Non-Catalytic Dissolution of Biochar Obtained by Hydrothermal Carbonization of Sawdust in Hydrogen Donor Solvent
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Biochar and Its Characteristics
3.2. Texture
3.3. TGA–DTG–DSC Data
3.4. Raman Spectroscopy of Sawdust and Biochar
3.5. Thermal Dissolution of Biochar
3.6. Composition of the Liquid Products of the Thermal Dissolution of Biochar
3.7. Composition of Gases Obtained after the Thermal Dissolution of Biochar
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yılmaz, S.; Selim, H. A review on the methods for biomass to energy conversion systems design. Renew. Sustain. Energy Rev. 2013, 25, 420–430. [Google Scholar] [CrossRef]
- Stranges, A.N. Friedrich bergius and the rise of the german synthetic fuel industry. Isis 1984, 75, 643–667. Available online: www.jstor.org/stable/232411 (accessed on 5 August 2021). [CrossRef]
- Alagidede, P.; Adu, G.; Frimpong, P.B. The effect of climate change on economic growth: Evidence from Sub-Saharan Africa. Environ. Econ. Pol. Stud. 2016, 183, 417–436. [Google Scholar] [CrossRef] [Green Version]
- Angulo-Mosquera, L.S.; Alvarado-Alvarado, A.A.; Rivas-Arrieta, M.J.; Cattaneo, C.R.; Rene, E.R.; García-Depraect, O. Production of solid biofuels from organic waste in developing countries: A review from sustainability and economic feasibility perspectives. Sci. Total Environ. 2021, 795, 148816. [Google Scholar] [CrossRef]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct liquefaction of biomass. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Krysanova, K.O.; Krylova, A.Y.; Zaichenko, V.M. Application of hydrothermal carbonization to improve the energy properties of peat. Solid Fuel Chem. 2021, 55, 123–128. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Nimz, H.H.; Schmitt, U.; Schwab, E.; Wittmann, O.; Wolf, F. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000. [Google Scholar]
- Baruya, P. World Forest and Agricultural Crop Residue Resources for Cofiring; IEA Clean Coal Centre: London, UK, 2015. [Google Scholar]
- Koopmans, A.; Koppejan, J. Agricultural and forest residues: Generation, utilization and availability. In Proceedings of the Regional Consultation on Modern Applications of Biomass Energy, Kuala Lumpur, Malaysia, 6–10 January 1997. [Google Scholar]
- Cocchi, M. Global Wood Pellet Industry Market and Trade Study. IEA Bioenergy Task 40: Sustainable International Bioenergy Trade; International Energy Agency: Paris, France, 2011. [Google Scholar]
- Bergius, F. Die Anwendung Hoher Drücke Bei Chemischen Vorgängen und Eine Nachbildung des Entstehungsprozesses der Steinkohle; W. Knapp: Berlin, Germany, 1913; pp. 2–58. [Google Scholar]
- Leibniz, E.; Simon, A. Zum fünfundsechzigsten Geburtstage. Prakt. Chem. 1958, 5, 209–211. [Google Scholar] [CrossRef]
- Method of Obtaining Coal from Biowaste. Available online: http://www.ecotoc.ru/traditional/ugol/d742/ (accessed on 5 August 2021).
- Lu, X.; Yamauchi, K.; Phaiboonsilpa, N. Two-step hydrolysis of Japanese beech as treated by semi-flow hot-compressed water. J. Wood Sci. 2009, 55, 367–375. [Google Scholar] [CrossRef]
- Brown, R.C. Biorenewable Resources: Engineering New Products from Agriculture; Iowa State Press: Ames, IA, USA, 2003; pp. 30–37. [Google Scholar]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, N.; Feiner, R.; Pucher, H.; Ellmaier, L.; Ritzberger, J.; Treusch, K.; Pucher, P.; Siebenhofer, M. Biomass pyrolysis refinery. Chem. Ing. Technol. 2015, 87, 803–809. [Google Scholar] [CrossRef]
- Li, C.; Ashida, S.; Iino, M.; Takanohashi, T. Coal dissolution by heat treatments in nmethyl-2-pyrrolidinone, 1, 4, 5, 8, 9, 10-hexahydroanthracene, and their mixed solvents: A large synergistic effect of the mixed solvents. Energy Fuels 2000, 14, 190–196. [Google Scholar] [CrossRef]
- Guin, J.; Tarrer, A.; Taylor, L.; Prather, J.; Green, S. Mechanisms of coal particle dissolution. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 490–494. [Google Scholar] [CrossRef]
- Shalabi, M.A.; Baldwin, R.M.; Bain, R.L.; Gary, J.H.; Golden, J.O. Noncatalytic coal liquefaction in a donor solvent. Rate of formation of oil, asphaltenes, and preasphaltenes. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 474–479. [Google Scholar] [CrossRef]
- Connors, W.; Johanson, L.; Sarkanen, K.; Winslow, P. Thermal degradation of kraft lignin in tetralin. Holzforschung 1980, 34, 29–37. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kuznetsova, L.I.; Buryukin, F.A.; Marakushina, E.N.; Frizorger, V.K. Methods for the preparation of coal-tar pitch. Solid Fuel Chem. 2015, 49, 213–225. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Marakushina, E.N.; Kazbanova, A.V.; Kolesnikova, S.M.; Kuznetsova, L.I.; Buryukin, F.A.; Kositcyna, S.S. Getting an alternative pitch binder by thermal dissolution of coal. Am. J. Appl. Sci. 2016, 13, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Weller, S.; Pelipetz, M.G.; Friedman, S. Kinetics of coal hydrogenation conversion of asphalt. Ind. Eng. Chem. 1951, 43, 1572–1575. [Google Scholar] [CrossRef]
- Weller, S.; Clark, E.L.; Pelipetz, M.G. Mechanism of coal hydrogenation. Ind. Eng. Chem. 1950, 42, 334–336. [Google Scholar] [CrossRef]
- Weller, S.; Pelipetz, M.G. Coal hydrogenation catalysts studies of catalyst distribution. Ind. Eng. Chem. 1951, 43, 1243–1246. [Google Scholar] [CrossRef]
- Ancheyta, J.; Trejo, F.; Rana, M.S. Asphaltenes: Chemical Transformation during Hydroprocessing of Heavy Oils, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 213–231. [Google Scholar]
- Kaneko, T.; Derbyshire, F.; Makino, E.; Gray, D.; Tamura, M. Coal Liquefaction. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: New York, NY, USA, 2001; pp. 1–83. [Google Scholar]
- Vasireddy, S.; Morreale, B.; Cugini, A. Clean liquid fuels from direct coal liquefaction: Chemistry, catalysis, technological status and challenges. Energy Environ. Sci. 2011, 4, 311–345. [Google Scholar] [CrossRef]
- Haenel, M.W. Catalysis in direct coal liquefaction. In Handbook of Heterogeneous Catalysis; Wiley: New York, NY, USA, 2008; pp. 3023–3036. [Google Scholar]
- Feiner, R.; Schwaiger, N.; Pucher, H.; Ellmaier, L.; Pucher, P.; Siebenhofer, M. Liquefaction of pyrolysis derived biochar: A new step towards biofuel from renewable resources. RSC Adv. 2013, 3, 17898–17903. [Google Scholar] [CrossRef]
- Kundu, R.; Ramsurn, H. Non-catalytic dissolution of biochar in hydrogen donor solvent. J. Anal. Appl. Pyrolysis 2019, 140, 227–238. [Google Scholar] [CrossRef]
- Trautmann, M.; Lang, S.; Traa, Y. Direct liquefaction of lower-rank coals and biocoals with magnetically separable catalysts as a sustainable route to fuels. Fuel 2015, 151, 102–109. [Google Scholar] [CrossRef]
- Wang, C.; Pan, J.; Li, J.; Yang, Z. Comparative studies of products produced from four different biomass samples via deoxy-liquefaction. Bioresour. Technol. 2009, 99, 2778–2786. [Google Scholar] [CrossRef]
- Trautmann, M.; Löwe, A.; Traa, Y. An alternative method for the production of second-generation biofuels. Green Chem. 2014, 16, 3710–3714. [Google Scholar] [CrossRef]
- Feiner, R.; Schwaiger, N.; Pucher, H.; Ellmaier, L.; Reiter, A.; Derntl, M.; Glatz, T.; Pucher, P.; Siebenhofer, M. Kinetics of biochar liquefaction. BioEnergy Res. 2014, 7, 1343–1350. [Google Scholar] [CrossRef]
- Kundu, R.; Ramsurn, H. Kinetic study of noncatalytic dissolution of cellulose biochar in hydrogen donor solvent. ACS Sustain. Chem. Eng. 2020, 8, 11606–11617. [Google Scholar] [CrossRef]
- Poudel, S.; Oh, S.J. Effect of torrefaction on the properties of corn stalk to enhance solid fuel qualities. Energies 2014, 7, 5586–5600. [Google Scholar] [CrossRef] [Green Version]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–555. [Google Scholar] [CrossRef]
- Huang, Y.; Rolfe, A.; Rezvani, S.; Hidalgo, H.; José, M.; Franco, F.; Pinto, F.; Snape, C.; Hewitt, N. Converting brown coal to synthetic liquid fuels through direct coal liquefaction technology: Techno-economic evaluation. Int. J. Energy Res. 2020, 44, 11827–11839. [Google Scholar] [CrossRef]
- Koriakin, A.; Moon, S.; Kim, D.W.; Lee, C.H. Liquefaction of oil palm empty fruit bunch using sub- and supercritical tetralin, n-dodecane, and their mixture. Fuel 2017, 208, 184–192. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Liang, M.; Lu, W.; Lei, P.; Wang, L.; Wang, B.; Li, B.; Shen, Y.; Zhang, K. Physical and combustion properties of binder-assisted hydrochar pellets from hydrothermal carbonization of tobacco stem. Waste Biomass Valorization 2020, 11, 6369–6382. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, Q.; Zhu, M.; Sun, G.; Zhang, T.; Kang, K. Comparative evaluation of hydrothermal carbonization and low temperature pyrolysis of eucommia ulmoides oliver for the production of solid biofuel. Sci. Rep. 2019, 9, 5535. [Google Scholar] [CrossRef] [Green Version]
- Kambo, H.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass—Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Maryandyshev, P.A.; Popova, E.I.; Chernov, A.A.; Popov, M.S.; Lyubov, V.K.; Trouve, G.; Kehrli, D.; Brillard, A.; Brilhac, J.-F. Thermal decomposition and combustion of biofuels. Solid Fuel Chem. 2017, 51, 370–378. [Google Scholar] [CrossRef]
- Edwards, G.M.; de Olivieira, L.F.C.; Nesbitt, M. Fourier-transform Raman characterization of brazilwood trees and substitutes. Analyst 2003, 128, 82–87. [Google Scholar] [CrossRef]
- Quirico, E.; Bonal, L.; Montagnas, G.; Beck, P.; Reynard, B. New insights into the structure and formation of coals, terrestrial and extraterrestrial kerogens from resonant UV Raman spectroscopy. Geochim. Cosmoscim. Acta 2020, 282, 156–176. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Du, S.; Shen, K.; Yang, Q.; Yi, H.; Zhang, J. Transformation Characteristics of Hydrogen-Donor Solvent Tetralin in the Process of Direct Coal Liquefaction. Front. Chem. 2019, 7, 737. [Google Scholar] [CrossRef]
- Norlin, L.-H. Tall Oil. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Dorrestijn, E.; Mulder, P. The radical-induced decomposition of 2-methoxyphenol. J. Chem. Soc. Perkin Trans. 1999, 2, 777–780. [Google Scholar] [CrossRef]
- Hatate, Y. Decomposition Behavior of Plant Biomass in Hot-Compressed Water. Ind. Eng. Chem. Res. 2009, 39, 3688–3693. [Google Scholar]
- Yu, Y.; Lou, X.; Wu, H. Some recent advances in hydrolysis of biomass in hot-compressed water and its comparisons with other hydrolysis methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Loppinet-Serani, A.; Aymonier, C.; Cansell, F. Supercritical water for environmental technologies. J. Chem. Technol. Biotechnol. 2010, 85, 583–589. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, H.J.; Ramaswamy, S. Reaction kinetics of the hydrothermal treatment of lignin. Appl. Biochem. Biotechnol. 2008, 147, 119–131. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Robert, J.H.; Hendrik, A.J.B.; David, G.E. Thermal dissociation of tetralin between 300 and 450 °C. Fuel 1979, 58, 132–138. [Google Scholar]
- Harris, G.C.; Sanderson, T.F. Resin acids; the position of the ring double bond in dextropimaric acid and the structure of isodextropimaric acid. J. Am. Chem. Soc. 1948, 70, 2081–2085. [Google Scholar] [CrossRef]
- Shui, H.; Cai, Z.; Xu, C. Recent Advances in Direct Coal Liquefaction. Energies 2010, 3, 155–170. [Google Scholar] [CrossRef]
- De Marco Rodriguez, I.; Chomón, M.J.; Caballero, B.; Arias, P.L.; Legarreta, J.A. Liquefaction behavior of a Spanish subbituminous coal under different conditions of hydrogen availability. Fuel Proc. Technol. 1998, 58, 17–24. [Google Scholar] [CrossRef]
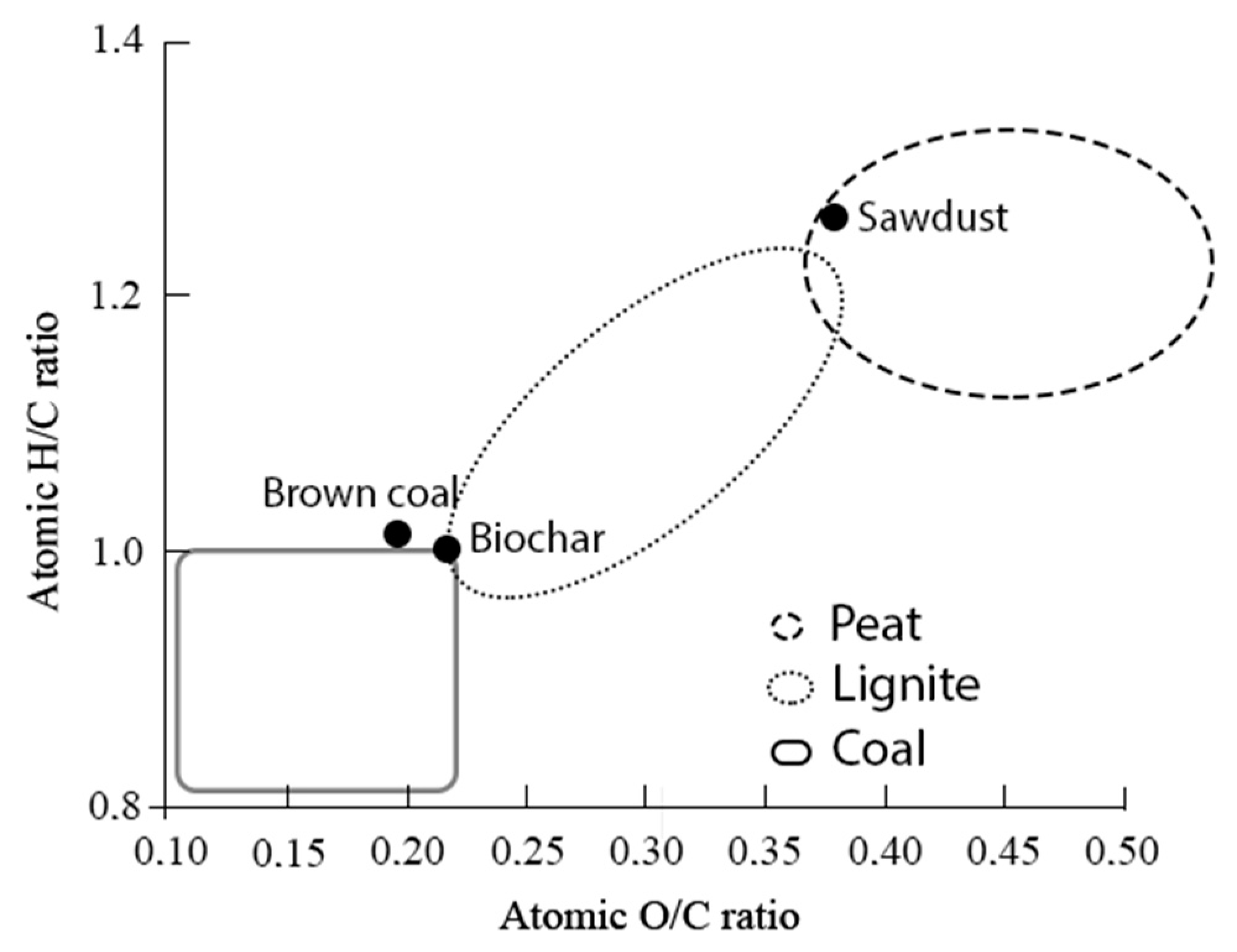
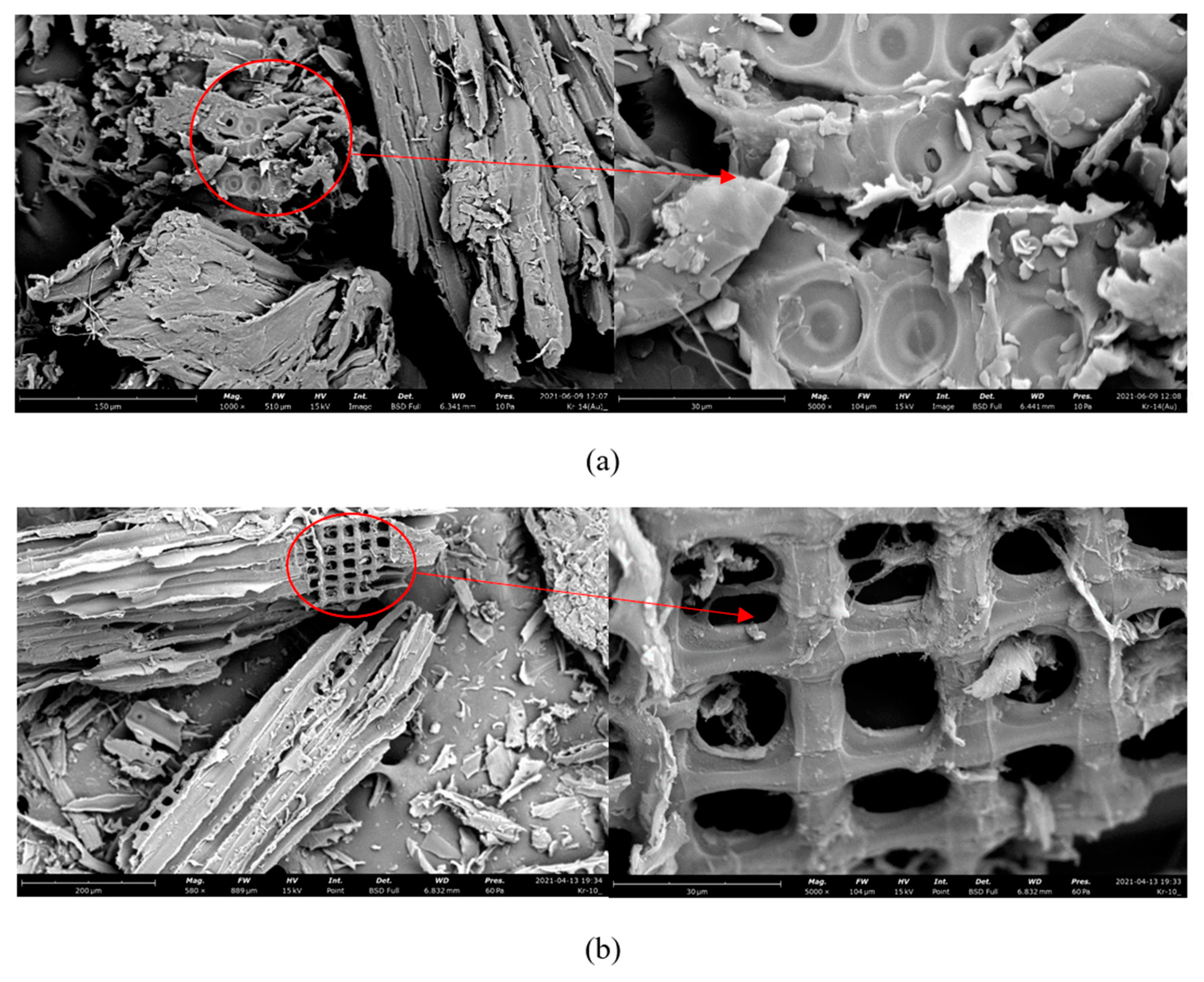
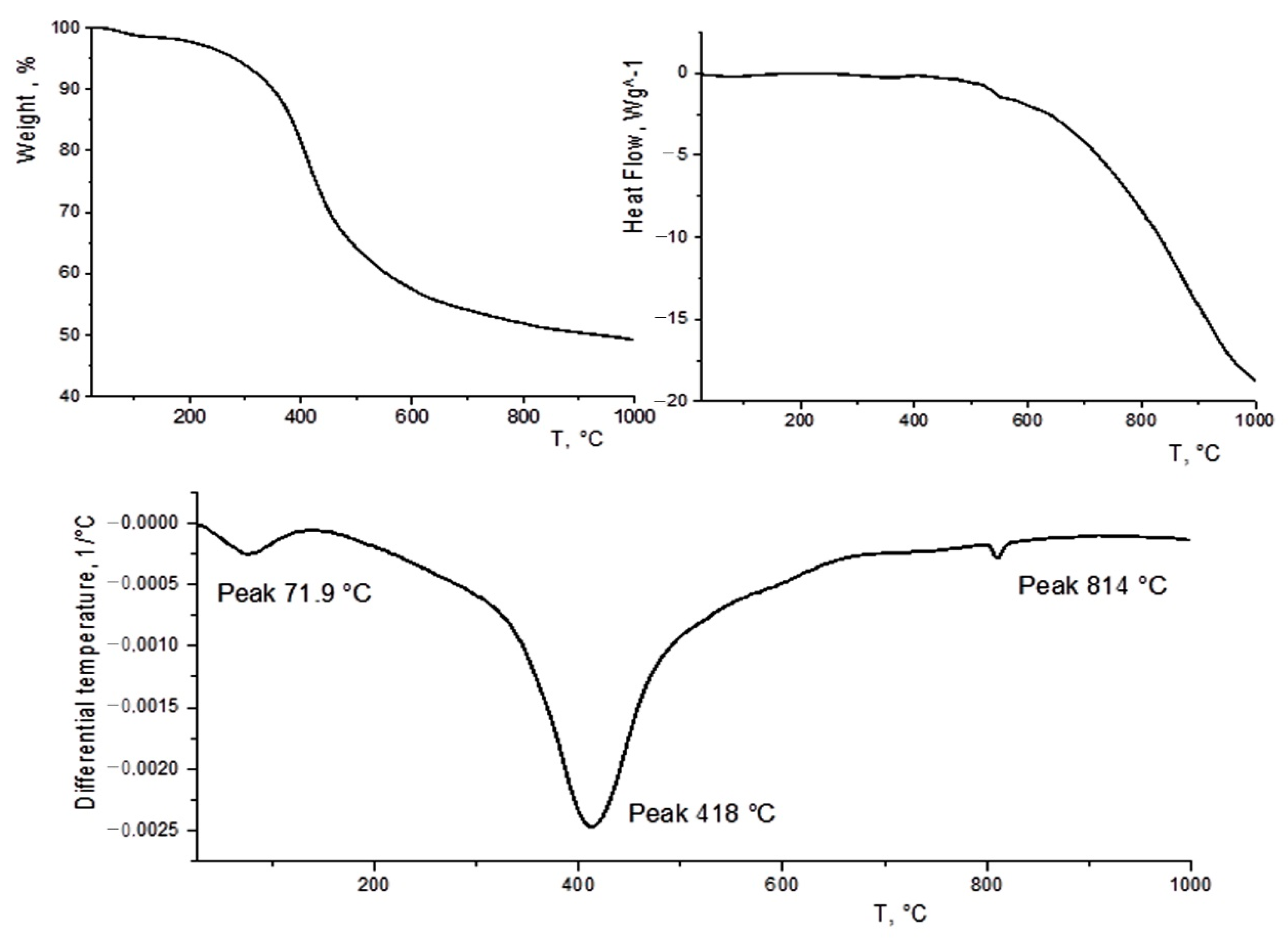

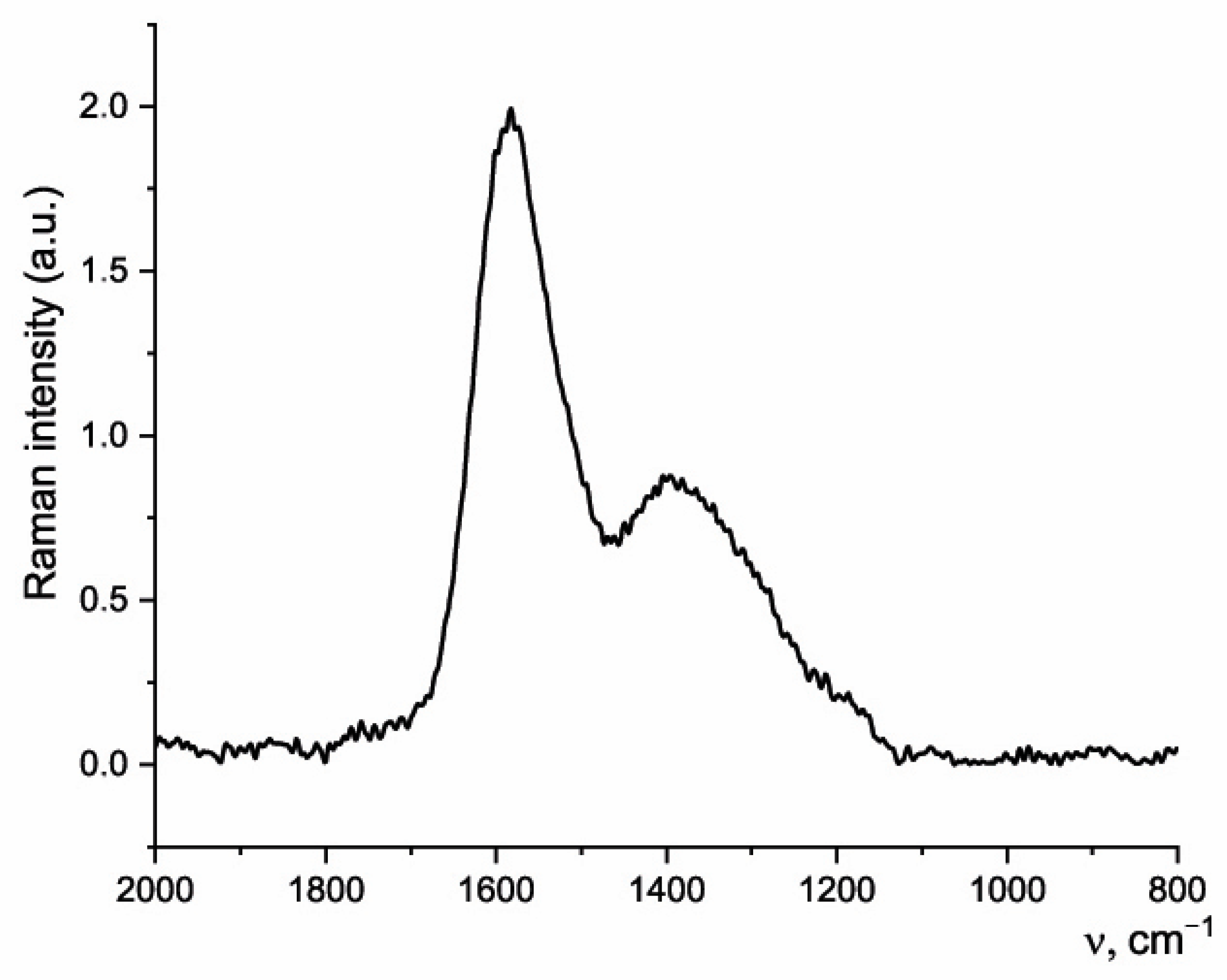
| Properties | Sawdust | Biochar | Brown Coal [46] |
|---|---|---|---|
| Proximate analysis (wt.% on dry basis). | |||
| Volatile matter (%) | 71.53 | 54.71 | 53.86 |
| Fixed carbon (%) | 26.33 | 42.67 | 41.92 |
| Ash content (%) | 1.60 | 2.62 | 4.23 |
| Ultimate analysis (wt.% daf). | |||
| Carbon (%) | 64.35 | 73.13 | 75.3 |
| Hydrogen (%) | 5.95 | 5.27 | 6.4 |
| Nitrogen (%) | 0.66 | 0.15 | 1.3 |
| Sulfur (%) | 0.07 | 0.11 | 1 |
| Oxygen (%) | 28.96 | 21.34 | 16.0 |
| HHV | 25.72 | 28.33 | 24.58 |
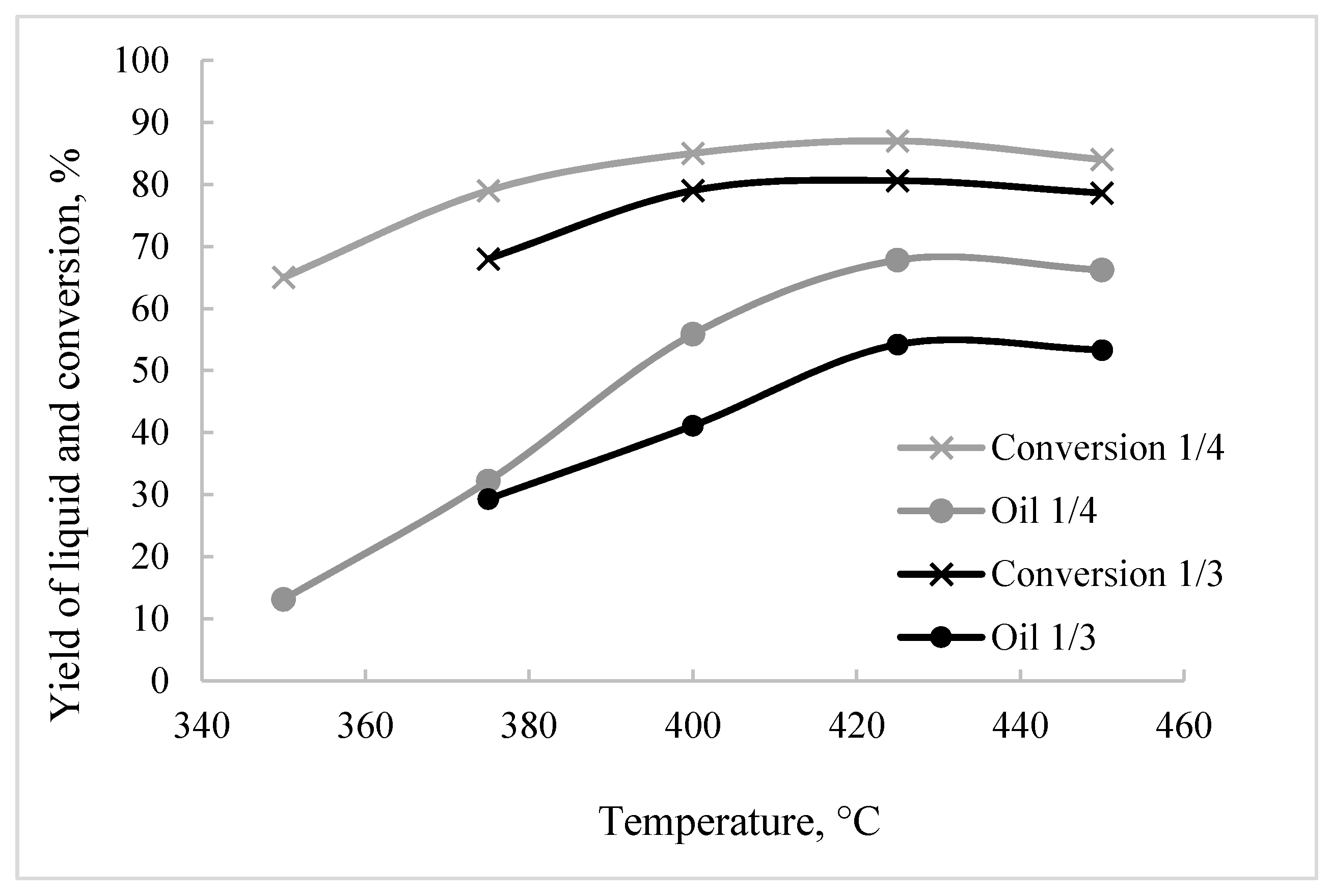
| Raw Materials/Tetralin wt./wt. | T, °C | Conversion, % | Product Composition, % | |||
|---|---|---|---|---|---|---|
| A | PA | Oil | Gases | |||
| 1/4 | 350 | 65 | 25 | 27 | 13 | 0.03 |
| 375 | 79 | 18 | 29 | 32 | 0.09 | |
| 400 | 82 | 16 | 27 | 42 | 0.17 | |
| 425 | 87 | 12 | 7 | 68 | 0.23 | |
| 450 | 84 | 17 | 2 | 66 | 0.29 | |
| 1/3 | 375 | 68 | 24 | 32 | 29 | 0.06 |
| 400 | 79 | 20 | 28 | 41 | 0.16 | |
| 425 | 80 | 18 | 16 | 54 | 0.22 | |
| 450 | 78 | 16 | 14 | 53 | 0.29 | |
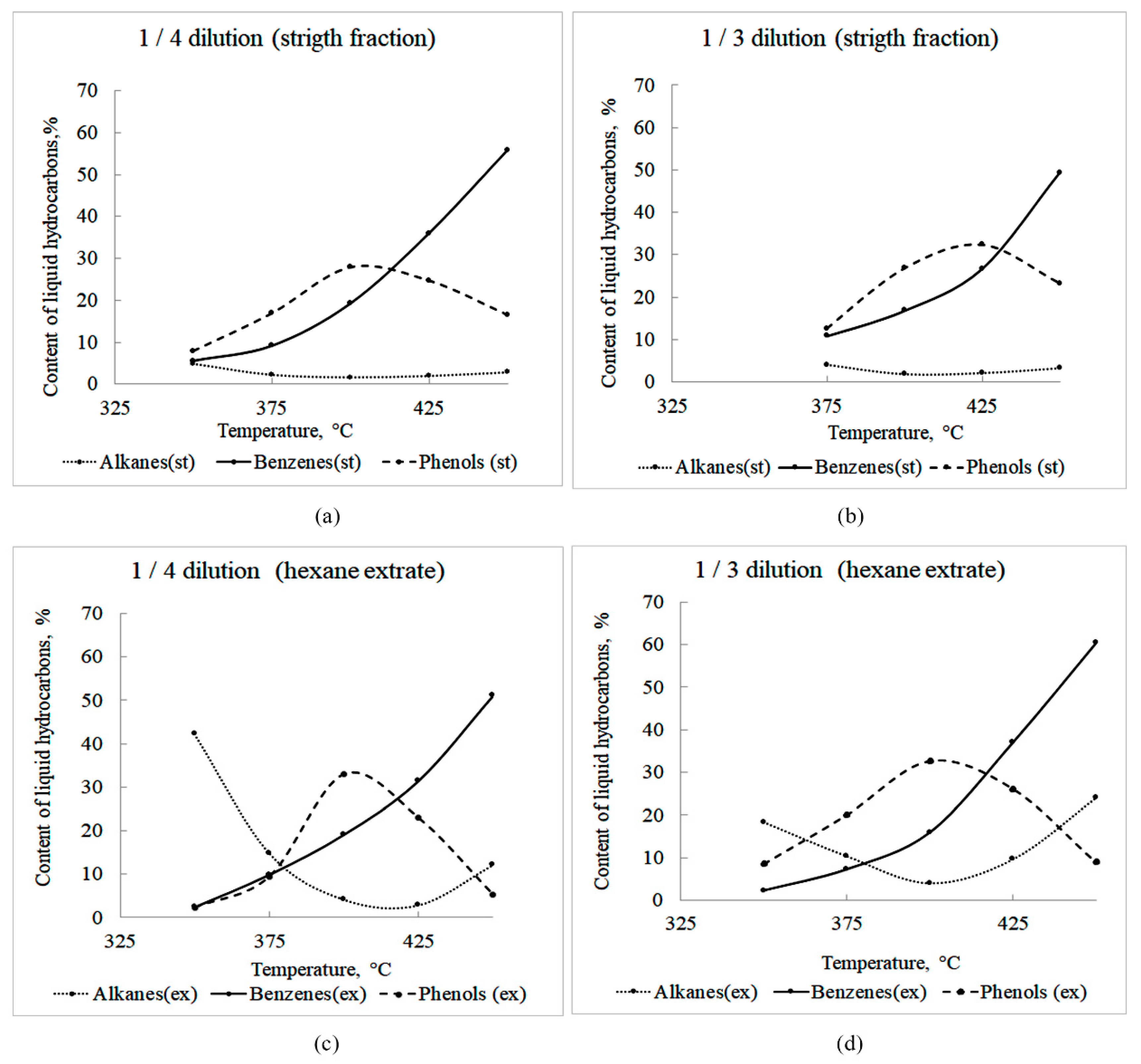
| Raw Materials/Tetralin wt./wt. | Fraction | Substance | C (%) at Temperature, °C | ||||
|---|---|---|---|---|---|---|---|
| 350 | 375 | 400 | 425 | 450 | |||
| 1/4 | Direct | Alkanes | 4.8 | 2.2 | 1.6 | 1.9 | 2.8 |
| Alkenes + napthenes | 1.2 | 1.5 | 1.4 | 2.0 | 3.2 | ||
| Decalines. bicyclicls. dienes | 29 | 15 | 11 | 7.4 | 6.6 | ||
| Benzenes | 5.5 | 9.1 | 19 | 35 | 55 | ||
| Biphenyls | 2.3 | 2.6 | 2.9 | 2.7 | 2.6 | ||
| Fluorenes | 0 | 0.2 | 0 | 0.01 | 0 | ||
| Polyaromatics | 2.1 | 5.9 | 7.9 | 6.5 | 2.7 | ||
| Phenols | 7.9 | 16 | 27 | 24 | 16 | ||
| Benzenediols +gauiacols | 19 | 11 | 5.4 | 4.8 | 0.1 | ||
| Benzofuranes | 0.9 | 1.0 | 0.2 | 0.13 | 0 | ||
| Abietates | 10 | 8.5 | 2.7 | 2.0 | 1.2 | ||
| Naphthols | 17 | 11 | 7.3 | 5.8 | 5.1 | ||
| Naphthalenones | 0 | 14 | 10 | 6.1 | 2.5 | ||
| Alk + Ben/Ph Ratio | 3.4 | 0.7 | 0.7 | 1.5 | 3.6 | ||
| 1/4 | Extract | Alkanes | 42 | 15 | 4.1 | 2.8 | 12 |
| Alkenes + napthenes | 1.7 | 0.5 | 1.9 | 1 | 0 | ||
| Decalines, bicyclicls, dienes | 6.3 | 3.1 | 4.1 | 11 | 14 | ||
| Benzenes | 2.5 | 9.9 | 19 | 31 | 51 | ||
| Biphenyls | 1.4 | 1.9 | 2.3 | 6 | 7.3 | ||
| Fluorenes | 0.1 | 0.2 | 0 | 0 | 0 | ||
| Polyaromatics | 2.3 | 8.2 | 2.4 | 2.1 | 0 | ||
| Phenols | 2.3 | 9.4 | 33 | 23 | 5.3 | ||
| Benzenediols +gauiacols | 8.1 | 15 | 3.9 | 0.01 | 0 | ||
| Benzofuranes | 0.9 | 0.8 | 0.29 | 0.7 | 0 | ||
| Abietates | 27 | 17 | 13 | 8.3 | 0 | ||
| Naphthols | 5.1 | 18 | 14 | 8.6 | 3.8 | ||
| Naphthalenones | 0 | 0 | 1.3 | 4.3 | 6.4 | ||
| Alk + Ben/Ph Ratio | 19.2 | 2.6 | 0.7 | 1.4 | 9.9 | ||
| 1/3 | Direct | Alkanes | 4.0 | 1.8 | 2.1 | 3.2 | |
| Alkenes + napthenes | 0.62 | 0.89 | 2.3 | 2.1 | |||
| Decalines, bicyclicls, dienes | 9 | 8 | 2.6 | 7.0 | |||
| Benzenes | 10 | 17 | 27 | 49 | |||
| Biphenyls | 3.0 | 3.2 | 2.5 | 2.8 | |||
| Fluorenes | 0.13 | 0 | 0.06 | 0 | |||
| Polyaromatics | 5.9 | 8.5 | 4.9 | 1.4 | |||
| Phenols | 13 | 27 | 32 | 23 | |||
| Benzenediols +gauiacols | 23 | 5.1 | 0.91 | 0 | |||
| Benzofuranes | 0.85 | 0.28 | 0.17 | 0 | |||
| Abietates | 12 | 11 | 12 | 2.5 | |||
| Naphthols | 16 | 19 | 10 | 5. | |||
| Naphthalenones | 0 | 0 | 2.3 | 2.0 | |||
| Alk + Ben/Ph Ratio | 1.2 | 0.7 | 0.9 | 2.3 | |||
| 1/3 | Extract | Alkanes | 18 | 11 | 4.1 | 9.7 | 24 |
| Alkenes + napthenes | 0.3 | 1.1 | 1.6 | 0.8 | 0 | ||
| Decalines, bicyclicls, dienes | 13 | 6.9 | 4.8 | 1.3 | 0 | ||
| Benzenes | 2.4 | 7.4 | 16 | 37 | 60 | ||
| Biphenyls | 0.7 | 1.9 | 2.3 | 1.14 | 3.5 | ||
| Fluorenes | 0 | 0.23 | 0 | 0 | 0 | ||
| Polyaromatics | 0.6 | 4.9 | 5.1 | 1.5 | 0 | ||
| Phenols | 8.6 | 20. | 32 | 26 | 9.1 | ||
| Benzenediols +gauiacols | 11 | 7.6 | 5.2 | 0 | 0 | ||
| Benzofuranes | 1.2 | 1.4 | 0.31 | 0 | 0 | ||
| Abietates | 11 | 10 | 6.0 | 5.8 | 0 | ||
| Naphthols | 1.93 | 9.3 | 14 | 10 | 2.3 | ||
| Naphthalenones | 30 | 18 | 7.04 | 5.3 | 0 | ||
| Alk + Ben/Ph Ratio | 2.4 | 0.9 | 0.6 | 1.8 | 9.4 | ||
| Temperature, °C | 300 | 350 | 375 | 400 | 425 | 450 |
|---|---|---|---|---|---|---|
| Raw materials/tetralin wt./wt. | 1/4 | |||||
| CO | 11.8 | 12.6 | 12.5 | 12.5 | 14.4 | 9.3 |
| CH4 | 3.5 | 17.0 | 29.4 | 38.0 | 45.7 | 32.0 |
| CO2 | 28.7 | 26.2 | 24.1 | 19.4 | 16.4 | 9.8 |
| C2-en | 0.14 | 0.28 | 0.37 | 0.44 | 0.47 | 0.36 |
| C2-an | 0.17 | 0.80 | 1.73 | 2.88 | 4.64 | 4.59 |
| C3 | 0.06 | 0.21 | 0.11 | 0.18 | 0.22 | 0.17 |
| C4 | 0.00 | 0.03 | 0.04 | 0.09 | 0.18 | 0.14 |
| H2 | 55.6 | 42.9 | 31.7 | 26.5 | 18.0 | 43.6 |
| Raw materials/tetralin wt./wt. | 1/3 | |||||
| CO | 13.0 | 9.7 | 14.0 | 10.0 | 11.0 | 8.5 |
| CH4 | 7.1 | 14.9 | 26.5 | 33.6 | 37.9 | 32.0 |
| CO2 | 28.1 | 25.7 | 23.7 | 23.4 | 17.0 | 13.4 |
| C2-en | 0.22 | 0.32 | 0.40 | 0.47 | 0.52 | 0.39 |
| C2-an | 0.37 | 0.87 | 1.72 | 3.14 | 4.56 | 5.23 |
| C3 | 0.05 | 0.25 | 0.59 | 0.21 | 0.22 | 0.21 |
| C4 | 0.02 | 0.03 | 0.06 | 0.13 | 0.18 | 0.22 |
| H2 | 51.1 | 48.2 | 32.9 | 29.1 | 30.8 | 40.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krylova, A.; Krysanova, K.; Kulikova, M.; Kulikov, A. Non-Catalytic Dissolution of Biochar Obtained by Hydrothermal Carbonization of Sawdust in Hydrogen Donor Solvent. Energies 2021, 14, 5890. https://doi.org/10.3390/en14185890
Krylova A, Krysanova K, Kulikova M, Kulikov A. Non-Catalytic Dissolution of Biochar Obtained by Hydrothermal Carbonization of Sawdust in Hydrogen Donor Solvent. Energies. 2021; 14(18):5890. https://doi.org/10.3390/en14185890
Chicago/Turabian StyleKrylova, Alla, Kristina Krysanova, Mayya Kulikova, and Albert Kulikov. 2021. "Non-Catalytic Dissolution of Biochar Obtained by Hydrothermal Carbonization of Sawdust in Hydrogen Donor Solvent" Energies 14, no. 18: 5890. https://doi.org/10.3390/en14185890
APA StyleKrylova, A., Krysanova, K., Kulikova, M., & Kulikov, A. (2021). Non-Catalytic Dissolution of Biochar Obtained by Hydrothermal Carbonization of Sawdust in Hydrogen Donor Solvent. Energies, 14(18), 5890. https://doi.org/10.3390/en14185890





