high-temperature secondary methods for HCl removal are needed; therefore, the research of recent years has focused on gas cleaning downstream of the gasifier, in order to reach the tolerance limit under 1 ppm.
3.3. High Temperature Secondary Methods for HCl Removal
Na2CO3, K2CO3, and NaAlO2 are among the most efficacious sorbents for HCl removal [38,98].
Krishnan et al. [99] investigated the adsorption capacity of nahcolite (NaHCO3), shortite (Na2CO3 2CaCO3) and dawsonite (NaAl(OH)2CO3) in a fixed bed reactor in the temperature range of 400–600 °C, fixing the space velocity at 3000 h−1. Nacholite over 150 °C became porous Na2CO3, and reacts quickly with HCl to be converted into NaCl. Reactions (4) and (5) resulted, in addition to reaction (1).
NaHCO3(s) + HCl(g) → NaCl(s) + H2O(g) + CO2(g)
2NaHCO3(s) → Na2CO3(s) + H2O(g) + CO2(g)
Nunokawa et al. [100] studied the capacity of sorbent containing NaAlO2 to adsorb HCl. The experiment was conducted at 550 °C, with a space velocity of 3000 h−1 and 1000 mg/m3 as the initial concentration of HCl. The raw materials of the sorbent were sodium carbonate (Na2CO3), alumina-sol (Al2O3-sol), and alumina powder(-Al2O3). The results indicated that the sorbent is able to reduce the level of HCl under 1 ppm.
Verdone et al. [101] performed an experiment in order to investigate the behavior of Na2CO3 sorbent. The feeding ensured a constant rate of HCl during the run time. A mixture of hydrogen chloride and steam in a nitrogen flow were the reactor feed. This mixture was prepared by injecting a calibrated amount of an aqueous solution of HCl 0.1N into a measured nitrogen flow. Using nitrogen rather than air allowed avoiding the oxidation of HCl to Cl2, according to the Deacon reaction. The mixture, before entering the reactor, was vaporized and heated up to a temperature of 200 °C. The temperature of the reactor was investigated in a range from 200 to 600 °C, with steps of 100 °C. Concentrations of 3000, 6000, and 9000 ppm of HCl were considered, in a constant nitrogen flow (1.10 L(SPT)/ min). The sorbent particle size was between 120 and 209 µm. The molar ratio HCl/Na2CO3 was fixed at 0.5. The best results were those obtained at temperature of 400 and 500 °C. In fact, in this temperature window, all the HCl that entered the reactor was captured and removed when it reached the carbonate layer, and the saturation was higher than 90%. The second carbonate layer was reached by HCl after the complete saturation of the first layer and so on. The results changed drastically outside the “windows” of 400–500 °C. Lower than 400 °C, the conversion of each layer was very small and almost the same throughout all the layers. When the temperature was 300 °C, the conversion of the carbonate layer was around 40% and reduced to 20% at 200 °C. In fact, at such low temperatures, the solid carbonate could not remove all the HCl. These kinds of results can be explained by noticing that under 400 °C, even if the thermodynamics of the reaction between sodium carbonate and HCl are favorable, the overall kinetics of the reaction are too slow to guarantee a considerable reduction of hydrogen chloride. Instead, the explanation for the behavior of the layer at 600 °C and at 300 °C, which is almost constant, can be attributed to sintering phenomena, which lower the particle porosity and then the surface area where the gas–solid reactions happen. Since the melting point temperature of sodium carbonate is 851 °C, there are not negative consequences correlated to sintering, which occurred under 426 °C. This paper indicated that the sintering phenomena provoke a moderate effect at temperatures higher than 400 °C and it are evident over 600 °C. The investigations conducted at 500 °C did not directly show the presence of sintering phenomena, but by interpretation of the results by means of a mathematical model, it is possible to infer indirect information about its occurrence. These analyses [102] indicated that the content of water vapor in the gas affected by a definite amount the equilibrium concentration of the gaseous HCl over an NaCl/Na2CO3 solid system. Furthermore, the hydrogen chloride equilibrium concentration between 20 and 600 °C is very low, and the effect of the presence of water or changes in its concentration in the gas phase can be neglected [103,104].
Fellows and Pilat [105] investigated the influence of the particle size of NaHCO3 on adsorption capacity and pointed out that the adsorption capacity does not change with an increase of particle diameter and the increase of temperature. Their experiments were carried out at 135 °C and at 190 °C; at the lower temperature NaHCO3 was decomposes, while, reacting with HCl and at the higher temperature, the NaHCO3 was almost totally decomposed, but this factor seemed not to influence the effect of particle diameter on the adsorption capacity.
Dou et al. [106,107] investigated experimentally the performance of a sorbent mix of Na- Ca- and Mg- based compounds in a fixed-bed reactor at 550 °C, with a space velocity of 2000 h−1. The inlet concentration of HCl was 640 ppm. The reaction rate remained constant for almost 20 h before decreasing gradually. The chemical reaction between HCl and Na-,Ca-, and Mg- based sorbent was evaluated to become a first-order reaction.
Weinlaender et al. [108] demonstrated that an increase of HCl concentration in the inlet stream allows achieving a 15% higher sorbent uptake; achieved because of the increased concentration gradient, which is a driving force of adsorptive separation. Moreover, the test run time was reduced by a factor of 10 when the inlet concentration of HCl was increased by a factor 10.
Liu et al. [109] synthesized an Na-Mg-Al layered double hydroxide based sorbent, indicated here as LDH or cLDH in its calcined form, keeping Mg:Na:Al ratios of 3.1:3.5:1 and 3.1:2.9:1 for the uncalcined and calcined forms, respectively. The synthesized sorbent was then benchmarked against a commercially available Mg-Al LDH, indicated here as ComLDH or cComLDH in its calcined form. The aim was to investigate the capacity of hydrogen chloride from 400 to 600 °C in a fixed-bed reactor. In Figure 5, the thermogravimetric (TGA) profiles show the thermal stability of the sorbents. Calcined Na2CO3 showed the highest stability, with a maximum mass loss of 0.11 wt%, followed by calcined NaAlO2 and Na-Mg-Al-LDH, with less than 1.84 and 3.33 wt% mass loss, respectively. The calcined commercial LDH (cComLDH) had the lowest thermal stability, with less than 9.85 wt% mass loss.
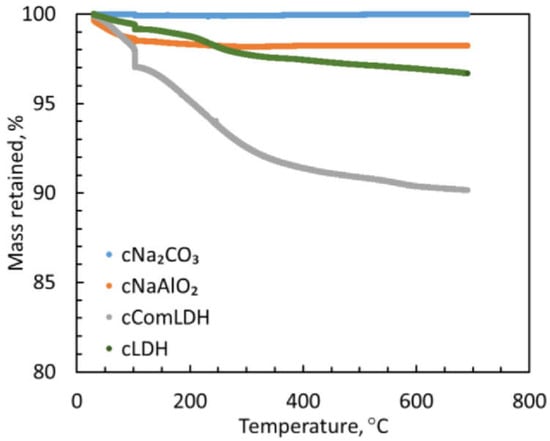
Figure 5.
TGA of the calcined sorbents as a function of temperature [109].
The study also evaluated the concentration of HCl in the effluent for each sorbent at 400, 500, and 600 °C (see Figure 6). Figure 5 shows that at the output of cComLDH there was the highest concentration of HCl, and this sorbent was the one with the shortest breakthrough time, this means it is ineffective for HCl removal. The most effective in the study was cLDH, which had a breakthrough time greater than 14 h for all the temperatures considered.
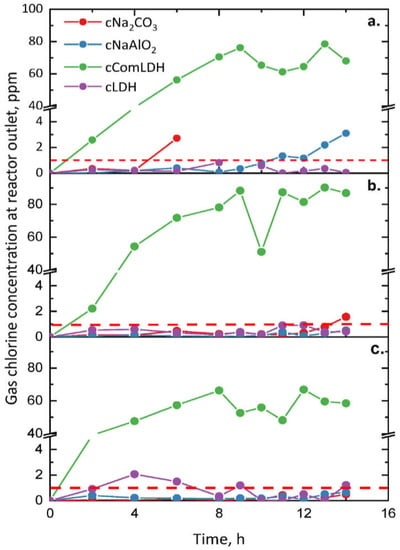
Figure 6.
Concentration of HCl in the effluent at 400 (a), 500 (b), and 600 °C (c) for each sorbent. (The horizontal red dashed line represents the 1 ppm breakthrough threshold) [109].
In the study, they also evaluated the breakthrough time of the sorbents at the three different temperatures considered, see Table 2.

Table 2.
Summary of the 1 ppm HCl breakthrough times of sorbents.
Ohtsuka et al. [58] analyzed the capacity of NaAlO2 to adsorb HCl in a fixed-bed reactor and showed that the concentration of HCl passed from 200 ppm to below 1 ppm at 400 °C. They also observed that the adsorption capacity of NaAlO2 was slightly higher than Na2CO3, and both reduced their adsorption capacity when H2S was present.
Baek et al. [38] analyzed the performance of unprocessed and processed potassium-based CO2 sorbents for removing HCl by means of a micro fluidized-bed reactor and by means of a bench-scale bubbling fluidized-bed reactor, which worked, respectively, at 300 and 540 °C and 20 bar. They showed that the concentrations of HCl decreased from 150–900 ppmv to 5 ppmv and from 130–390 ppmv to 1 ppmv.
Stemmler et al. [110] investigated HCl removal using an alkali (Na and K) biomass ash from DDGS, in comparison with pure sodium and potassium carbonate. According to thermodynamic equilibrium calculations, both alkali carbonates should be able to reduce the HCl concentration in flue gas from a Güssing gasifier to <1 ppm at temperatures <550 °C, with potassium carbonate achieving even lower concentrations than sodium carbonate. The experimental investigations showed that the limit of 1 ppmv HCl was achieved at a space velocity of 4900 h−1 for the Na2CO3 sorbent, 3750 h−1 for the K2CO3, and 7350 h−1 for the biomass ash. The much better performance of the latter was mainly attributed to a high specific surface area and porosity, resulting from the release of volatiles during gasification of the biomass.
4. Simultaneous Removal of Hydrogen Chloride and Hydrogen Sulfide
The possibility of removing hydrogen sulfide and hydrogen chloride at the same time is very attractive, because it would allow reducing gas cleaning costs (unifying two steps in a single reactor) and simplify the process [111,112]. Therefore, some researchers investigated this possibility.
Gupta et al. [31] studied the simultaneous removal of HCl and H2S using zinc-ferrite and zinc-titanate based sorbents, and they highlighted that HCl has a deleterious short-term effect on the desulfurization of these kinds of sorbents.
Nunokawa et al. [100,113,114] investigated the influence of H2S on NaAlO2 sorbent during the adsorption of HCl at 400 °C and showed that the capacity for adsorption of HCl reduced drastically when H2S was added. The time needed for the breakthrough of 1 ppm HCl passed from 1100 min without any H2S, to 930 min when 2000 ppm of H2S was added, and the Na conversion decreased to 30%, as shown in Figure 7.
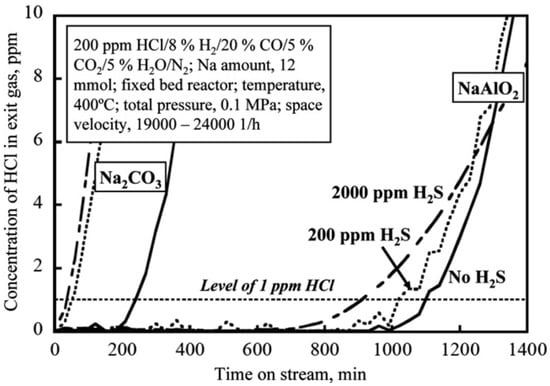
Figure 7.
Breakthrough profiles for HCl sorption with Na2CO3 and NaAlO2 at 400 °C in a simulated fuel gas, without and with H2S added [113].
Figure 7 shows that H2S causes a more severe deactivation of the Na2CO3 sorbent rather than the NaAlO2 sorbent.
Ohtsuka et al. [58] showed that it is possible to minimize the sulfur influence on Na2CO3 by the addition of Al2O3, in order to form NaAlO2. In this way, the sorbent could tolerate 0.2 mL l−1 of sulfur and reduced 0.2 mL l−1 of HCl under 1 µL l−1 at 400 °C. However, this strategy is not always effective [115].
Therefore, even if a multifunctional reactor, able to remove H2S and HCl at the same time is preferable, this choice seems to be not practical, since the contaminants may interfere in the adsorption of each other (e.g., HCl inhibits the adsorption of H2S in many metal oxides).
5. Conclusions
In the actual context of sustainable energy, the application of biomass waste for combined heat and power production has acquired great interest. However, it is not possible to directly feed downstream applications (such as gas turbines, SOFC, etc.) with the gas produced by the gasifier. In fact, the syngas contains contaminants that could severely affect the equipment. The scope of this review was to investigate the removal of hydrogen chloride, an inorganic contaminant with a corrosive nature, which is present in syngas. The tolerance limit, for most syngas applications, established by the experimental literature is <1 ppmv for HCl. The literature research indicated that hot gas cleaning is the preferred method, ensuring the advantage of improved thermal efficiency. Calcium oxide sorbents demonstrated effectiveness for binding HCl, but they decomposed over 500 °C, resulting in a reduction of adsorption capacity and, eventually, in the release of the adsorbed HCl. NaAlO2 and Na2CO3 have been demonstrated as very good sorbents for HCl removal, showing adsorption capacities of 40 and 10%, respectively, at 400 °C. However, their adsorption rate decreased when H2S was present. This is the reason why the adsorption of HCl and the adsorption of H2S have to be considered as two separated steps, in two different reactors.
Author Contributions
Conceptualization, V.M. and E.B.; methodology V.M. and E.B.; experimental data curation, M.M.; writing—original draft preparation, V.M.; writing—review and editing, V.M., E.B. and M.M.; supervision, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are reported in the paper.
Acknowledgments
This project belongs to the European Union’s Horizon 2020 research and innovation program, under grant agreement No. 101006656 GICO Project.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Balat, M. Political, Economic and Environmental Impacts of Biomass-Based Hydrogen. Int. J. Hydrog. Energy 2009, 34, 3589–3603. [Google Scholar] [CrossRef]
- La Villetta, M.; Costa, M.; Cirillo, D.; Massarotti, N.; Vanoli, L. Performance Analysis of a Biomass Powered Micro-Cogeneration System Based on Gasi Fi Cation and Syngas Conversion in a Reciprocating Engine. Energy Convers. Manag. 2018, 175, 33–48. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Turrion-Gomez, J.L. Second-Generation Biofuels and Local Bioenergy Systems. Biofuels Bioprod. Biorefin. 2008, 2, 455–469. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of Agricultural Residues in a Demonstrative Plant: Vine Pruning and Rice Husks. Bioresour. Technol. 2015, 194, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Martis, R.; Al-Othman, A.; Tawalbeh, M.; Alkasrawi, M. Energy and Economic Analysis of Date Palm Biomass Feedstock for Biofuel Production in UAE: Pyrolysis, Gasification and Fermentation. Energies 2020, 13, 5877. [Google Scholar] [CrossRef]
- Brunerová, A.; Roubík, H.; Brožek, M.; Haryanto, A.; Hasanudin, U.; Iryani, D.; Herák, D. Valorization of Bio-Briquette Fuel by Using Spent Coffee Ground as an External Additive. Energies 2019, 13, 54. [Google Scholar] [CrossRef]
- Colantoni, A.; Villarini, M.; Marcantonio, V.; Gallucci, F.; Cecchini, M. Performance Analysis of a Small-Scale ORC Trigeneration System Powered by the Combustion of Olive Pomace. Energies 2019, 12, 2273. [Google Scholar] [CrossRef]
- Villarini, M.; Marcantonio, V.; Colantoni, A.; Bocci, E. Sensitivity Analysis of Different Parameters on the Performance of a CHP Internal Combustion Engine System Fed by a Biomass Waste Gasifier. Energies 2019, 12, 688. [Google Scholar] [CrossRef]
- Li, X.T.; Grace, J.R.; Lim, C.J.; Watkinson, A.P.; Chen, H.P.; Kim, J.R. Biomass Gasification in a Circulating Fluidized Bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Jand, N.; Foscolo, P.U. Decomposition of Wood Particles in Fluidized Beds. Ind. Eng. Chem. Res. 2005, 44, 5079–5089. [Google Scholar] [CrossRef]
- Begum, S.; Rasul, M.G.; Akbar, D.; Ramzan, N. Performance Analysis of an Integrated Fixed Bed Gasifier Model for Different Biomass Feedstocks. Energies 2013, 6, 6508–6524. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Labaki, M. Energy Applications of Coffee Processing By-Products. In Handbook of Coffee Processing By-Products: Sustainable Applications; Elsevier Inc.: Galanakis Laboratories, Chania, Greece, 2017; pp. 323–367. ISBN 9780128112915. [Google Scholar]
- Andrea, M.F.; Sara, R.H.; Luca, D.Z.; Giovanni, S.S.; Enrico, B. Techno-Economic Analysis of in-Situ Production by Electrolysis, Biomass Gasification and Delivery Systems for Hydrogen Refuelling Stations: Rome Case Study. Energy Procedia 2018, 148, 82–89. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and Analysis of Biomass Gasification Models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Rapagnà, S.; Jand, N.; Kiennemann, A.; Foscolo, P.U. Steam-Gasification of Biomass in a Fluidised-Bed of Olivine Particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- An, H.; Song, T.; Shen, L.; Qin, C.; Yin, J.; Feng, B. Coal Gasification with in Situ CO2 Capture by the Synthetic CaO Sorbent in a 1 KWth Dual Fluidised-Bed Reactor. Int. J. Hydrog. Energy 2012, 37, 14195–14204. [Google Scholar] [CrossRef]
- Sadaka, S.S.; Ghaly, A.E.; Sabbah, M.A. Two-Phase Biomass Air-Steam Gasification Model for Fluidized Bed Reactors: Part III—Model Validation. Biomass Bioenergy 2002, 22, 479–487. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The Study of Reactions Influencing the Biomass Steam Gasification Process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Cuellar, A.D.; Herzog, H. A Path Forward for Low Carbon Power from Biomass. Energies 2015, 8, 1701–1715. [Google Scholar] [CrossRef]
- Dascomb, J.; Krothapalli, A.; Fakhrai, R. Thermal Conversion Efficiency of Producing Hydrogen Enriched Syngas from Biomass Steam Gasification. Int. J. Hydrog. Energy 2013, 38, 11790–11798. [Google Scholar] [CrossRef]
- Násner, A.M.L.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Restrepo, J.C.; Venturini, O.J.; Ratner, A. Refuse Derived Fuel (RDF) Production and Gasification in a Pilot Plant Integrated with an Otto Cycle ICE through Aspen PlusTM Modelling: Thermodynamic and Economic Viability. Waste Manag. 2017, 69, 187–201. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Marcantonio, V.; Monforti Ferrario, A.; Di Carlo, A.; Del Zotto, L.; Monarca, D.; Bocci, E. Biomass Steam Gasification: A Comparison of Syngas Composition between a 1-D MATLAB Kinetic Model and a 0-D Aspen Plus Quasi-Equilibrium Model. Computation 2020, 8, 86. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Monarca, D. Development of a Chemical Quasi-Equilibrium Model of Biomass Waste Gasification in a Fluidized-Bed Reactor by Using Aspen Plus. Energies 2019, 13, 53. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Analysis of Organic and Inorganic Contaminants in Dried Sewage Sludge and By-Products of Dried Sewage Sludge Gasification. Energies 2014, 7, 462–476. [Google Scholar] [CrossRef]
- Marseglia, G.; Medaglia, C.M.; Petrozzi, A.; Nicolini, A.; Cotana, F.; Sormani, F. Experimental Tests and Modeling on a Combined Heat and Power Biomass Plant. Energies 2019, 12, 2615. [Google Scholar] [CrossRef]
- Marcantonio, V.; Monarca, D.; Villarini, M.; Di Carlo, A.; Del Zotto, L.; Bocci, E. Cleaning, and SOFC Model: A Parametric Analysis. Energies 2020, 13, 5936. [Google Scholar] [CrossRef]
- Marcantonio, V.; Monarca, D.; Colantoni, A.; Cecchini, M. Ultrasonic Waves for Materials Evaluation in Fatigue, Thermal and Corrosion Damage: A Review. Mech. Syst. Signal Process. 2019, 120, 32–42. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Han, L.; Chang, L.; Bao, W. Simultaneous Removal of Hydrogen Sulfide and Mercury from Simulated Syngas by Iron-Based Sorbents. Fuel 2013, 103, 73–79. [Google Scholar] [CrossRef]
- Gupta, R.P.; O’Brien, W.S. Desulfurization of Hot Syngas Containing Hydrogen Chloride Vapors Using Zinc Titanate Sorbents. Ind. Eng. Chem. Res. 2000, 39, 610–619. [Google Scholar] [CrossRef]
- Xu, C.C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent Advances in Catalysts for Hot-Gas Removal of Tar and NH3 from Biomass Gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594. [Google Scholar] [CrossRef]
- Sadegh-Vaziri, R.; Amovic, M.; Ljunggren, R.; Engvall, K. A Medium-Scale 50 MWfuel Biomass Gasification Based Bio-SNG Plant: A Developed Gas Cleaning Process. Energies 2015, 8, 5287–5302. [Google Scholar] [CrossRef]
- Kuramoto, K.; Hosokai, S.; Matsuoka, K.; Ishiyama, T.; Kishimoto, H.; Yamaji, K. Degradation Behaviors of SOFC Due to Chemical Interaction between Ni-YSZ Anode and Trace Gaseous Impurities in Coal Syngas. Fuel Process. Technol. 2017, 160, 8–18. [Google Scholar] [CrossRef]
- Ephraim, A.; Ngo, L.D.; Pham Minh, D.; Lebonnois, D.; Peregrina, C.; Sharrock, P.; Nzihou, A. Valorization of Waste-Derived Inorganic Sorbents for the Removal of HCl in Syngas. Waste Biomass Valorization 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Cheah, S.; Parent, Y.O.; Jablonski, W.S.; Vinzant, T.; Olstad, J.L. Manganese and Ceria Sorbents for High Temperature Sulfur Removal from Biomass-Derived Syngas—The Impact of Steam on Capacity and Sorption Mode. Fuel 2012, 97, 612–620. [Google Scholar] [CrossRef]
- Baek, J.I.; Eom, T.H.; Lee, J.B.; Jegarl, S.; Ryu, C.K.; Park, Y.C.; Jo, S.H. Cleaning of Gaseous Hydrogen Chloride in a Syngas by Spray-Dried Potassium-Based Solid Sorbents. Korean J. Chem. Eng. 2015, 32, 845–851. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A Review on Biomass Gasification Syngas Cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Cayan, F.N.; Zhi, M.; Pakalapati, S.R.; Celik, I.; Wu, N.; Gemmen, R. Effects of Coal Syngas Impurities on Anodes of Solid Oxide Fuel Cells. J. Power Sources 2008, 185, 595–602. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A Review of Cleaning Technologies for Biomass-Derived Syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Torres, W.; Pansare, S.S.; Goodwin, J.G. Hot Gas Removal of Tars, Ammonia, and Hydrogen Sulfide from Biomass Gasification Gas. Catal. Rev. Sci. Eng. 2007, 49, 407–456. [Google Scholar] [CrossRef]
- Dayton, D. Review of the Literature on Catalytic Biomass Tar Destruction: Milestone Completion Report; National Renewable Energy Lab.: Golden, CO, USA, 1 December 2002; p. 28. [Google Scholar]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Review of Catalysts for Tar Elimination in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Gas cleaning for IC engine applications from fixed bed biomass gasification. Biomass Bioenergy 1999, 16, 385–395. [Google Scholar] [CrossRef]
- Cheah, S.; Carpenter, D.L.; Magrini-Bair, K.A. Review of Mid- to High-Temperature Sulfur Sorbents for Desulfurization of Biomass- and Coal-Derived Syngas. Energy Fuels 2009, 23, 5291–5307. [Google Scholar] [CrossRef]
- Husmann, M.; Hochenauer, C.; Meng, X.; De Jong, W.; Kienberger, T. Evaluation of Sorbents for High Temperature in Situ Desulfurization of Biomass-Derived Syngas. Energy Fuels 2014, 28, 2523–2534. [Google Scholar] [CrossRef]
- Dou, B.; Wang, C.; Chen, H.; Song, Y.; Xie, B.; Xu, Y.; Tan, C. Research Progress of Hot Gas Filtration, Desulphurization and HCl Removal in Coal-Derived Fuel Gas: A Review. Chem. Eng. Res. Des. 2012, 90, 1901–1917. [Google Scholar] [CrossRef]
- Kim, Y.D.; Yang, C.W.; Kim, B.J.; Kim, K.S.; Lee, J.W.; Moon, J.H.; Yang, W.; Yu, T.U.; Lee, U. Do Air-Blown Gasification of Woody Biomass in a Bubbling Fluidized Bed Gasifier. Appl. Energy 2013, 112, 414–420. [Google Scholar] [CrossRef]
- Kern, S.; Pfeifer, C.; Hofbauer, H. Gasification of Wood in a Dual Fluidized Bed Gasifier: Influence of Fuel Feeding on Process Performance. Chem. Eng. Sci. 2013, 90, 284–298. [Google Scholar] [CrossRef]
- Karmakar, M.K.; Mandal, J.; Haldar, S.; Chatterjee, P.K. Investigation of Fuel Gas Generation in a Pilot Scale Fluidized Bed Autothermal Gasifier Using Rice Husk. Fuel 2013, 111, 584–591. [Google Scholar] [CrossRef]
- Bocci, E.; Sisinni, M.; Moneti, M.; Vecchione, L.; Di Carlo, A.; Villarini, M. State of Art of Small Scale Biomass Gasification Power Systems: A Review of the Different Typologies. Energy Procedia 2014, 45, 247–256. [Google Scholar] [CrossRef]
- Paniagua, S.; García-Pérez, A.I.; Calvo, L.F. Biofuel Consisting of Wheat Straw–Poplar Wood Blends: Thermogravimetric Studies and Combustion Characteristic Indexes Estimation. Biomass Convers. Biorefin. 2019, 9, 433–443. [Google Scholar] [CrossRef]
- Del Zotto, L.; Bocci, E.; Fontana, F.; Martini, M.; Meola, S.; Amoruso, C. DELIVERABLE D2.1 Biomass Selection and Characterization for Small-to-Medium Scale Gasification-SOFC CHP Plants; 2019; Available online: https://www.blazeproject.eu/wp-content/uploads/2020/04/D2.1_Biomass_20_12_19_for-Website.pdf (accessed on 2 September 2021).
- Albina, D.O.; Millrath, K.; Themelis, N.J. Effects of Feed Composition on Boiler Corrosion in Waste-to-Energy Plants. In Proceedings of the 12th Annual North American Waste-to-Energy Conference, Savannah, GA, USA, 17–19 May 2004; pp. 99–109. [Google Scholar]
- Bajpai, P. Advances in Bioethanol; Springer: New Delhi, India, 2013; Volume 44, ISBN 978-81-322-1583-7. [Google Scholar] [CrossRef]
- Miranda, T.; Esteban, A.; Rojas, S.; Montero, I.; Ruiz, A. Combustion Analysis of Different Olive Residues. Int. J. Mol. Sci. 2008, 9, 512–525. [Google Scholar] [CrossRef]
- Barisano, D.; Canneto, G.; Nanna, F.; Villone, A.; Fanelli, E.; Freda, C.; Grieco, M.; Cornacchia, G.; Braccio, G.; Marcantonio, V.; et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Performance—Part I. Processes 2021, 9, 1104. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Tsubouchi, N.; Kikuchi, T.; Hashimoto, H. Recent Progress in Japan on Hot Gas Cleanup of Hydrogen Chloride, Hydrogen Sulfide and Ammonia in Coal-Derived Fuel Gas. Powder Technol. 2009, 190, 340–347. [Google Scholar] [CrossRef]
- Hermann, H.; Reinhard, R.; Klaus, B.; Koch, R.B.; Kraftwerk, G.G.M.B.H. Biomass CHP Plant Güssing-A Success Story. In Proceedings of the Expert Meeting on Pyrolysis and Gasification of Biomass and Waste, Strasbourg, France, 30 September–1 October 2002; Volume 30. [Google Scholar]
- Karmakar, M.K.; Chandra, P.; Chatterjee, P.K. A Review on the Fuel Gas Cleaning Technologies in Gasification Process. J. Environ. Chem. Eng. 2015, 3, 689–702. [Google Scholar] [CrossRef]
- Gullett, B.K.; Bruce, K.R.; Beach, L.O. Formation of Chlorinated Organics during Solid Waste Combustion. Waste Manag. Res. 1990, 8, 203–214. [Google Scholar] [CrossRef]
- Stemmler, M.; Tamburro, A.; Müller, M. Thermodynamic Modelling of Fate and Removal of Alkali Species and Sour Gases from Biomass Gasification for Production of Biofuels. Biomass Convers. Biorefin. 2013, 3, 187–198. [Google Scholar] [CrossRef]
- Sharma, S.D.; Dolan, M.; Park, D.; Morpeth, L.; Ilyushechkin, A.; McLennan, K.; Harris, D.J.; Thambimuthu, K.V. A Critical Review of Syngas Cleaning Technologies-Fundamental Limitations and Practical Problems. Powder Technol. 2008, 180, 115–121. [Google Scholar] [CrossRef]
- Epikhin, A.N.; Krylov, I.O.; Somov, A.A.; Suchkov, S.I.; Timashkov, K.V.; Strokov, A.A.; Ligovskaya, I.G. Prospects for the Use of Natural Ferromanganese Sorbents of Hydrogen Sulfide for High-Temperature Cleaning of Syngas. Power Technol. Eng. 2012, 46, 143–148. [Google Scholar] [CrossRef]
- Ptasinski, K.J. Thermodynamic Efficiency of Biomass Gasification and Biofuels Conversion. Biofuels Bioprod. Biorefin. 2008, 2, 239–253. [Google Scholar] [CrossRef]
- Ngoc Lan Thao, N.T.; Chiang, K.-Y.; Wan, H.-P.; Hung, W.-C.; Liu, C.-F. Nhanced Trace Pollutants Removal Efficiency and Hydrogen Production in Rice Straw Gasification Using Hot Gas Cleaning System. Int. J. Hydrog. Energy 2019, 44, 3363–3372. [Google Scholar] [CrossRef]
- Aravind, P.V.; De Jong, W. Review Evaluation of High Temperature Gas Cleaning Options for Biomass Gasification Product Gas for Solid Oxide Fuel Cells. Prog. Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar Reduction in Biomass Producer Gas via Mechanical, Catalytic and Thermal Methods: A Review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Pinto, F.; Lopes, H.; André, R.N.; Gulyurtlu, I.; Cabrita, I. Effect of Catalysts in the Quality of Syngas and By-Products Obtained by Co-Gasification of Coal and Wastes. 1. Tars and Nitrogen Compounds Abatement. Fuel 2007, 86, 2052–2063. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass Gasification Gas Cleaning for Downstream Applications: A Comparative Critical Review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Richardson, Y.; Blin, J.; Julbe, A. A Short Overview on Purification and Conditioning of Syngas Produced by Biomass Gasification: Catalytic Strategies, Process Intensification and New Concepts. Prog. Energy Combust. Sci. 2012, 38, 765–781. [Google Scholar] [CrossRef]
- Buentello-Montoya, D.; Zhang, X.; Li, J.; Ranade, V.; Marques, S.; Geron, M. Performance of Biochar as a Catalyst for Tar Steam Reforming: Effect of the Porous Structure. Appl. Energy 2020, 259, 114176. [Google Scholar] [CrossRef]
- Sharma, S.D.; Dolan, M.; Ilyushechkin, A.Y.; McLennan, K.G.; Nguyen, T.; Chase, D. Recent developments in dry hot syngas cleaning processes. Fuel 2010, 89, 817–826. [Google Scholar] [CrossRef]
- Turn, S.Q.; Kinoshita, C.M.; Ishimura, D.M.; Zhou, J. The Fate of Inorganic Constituents of Biomass in Fluidized Bed Gasification. Fuel 1998, 77, 135–146. [Google Scholar] [CrossRef]
- Kuramochi, H.; Wu, W.; Kawamoto, K. Prediction of the Behaviors of H2S and HCl during Gasification of Selected Residual Biomass Fuels by Equilibrium Calculation. Fuel 2005, 84, 377–387. [Google Scholar] [CrossRef]
- Stemmler, M.; Müller, M. Theoretical Evaluation of Feedstock Gasification Using H2/C Ratio and ROC as Main Input Variables. Ind. Eng. Chem. Res. 2010, 49, 9230–9237. [Google Scholar] [CrossRef]
- Nunokawa, M.; Kobayashi, M.; Akiho, H. Development of Halide Removal Sorbent for Hot Gas Cleaning Technology. J. Soc. Powder Technol. Jpn. 2009, 46, 442–447. [Google Scholar] [CrossRef][Green Version]
- Orío, A.; Corella, J.; Narváez, I. Performance of Different Dolomites on Hot Raw Gas Cleaning from Biomass Gasification with Air. Ind. Eng. Chem. Res. 1997, 36, 3800–3808. [Google Scholar] [CrossRef]
- Delgado, J.; Aznar, M.P.; Corella, J. Biomass Gasification with Steam in Fluidized Bed: Effectiveness of CaO, MgO, and CaO-MgO for Hot Raw Gas Cleaning. Ind. Eng. Chem. Res. 1997, 36, 1535–1543. [Google Scholar] [CrossRef]
- Pallozzi, V.; Di Carlo, A.; Bocci, E.; Carlini, M. Combined Gas Conditioning and Cleaning for Reduction of Tars in Biomass Gasification. Biomass Bioenergy 2018, 109, 85–90. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Dohrup, J.; Sørensen, H.R.; Girio, F. Effects of Experimental Conditions and of Addition of Natural Minerals on Syngas Production from Lignin by Oxy-Gasification: Comparison of Bench- and Pilot Scale Gasification. Fuel 2015, 140, 62–72. [Google Scholar] [CrossRef]
- Roche, E.; De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and Air-Steam Gasification of Sewage Sludge. The Influence of Dolomite and Throughput in Tar Production and Composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- Simell, P.A.; Leppälahti, J.K.; Bredenberg, J.S. Catalytic Purification of Tarry Fuel Gas with Carbonate Rocks and Ferrous Materials. Fuel 1992, 71, 211–218. [Google Scholar] [CrossRef]
- Taralas, G.; Vassilatos, V.; Sjöström, K.; Delgado, J. Thermal and Catalytic Cracking of n -Heptane in Presence of CaO, MgO and Calcined Dolomites. Can. J. Chem. Eng. 1991, 69, 1413–1419. [Google Scholar] [CrossRef]
- Taralas, G. Catalytic Steam Cracking of N-Heptane with Special Reference to the Effect of Calcined Dolomite. Ind. Eng. Chem. Res. 1996, 35, 2121–2126. [Google Scholar] [CrossRef]
- Orío, A.; Corella, J.; Narváez, I. Characterization and Activity of Different Dolomites for Hot Gas Cleaning in Biomass Gasification. In Developments in Thermochemical Biomass Conversion; Bridgwater, A.V., Boocock, D.G.B., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 1144–1157. [Google Scholar]
- Gil, J.; Caballero, M.A.; Martín, J.A.; Aznar, M.P.; Corella, J. Biomass Gasification with Air in a Fluidized Bed: Effect of the in-Bed Use of Dolomite under Different Operation Conditions. Ind. Eng. Chem. Res. 1999, 38, 4226–4235. [Google Scholar] [CrossRef]
- Narváez, I.; Orío, A.; Aznar, M.P.; Corella, J. Biomass Gasification with Air in an Atmospheric Bubbling Fluidized Bed. Effect of Six Operational Variables on the Quality of the Produced Raw Gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Mura, G.; Lallai, A. On the Kinetics of Dry Reaction between Calcium Oxide and Gas Hydrochloric Acid. Chem. Eng. Sci. 1992, 47, 2407–2411. [Google Scholar] [CrossRef]
- Weinell, C.E.; Jensen, P.I.; Dam-Johansen, K.; Livbjerg, H. Hydrogen Chloride Reaction with Lime and Limestone: Kinetics and Sorption Capacity. Ind. Eng. Chem. Res. 1992, 31, 164–171. [Google Scholar] [CrossRef]
- Lin, G.M.; Chyang, C.S. Removal of HCl in Flue Gases by Calcined Limestone at High Temperatures. Energy Fuels 2017, 31, 12417–12424. [Google Scholar] [CrossRef]
- Partanen, J.; Backman, P.; Backman, R.; Hupa, M. Absorption of HCl by Limestone in Hot Flue Gases. Part II: Importance of Calcium Hydroxychloride. Fuel 2005, 84, 1674–1684. [Google Scholar] [CrossRef]
- Gullett, B.K.; Jozewicz, W.; Stefanski, L.A. Reaction Kinetics of Ca-Based Sorbents with HCl. Ind. Eng. Chem. Res. 1992, 31, 2437–2446. [Google Scholar] [CrossRef]
- Li, M.; Shaw, H.; Yang, C.L. Reaction Kinetics of Hydrogen Chloride with Calcium Oxide by Fourier Transform Infrared Spectroscopy. Ind. Eng. Chem. Res. 2000, 39, 1898–1902. [Google Scholar] [CrossRef]
- Petrini, S.; Eklund, H.; Bjerle, I. HCl Absorption with Limestone. Aufbereitungstechnik 1979, 6, 309–315. [Google Scholar]
- Wang, W.; Ye, Z.; Bjerle, I. The Kinetics of the Reaction of Hydrogen Chloride with Fresh and Spent Ca-Based Desulfurization Sorbents. Fuel 1996, 75, 207–212. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of Sorbents for High Temperature Removal of Tars, Hydrogen Sulphide, Hydrogen Chloride and Ammonia from Biomass-Derived Syngas by Using Aspen Plus. Int. J. Hydrog. Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Krishnan, G.; Gupta, R. Development of Disposable Sorbents for Chloride Removal from High Temperature Coal-Derived Gases; US DOE Report, AC21-93MC30005-02; US DOE: Washington, DC, USA, 1999; p. 122.
- Nunokawa, M.; Kobayashi, M.; Shirai, H. Halide Compound Removal from Hot Coal-Derived Gas with Reusable Sodium-Based Sorbent. Powder Technol. 2008, 180, 216–221. [Google Scholar] [CrossRef]
- Verdone, N.; De Filippis, P. Reaction Kinetics of Hydrogen Chloride with Sodium Carbonate. Chem. Eng. Sci. 2006, 61, 7487–7496. [Google Scholar] [CrossRef]
- Verdone, N.; De Filippis, P. Thermodynamic Behaviour of Sodium and Calcium Based Sorbents in the Emission Control of Waste Incinerators. Chemosphere 2004, 54, 975–985. [Google Scholar] [CrossRef]
- Mocek, K.; Lippert, E.; Erdös, E. Reactivity of the Solid Sodium Carbonate towards the Gaseous Hydrogen Chloride and the Sulphur Dioxide. Collect. Czechoslov. Chem. Commun. 1983, 48, 3500–3507. [Google Scholar] [CrossRef]
- Duo, W.; Kirkby, N.F.; Seville, J.P.K.; Kiel, J.H.A.; Bos, A.; Den Uil, H. Kinetics of HCl Reactions with Calcium and Sodium Sorbents for IGCC Fuel Gas Cleaning. Chem. Eng. Sci. 1996, 51, 2541–2546. [Google Scholar] [CrossRef]
- Fellows, K.T.; Pilat, M.J. Hci Sorption by Dry Nahc03 for Incinerator Emissions Control. J. Air Waste Manag. Assoc. 1990, 40, 887–893. [Google Scholar] [CrossRef]
- Dou, B.L.; Gao, J.S.; Sha, X.Z. A Study on the Reaction Kinetics of HCl Removal from High-Temperature Coal Gas. Fuel Process. Technol. 2001, 72, 23–33. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Wu, J.; Liu, Y. A review of sorbents for high-temperature hydrogen sulfide removal from hot coal gas. Environ. Chem. Lett. 2019, 17, 259–276. [Google Scholar] [CrossRef]
- Weinlaender, C.; Neubauer, R.; Hauth, M.; Hochenauer, C. Adsorptive Hydrogen Chloride and Combined Hydrogen Chloride–Hydrogen Sulphide Removal from Biogas for Solid Oxide Fuel Cell Application. Adsorpt. Sci. Technol. 2018, 36, 1215–1232. [Google Scholar] [CrossRef]
- Liu, Q.; Ehite, E.; Houston, R.; Li, Y.; Pope, C.; Labbé, N.; Abdoulmoumine, N. Materials Science for Energy Technologies Synthesis and Evaluation of Layered Double Hydroxide Based Sorbent for Hot Gas Cleanup of Hydrogen Chloride. Mater. Sci. Energy Technol. 2021, 4, 46–53. [Google Scholar] [CrossRef]
- Stemmler, M.; Tamburro, A.; Müller, M. Chemical Hot Gas Cleaning of Syngas from Biomass Gasification for Production of Biofuels. In Proceedings of the ICPS10, International Conference on Polygeneration Strategies, Leipzig, Germany, 7–9 September 2010. [Google Scholar]
- Sharma, S.D.; McLennan, K.; Dolan, M.; Nguyen, T.; Chase, D. Design and Performance Evaluation of Dry Cleaning Process for Syngas. Fuel 2013, 108, 42–53. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Han, Y.; Sun, Y.; Fang, Y.; Zhao, J.; Qin, Z. Coal to Liquid Fuels by Gasification and the Associated Hot Gas Cleanup Challenges. Chin. J. Catal. 2009, 30, 770–775. [Google Scholar] [CrossRef]
- Nunokawa, M.; Kobayashi, M.; Shirai, H. Summaries 5 (Systems and Technology). In Proceedings of the 15th International Congress of Chemical and Process Engineering, Praha, Czech, 25–29 August 2002; pp. 1–13. [Google Scholar]
- Kobayashi, M.; Shirai, H.; Nunokawa, M. Estimation of Multiple-Cycle Desulfurization Performance for Extremely Low-Concentration Sulfur Removal with Sorbent Containing Zinc Ferrite-Silicon Dioxide Composite Powder. Energy Fuels 2002, 16, 1378–1386. [Google Scholar] [CrossRef]
- Dou, B.; Chen, B.; Gao, J.; Sha, X. Reaction of Solid Sorbents with Hydrogen Chloride Gas at High Temperature in a Fixed-Bed Reactor. Energy Fuels 2005, 19, 2229–2234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).