Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland)
Abstract
1. Introduction
2. Geological Setting
3. Methods
4. Results and Discussion
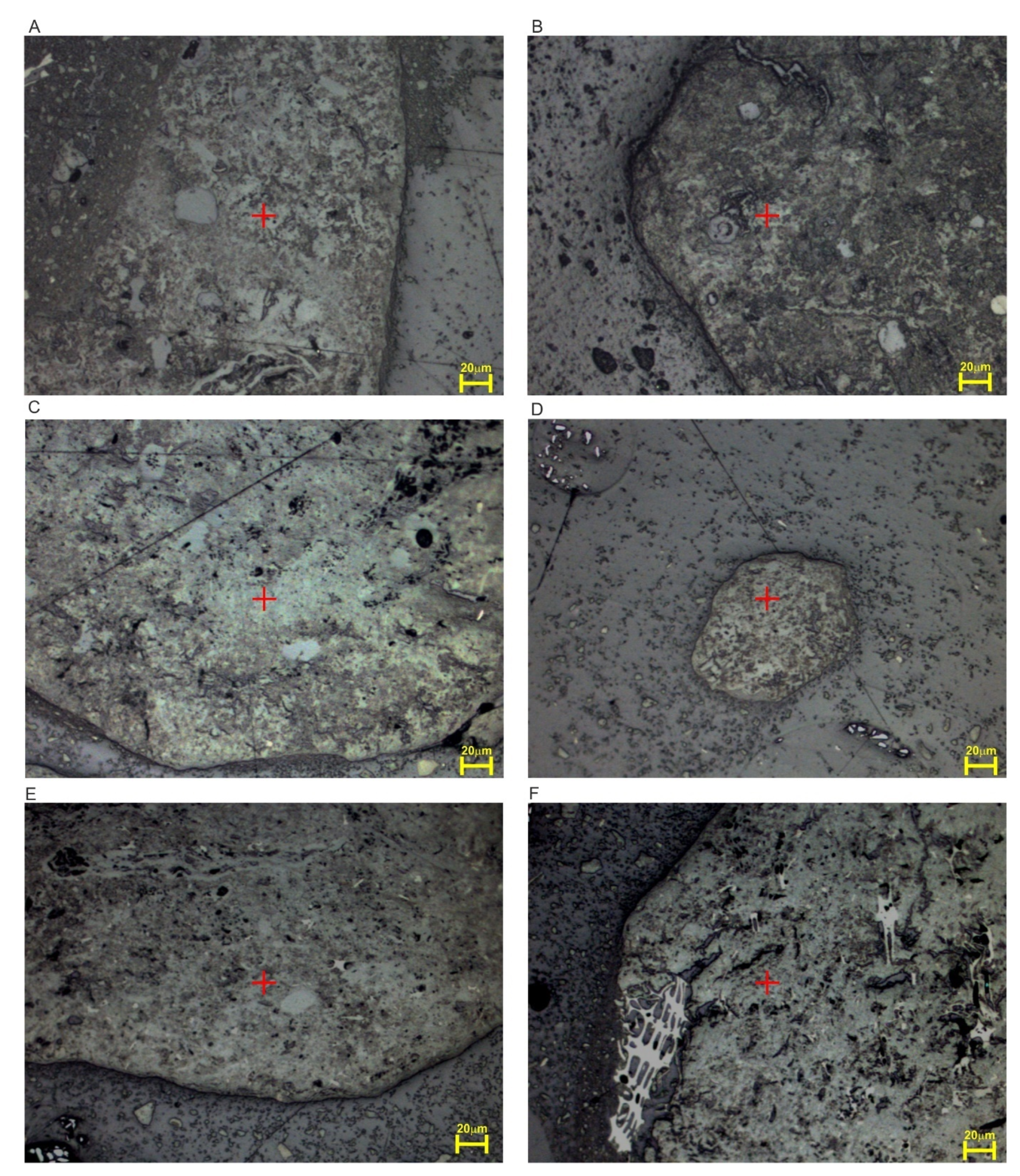
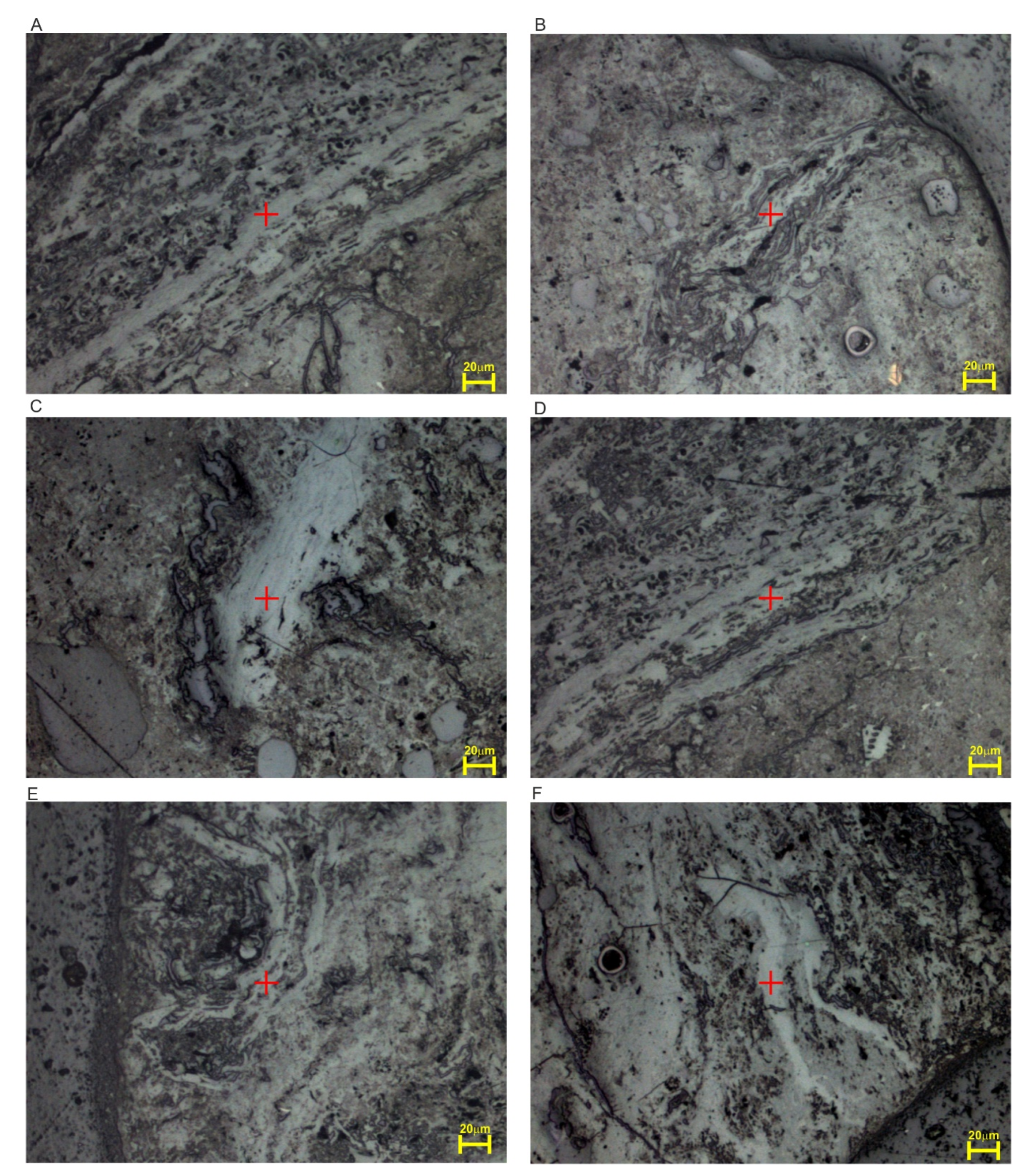
4.1. Petrographic Composition
4.2. Random Reflectance and Proximate and Ultimate Parameters of Lignite from the Szczerców Deposit
4.3. Micro-Raman Spectroscopy
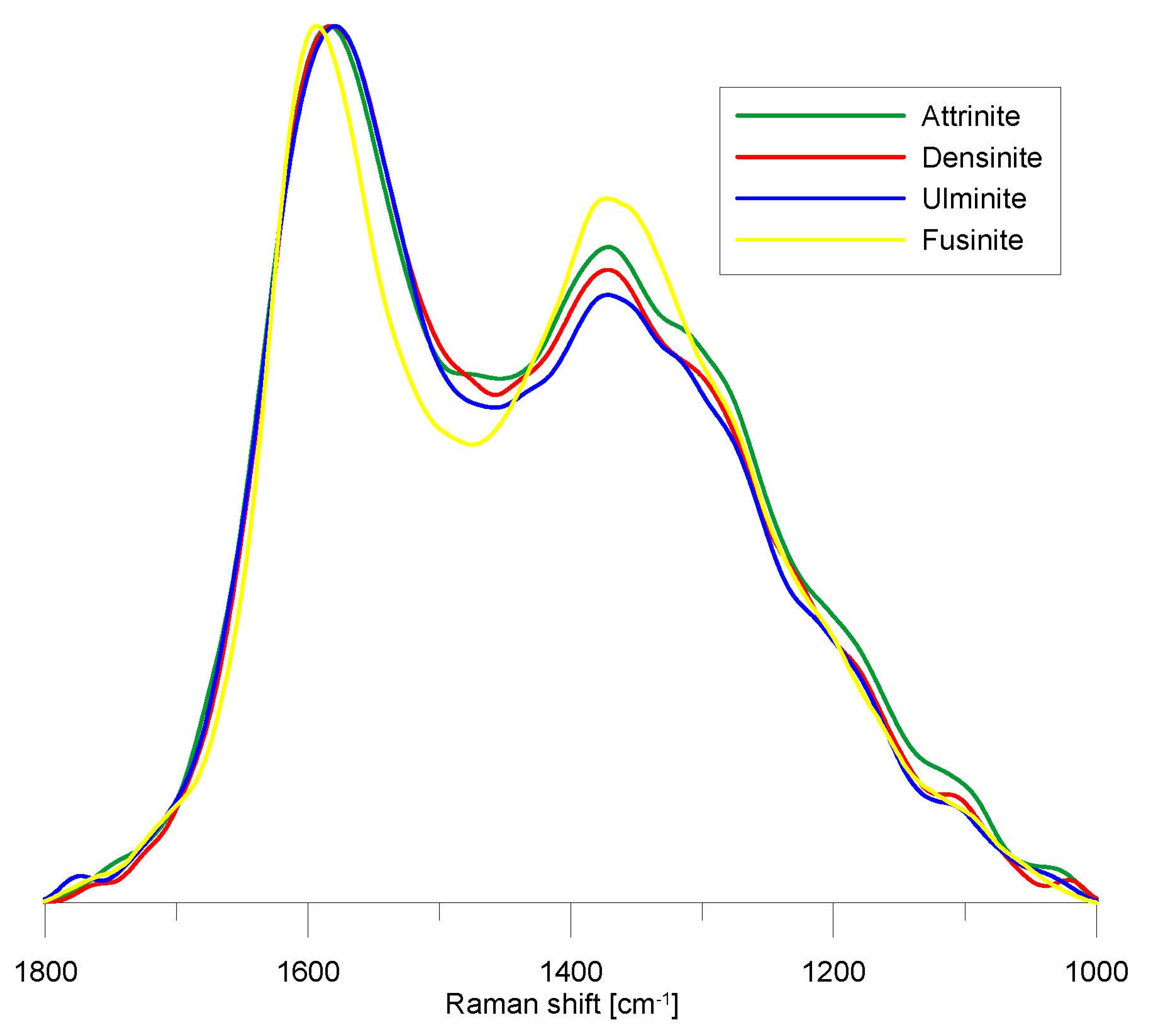
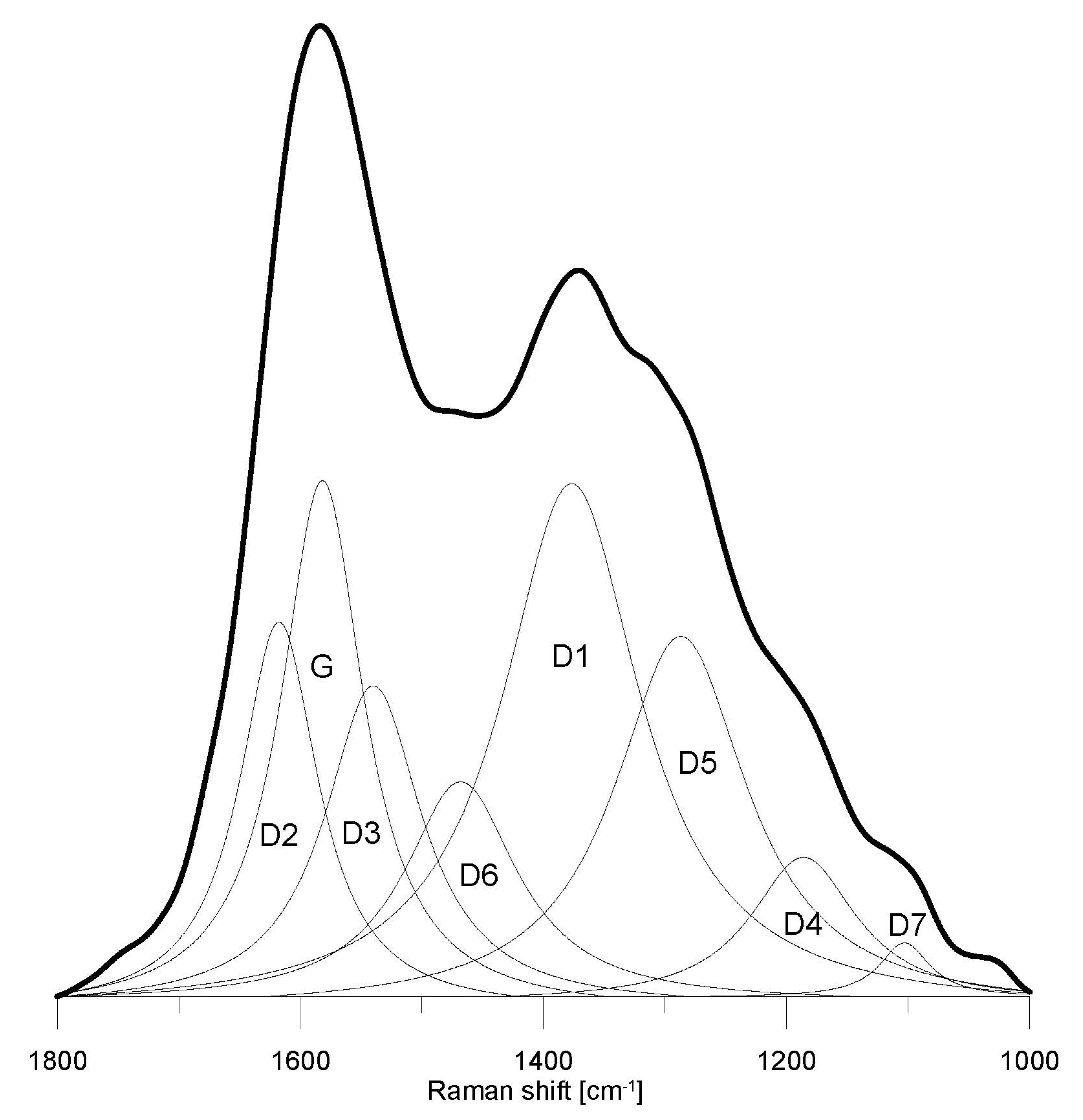
4.3.1. General Characteristics of the Raman Spectra
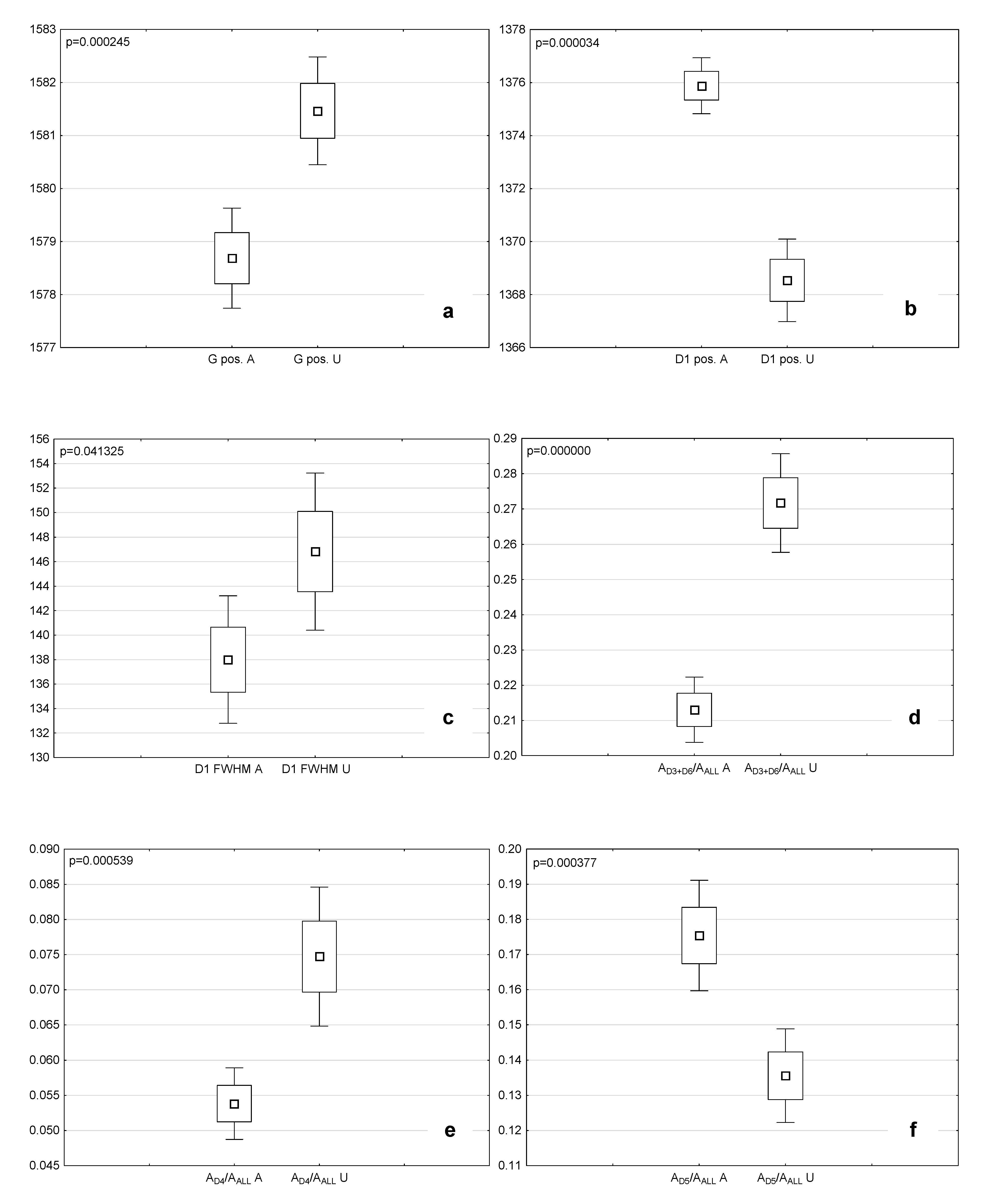
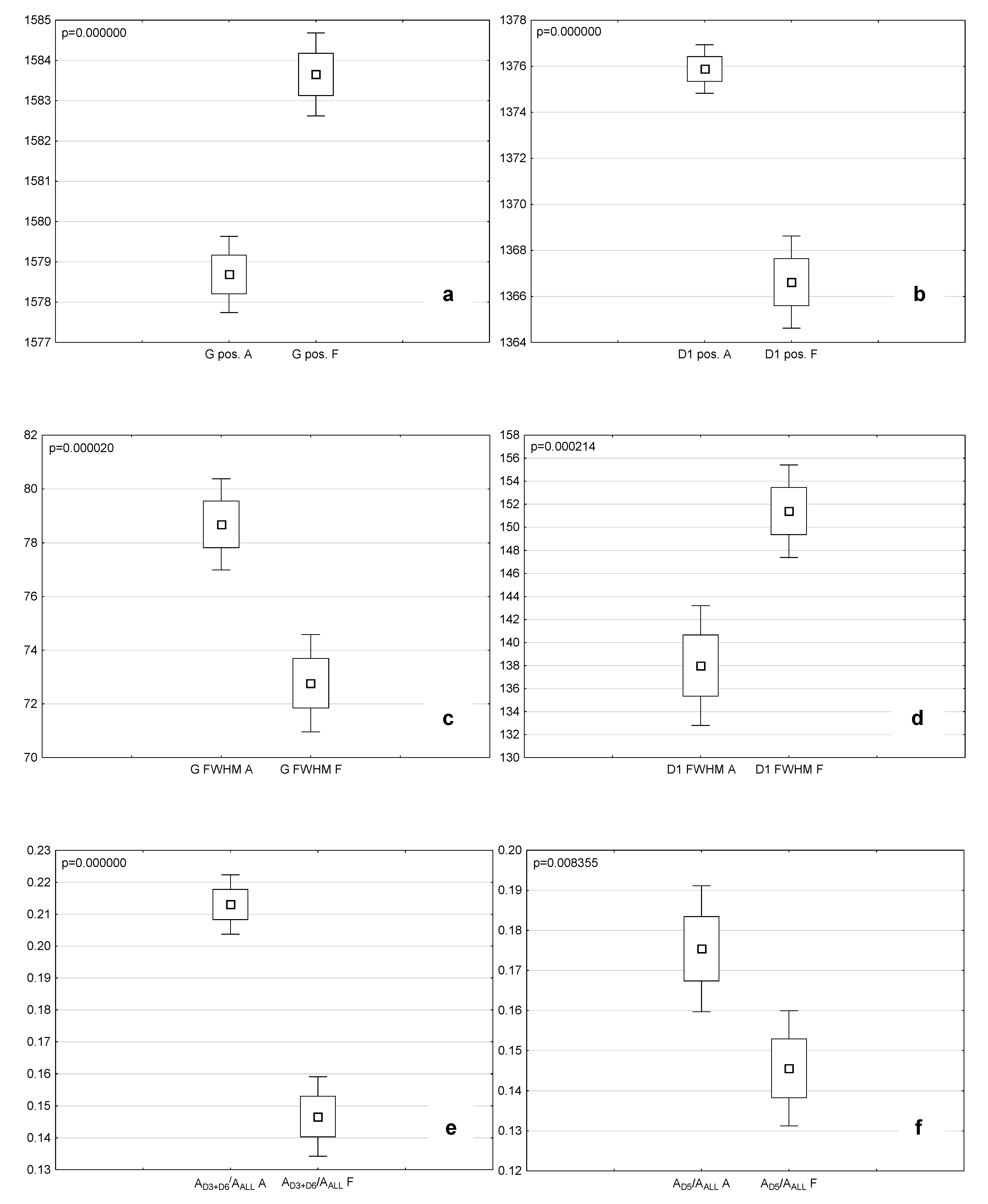
4.3.2. Comparison of the Microstructural and Chemical Properties of the Analyzed Macerals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Suárez-Ruiz, I.; Crelling, J. Applied Coal Petrology; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; ISBN 9780080450513. [Google Scholar]
- Suárez-Ruiz, I.; Rubiera, F.; Diez, M. New Trends in Coal Conversion: Combustion, Gasification, Emissions, and Coking; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Roberts, M.J.; Everson, R.C.; Neomagus, H.W.J.P.; Van Niekerk, D.; Mathews, J.P.; Branken, D.J. Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals. Fuel 2015, 142, 9–20. [Google Scholar] [CrossRef]
- Bielowicz, B.; Baran, P. CO2 sorption properties of selected lithotypes of lignite from Polish deposits. Geol. Q. 2019, 63, 786–800. [Google Scholar] [CrossRef]
- Wagner, M. The character of the IR absorption in the spectral rang 1700–1500 cm−1 of some macerals of the huminite group of brown coal. Bull. L’Acad. Pol. Sci. 1981, 29, 321–330. [Google Scholar]
- Li, X.; Hamashi, J.I.; Li, C.Z.; Hayaschi, J.; Li, C.Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Liu, X.; You, J.; Wang, Y.; Lu, L.; Xie, Y.; Yu, I.; Fu, Q. Raman spectroscopic study on the pyrolysis of Australian bituminous coal. J. Fuel Chem. Technol. 2014, 42, 270–276. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Rodrigues, S.; Noronha, F. Micro-Raman spectroscopy of collotelinite, fusinite and macrinite. Int. J. Coal Geol. 2010, 83, 415–422. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Xu, J.; Tang, H.; Su, S.; Liu, J.; Xu, K.; Qian, K.; Wang, Y.; Zhou, Y.; Hu, S.; Zhang, A.; et al. A study of the relationships between coal structures and combustion characteristics: The insights from micro-Raman spectroscopy based on 32 kinds of Chinese coals. Appl. Energy 2018, 212, 46–56. [Google Scholar] [CrossRef]
- Kasiński, J.; Piwocki, M.; Sadowska, E.; Ziembińska-Tworzydło, M. Lignite of the Polish Lowlands Miocene: Characteristics on a base of selected profiles. Biul. Państw. Inst. Geol. 2010, 439, 99–154. [Google Scholar]
- Czarnecki, L.; Frankowski, R.; Ślusarczyk, G. Syntetyczny profil litostratygraficzny rejonu złoża Bełchatów dla potrzeb Bazy Danych Geologicznych. Gór. Odkryw. 1992, 3, 12–22. [Google Scholar]
- Wagner, M. Zmienność Petrologiczno-Sedymentologiczna i Własności Technologiczne Kredy Jeziornej w Osadach Neogenu Typu Wapiennego Zapadliska Tektonicznego na Przykładzie Zloża Węgla Brunatnego “Szczerców” (Petrological and Sedimentological Variability and Tech; AGH Uczelniane Wydawn. Naukowo-Dydaktyczne: Kraków, Poland, 2007; ISBN 9788374641289. [Google Scholar]
- Wagner, M.; Matl, K. Stratygrafia Kredy Jeziornej ze Złoża Węgla Brunatnego “Szczerców”. (Stratigraphy of Lacustrine Chalk from the “Szczerców” Lignite Deposit); Wydawnictwa AGH: Kraków, Poland, 2007; Volume 33. [Google Scholar]
- Piwocki, M.; Ziembińska-Tworzydło, M. Neogene of the polish lowlands—Lithostratigraphy and pollen-spore zones. Kwart. Geol. 1997, 41, 21–40. [Google Scholar]
- Widera, M. An overview of lithotype associations of Miocene lignite seams exploited in Poland. Geologos 2016, 22, 213–225. [Google Scholar] [CrossRef]
- Słomka, T.; Wagner, M. Charakter Petrograficzny I Warunki Sedymentacji Wybranych Kompleksów Litologicznych Z Profilu Miocenu W złożu węGla Brunatnego Bełchatów (Petrological Studies and Sedimentological Conditions of Select Lithologic Series in Miocene from Bełcható W Lign. Prace Geol. PAN 2000, 147. Available online: https://integro.ciniba.edu.pl/integro/191800753249/ksiazka/charakter-petrograficzny-i-warunki-sedymentacji-wybranych-kompleksow-litologicznych-z-profilu-miocenu?autor=ksika&ln=gb&_lang=en (accessed on 24 December 2020).
- Kasiński, J.R.; Piwocki, M. Low-rank coals in Poland: Prospection—Mining—Progress. Pol. Geol. Inst. Spec. Pap. 2002, 7, 17–30. [Google Scholar]
- ISO 7404-2. Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 7404-5. Methods for the Petrographic Analysis of Coals—Part 5: Method of Determining Microscopically the Reflectance of Vitrinite; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ICCP. The new inertinite classification (ICCP System 1994). Fuel 2001, 80, 459–471. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D.; Kalaitzidis, S.; Christanis, K.; Cardott, B.J.; Misz-Kennan, M.; Rodrigues, S.; Hentschel, A.; Hamor-Vido, M.; et al. Classification of liptinite—ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- Sýkorová, I.; Pickel, W.; Christanis, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite—ICCP System 1994. Int. J. Coal Geol. 2005, 62, 85–106. [Google Scholar] [CrossRef]
- ISO 7404-3. Methods for the Petrographic Analysis of Coals—Part 3: Method of Determining Maceral Group Composition; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 579. Coke—Determination of Total Moisture; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- ISO 1171. Solid Mineral Fuels—Determination of Ash; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- ISO 562. Hard Coal and Coke—Determination of Volatile Matter; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- ISO 351. Solid Mineral Fuels—Determination of Total Sulfur—High Temperature Combustion Method; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- ISO 1928. Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 609. Solid Mineral Fuels—Determination of Carbon and Hydrogen—High Temperature Combustion Method; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Henry, D.G.; Jarvis, I.; Gillmore, G.; Stephenson, M. Raman spectroscopy as a tool to determine the thermal maturity of organic matter: Application to sedimentary, metamorphic and structural geology. Earth Sci. Rev. 2019, 198, 102936. [Google Scholar] [CrossRef]
- ISO 11760. Classification of Coals; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- UN ECE. International Classification of in-Seam Coals; UN ECE: Geneva, Switzerland, 1998. [Google Scholar]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Green, P.D.; Johnson, C.A.; Thomas, K.M. Applications of laser Raman microprobe spectroscopy to the characterization of coals and cokes. Fuel 1983, 62, 1013–1023. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.P.; Froigneux, E.; Moreau, M.; Rouzaud, J.N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. In Spectrochimica Acta—Part A: Molecular and Biomolecular Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2003; Volume 59, pp. 2267–2276. [Google Scholar]
- Rouzaud, J.N.; Oberlin, A.; Beny-Bassez, C. Carbon films: Structure and microtexture (optical and electron microscopy, Raman spectroscopy). Thin Solid Film. 1983, 105, 75–96. [Google Scholar] [CrossRef]
- Beny-Bassez, C.; Rouzaud, J.N. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectrometry. Scanning Electron Microsc. 1985, 1, 119–132. [Google Scholar]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Ferralis, N.; Matys, E.D.; Knoll, A.H.; Hallmann, C.; Summons, R.E. Rapid, direct and non-destructive assessment of fossil organic matter via microRaman spectroscopy. Carbon 2016, 108, 440–449. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Chabalala, V.P.; Wagner, N.; Potgieter-Vermaak, S. Investigation into the evolution of char structure using Raman spectroscopy in conjunction with coal petrography; Part 1. Fuel Process. Technol. 2011, 92, 750–756. [Google Scholar] [CrossRef]
- Morga, R. Reactivity of semifusinite and fusinite in the view of micro-Raman spectroscopy examination. Int. J. Coal Geol. 2011. [Google Scholar] [CrossRef]
- Morga, R.; Jelonek, I.; Kruszewska, K. Relationship between coking coal quality and its micro-Raman spectral characteristics. Int. J. Coal Geol. 2014, 134–135, 17–23. [Google Scholar] [CrossRef]
- Ulyanova, E.V.V.; Molchanov, A.N.N.; Prokhorov, I.Y.Y.; Grinyov, V.G.G. Fine structure of Raman spectra in coals of different rank. Int. J. Coal Geol. 2014, 121, 37–43. [Google Scholar] [CrossRef]
- Morga, R. Changes of Semifusinite and Fusinite Microstructure during Carbonization Inferred from the Raman Spectroscopy Examination (Monograph. In Polish, English Abstract); Silesian University of Technology Publishing House: Gliwice, Poland, 2013. [Google Scholar]
- Gaines, A.F.; Özyildiz, G.; Wolf, M. Infrared spectra of humodetrinite from the Fortuna-Garsdorf mine. Fuel 1981, 60, 615–618. [Google Scholar] [CrossRef]
- Russell, N.J. Gelification of Victorian tertiary soft brown coal wood. I. Relationship between chemical composition and microscopic appearance and variation in the degree of gelification. Int. J. Coal Geol. 1984, 4, 99–118. [Google Scholar] [CrossRef]
- Drobniak, A.; Mastalerz, M. Chemical evolution of Miocene wood: Example from the Belchatow brown coal deposit, central Poland. Int. J. Coal Geol. 2006, 66, 157–178. [Google Scholar] [CrossRef]
- Mastalerz, M.; Hower, J.C.; Taulbee, D.N. Variations in chemistry of macerals as refl ected by micro-scale analysis of a Spanish coal. Geol. Acta 2013, 11, 483–493. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Fang, H.L. Maturity trends in Raman spectra from kerogen and coal. Energy Fuels 2001, 15, 653–658. [Google Scholar] [CrossRef]
- Liu, D.H.; Xiao, X.M.; Tian, H.; Min, Y.S.; Zhou, Q.; Cheng, P.; Shen, J.G. Sample maturation calculated using Raman spectroscopic parameters for solid organics: Methodology and geological applications. Chin. Sci. Bull. 2013, 58, 1285–1298. [Google Scholar] [CrossRef]
- Sauerer, B.; Craddock, P.R.; AlJohani, M.D.; Alsamadony, K.L.; Abdallah, W. Fast and accurate shale maturity determination by Raman spectroscopy measurement with minimal sample preparation. Int. J. Coal Geol. 2017, 173, 150–157. [Google Scholar] [CrossRef]
- Luo, Q.; Fariborz, G.; Zhong, N.; Wang, Y.; Qiu, N.; Skovsted, C.B.; Suchý, V.; Hemmingsen Schovsbo, N.; Morga, R.; Xu, Y.; et al. Graptolites as fossil geo-thermometers and source material of hydrocarbons: An overview of four decades of progress. Earth Sci. Rev. 2020, 200, 103000. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Fritz, L.P.; Parris, T.M.; Hackley, P.C.; Solotky, L.; Eble, C.F.; Schlaegle, S. Assessment of thermal maturity trends in devonian–mississippian source rocks using raman spectroscopy: Limitations of peak-fitting method. Front. Energy Res. 2017, 5, 27. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Georgakopoulos, A.; Christanis, K.; Iordanidis, A. Early coalification features as approached by solid state 13C CP/MAS NMR spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 947–959. [Google Scholar] [CrossRef]
- Quirico, E.; Rouzaud, J.N.; Bonal, L.; Montagnac, G. Maturation grade of coals as revealed by Raman spectroscopy: Progress and problems. In Spectrochimica Acta—Part A: Molecular and Biomolecular Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hinrichs, R.; Brown, M.T.; Vasconcellos, M.A.Z.Z.; Abrashev, M.V.; Kalkreuth, W. Simple procedure for an estimation of the coal rank using micro-Raman spectroscopy. Int. J. Coal Geol. 2014, 136, 52–58. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]


| Component | Content (%) | ||
|---|---|---|---|
| Macerals | Huminite | Textinite | 11.7 |
| Ulminite | 12.1 | ||
| Attrinite | 29.9 | ||
| Densinite | 23.9 | ||
| Corpohuminite | 1.6 | ||
| Gelinite | 1.5 | ||
| Liptinite | Sporinite | 0.6 | |
| Cutinite | 0.1 | ||
| Resinite | 1.1 | ||
| Suberinite | 0.2 | ||
| Alginite | 0.1 | ||
| Liptodetrinite | 3.6 | ||
| Inertinite | Fusinite | 1 | |
| Semifusinite | 0.2 | ||
| Funginite | 0.1 | ||
| Micrinite | 0.0 | ||
| Inertodetrinite | 1.8 | ||
| Minerals | Pyrite | 0.8 | |
| Carbonates | 0.5 | ||
| Quartz + Clays | 9.2 |
| Maceral | Random Reflectance | Standard Deviation |
|---|---|---|
| (%] | (%] | |
| Textinite | 0.20 | 0.034 |
| Ulminite | 0.27 | 0.056 |
| Attrinite | 0.26 | 0.039 |
| Densinite | 0.31 | 0.060 |
| Fusinite | 0.95 | 0.067 |
| Proximate Analysis | ||
|---|---|---|
| Total moisture, as received basis, Mt ar | (%) | 51.3 |
| Total moisture, moisture, ash free basis of Mt maf | (%) | 43.8 |
| Ash, dry basis A db | (%) | 18.6 |
| Total sulphur, dry basis, St db | (%) | 3.89 |
| Gross calorific value, moisture, ash free basis GCV maf | KJ/kg | 13.9 |
| Gross calorific value, dry, ash free basis GCV daf | KJ/kg | 24.70 |
| Volatile matter, dry, ash free basis V daf | (%) | 49.37 |
| Ultimate analysis of lignite | ||
| Carbon content, dry, ash free basis C daf | (%) | 67.39 |
| Hydrogen content, dry, ash free basis H daf | (%) | 5.18 |
| Nitrogen content, dry, ash free basis N daf | (%) | 0.89 |
| Oxygen content, dry, ash free basis O daf | (%) | 21.47 |
| Maceral | D2 pos. cm−1 | D2 FWHM cm−1 | G pos. cm−1 | G FWHM cm−1 | D3 pos. cm−1 | D3 FWHM cm−1 | D6 pos. cm−1 | D6 FWHM cm−1 | D1 pos. cm−1 | D1 FWHM cm−1 | D5 pos. cm−1 | D5 FWHM cm−1 | D4 pos. cm−1 | D4 FWHM cm−1 | D7 pos. cm−1 | D7 FWHM cm−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attrinite | 1613.3 | 76.3 | 1578.7 | 78.7 | 1541.1 | 93.3 | 1471.5 | 127.0 | 1375.9 | 138.0 | 1287.7 | 137.5 | 1191.9 | 105.6 | 1101.7 | 74.7 |
| n = 28 | 2.3 | 5.0 | 2.6 | 4.6 | 4.2 | 10.6 | 6.6 | 13.8 | 2.9 | 14.1 | 4.8 | 18.2 | 4.2 | 15.9 | 6.3 | 25.00 |
| Densinite | 1614.6 | 74.0 | 1579.7 | 77.8 | 1541.6 | 95.8 | 1468.1 | 139.7 | 1374.1 | 140.7 | 1289.9 | 138.0 | 1197.1 | 126.9 | 1098.8 | 105.0 |
| n = 30 | 2.4 | 4.3 | 2.9 | 4.0 | 3.9 | 10.5 | 8.5 | 13.5 | 3.2 | 16.3 | 5.0 | 20.5 | 7.3 | 37.9 | 8.3 | 77.3 |
| Ulminite | 1616.8 | 73.6 | 1581.5 | 79.7 | 1539.2 | 103.3 | 1461.4 | 142.1 | 1368.5 | 146.8 | 1289.4 | 131.9 | 1199.0 | 134.6 | 1094.6 | 87.1 |
| n = 28 | 3.0 | 5.2 | 2.7 | 4.5 | 3.1 | 6.9 | 7.00 | 14.4 | 4.2 | 17.3 | 5.8 | 17.1 | 7.6 | 34.5 | 11.1 | 37.8 |
| Fusinite | 1612.0 | 52.9 | 1583.7 | 72.8 | 1538.4 | 123.8 | 1458.4 | 132.8 | 1366.6 | 151.4 | 1278.5 | 153.6 | 1189.2 | 124.5 | 1088.4 | 108.0 |
| n = 27 | 2.2 | 6.5 | 2.7 | 4.8 | 5.7 | 15.4 | 10.9 | 26.7 | 5.3 | 10.6 | 8.7 | 23.8 | 9.3 | 31.8 | 8.8 | 29.5 |
| Maceral | ID1/IG | AD3+D6/AALL | AD5/AALL | AD4/AALL | RBS cm−1 |
|---|---|---|---|---|---|
| Attrinite | 1.05 | 0.21 | 0.18 | 0.05 | 202.8 |
| n = 28 | 0.08 | 0.03 | 0.04 | 0.01 | 4.2 |
| Densinite | 1.04 | 0.23 | 0.16 | 0.06 | 205.6 |
| n = 30 | 0.14 | 0.03 | 0.04 | 0.02 | 4.1 |
| Ulminite | 0.95 | 0.27 | 0.14 | 0.07 | 212.9 |
| n = 28 | 0.12 | 0.04 | 0.04 | 0.03 | 5.4 |
| Fusinite | 1.09 | 0.15 | 0.15 | 0.05 | 217.0 |
| n = 27 | 0.16 | 0.03 | 0.04 | 0.02 | 6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielowicz, B.; Morga, R. Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland). Energies 2021, 14, 281. https://doi.org/10.3390/en14020281
Bielowicz B, Morga R. Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland). Energies. 2021; 14(2):281. https://doi.org/10.3390/en14020281
Chicago/Turabian StyleBielowicz, Barbara, and Rafał Morga. 2021. "Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland)" Energies 14, no. 2: 281. https://doi.org/10.3390/en14020281
APA StyleBielowicz, B., & Morga, R. (2021). Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland). Energies, 14(2), 281. https://doi.org/10.3390/en14020281





