The Influence of the Nature of Redox-Active Moieties on the Properties of Redox-Active Ionic Liquids and on Their Use as Electrolyte for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
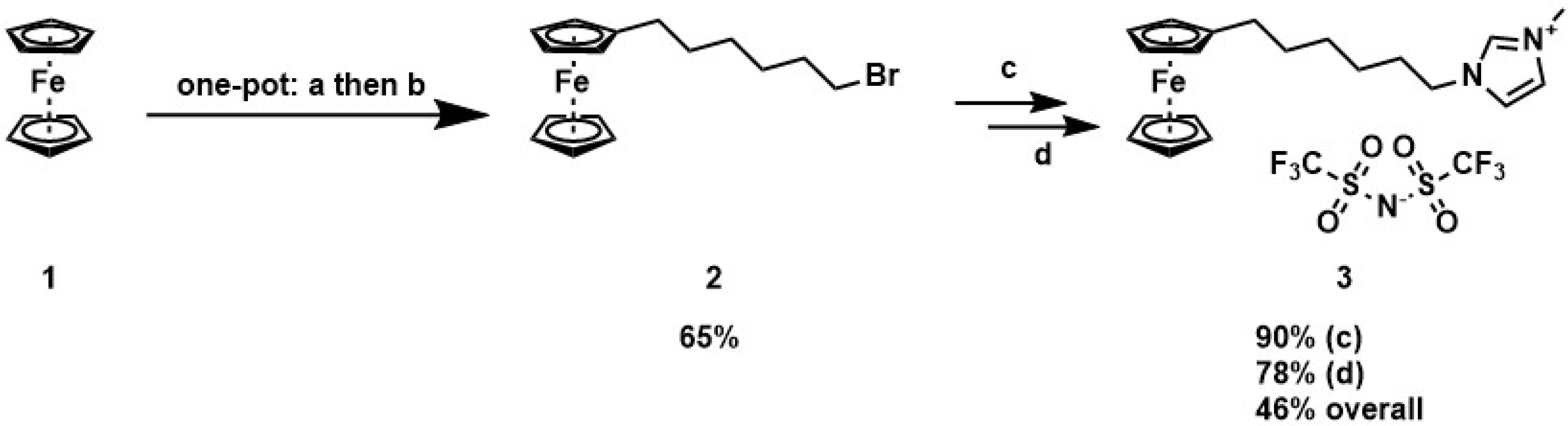
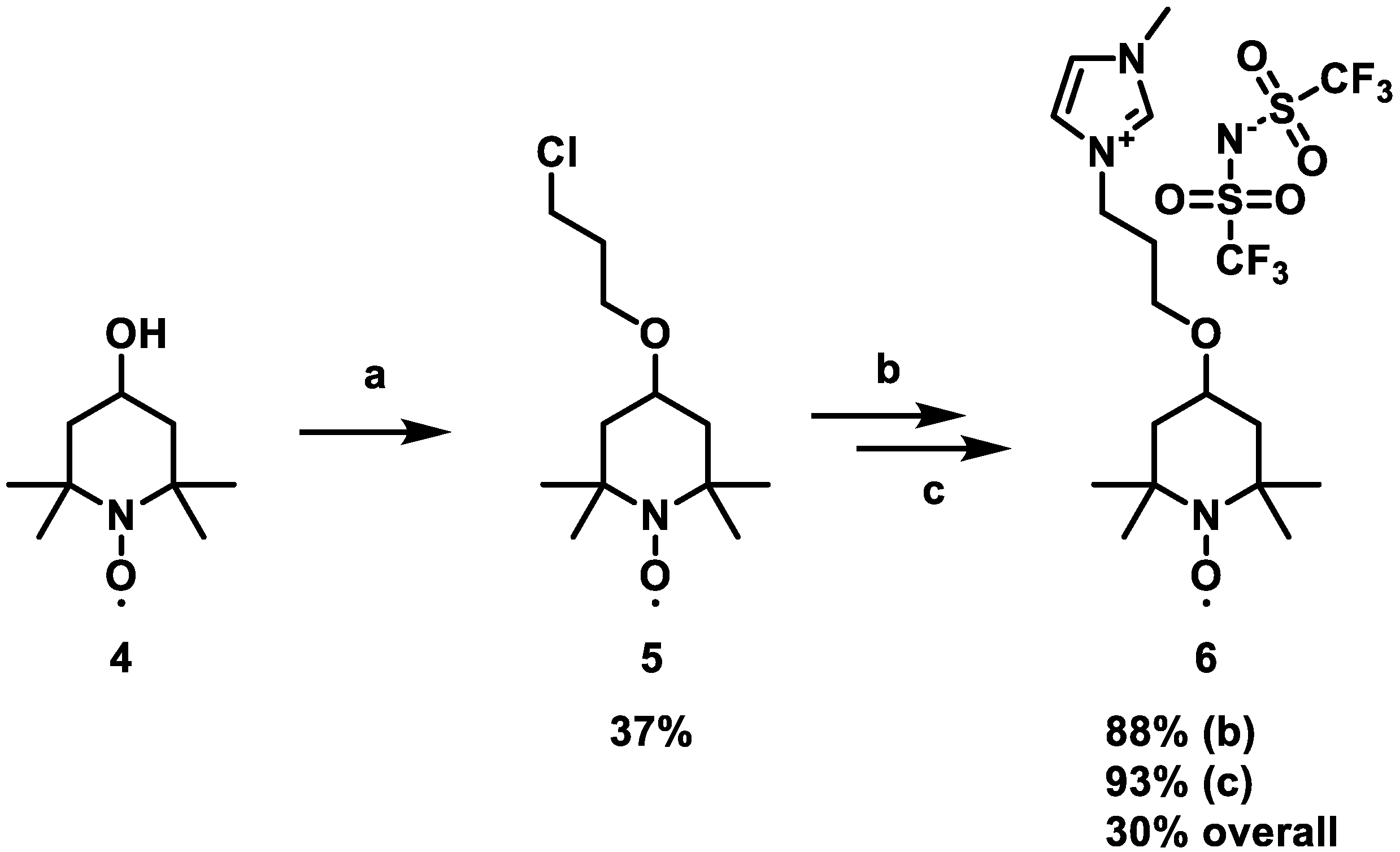
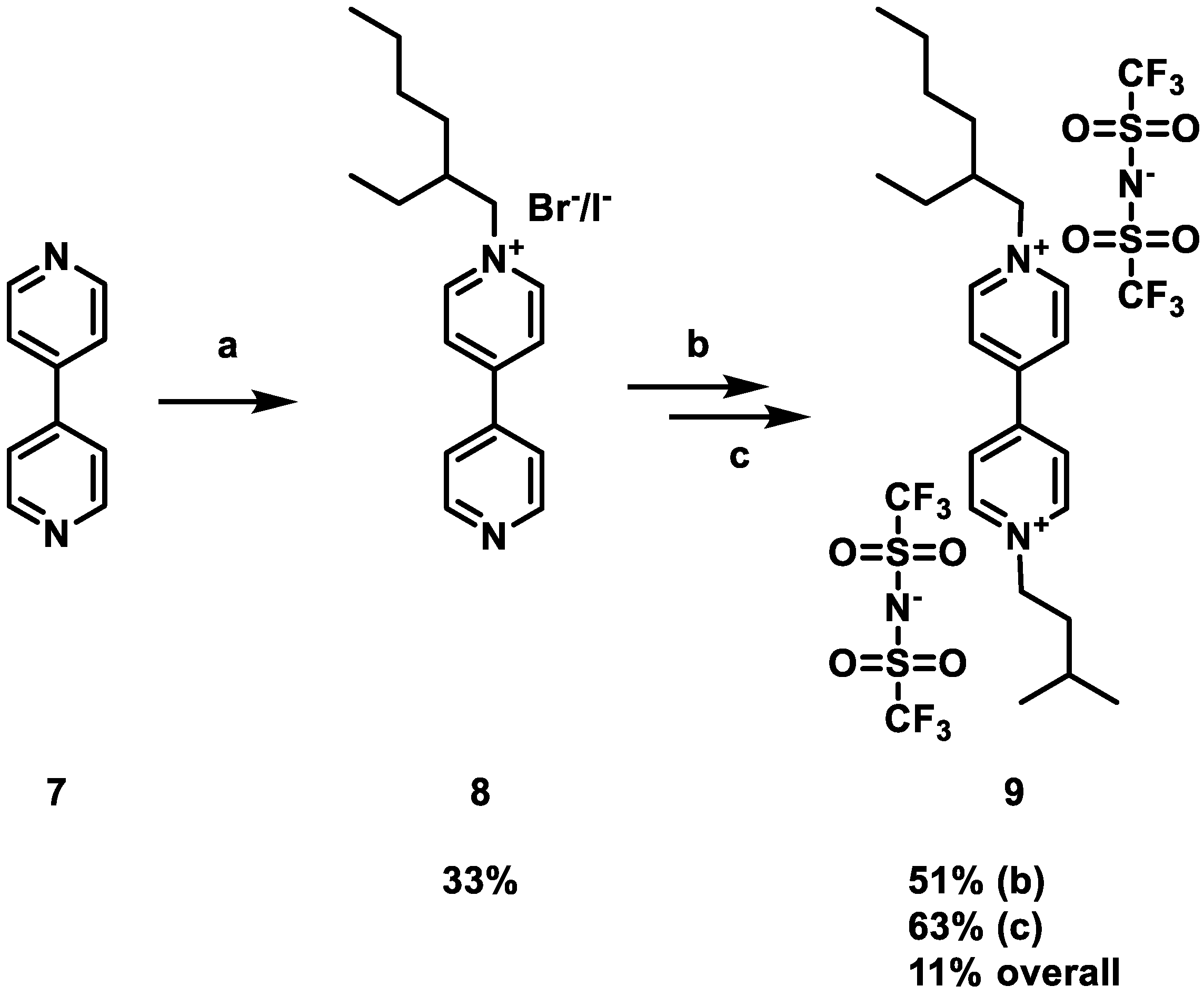
2.2. Synthesis of the Ionic Liquis
2.3. Differential Scanning Calorimetry
2.4. Electrochemical Characterization of the Redox Active Ionic Liquids
3. Results
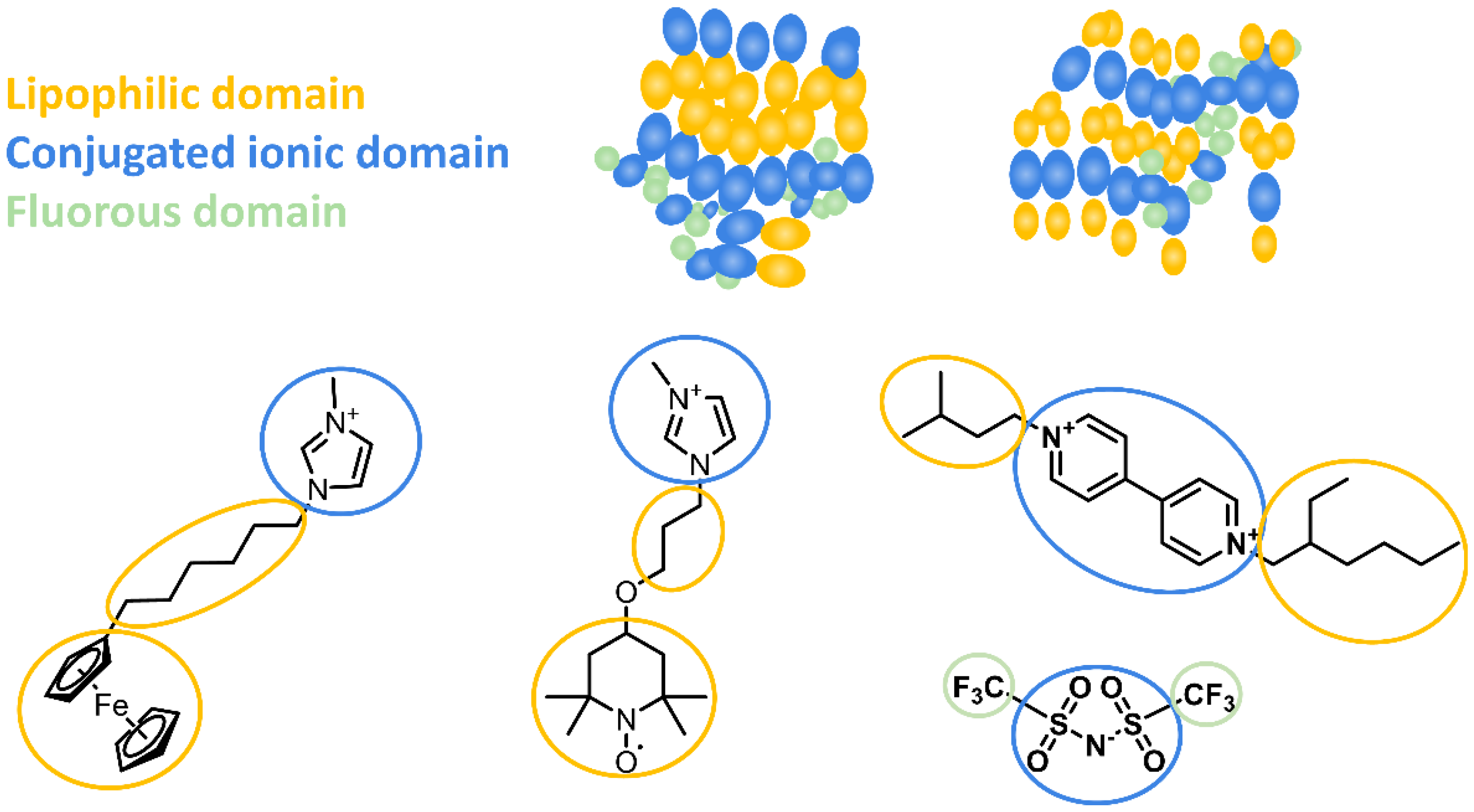
3.1. Synthesis and Characterization of the Redox Active Ionic Liquids
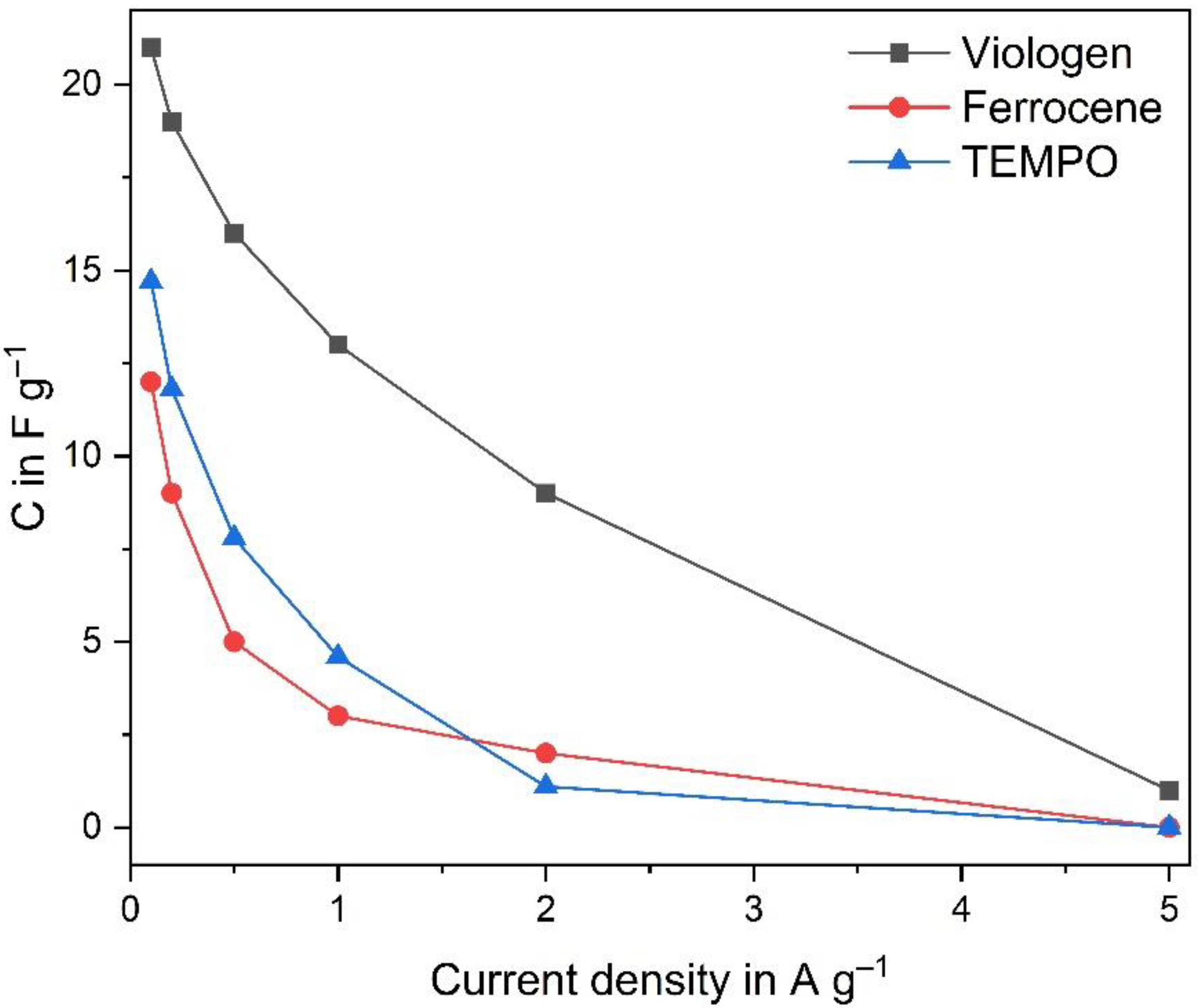
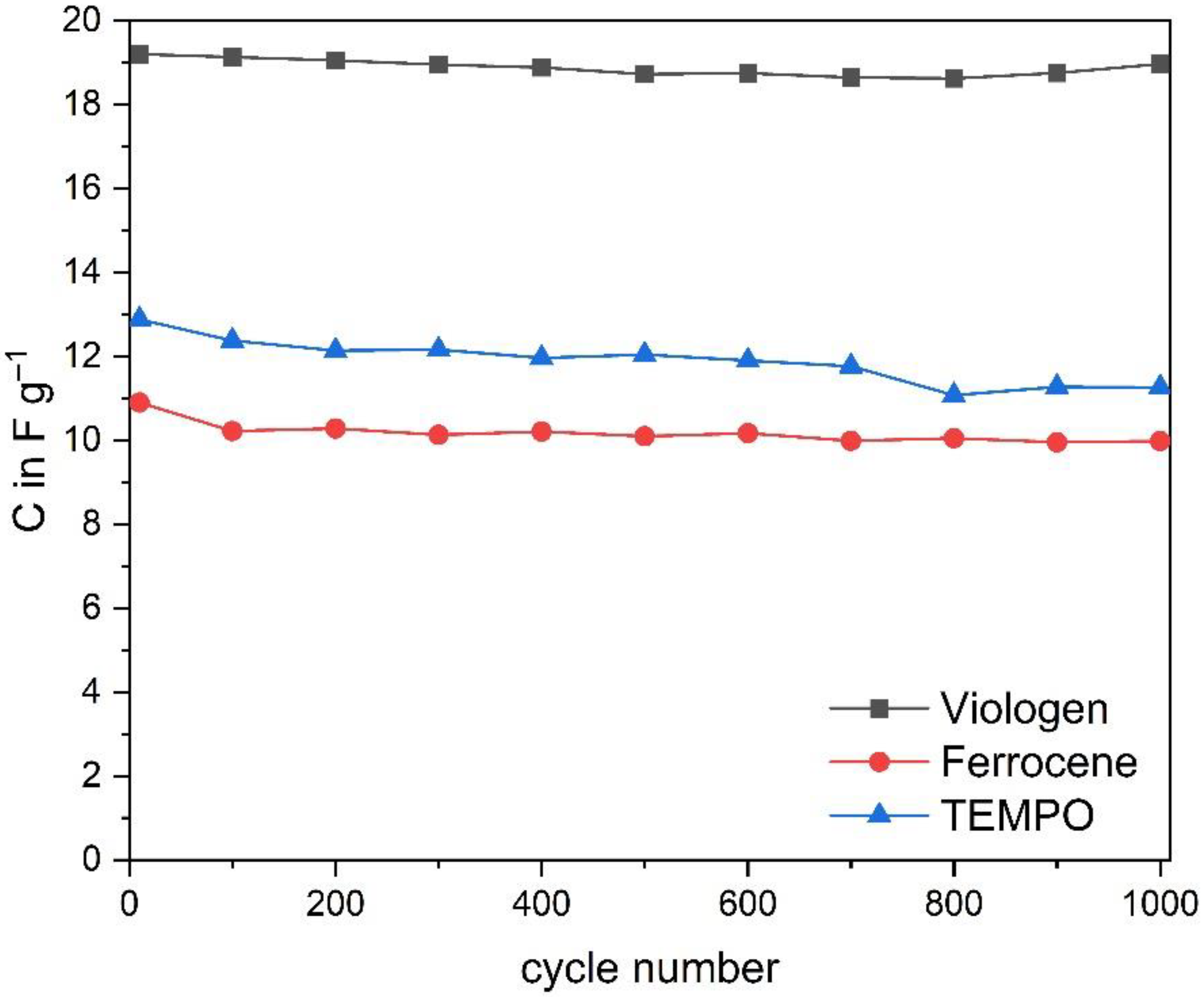
3.2. Electrochemical Characterization of the Redox Active Ionic Liquids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H.; Rogers, R.D. Task-Specific Ionic Liquids Incorporating Novel Cations for the Coordination and Extraction of Hg2+ and Cd2+: Synthesis, Characterization, and Extraction Studies. Environ. Sci. Technol. 2002, 36, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Giernoth, R. Task-Specific Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Fang, D.; Liu, L.; Yi, T.-F. Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J. Mol. Liq. 2011, 163, 99–121. [Google Scholar] [CrossRef]
- Mandal, B.; Ghosh, S.; Basu, B. Task-Specific Properties and Prospects of Ionic Liquids in Cross-Coupling Reactions. Top. Curr. Chem. 2019, 377, 30. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic liquids for electrochemical energy storage devices applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Kar, M.; Tutusaus, O.; MacFarlane, D.R.; Mohtadi, R. Novel and versatile room temperature ionic liquids for energy storage. Energy Environ. Sci. 2019, 12, 566–571. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Ionic Liquids for Supercapacitive Energy Storage: A Mini-Review. Energy Fuels 2021, 35, 8443–8455. [Google Scholar] [CrossRef]
- Van Aken, K.L.; Beidaghi, M.; Gogotsi, Y. Formulation of Ionic-Liquid Electrolyte To Expand the Voltage Window of Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef] [PubMed]
- Balducci, A.; Dugas, R.; Taberna, P.L.; Simon, P.; Plée, D.; Mastragostino, M.; Passerini, S. High temperature carbon—Carbon supercapacitor using ionic liquid as electrolyte. J. Power Sources 2007, 165, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Balducci, A.; Bardi, U.; Caporali, S.; Mastragostino, M.; Soavi, F. Ionic liquids for hybrid supercapacitors. Electrochem. Commun. 2004, 6, 566–570. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic ionic liquids in energy storage devices: Past, present and future perspective. Energy Storage Mater. 2021, 40, 402–414. [Google Scholar] [CrossRef]
- Park, J.-W.; Ueno, K.; Tachikawa, N.; Dokko, K.; Watanabe, M. Ionic Liquid Electrolytes for Lithium–Sulfur Batteries. J. Phys. Chem. C 2013, 117, 20531–20541. [Google Scholar] [CrossRef]
- Kühnel, R.S.; Böckenfeld, N.; Passerini, S.; Winter, M.; Balducci, A. Mixtures of ionic liquid and organic carbonate as electrolyte with improved safety and performance for rechargeable lithium batteries. Electrochim. Acta 2011, 56, 4092–4099. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sugimoto, T.; Kikuta, M.; Ishiko, E.; Kono, M. Pure ionic liquid electrolytes compatible with a graphitized carbon negative electrode in rechargeable lithium-ion batteries. J. Power Sources 2006, 162, 658–662. [Google Scholar] [CrossRef]
- Wylie, L.; Seeger, Z.L.; Hancock, A.N.; Izgorodina, E.I. Increased stability of nitroxide radicals in ionic liquids: More than a viscosity effect. Phys. Chem. Chem. Phys. 2019, 21, 2882–2888. [Google Scholar] [CrossRef]
- Wang, X.; Shang, Z.; Yang, A.; Zhang, Q.; Cheng, F.; Jia, D.; Chen, J. Combining Quinone Cathode and Ionic Liquid Electrolyte for Organic Sodium-Ion Batteries. Chem 2019, 5, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Lan, Q.; Liu, N.; Men, F.; Wang, X.; Song, Z.; Zhan, H. A Metal-free Battery with Pure Ionic Liquid Electrolyte. iScience 2019, 15, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, P.; Burges, R.; Lex-Balducci, A.; Schubert, U.S.; Balducci, A. The influence of the electrolyte composition on the electrochemical behaviour of cathodic materials for organic radical batteries. J. Power Sources 2018, 405, 142–149. [Google Scholar] [CrossRef]
- Kim, J.-K.; Matic, A.; Ahn, J.-H.; Jacobsson, P. Improving the stability of an organic battery with an ionic liquid-based polymer electrolyte. RSC Adv. 2012, 2, 9795–9797. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Li, C.-C.; Lee, J.-T. Electrochemical behavior of organic radical polymer cathodes in organic radical batteries with N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquid electrolytes. Electrochim. Acta 2012, 66, 332–339. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Seddon, K.R. Ionic LiquidsProgress on the Fundamental Issues. Aust. J. Chem. 2007, 60, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Pérez, G.; Ortiz, A.; Ortiz, I. The roles of ionic liquids as new electrolytes in redox flow batteries. Sep. Purif. Technol. 2020, 252, 117436. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, Y.; Ding, Y.; Zhang, L.; Guo, X.; Zhao, Y.; Yu, G. Biredox Eutectic Electrolytes Derived from Organic Redox-Active Molecules: High-Energy Storage Systems. Angew. Chem. Int. Ed. 2019, 58, 7045–7050. [Google Scholar] [CrossRef]
- Small, L.J.; Pratt, H.D., III; Staiger, C.L.; Anderson, T.M. MetILs3: A Strategy for High Density Energy Storage Using Redox-Active Ionic Liquids. Adv. Sustain. Syst. 2017, 1, 1700066. [Google Scholar] [CrossRef]
- Hernández, G.; Işik, M.; Mantione, D.; Pendashteh, A.; Navalpotro, P.; Shanmukaraj, D.; Marcilla, R.; Mecerreyes, D. Redox-active poly(ionic liquid)s as active materials for energy storage applications. J. Mater. Chem. A 2017, 5, 16231–16240. [Google Scholar] [CrossRef]
- Rochefort, D. Enabling new electrochemical methods with redox-active ionic liquids. Curr. Opin. Electrochem. 2019, 15, 125–132. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Tu, Q.-M.; Geng, C.-L.; Wang, Y.-L.; Sun, S.-J.; Huang, Y.-F.; Wu, J.-H. Improved redox-active ionic liquid-based ionogel electrolyte by introducing carbon nanotubes for application in all-solid-state supercapacitors. Int. J. Hydrog. Energy 2020, 45, 17131–17139. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhao, S.; Wang, Z.; Wang, S. The high performance of polyaniline-gel network modified electrode in 3-(2,2,6,6-tetramethyl-piperidiynl-1-oxyl)-1-methylylimidazoliumbromide biredox electrolyte used for supercapacitor. J. Power Sources 2019, 434, 226745. [Google Scholar] [CrossRef]
- Bodin, C.; Mourad, E.; Zigah, D.; Le Vot, S.; Freunberger, S.A.; Favier, F.; Fontaine, O. Biredox ionic liquids: New opportunities toward high performance supercapacitors. Faraday Discuss. 2018, 206, 393–404. [Google Scholar] [CrossRef] [PubMed]
- You, D.-J.; Yin, Z.; Ahn, Y.-K.; Lee, S.-H.; Yoo, J.; Kim, Y.S. Redox-active ionic liquid electrolyte with multi energy storage mechanism for high energy density supercapacitor. RSC Adv. 2017, 7, 55702–55708. [Google Scholar] [CrossRef] [Green Version]
- Salanne, M. Ionic Liquids for Supercapacitor Applications. Top. Curr. Chem. 2017, 375, 63. [Google Scholar] [CrossRef] [PubMed]
- Mourad, E.; Coustan, L.; Lannelongue, P.; Zigah, D.; Mehdi, A.; Vioux, A.; Freunberger, S.A.; Favier, F.; Fontaine, O. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 2017, 16, 446–453. [Google Scholar] [CrossRef]
- Xie, H.J.; Gélinas, B.; Rochefort, D. Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 2016, 66, 42–45. [Google Scholar] [CrossRef]
- Navalpotro, P.; Palma, J.; Anderson, M.; Marcilla, R. High performance hybrid supercapacitors by using para-Benzoquinone ionic liquid redox electrolyte. J. Power Sources 2016, 306, 711–717. [Google Scholar] [CrossRef]
- Sathyamoorthi, S.; Suryanarayanan, V.; Velayutham, D. Organo-redox shuttle promoted protic ionic liquid electrolyte for supercapacitor. J. Power Sources 2015, 274, 1135–1139. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Sun, G.; Li, K.; Sun, C. Electrochemical performance of electrochemical capacitors using Cu(II)-containing ionic liquid as the electrolyte. Microporous Mesoporous Mater. 2010, 128, 56–61. [Google Scholar] [CrossRef]
- Beh, E.S.; De Porcellinis, D.; Gracia, R.L.; Xia, K.T.; Gordon, R.G.; Aziz, M.J. A Neutral pH Aqueous Organic–Organometallic Redox Flow Battery with Extremely High Capacity Retention. ACS Energy Lett. 2017, 2, 639–644. [Google Scholar] [CrossRef]
- Liu, Y.; Goulet, M.-A.; Tong, L.; Liu, Y.; Ji, Y.; Wu, L.; Gordon, R.G.; Aziz, M.J.; Yang, Z.; Xu, T. A Long-Lifetime All-Organic Aqueous Flow Battery Utilizing TMAP-TEMPO Radical. Chem 2019, 5, 1861–1870. [Google Scholar] [CrossRef]
- Krause, A.; Kossyrev, P.; Oljaca, M.; Passerini, S.; Winter, M.; Balducci, A. Electrochemical double layer capacitor and lithium-ion capacitor based on carbon black. J. Power Sources 2011, 196, 8836–8842. [Google Scholar] [CrossRef]
- Schütter, C.; Husch, T.; Viswanathan, V.; Passerini, S.; Balducci, A.; Korth, M. Rational design of new electrolyte materials for electrochemical double layer capacitors. J. Power Sources 2016, 326, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, B.; Hirsch, A. Synthesis and Characterization of New Ferrocene-Containing Ionic Liquids. Eur. J. Org. Chem. 2014, 2014, 4123–4136. [Google Scholar] [CrossRef]
- Tahara, H.; Furue, Y.; Suenaga, C.; Sagara, T. A Dialkyl Viologen Ionic Liquid: X-ray Crystal Structure Analysis of Bis(trifluoromethanesulfonyl)imide Salts. Cryst. Growth Des. 2015, 15, 4735–4740. [Google Scholar] [CrossRef]
- Miao, C.-X.; He, L.-N.; Wang, J.-Q.; Wang, J.-L. TEMPO and Carboxylic Acid Functionalized Imidazolium Salts/Sodium Nitrite: An Efficient, Reusable, Transition Metal-Free Catalytic System for Aerobic Oxidation of Alcohols. Adv. Synth. Catal. 2009, 351, 2209–2216. [Google Scholar] [CrossRef]
- Qian, W.; Jin, E.; Bao, W.; Zhang, Y. Clean and selective oxidation of alcohols catalyzed by ion-supported TEMPO in water. Tetrahedron 2006, 62, 556–562. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Noémi, J.; Luis, C.; Fernando, P.; Branco, L.C. Novel Bipyridinium Ionic Liquids as Liquid Electrochromic Devices. Chem. Eur. J. 2014, 20, 3982–3988. [Google Scholar]
- Bhowmik, P.K.; Noori, O.; Chen, S.L.; Han, H.; Fisch, M.R.; Robb, C.M.; Variyam, A.; Martinez-Felipe, A. Ionic liquid crystals: Synthesis and characterization via NMR, DSC, POM, X-ray diffraction and ionic conductivity of asymmetric viologen bistriflimide salts. J. Mol. Liq. 2021, 328, 115370. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Al-Karawi, M.K.M.; Killarney, S.T.; Dizon, E.J.; Chang, A.; Kim, J.; Chen, S.L.; Principe, R.C.G.; Ho, A.; Han, H.; et al. Thermotropic Liquid-Crystalline and Light-Emitting Properties of Bis(4-aalkoxyphenyl) Viologen Bis(triflimide) Salts. Molecules 2020, 25, 2435. [Google Scholar] [CrossRef]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Viologen-based ionic liquid crystals: Induction of a smectic A phase by dimerisation. Phys. Chem. Chem. Phys. 2014, 16, 5048–5051. [Google Scholar] [CrossRef]
- Causin, V.; Saielli, G. Effect of asymmetric substitution on the mesomorphic behaviour of low-melting viologen salts of bis(trifluoromethanesulfonyl)amide. J. Mater. Chem. 2009, 19, 9153–9162. [Google Scholar] [CrossRef]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Arbizzani, C.; Beninati, S.; Lazzari, M.; Soavi, F.; Mastragostino, M. Electrode materials for ionic liquid-based supercapacitors. J. Power Sources 2007, 174, 648–652. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borchers, P.S.; Gerlach, P.; Liu, Y.; Hager, M.D.; Balducci, A.; Schubert, U.S. The Influence of the Nature of Redox-Active Moieties on the Properties of Redox-Active Ionic Liquids and on Their Use as Electrolyte for Supercapacitors. Energies 2021, 14, 6344. https://doi.org/10.3390/en14196344
Borchers PS, Gerlach P, Liu Y, Hager MD, Balducci A, Schubert US. The Influence of the Nature of Redox-Active Moieties on the Properties of Redox-Active Ionic Liquids and on Their Use as Electrolyte for Supercapacitors. Energies. 2021; 14(19):6344. https://doi.org/10.3390/en14196344
Chicago/Turabian StyleBorchers, Philipp S., Patrick Gerlach, Yihan Liu, Martin D. Hager, Andrea Balducci, and Ulrich S. Schubert. 2021. "The Influence of the Nature of Redox-Active Moieties on the Properties of Redox-Active Ionic Liquids and on Their Use as Electrolyte for Supercapacitors" Energies 14, no. 19: 6344. https://doi.org/10.3390/en14196344
APA StyleBorchers, P. S., Gerlach, P., Liu, Y., Hager, M. D., Balducci, A., & Schubert, U. S. (2021). The Influence of the Nature of Redox-Active Moieties on the Properties of Redox-Active Ionic Liquids and on Their Use as Electrolyte for Supercapacitors. Energies, 14(19), 6344. https://doi.org/10.3390/en14196344







