Abstract
Energy consumption is on the rise due to rapid technological progress and a higher standard of living. The use of alternative energy resources is essential to meet the rising energy demand and mitigate the carbon emissions caused due to use of fossil-based fuels. Biodiesel produced from non-edible oils such as castor seed oil (CO) can be used in diesel engines to replace fossil diesel. However, the quality and yields for CO biodiesel is low due to the presence of ricinolic acid C18:1OH (79%). In this study, two-stage conversion techniques were used to improve the yields and properties of CO biodiesel. The catalyst CaCu(OCH3)2 was prepared from waste eggshell and synthesized with copper oxide in the presence of methanol. The castor oil was subjected to pyrolysis at 450–500 °C and then transesterified in the presence of modified catalyst. The reaction parameters such as methanol-to-oil ratio and catalyst and reaction time were investigated, and the optimum combination was used to produce castor biodiesel from pyrolysis castor oil. Results showed that the cetane number and oxidation stability were increased by 7% and 42% respectively. The viscosity, density, flash point, and iodine value were decreased by 52%, 3%, 5% and 6%, respectively. The calorific values remained the same. This study suggests that pyrolyzed castor seed oil followed by transesterification in the presence of a modified catalyst gave better fuel properties and yields than the conventional transesterification process for biodiesel fuel production.
1. Introduction
The global energy demand has increased rapidly due to continuous growth in industrialization and the world population [1]. The International Energy Agency (2015) reported that global energy consumption is powered by 31.1% oil, 28.9% coal/peat, 21.4% natural gas, 2.4% hydro and 1.2% other renewable energy source [2]. According to the United States Energy Information Administration (EIA), worldwide, fossil fuels fulfill the energy demand of 80% countries [3]. This demand will increase from 95 barrels (in 2015) to 113 barrels/day by 2040, while by 2030, it is projected to increase by 53% from 2001 [1,3]. At this higher rate, fossil fuels such as coal, oil, and gas will deplete soon in another 200, 40, and 70 years, respectively [1,3]. The transportation sector consumed 60% of fossil fuels as compared to manufacturing and power generation sectors [4]. These sectors are major concerns that burn fossil fuel and generate greenhouse gas (GHG) and CO2 up to 0.5–1.1 kg-eq/kWh [3,4]. To control global CO2 emission by 2050, international organizations signed the Paris Agreement (2015) to reduce a global temperature rise of below 1.5 °C [1,5]. The continuous increase of global energy demand and climate change explores the need to refine the environmental policies by adopting sustainable clean energy sources [6]. The sustainable alternative fuel should be readily available, affordable, eco-friendly, and technically viable [7]. In this search, it is found that biodiesel is the most suitable sustainable fuel for diesel engine due to its similar fuel properties and clean burning [7]. The biodiesel is a mono-alkyl ester of long chain fatty acids [7]. It can be produced from the vegetable oil and animal fats through a transesterification process by separating glycerol from vegetable oil to form ester [7]. The production of methyl or ethyl ester is mainly dependent on the type of alcohol used [7].
The biodiesel production is nearly 21 million tonnes per annum globally [7]. The expected potential varies from 0.1 to 20 million tonnes in 2019, out of which 20% to 25% has been consumed [8]. To avoid food vs. fuel debates, about 150 non-edible oil seed tree species exist in south Asian countries [9]. The most widely known non-edible oil seed for biodiesel production are Jatropha (Jatropha curcas), Karanja (Pongamia pinnata), Mahua (Madhuca Indica), Jojoba (Simmondsia chinensis) and castor seed (Ricinus commu). The oil content in these non-edible oil seeds varies from 21% to 73% [9]. The castor plant grows easily with a suitable climate [10]. About 43 countries produce castor oil, and in about 30 countries, cultivation of castor plant is at a commercial scale [10]. Due to climate feasibility, India is one of the third largest producers of castor oil and meets 86% of the global market demand. The castor plant seed contains approximately 45–55% oil [9,10]. Due to high oil content, researchers are considering castor oil as a preferable candidature for biodiesel feedstocks [11]. Furthermore, castor oil seed production does not compete with the cultivation of other crops. They can grow on dry lands and in forest areas. Moreover, the water and fertilizer requirement of the castor plant are low [11]. The chemical compositions present in castor oil are C16:0 (1%), C18:0 (3%), C18:1 (8%), and C18:1OH (79%). The presence of ricinolic acid C18:1OH (79%) makes this oil highly viscous [12].
Researchers investigated techniques to optimize the biodiesel production from castor seed oil. Karmakar et al. (2018) optimized the castor oil biodiesel fuel properties through Taguchi methods, which refers to optimization of design parameters for minimizing defects before the experiment [12]. They developed a cost-effective technique for biodiesel production from castor seed oil. The esterification process was suggested by L16 Taguchi orthogonal array [12]. The maximum biodiesel yield was about 90.85% using the parameters: (i) reaction time 1 h, (ii) temperature 50 °C, (iii) catalyst (H2SO4) concentrated 1%w/w, oil-to-methanol ratio 1:20, and (iv) stirrer speed 700 rpm. They found optimized conditions for castor oil and achieved about 90.85% yield by using L16 Taguchi approaches [12]. The effectiveness of Pt/HAP as a catalyst was studied by Lei et al. (2021) [13]. The authors reported that Pt/HAP-IE catalyst may be used in hydrogen-free decarboxylation processes, particularly for the deoxygenation of molecules with unsaturated C=O and hydrogen-sensitive O=H bonds. They found that under hydrogen-free circumstances, this multi-functional synergistic catalysis creates an effective and selective deoxygenation catalyst [13].
The castor oil biodiesel is highly viscous and unable to meet the ASTM and EN biodiesel standards, hence reducing the viscosity is essential to meet the standards [14]. The thermo-chemical cracking (known as pyrolysis process) of biomass (and waste) to liquid biofuel is one of the fastest-growing technologies [15]. Many researchers have used different types of pyrolysis processes to convert castor seed into liquid fuel. The castor seed was pyrolyzed at 380 °C and characterizes the castor pyrolysis oil [16]. The yields from the pyrolysis process were oil 50%, char 29%, water 13% and gas 8% [16]. They reported that castor pyrolysis oil possesses a polar component and long-chain hydrocarbon [16]. The compounds consist of positive and negative charge mainly known as polar compounds such as N-, O-, S-, and trace metals [16]. Table 1 shows the effects of different pyrolysis processes on viscosity of castor oil. It was found out that the catalytic pyrolysis provides lower viscosity oils than non-catalytic process (Table 1).

Table 1.
Effect of various pyrolysis process on viscosity of castor oil.
Fuels with higher viscosity produce larger droplet size, which ultimately affect the atomization and air/fuel mixing. In addition, high viscous fuel results in shorter spray penetration and poor evaporation [19]. The consequences of higher viscosity of a fuel are incomplete combustion, increased fuel consumption and higher smoke emissions [19]. The fuel properties and engine performance of castor oil (and other vegetable oil) were improved through triple and double blending techniques. Oxygenated additives such as Di-ethyl ether (DEE) and diesel were used to reduce the viscosity [20]. The authors reported that up to 40% replacement of fossil diesel is possible through these blending options [20]. A comparison on the engine performance and emission characteristics of various non-edible biodiesels are shown in Table 2. The increase or decrease of parameters shown in Table 2 are based on the reference fuel (diesel). The fuel consumption of castor oil biodiesel is higher than other biodiesels (Table 2). Shu-Yao et al., (2014), investigated the biodiesel production from the dwarf castor oil [21]. They studied biodiesel production and their combustion and emission characteristics in the engine. They prepared seven different castor biodiesel-diesel blends and observed that blend B20 met the off-road diesel engine emissions norms [21]. Mithun et al. (2018), studied castor biodiesel-diesel blends effect on diesel engine performance, combustion, and emissions characteristics [22]. The mass burn rate and transition velocity were also investigated by a porous sphere experiment. They reported that the castor biodiesel blend has a slower evaporation rate than diesel fuel. In the case of diesel-biodiesel blend, combustion started earlier, and the rate of pressure rise was higher than diesel fuel [22]. When compared to pure diesel, no significant differences were observed in engine performance and exhaust gas emission characteristics with castor biodiesel–diesel blends [22].

Table 2.
Comparison of engine performance and emissions of castor biodiesel with other biodiesels.
The above literature shows that the viscosity of the CO biodiesel is still higher than other biodiesel types. In this study, an attempt was made to reduce the viscosity through combined pyrolysis and transesterification process, which has been not applied before. The castor oil was pyrolyzed to form pyrolysis castor oil (PCO) and afterward, the PCO was converted to castor biodiesel through transesterification process. The pyrolysis of castor seed was conducted between 450–500 °C. The transesterification process was carried out using a heterogeneous catalyst due to having the various advantages over a homogeneous catalyst (KOH) [26,27]. The heterogeneous catalyst does not dissolve with the mixture and was easy to separate during glycerol and biodiesel separation [21,22]. It can be reused and does not involve the soap formation process. Because of this, the heterogeneous catalyst reduced the post-treatment process. The objectives of the present study are (i) synthesis of heterogeneous catalyst, (ii) setting up of combined pyrolysis-transesterification process for castor biodiesel production, (iii) conducting pyrolysis-transesterification experiment of castor seeds (iv) optimization of the transesterification reaction parameters for high quality castor biodiesel production, and (v) fuel characterization of castor oil biodiesel fuel.
2. Materials and Methods
The castor oil was procured from the local supplier Ganapati Oil Mill, Krishnageri, Tamilnadu, India. Methanol (98% pure) and other chemicals were procured from the Lab Chemicals, Chennai, Tamilnadu, India. The experiments were conducted by varying catalysts and other reaction conditions. The measurements of fuel properties were repeated, and average of the measurement’s readings was used in the study. During biodiesel production, mixing is important. Therefore, stirrer speed (600 rpm) was kept constant throughout the experiments.
2.1. Catalyst and Fuel Characterization
The synthesis composite catalyst was subjected to Perkin Elmer, Fourier transform infrared (FTIR) spectroscopy to study the presence of functional groups. Scanning electron microscopy (SEM) was used to study the surface morphology of the catalyst by using apparatus Ziess (Model: EVO 18). For reuse of the catalyst, glycerol should be separated through chemical dilution and purification process. In this study, the suitability of catalyst repeatability was not studied. The characterization of prepared biodiesel fuel was done in the fuel characterization laboratory at Anna University (India). The equipment and standards used for fuel characterization is shown in Table 3. Several tests such as cloud point, and gas chromatography were not performed due to the lack of the facility available in the University.

Table 3.
List of the instruments used.
2.2. Catalyst Preparation
The waste eggshell was collected from the college canteen. The method of preparation of eggshell powder is shown in Figure 1. The collected eggshell was first washed in water and later boiled in a distilled water for 1 h to remove all the impurities [31]. It was dried in a sunlight then kept in an oven for overnight at a temperature of 110 °C. Initially, normal grinder was used to make eggshell powder; later, planetary ball mill machine was used to make fine calcium oxide (CaO) powder as shown in Figure 2. CaO powder was synthesized with copper oxide (CuO) to prepare composite methoxide powder as shown in Figure 2. The chemical reaction is represented in Equation (1) [32]. Both the catalyst was taken in weight basis (70/30 ratio), 70% CaO and 30 % CuO and dispersed rapidly into 20 mL of methanol (99% pure) in a 200 mL three-neck round glass bottle reactor (Figure 2) equipped with glass reflux condenser and magnetic stirrer with heater. The reaction was conducted at a temperature of 65 °C under atmospheric pressure with continuous stirring at 600 rpm for 10 h. After completion of the reaction, the gray color slurry formed and dried in oven at 110 °C for 12 h. Finally, dried composite powder was obtained, which was used as catalyst [32].
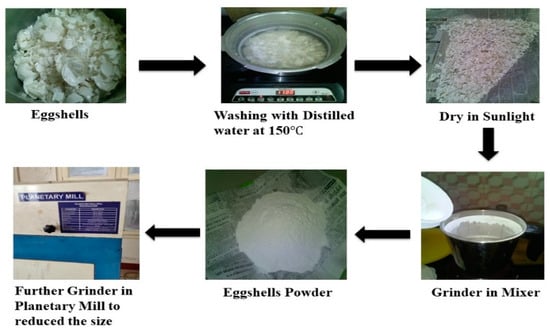
Figure 1.
Eggshell powder preparation.
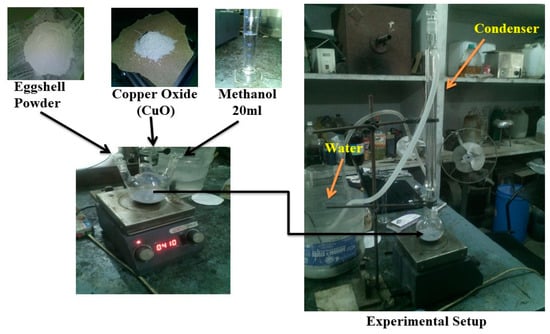
Figure 2.
Preparation of calcium-copper oxide catalyst.
2.3. Pyrolysis of Castor Oil
Thermal pyrolysis of castor oil was conducted at a temperature range 450–500 °C, residence time of 180 min at a rate of 20 °C/min as shown in Figure 3. The pyrolysis experiment was performed in a vertical semi-batch reactor (10 kg capacity) with electrical heating. The temperature was monitored through PID controller. The specification of the reactor is given in Table 4. A total of 8 kg of castor oil was fed and pyrolyzed for generating vapors, which was condensed by passing them through water cooled condenser. The condensed pyrolysis castor oil (PCO) was collected in a beaker. A small amount of ash was found as a by-product of pyrolysis after cooldown of the reactor.

Figure 3.
In total, 10 kg capacity laboratory scale pyrolysis setup.

Table 4.
Specification of the pyrolysis rector.
2.4. Biodiesel Production
2.4.1. Esterification of Castor Oil
Biodiesel production was conducted in the biofuel laboratory using 5 L capacity biodiesel plant available in the laboratory. The various components of the test rig are shown in Figure 4. Prior to the esterification, acid value (AV) test was performed for castor oil [33]. According to Equation (2), AV of raw castor oil was measured to be 6.5 mgKOH/g which correspond to 3.25 of free fatty acids (FFA %) calculated from Equation (3) [33]. Since FFA value is greater than 2.5, which is not preferable for transesterification process. Therefore, esterification (Acid catalyst) reaction is required to minimize the AV [34]. A molar ratio (oil to methanol) of 1:6 was used with 2% (v/v) sulfuric acid (H2SO4) [34]. The reaction time of 3 h, temperature of 65 °C and a stirrer speed of 600 rpm was maintained. After the reaction, the resultant mixture was collected to separating flask and kept to 10 h for phase separation of glycerol and esterified oil (Figure 4). Later, esterified oil was collected in a beaker and placed in an oven at 110 °C for 2 h to remove the excess methanol and moisture. The FFA % was calculated and found to be 1.9% which is lower than 2.5. Therefore, a dried esterified castor oil was used for further transesterification process.
where, V—titration volume (ml), N—normality of the titration solution (mol/L), Mw—molecular weight of the base catalyst and m—sample oil weight (g).
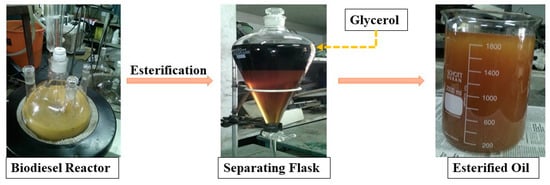
Figure 4.
Esterified castor oil.
2.4.2. Transesterification of Esterified Oil
Esterified castor oil was shifted to biodiesel reactor (Figure 5) for transesterification reaction. An oil to methanol molar ratio (1:4) was used with 1% (wt./wt.) base catalyst (KOH). The methanol and KOH solution was added to the preheated oil. The reaction temperature of 65 °C and stirrer speed of 600 rpm was maintained for 1 h. After the reaction, the resultant mixture was transferred to separating flask and kept for 10 h for phase separation of glycerol and raw castor oil biodiesel (COB). In this process, biodiesel floats at the top and glycerol is settled down at the bottom. Later, the glycerol was drained, and raw biodiesel was subjected to post treatment process with hot distilled water (90 °C). After water wash, it was kept for overnight for settle down of all the water molecules. Biodiesel was placed in an oven at 110 °C temperature for 2 h to remove the excess methanol and moisture. Finally, a dried castor oil biodiesel (COB) was obtained. The methyl ester (Biodiesel) yield was calculated from Equation (4) [33].
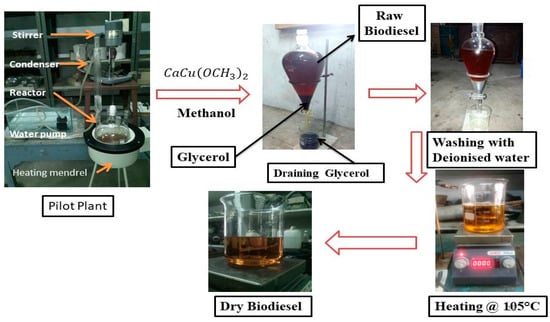
Figure 5.
Five-liter capacity biodiesel production plant.
2.4.3. Transesterification of Pyrolysis Castor Oil (PCO)
The transesterification of PCO was performed with CaCu(OCH3)2 catalyst to produce pyrolyzed castor oil biodiesel (PCOB) as shown in Figure 5. To find the optimized condition for modified catalyst [CaCu(OCH3)2], an experiment was performed in terms of oil-to-methanol ratio, catalyst concentration, reaction time, and temperature. The stirrer speed (600 rpm) was kept constant for all test runs. The production process is detailed in Section 2.4.2.
3. Results and Discussions
3.1. SEM and FTIR Analysis
Figure 6 shows the morphology of the synthesis [CaCu(OCH3)2] catalyst. The particle was found to be stone-type and irregular shaped (Figure 6a). The particle size was not found less than 100 nm as shown Figure 6b; therefore, it cannot be considered as nanoparticle. The particle resembles a cluster due to heating at high temperature for long time. The FTIR spectrum of synthesis [CaCu(OCH3)2] catalyst is shown in Figure 7. It indicates that the important functional groups appeared in the -OH stretching vibration primary free alcohol (3640.972 cm−1), -C-H stretching alkane (3051–2905 cm−1), C=O stretching vibration and -C-C bond stretching (8630 cm−1). Furthermore, it was observed that the unusual peak appeared at 1256 cm−1.
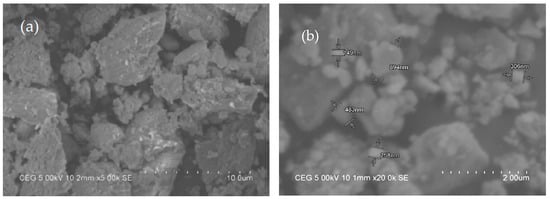
Figure 6.
Morphology of the [CaCu(OCH3)2] catalyst—(a) structure of catalyst, (b) identification of catalyst particle sizes.
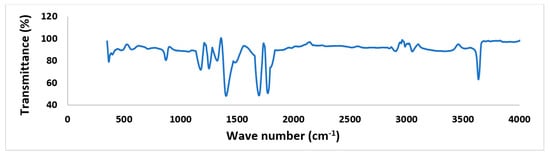
Figure 7.
FTIR spectrum [CaCu(OCH3)2] catalyst.
3.2. Optimization of Operating Parameters
The biodiesel yields and fuel properties of the prepared castor biodiesel from the castor pyrolysis oil are dependent on the type and quantity of catalyst used, methanol-to-oil molar ratio, reaction temperature, reaction time and mixing speed. It is necessary to analyze the effect of a catalyst on yields such as methanol molar ratio, reaction temperature, reaction time and catalyst percentage [33,34]. It was observed that 16:1 methanol molar ratio gave the highest yields and beyond that point, the yields started to reduce (Figure 8a). The required catalyst percentage was measured on weight basis (wt./wt.%), and it was observed that 3% catalyst (i.e., 3% of oil weight) provides maximum yields (Figure 8b). The reaction temperature always plays an important role in the heterogeneous catalyst. It was observed that reaction temperature of 65 °C was optimum to obtain higher yields, beyond that, methanol starts to vaporize and lead to lower yield (Figure 8c). Reaction time is also an important parameter for heterogeneous catalyst to obtain a higher yield. Reaction time of 90 min was found to be best for maximum biodiesel yields (Figure 8d).
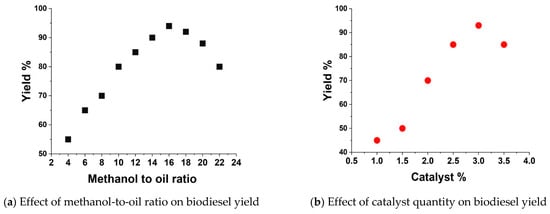
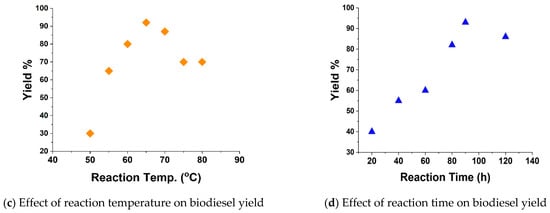
Figure 8.
Optimization of parameters for transesterification process.
3.3. Biodiesel Properties Analysis
The physical and chemical properties of the fuel are important, as they affect the combustion and emission characteristics of the internal combustion engine. The important properties of a fuel for diesel engine application are cetane number, density, viscosity, and calorific value. The oxidation stability is an important parameter, which refers to the long-term storage ability of the fuel without affecting the properties of the fuel.
3.3.1. Cetane Number (CN)
The cetane number (CN) represents the ignition quality of the fuel for diesel engine application. In this study, CN was estimated from the cetane index (CI) value, which was calculated using Equations (5) and (6) [35]. The CN is dimensionless property of diesel fuel; the higher the CN means better combustion inside the engine [35]. Lower CN fuels refer to longer ignition delay (ID), which results in more fuel accumulation and increased energy release in a pre-mixed combustion phase, which promotes thermal NO× [36]. Generally, biodiesel has higher a cetane number than fossil diesel fuel [36]. The cetane index of diesel fuel is in the range of 40–50 [29].
Cetane Index (CI) = (Aniline Point (℉) × Degree of API (60 ℉))/100
Cetane Number = 0.72 × CI + 10
The CN of the PCOB and COB fuels were 45 and 42, which is found within the fuel standards (Table 5). The CN of PCOB was observed to be 7% higher than COB sample due to the presence of higher percentage of SFA in PCOB as shown in Figure 9a. The CN was increased when the saturation level (number of single C-C bond) of the fuel was increased [37]. Sharma et al. (2019) studied the effect of varying saturation level of oil and fuels on the physicochemical properties [37]. They produced biodiesel by mixing edible oil (high in saturation level) into non-edible oil (high in unsaturation level, double C=C bond) and referred to it as “bio-mix” fuel [37]. The authors concluded that the saturation level and cetane number of the bio-mix fuels could be increased by up to 10% and 12% as compared to single feedstock non-edible oil biodiesel [37]. The CN of PCOB was found to be higher than the minimum limit of biodiesel and petroleum standards (Table 5).

Table 5.
Comparison of the biodiesel fuel properties with and without pyrolysis process.
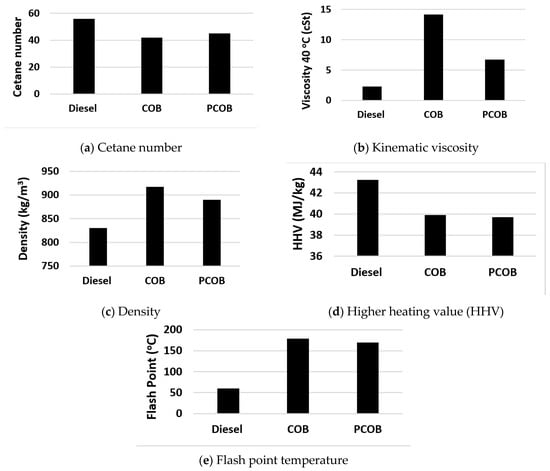
Figure 9.
Fuel properties analysis.
3.3.2. Kinematic Viscosity
Higher viscosity creates resistance to fluid flow [20,37]. The viscosity of the fuel affects fuel injection parameters such as spray penetration, spray cone angle, atomization, and vaporization [38]. High viscosity of fuel causes poor injection performance, which then leads to poor combustion inside the engine cylinder. The viscosity of neat castor oil is high (about 38 mm2/s) due to its higher viscosity; neat CO cannot be used as fuel in the internal combustion (IC) engine. The viscosity of the neat CO can be reduced either by preheating the CO before injecting into the engine or by converting neat CO into COB [20,37]. The viscosity of the PCOB was measured as 4.8 cSt, which is 52% lower than the COB (Figure 9b). The viscosity of PCOB was found within the biodiesel standards limit, but it is higher than the petroleum standard limits as shown in Table 5. The decrease in viscosity of PCOB is mainly due to the breakdown of longer carbon–carbon chains into shorter carbon chains during pyrolysis [27,37].
3.3.3. Density
The density of biodiesel is higher than fossil diesel fuel. This means higher mass of biodiesel is injected per cycle when compared to fossil diesel operation. The fuel density affects the air–fuel ratio, ignition delay, in-cylinder temperature, and engine out emissions [24,37]. The density of the PCOB was measured at 890 kg/m3, which is 3% lower than that of COB (Figure 9c). The density of PCOB was also found within the biodiesel standards limits, but it is higher than petroleum standard limits as shown in Table 5.
3.3.4. Higher Heating Value (HHV)
HHV refers to the heat of combustion of fuel. The HHV of biodiesel is lower than fossil diesel due to the presence of additional oxygen in biodiesel fuel [27,37]. The specific fuel consumption is directly affected by the HHV of the fuel; the lower the HHV, the higher the fuel consumption will be, as more fuels need to be combusted to produce the same engine power output [27,37]. No significant difference was observed in HHV (Figure 9d) as given in Table 5.
3.3.5. Flash Point and Iodine Value
Flash point (FP) is mainly referred to as the storage and handling of fuels. It is observed that flash point of PCOB is 170 °C, which is higher than the minimum the biodiesel standard limits (EN3679). FP of PCOB was observed to be 5% lower than COB (Figure 9e). The reduction in FP is mainly due to breakdown of long carbon chains into small ones [24,37]. Iodine value (IV) generally defines the unsaturation fatty acids present (USFA) in the oil. The higher the IV, the higher the USFA, which is normally discovered through a titration method. It is observed that IV of PCOB is 80 gl2/100 g, which is within the standard limit. The IV of PCOB was found to be 6% lower than COB. This 6% reduction in IV refers to the decreases in USFA comply with COB as shown in Table 5.
3.3.6. Oxidation Stability
Oxidation stability (OS) of the prepared biodiesel was measured through the Rancimat apparatus using EN14112 standard [38]. The number of double bonds or higher unsaturation property of the fuels [38] accelerates oxidation stability. Higher unsaturated fatty acids (USFA) react with oxygen molecules and increase the moisture content in the biodiesel [38]. The OS increases with the increase in amount of saturated fatty acids (SFA) and the low percentage of fuel bound oxygen molecules [38]. The OS of PCOB was measured to be 18.5 h, which is 42% higher than that of COB. The OS of PCOB was found to be higher than the minimum limit of biodiesel standards. It was observed that PCOB provides 10% higher OS as compared to COB (Table 5).
4. Conclusions
In this study, a combined pyrolysis and transesterification process was used to produce high quality castor oil biodiesel fuel. For pyrolysis of castor oil, a batch type electrically heated pyrolysis reactor was used. Then, the pyrolyzed oil was transesterified using a synthesis heterogeneous catalyst. The transesterification reaction parameters were optimized for maximum biodiesel yield. Generally, in India, mechanical extraction is preferable to the extract oil from the seeds. In the mechanical extraction process, the waste of the oil seed, which emerges in the form of dry chips, is used as animal feed. Therefore, the oil was directly used for pyrolysis. The main findings of the study are summarized below:
- (1)
- Heterogeneous catalyst was prepared from waste eggshell and synthesis with CuO. The size was studied through SEM and found to be larger than 100 nm; therefore, it was used as catalyst instead of nano-catalyst;
- (2)
- Castor biodiesel fuel properties were improved through the combined pyrolysis and transesterification process;
- (3)
- Compared to un-pyrolysed condition, the cetane number and oxidation stability of PCOB were improved, while viscosity, density, flash point, and iodine value were reduced;
- (4)
- The viscosity of PCOB is higher than the automotive biodiesel standard; hence, it can be used in marine engine application.
This study suggested that thermal cracking of castor oil prior to biodiesel conversion is a suitable method to produce CO biodiesel fuel for diesel engine application. The heterogeneous catalyst can be reused; therefore, study on the reusability of the CaCu(OCH3)2 catalyst is recommended as future work. Furthermore, characterization of synthesis CaCu(OCH3)2 catalyst such as XRD, HR-TEM, BET, and CO2-TPD is also suggested as other items for future investigation. Investigation of pyrolysis process at different operating conditions and use of various catalyst are recommended. Use of PCOB in the engine is also recommended as further work.
Author Contributions
Conceptualization, V.S.; Data curation, V.S.; Formal analysis, V.S., A.K.H. and M.V.; Funding acquisition, A.K.H. and G.D.; Investigation, V.S. and G.D.; Methodology, V.S., A.K.H. and G.D.; Project administration, M.V.; Resources, G.D. and M.V.; Supervision, A.K.H. and G.D.; Visualization, V.S. and M.V.; Writing—original draft, V.S.; Writing—review & editing, A.K.H. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the DST-UKIERI project (grant number: DST-UKIERI 18-19-04): Waste to Energy-Low Temperature Combustion of Sustainable Green Fuels.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
There are no conflict of interests. The funder was not involved in the study’s design, data collection, analysis, or interpretation; manuscript preparation; or the decision to publish the findings.
Abbreviations
| AOCS | American Oil Chemists’ Society |
| BSFC | brake specific fuel consumption |
| BTE | brake thermal efficiency |
| CN | cetane number |
| COB | castor oil biodiesel |
| CO | castor oil |
| CV | calorific value |
| FP | flash point |
| HHV | higher heating value |
| IV | iodine value |
| NOx | oxides of nitrogen |
| OS | oxidation stability |
| SFA% | saturated fatty acids |
| TCOB | thermal cracked castor oil biodiesel |
| USFA | unsaturated fatty acids |
References
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Chen, G.-B.; Li, Y.-H.; Wu, W.-T. Effects of catalysts on pyrolysis of castor meal. Energy 2017, 119, 1–9. [Google Scholar] [CrossRef]
- Manojkumar, N.; Muthukumaran, C.; Sharmila, G. A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. J. King Saud Univ.-Eng. Sci. 2020. [Google Scholar] [CrossRef]
- Renish, R.R.; Selvam, M.A.J. A critical review on production process, physicochemical properties, performance, and emission characteristics of sea mango biodiesel-diesel blends. Mater. Today Proc. 2021, 44, 2600–2605. [Google Scholar] [CrossRef]
- Estevez, R.; Deblas, L.M.A.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Kochaphum, C.; Gheewala, S.H.; Vinitnantharat, S. Does biodiesel demand affect palm oil prices in Thailand? Energy Sustain. Dev. 2013, 17, 658–670. [Google Scholar] [CrossRef]
- Bin Mohiddin, M.N.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Parvizsedghy, R.; Sadrameli, S.M. Thermal Cracking Approach Investigation to Improve Biodiesel Properties. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2015, 9, 805–809. [Google Scholar] [CrossRef]
- India National Biofuel Policy 2018. 2020. Available online: http://www.cbip.org/Policies2019/PD_07_Dec_2018_Policies/1_MNRE/2-Biofuel/2OrderBio-FuelPolicy.pdf (accessed on 6 March 2020).
- Mishra, S.; Anand, K.; Mehta, P.S. Predicting the Cetane Number of Biodiesel Fuels from Their Fatty Acid Methyl Ester Composition. Energy Fuels 2016, 30, 10425–10434. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.H.; Abu-Elyazeed, O.S.M.; El Mawla, E.A.; Abdelazeem, M.A. On biodiesels from castor raw oil using catalytic pyrolysis. Energy 2018, 143, 950–960. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Lei, S.; Qin, S.; Li, B.; Zhao, C. Pt/HAP catalyzed direct decarboxylation of lipid to alkanes via stabilization and synergism effect. J. Catal. 2021, 400, 244–254. [Google Scholar] [CrossRef]
- Aboelazayem, O.; El-Gendy, N.S.; Abdel-Rehim, A.A.; Ashour, F.; Sadek, M.A. Biodiesel production from castor oil in Egypt: Process optimisation, kinetic study, diesel engine performance and exhaust emissions analysis. Energy 2018, 157, 843–852. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Figueiredo, M.-K.; Romeiro, G.; Damasceno, R. Low temperature conversion (LTC) of castor seeds—A study of the oil fraction (pyrolysis oil). J. Anal. Appl. Pyrolysis 2009, 86, 53–57. [Google Scholar] [CrossRef]
- Singh, R.; Shadangi, K. Liquid fuel from castor seeds by pyrolysis. Fuel 2011, 90, 2538–2544. [Google Scholar] [CrossRef]
- Koul, M.; Shadangi, K.P.; Mohanty, K. Effect of catalytic vapour cracking on fuel properties and composition of castor seed pyrolytic oil. J. Anal. Appl. Pyrolysis 2016, 120, 103–109. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.; Roberts, W.; Dibble, R. Feasibility of using less viscous and lower cetane (LVLC) fuels in a diesel engine: A review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A.; Estevez, R. Diethyl Ether as an Oxygenated Additive for Fossil Diesel/Vegetable Oil Blends: Evaluation of Performance and Emission Quality of Triple Blends on a Diesel Engine. Energies 2020, 13, 1542. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Tseng, W.-S.; Wu, C.-T.; Lin, C.-P. Dwarf-castor Oil made into a Suitable Biodiesel. Procedia Eng. 2014, 84, 940–947. [Google Scholar] [CrossRef][Green Version]
- Das, M.; Sarkar, M.; Datta, A.; Santra, A.K. An experimental study on the combustion, performance and emission characteristics of a diesel engine fuelled with diesel-castor oil biodiesel blends. Renew. Energy 2018, 119, 174–184. [Google Scholar] [CrossRef]
- Thakkar, K.; Kachhwaha, S.S.; Kodgire, P.; Srinivasan, S. Combustion investigation of ternary blend mixture of biodiesel/n-butanol/diesel: CI engine performance and emission control. Renew. Sustain. Energy Rev. 2021, 137, 110468. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.; Alam, M.A.; Fattah, I.M.R.; Ashraful, A.; Mobarak, H. Biodiesel production and performance evaluation of coconut, palm and their combined blend with diesel in a single-cylinder diesel engine. Energy Convers. Manag. 2014, 87, 250–257. [Google Scholar] [CrossRef]
- Patel, C.; Chandra, K.; Hwang, J.; Agarwal, R.A.; Gupta, N.; Bae, C.; Gupta, T.; Agarwal, A.K. Comparative compression ignition engine performance, combustion, and emission characteristics, and trace metals in particulates from Waste cooking oil, Jatropha and Karanja oil derived biodiesels. Fuel 2019, 236, 1366–1376. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Ganesan, S.; Kumar, B.R.; Saravanan, S. Utilization of waste cooking oil in a light-duty DI diesel engine for cleaner emissions using bio-derived propanol. Fuel 2019, 235, 832–837. [Google Scholar] [CrossRef]
- Rashed, M.; Kalam, A.; Masjuki, H.; Mofijur, M.; Rasul, M.; Zulkifli, N. Performance and emission characteristics of a diesel engine fueled with palm, jatropha, and moringa oil methyl ester. Ind. Crop. Prod. 2016, 79, 70–76. [Google Scholar] [CrossRef]
- Auti, S.; Rathod, W. Effect of hybrid blends of raw tyre pyrolysis oil, karanja biodiesel and diesel fuel on single cylinder four stokes diesel engine. Energy Rep. 2021, 7, 2214–2220. [Google Scholar] [CrossRef]
- Raman, L.A.; Deepanraj, B.; Rajakumar, S.; Sivasubramanian, V. Experimental investigation on performance, combustion and emission analysis of a direct injection diesel engine fuelled with rapeseed oil biodiesel. Fuel 2019, 246, 69–74. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Sassykova, L.R.; Prabhahar, M.; Bhaskar, K.; Kannayiram, G.; Subramanian, S.; Prakash, S. Influence of saturated fatty acid material composition in biodiesel on its performance in internal combustion engines. Mater. Today Proc. 2020, 33, 1181–1186. [Google Scholar] [CrossRef]
- Kamaronzaman, M.F.F.; Kahar, H.; Hassan, N.; Hanafi, M.F.; Sapawe, N. Biodiesel production from waste cooking oil using nickel doped onto eggshell catalyst. Mater. Today Proc. 2020, 31, 342–346. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Green nano-catalyst for methanolysis of non-edible Jatropha oil. Energy Convers. Manag. 2014, 87, 618–627. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Selvan, V.A.M. Experimental investigation on the effects of micro eggshell and nano-eggshell catalysts on biodiesel optimization from waste chicken fat. Bioresour. Technol. Rep. 2021, 14, 100658. [Google Scholar] [CrossRef]
- Sharma, V.; Duraisamy, G. Production and characterization of bio-mix fuel produced from a ternary and quaternary mixture of raw oil feedstock. J. Clean. Prod. 2019, 221, 271–285. [Google Scholar] [CrossRef]
- Niazmahmud. Cetane Number Prediction 2012. 2020. Available online: https://www.slideshare.net/NIAZMAHMUD/aniline-point-for-petroleum-productes (accessed on 10 September 2020).
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a waste cooking oil biodiesel mixture. Energy Convers. Manag. 2014, 87, 676–686. [Google Scholar] [CrossRef]
- Sharma, V.; Duraisamy, G. Production and characterization of bio-mix fuel produced from the mixture of raw oil feedstock, and its effects on performance and emission analysis in DICI diesel engine. Environ. Sci. Pollut. Res. 2019, 26, 16742–16761. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Li, F.; Wang, H.; Ma, X.; Li, J.; Zhou, L.; Xiao, Q. Effects of unsaturated fatty acid methyl esters on the oxidation stability of biodiesel determined by gas chromatography-mass spectrometry and information entropy methods. Renew. Energy 2021, 175, 880–886. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).