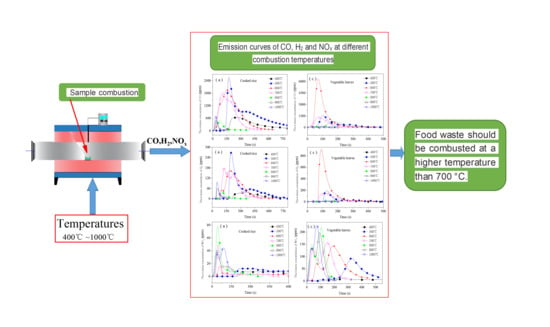
Emission Characteristics of Pollution Gases from the Combustion of Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
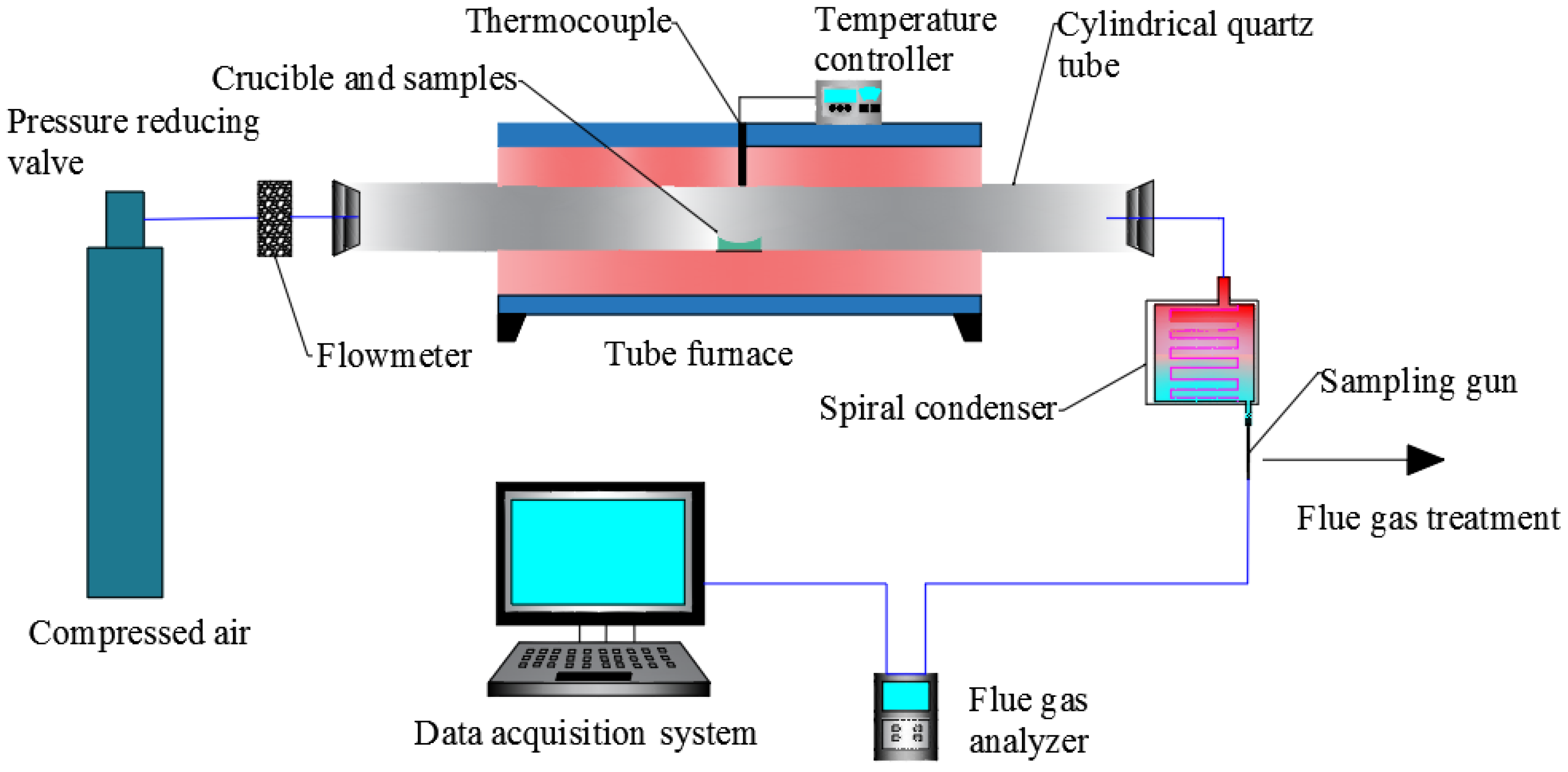
2.2. Experimental Equipment
2.3. Methods
2.3.1. Experimental Methods
2.3.2. Calculation Methods
- Peak concentration (ppm): the maximum concentration of gases produced.
- Peak time (s): the time taken to reach the peak concentration of gases.
- Burnout time t (s): the longer one of either the time taken for the H2 concentration to fall to zero or the time for CO concentration to decrease to 5% of its peak value.
- The average concentration (AC) is given bywhere the numerator on the right-hand side refers to the integral of the gas concentration for the reaction time, t represents the burnout time, and the AC is measured in ppm.
- The produced gas volume (GV) is given bywhere , , and refer to the injected air flow (L/s), the sum of AC of all gases, and the burnout time, respectively. The gas production is measured in L.Considering that is very small and can be ignored relative to 1, Equation (2) can be simplified to
- The yield of gas (Yg) is given bywhere m refers to the mass of the test sample.
3. Results and Discussion
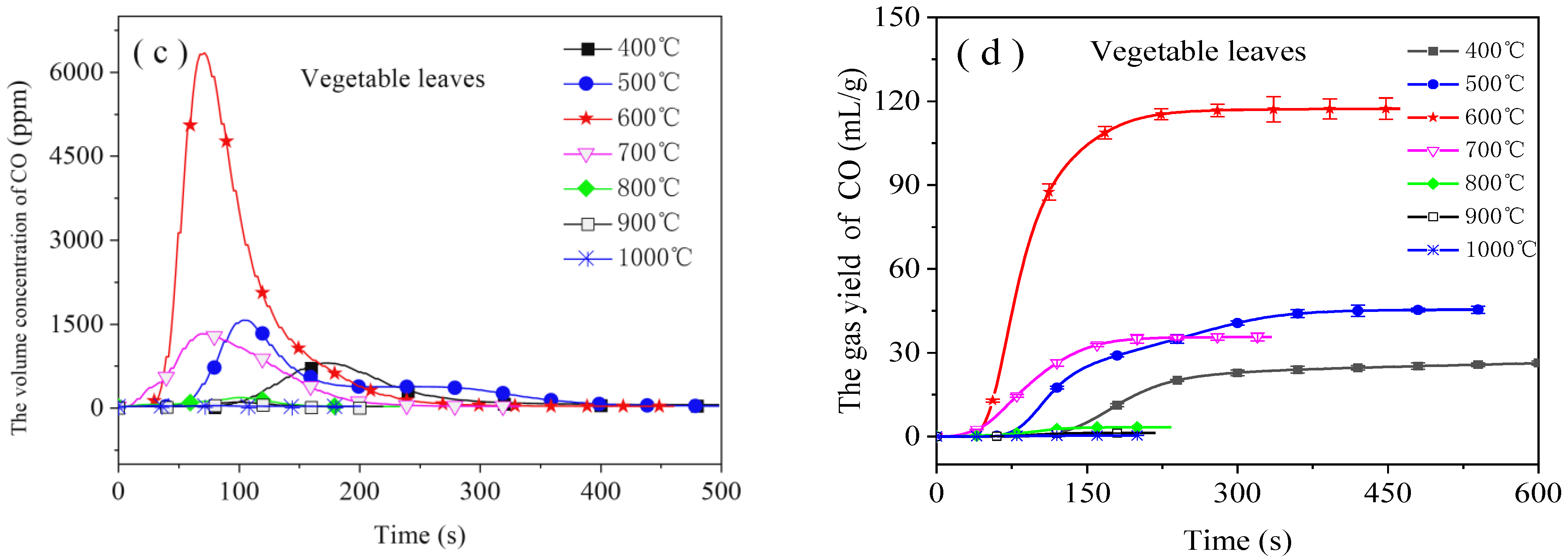
3.1. Emission Characteristics of CO
3.2. Emission Characteristics of H2
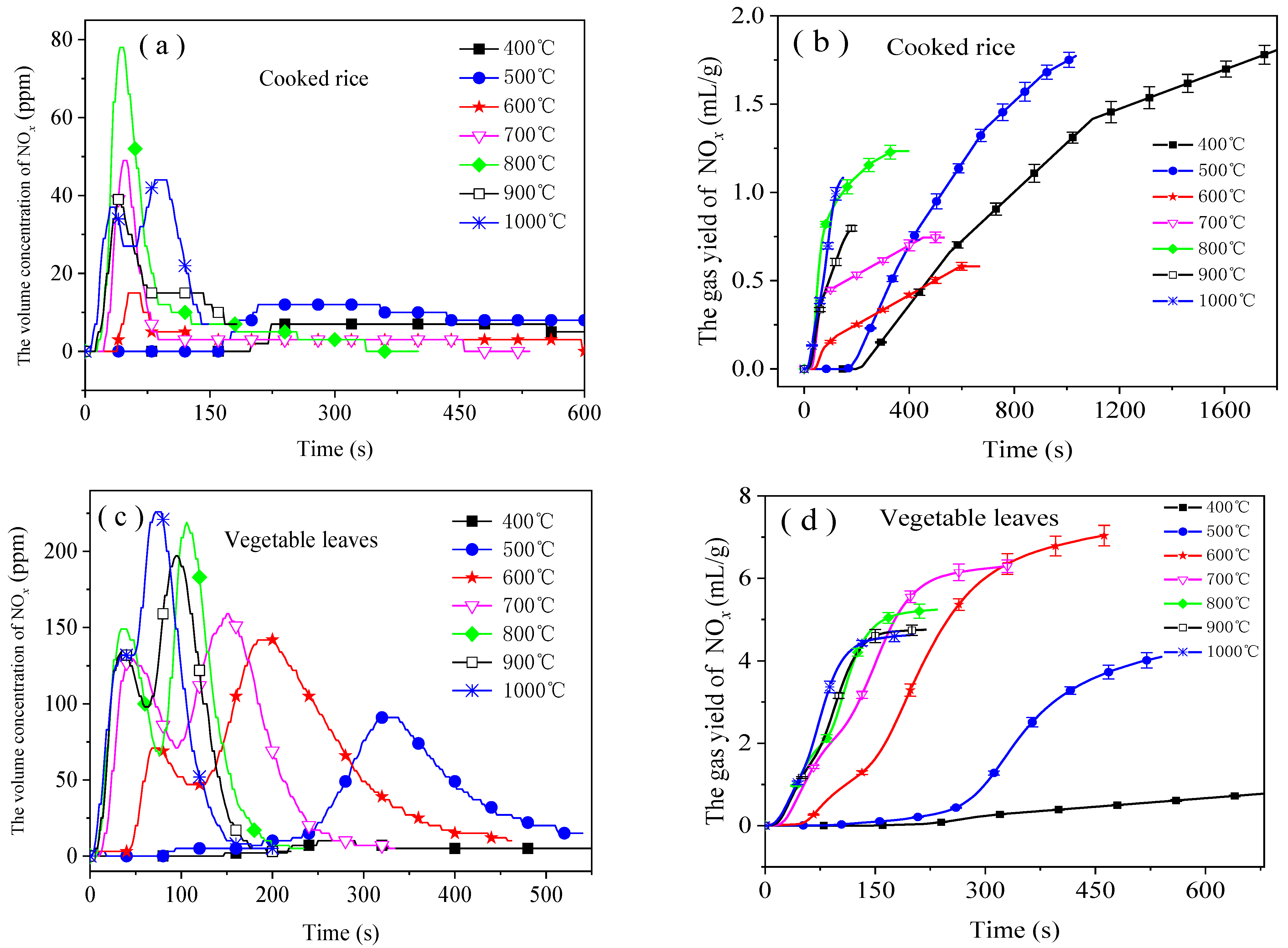
3.3. Emission Characteristics of NOx
4. Conclusions
- Each emission curve of CO had a peak. The peak increased at first and then decreased. The burnout time gradually decreased as the combustion temperature rose from 400 °C to 1000 °C. CO emissions were greatest from 400 °C to 700 °C; therefore, the combustion of food waste within that temperature range should be avoided from the perspective of controlling CO emissions.
- The emission of H2 resembled that of CO. If CO emissions were present in large amounts at a certain temperature, then those of H2 were also present in large amounts at this temperature. H2 emissions from the combustion of cooked rice and vegetable leaves were greatest (9.5 mL/g and 16.5 mL/g, respectively) at 500 °C and 600 °C, respectively.
- Two peaks occurred in the NOx emission curves within the range of high temperatures (1000 °C for the cooked rice and 600 °C to 1000 °C for the vegetable leaves). The higher the temperature, the higher the second emission peak. When being combusted from 400 °C to 500 °C, cooked rice emitted a large amount of NOx, while vegetable leaves emitted a large amount of NOx from 600 °C to 700 °C. Therefore, from the perspective of reducing NOx emissions, the combustion of food waste should be done at a temperature higher than 700 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AC | The average concentration of gas(ppm) |
| GV | The gas volume (L) |
| Q | The injected air flow (L/s) |
| t | The burnout time(s) |
| Yg | The yield of gas (mL/g) |
| Subscripts and Superscripts | |
| i | The type of gas |
References
- China’s National Bureau of Statistics. China Statistical Yearbook 2019; China Statistical Press: Beijing, China, 2019.
- Tang, Y.; Ma, X.; Lai, Z.; Fan, Y. Thermogravimetric analyses of co-combustion of plastic, rubber, leather in N2/O2 and CO2/O2 atmospheres. Energy 2015, 90, 1066–1074. [Google Scholar] [CrossRef]
- Lai, Z.; Ma, X.; Tang, Y.; Lin, H. A study on municipal solid waste (MSW) combustion in N2/O2 and CO2/O2 atmosphere from the perspective of TGA. Energy 2011, 36, 819–824. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C.; Bi, H.; Jiang, C.; Liu, Y.; Song, Z.; Lin, Q. Experimental Investigation on Combustion and Gas Emission of Scrap Tire Pellet under Various Concentrations of CO2/O2 Mixtures. Combust. Sci. Technol. 2019, 193, 1495–1515. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, L.; Lin, R.; Yue, L.; Liu, J.; Zhou, J.; Cen, K. Fermentative biohydrogen and biomethane co-production from mixture of food waste and sewage sludge: Effects of physiochemical properties and mix ratios on fermentation performance. Appl. Energy 2016, 184, 1–8. [Google Scholar] [CrossRef]
- Mahmood, R.; Parshetti, G.; Balasubramanian, R. Energy, exergy and techno-economic analyses of hydrothermal oxidation of food waste to produce hydro-char and bio-oil. Energy 2016, 102, 187–198. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, J.; Chi, Y.; Zhou, Z.; Ni, M. Energy and exergy optimization of food waste pretreatment and incineration. Environ. Sci. Pollut. Res. 2017, 24, 18434–18443. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Hong, J.; Chen, Y.; Wang, M.; Ye, L.; Qi, C.; Yuan, H.; Zheng, T.; Li, X. Intensification of municipal solid waste disposal in China. Renew. Sustain. Energy Rev. 2017, 69, 168–176. [Google Scholar] [CrossRef]
- Sotiropoulos, A.; Malamis, D.; Michailidis, P.; Krokida, M.; Loizidou, M. Research on the drying kinetics of household food waste for the development and optimization of domestic waste drying technique. Environ. Technol. 2015, 37, 929–939. [Google Scholar] [CrossRef]
- Boldrin, A.; Hartling, K.R.; Laugen, M.; Christensen, T.H. Environmental inventory modelling of the use of compost and peat in growth media preparation. Resour. Conserv. Recycl. 2010, 54, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Tsigkou, K.; Tsafrakidou, P.; Kopsahelis, A.; Zagklis, D.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew. Energy 2019, 147, 610–619. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yu, Z.; Zhang, X.; Ma, X. Co-combustion behavior of municipal solid waste and food waste anaerobic digestates: Combustion performance, kinetics, optimization, and gaseous products. J. Environ. Chem. Eng. 2021, 9, 106028. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, X.; Chen, Z.; Li, X.; Jiang, X.; Yan, J. Influence on gaseous pollutants emissions and fly ash characteristics from co-combustion of municipal solid waste and coal by a drop tube furnace. Waste Manag. 2018, 81, 33–40. [Google Scholar] [CrossRef]
- Dong, C.; Yang, Y.; Zhang, J.; Lu, X. Gaseous emissions from the combustion of a waste mixture containing a high concentration of N2O. Waste Manag. 2009, 29, 272–276. [Google Scholar] [CrossRef]
- Riaza, J.; Khatami, R.; Levendis, Y.A.; Álvarez, L.; Gil, M.V.; Pevida, C.; Rubiera, F.; Pis, J.J. Single particle ignition and combustion of anthracite, semi-anthracite and bituminous coals in air and simulated oxy-fuel conditions. Combust. Flame 2014, 161, 1096–1108. [Google Scholar] [CrossRef] [Green Version]
- Lasek, J.; Janusz, M.; Zuwała, J.; Głód, K.; Iluk, A. Oxy-fuel combustion of selected solid fuels under atmospheric and elevated pressures. Energy 2013, 62, 105–112. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, X.; Lai, Z.; Zhou, D.; Chen, Y. Thermogravimetric characteristics and combustion emissions of rubbers and polyvinyl chloride in N2/O2 and CO2/O2 atmospheres. Fuel 2013, 104, 508–514. [Google Scholar] [CrossRef]
- Ding, G.; He, B.; Cao, Y.; Wang, C.; Su, L.; Duan, Z.; Song, J.; Tong, W.; Li, X. Process simulation and optimization of municipal solid waste fired power plant with oxygen/carbon dioxide combustion for near zero carbon dioxide emission. Energy Convers. Manag. 2018, 157, 157–168. [Google Scholar] [CrossRef]
- Onenc, S.; Retschitzegger, S.; Evic, N.; Kienzl, N.; Yanik, J. Characteristics and synergistic effects of co-combustion of carbonaceous wastes with coal. Waste Manag. 2018, 71, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xie, C.; Liu, J.; Zhang, X.; Chang, K.; Kuo, J.; Sun, J.; Xie, W.; Zheng, L.; Sun, S.; et al. Influence of catalysts on co-combustion of sewage sludge and water hyacinth blends as determined by TG-MS analysis. Bioresour. Technol. 2018, 247, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Boumanchar, I.; Chhiti, Y.; M’Hamdi Alaoui, F.E.; Elkhouakhi, M.; Sahibed-Dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Investigation of (co)-combustion kinetics of biomass, coal and municipal solid wastes. Waste Manag. 2019, 97, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Namioka, T.; Yoshikawa, K. Effects of hydrothermal treatment on characteristics and combustion behaviors of municipal solid wastes. Appl. Energy 2011, 88, 3659–3664. [Google Scholar]
- Liu, H.; E, J.; Ma, X.; Xie, C. Influence of microwave drying on the combustion characteristics of food waste. Dry Technol. 2016, 34, 1397–1405. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Peng, H.; Li, Y.; Jiang, Z. Characteristics of flue gas emissions during large wood pellet combustion. Trans. Chin. Soc. Agric. Eng. 2015, 31, 215–220. [Google Scholar]
- Xu, Q.; Peng, W.; Ling, C. An Experimental Analysis of Soybean Straw Combustion on Both CO and NOX Emission Characteristics in a Tubular Furnace. Energies 2020, 13, 1587. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.H.; Chu, X.J.; Cheng, S.J. Sulfur Transfers from Pyrolysis and Gasification of Coal. Adv. Mater. Res. 2012, 512–515, 2526–2530. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Yi, H.; Yang, C.; Zhang, R.; Zhou, Y.; Tang, X. Recent advances in selective catalytic oxidation of nitric oxide (NO-SCO) in emissions with excess oxygen: A review on catalysts and mechanisms. Environ. Sci. Pollut. Res. 2020, 28, 2549–2571. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Fan, X.-H.; Gan, M.; Chen, X.-L. Effect of Ca-Fe oxides additives on NOx reduction in iron ore sintering. J. Iron Steel Res. Int. 2017, 24, 1184–1189. [Google Scholar] [CrossRef]
- Pillier, L.; Idir, M.; Molet, J.; Matynia, A.; De Persis, S. Experimental study and modelling of NOx formation in high pressure counter-flow premixed CH4/air flames. Fuel 2015, 150, 394–407. [Google Scholar] [CrossRef]
- Shamooni, A.; Debiagi, P.; Wang, B.; Luu, T.D.; Stein, O.T.; Kronenburg, A.; Bagheri, G.; Stagni, A.; Frassoldati, A.; Faravelli, T.; et al. Carrier-phase DNS of detailed NOx formation in early-stage pulverized coal combustion with fuel-bound nitrogen. Fuel 2021, 291, 119998. [Google Scholar] [CrossRef]
- Verma, P.; Zare, A.; Jafari, M.; Bodisco, T.; Rainey, T.; Ristovski, Z.; Brown, R.J. Diesel engine performance and emissions with fuels derived from waste tyres. Sci. Rep. 2018, 8, 2457. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zhou, M.; Liu, Z.; Cheng, M.; Chen, J. Modeling NOx emission of coke combustion in iron ore sintering process and its experimental validation. Fuel 2016, 179, 322–331. [Google Scholar] [CrossRef]
- Lane, D.J.; Ashman, P.J.; Zevenhoven, M.; Hupa, M.; van Eyk, P.J.; de Nys, R.; Karlström, O.; Lewis, D.M. Combustion Behavior of Algal Biomass: Carbon Release, Nitrogen Release, and Char Reactivity. Energy Fuel 2014, 28, 41–51. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, B.; Su, Y. Advanced control of NO emission from algal biomass combustion using loaded iron-based additives. Energy 2019, 185, 229–238. [Google Scholar] [CrossRef]
- Glarborg, P.; Jensen, A.D.; Johnsson, J.E. Fuel nitrogen conversion in solid fuel fired systems. Prog. Energy Combust. 2003, 29, 89–113. [Google Scholar] [CrossRef]
- Meng, X.; Sun, R.; Zhou, W.; Liu, X.; Yan, Y.; Ren, X. Effects of corn ratio with pine on biomass co-combustion characteristics in a fixed bed. Appl. Therm. Eng. 2018, 142, 30–42. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Cheng, Z.; Chan, S.; Kook, S.; Yeoh, G.H. Emissions characteristics of NOx and SO2 in the combustion of microalgae biomass using a tube furnace. J. Energy Inst. 2017, 90, 806–812. [Google Scholar] [CrossRef]
- Vermeulen, I.; Block, C.; Vandecasteele, C. Estimation of fuel-nitrogen oxide emissions from the element composition of the solid or waste fuel. Fuel 2012, 94, 75–80. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y.; Liu, D.; Zhang, H.; Liu, W.; Cui, G. SO2/NOx emissions and ash formation from algae biomass combustion: Process characteristics and mechanisms. Energy 2016, 113, 821–830. [Google Scholar] [CrossRef]


| Materials | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | LHV(MJ·kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| A | V | FC | C | H | N | S | ||
| Vegetable leaves | 13.62 | 76.22 | 10.16 | 40.86 | 6.638 | 4.219 | 0.389 | 14.938 |
| Cooked rice | 0.45 | 91.41 | 8.14 | 43.28 | 8.04 | 1.527 | 0.26 | 15.806 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, X.; Hong, Q. Emission Characteristics of Pollution Gases from the Combustion of Food Waste. Energies 2021, 14, 6439. https://doi.org/10.3390/en14196439
Liu H, Zhang X, Hong Q. Emission Characteristics of Pollution Gases from the Combustion of Food Waste. Energies. 2021; 14(19):6439. https://doi.org/10.3390/en14196439
Chicago/Turabian StyleLiu, Haili, Xu Zhang, and Qingchao Hong. 2021. "Emission Characteristics of Pollution Gases from the Combustion of Food Waste" Energies 14, no. 19: 6439. https://doi.org/10.3390/en14196439
APA StyleLiu, H., Zhang, X., & Hong, Q. (2021). Emission Characteristics of Pollution Gases from the Combustion of Food Waste. Energies, 14(19), 6439. https://doi.org/10.3390/en14196439