Abstract
The production of fuel hydrocarbons from CO2-neutral raw materials is a promising task at present. The thermal dissolution of biochar obtained by the method of hydrothermal carbonization of sawdust was studied. The dissolution of biochar in tetralin (hydrogen donor solvent) was studied at different temperatures (350–450 °C) and with two types of dilution of the mixture with tetralin: 1/3 and 1/4. The process proceeded without a catalyst. It was found that the samples subjected to thermal dissolution at temperatures of 425–450 °C had the highest conversion and yield of liquid products. The reaction temperature also had a significant effect on the composition of liquid products. It was found that an increase in the reaction temperature led to a significant increase in benzenes, both in the direct and in the hexane fraction. A benzene yield of more than 50% was observed for both fractions at a temperature of 450 °C. It was also suggested that the possible positive effect of abietates on the homogenization of the reaction mixture contributed to high conversion in the process. The biochar/tetralin ratio effects the yield and composition of the liquid products as well. An increase in the tetralin concentration in the mixture during thermal dissolution led to an increase in the conversion and yield of hydrocarbon fractions for fuel purposes. This is undoubtedly due to the large amount of elemental hydrogen involved in the hydrogenation of the reaction mixture.
1. Introduction
Biomass is currently considered as a renewable raw material for the production of heat and electric energy [1]. It, like fossil coal, is a solid energy fuel. However, as you know, the fields of application of natural coals are not limited to energetics. Before the oil era of the middle of the last century, coal was practically the only source of organic substances, including liquid and gas fuels [2].
Now, around the world, there is a noticeable decline in interest in coal chemistry as a science that ensures the development and practical application of processes for converting coal into valuable chemical products, in particular, into motor fuels. The main direction of the development of technologies for the production of synthetic motor fuels is the use of renewable biomass resources, of various origins, as a raw material base. At the same time, much attention is paid to the development of biotechnologies. According to existing estimates [3], the world biotechnology market will reach USD 2 trillion in 2025. A delay in the development and implementation of biotechnology can lead to the degradation of a number of industrial sectors (including coal chemistry), since their spread to world markets will not be possible without the use of biotechnologies.
One of the solutions to this problem is the gradual, and later complete, replacement of fossil coal with biochar in coal chemical processes. The interest in the production of biochar is due to the fact that biochar, in comparison with the initial biomass, has a number of positive characteristics, such as a higher calorific value, and the possibility of long term storage and transportation over long distances. Just like biomass, biochar is a CO2 neutral energy fuel [4].
All this makes it a promising energy source for distributed generation. The production of biochar as a power-generating fuel is an independent chemical process, in which biochar is a commercial product and can successfully compete with its natural analogue, occupying its own economic niche.
However, in order to replace traditional petroleum products with analogues obtained from biomass, it is necessary to overcome a number of obstacles. Most of the processes developed in the petrochemical and chemical industries are not suitable for converting biomass to biofuel. Biomass consists of 30–35 wt.% of cellulose, 15–35 wt.% of hemicellulose and 20–35 wt.% of lignin [5,6,7]. This is due to the fact that biomass is a raw material with a high oxygen content [8]: the average formula of dry wood can be represented as CH1.4O0.7 [5], that is, there is almost one oxygen atom for each carbon atom. This indicates the need to use hydrogen in the conversion of lignocellulosic biomass into liquid hydrocarbon products (biofuel). For the oxygenation of biomass, the well known slow pyrolysis (“dry distillation” of wood) [9], as well as its low temperature options (torrefaction [10] and hydrothermal carbonization [11]), can be used. All pyrolysis options are cheap and simple, which makes them especially attractive. The thermal treatment of biomass, in particular its mildest option, hydrothermal carbonization, allows obtaining biochar and bringing the properties of raw materials closer to those of fossil analogues. Thus, the use of biochar can be significantly expanded in the direction of processes that are traditionally used for the processing of fossil coal: for the production of liquid hydrocarbon products, artificial combustible gases and valuable chemical compounds. In this case, biomass will act as a raw material, and the process of obtaining biochar will be a stage of raw material upgrading. An unconditional advantage of biochar processing technologies, over similar processes used for fossil coal, is the ability to purposefully change the properties of biochar in accordance with the requirements of subsequent processing operations.
The most abundant renewable material in the world is lignocellulosic biomass [12]. Wood accounts for about 80% of the entire phytomass of our planet [13]. Every year, in the process of logging operations in the world, 715 Mt/year of wood waste is generated, which has a significant energy potential [14]. This waste appears mainly from primary processing and includes branch and bark trimmings, slabs/blocks/extra trimming (about 34%) and sawdust (about 12%). It is believed that there is a cubic meter of waste for every cubic meter of timber felled in the forest [15]. After the drying and processing of wood, additional waste appears—shavings (about 6%) and sawdust/trimming (about 2%) [16]. As a raw material with energy potential, wood waste has a number of advantages. They are usually characterized by a low content of mineral components and heteroatoms, which has a beneficial effect not only when they are directly burned, but also when it is possible to obtain products with a higher added value, for example, alcohol.
This allows us to consider wood and wood waste as a very promising raw material for chemical conversion into liquid biofuel.
Hydrothermal carbonization (HTC) is the most acceptable pyrolysis option in terms of introducing this process into the process flow of biochar conversions. This reaction was discovered by F. Bergius in the process of studying the thermal transformations of coal and peat (HTC) [17,18]. Hydrothermal carbonization is the process of obtaining biochar (or “hydrochar”) at a temperature of 180–220 °C and a pressure of up to 25 atm, in the presence of water without access to air and with the addition of a catalyst [19]. Hydrothermal carbonization is a thermochemical method that uses water as a reaction medium to convert wet biomass into a high carbon solid product. The reaction is carried out at a pressure higher than the saturated vapor pressure of water to ensure that it is in a liquid state [20]. During the process, the biomass is dehydrated and carbonized. The resulting biochar has a lower moisture content and hydrophobicity, therefore, it is more transportable and easier to store, in comparison with untreated biomass.
As a first approximation, the equation for the reaction of hydrothermal carbonization (as applied to glucose) can be expressed as follows [21]:
[C6H12O6]n → n[C6H2O + 5H2O] + ~950 kJ/mol,
Four to five water molecules are released from each carbohydrate in the biomass, depending on the reaction profile. At first glance, the removal of water in the presence of water seems unlikely, but the possibility of the reaction proceeding is largely explained by an increase in entropy (due to an increase in the number of molecules and degrees of freedom) and the exothermic effect of transformation.
Hydrothermal carbonization has not found practical application for almost a hundred years, and in the early 2000s, the reaction was practically rediscovered when it was found that it could be effectively used to process plant waste containing significant amounts of water.
Direct liquefaction can be used to obtain liquid biomass products. In general, the direct liquefaction of coal or biomass is heat treatment at a temperature of 250–450 °C and a pressure of 5–20 MPa, in the presence of a solvent and a reducing agent (hydrogen) [22]. The technology of such a process includes two stages [23]: (a) the thermal dissolution of raw materials in an organic solvent to obtain liquid products that are analogues of heavy crude oil; and (b) the hydroskimming of liquid products obtained in the first stage in order to obtain light and middle distillates, as well as to reduce the amount of oxygen containing products.
It is known that the thermal dissolution of fossil coal can be carried out in the presence of organic hydrogen donor solvents, such as tetralin or N-methyl-2-pyrrolidone (NMP) [24,25,26]. In general, the process can be described as follows [27]:
where R is carbon pieces, and R′ and R′H are radicals and products formed during dissolution, respectively.
Tetralin dehydration: C10H12 → C10H8 + 4H,
Coal dissolution: R → R′ → R′H,
At high temperatures, tetralin dehydrates to form naphthalene and four hydrogen radicals. Hydrogen radicals promote hydrocracking and the stabilization of hydrocarbon fragments obtained by the thermal decomposition of the original coal.
Traditionally, this process is focused on achieving the complete destruction of the organic mass of coal while obtaining light hydrocarbon fractions for fuel purposes [28,29].
According to modern concepts, the conversion of fossil coal into oil occurs due to the intermediate formation of asphaltenes (A) [30,31,32]. Asphaltenes are polycondensed, aromatic structures consisting of five to seven rings and aliphatic chains with a total number of carbon atoms up to 80 [33]. Asphaltenes are a black, amorphous solid, such as those extracted from crude oil using light hydrocarbons. Another class of intermediates are pre-asphaltenes (PA). They are formations that are an order of magnitude larger in size than asphaltenes (A). The conversion of coal via (PA) and (A) to liquid products—oil (O) and gas (G)—is currently the accepted mechanism for the direct liquefaction of coal [34,35,36].
Unfortunately, data on the thermal dissolution of biochar are very limited [37,38]. However, by analogy with coal, it can be assumed that dissolution occurs due to the thermal destruction of hydrocarbon bonds with the formation of radicals, which are stabilized by hydrogen released during heat treatment with a donor solvent [27]. It is known that biochar obtained from the hydrothermal carbonization of biomass can be thermally dissolved in tetralin at 350 °C, in the presence of a Ni/TiO2 catalyst [39]. This produces synthetic oil with a yield of 42%. Some criteria have been defined for “bio-oil” obtained by processing biomass [40]: (a) a molar ratio of H/C > 1.5; (b) a higher calorific value (GCV > 40 MJ/kg); and (c) an oxygen content of O < 6.0 wt.%. For example, the direct liquefaction of biochar, in the presence of Ni/TiO2 at a pressure of 21 MPa and a temperature of 673 K, made it possible to obtain 32 wt.% of bio-oil, the quality of which corresponded to the last two criteria [41].
The kinetics of the direct liquefaction of biochar obtained by the liquid-phase pyrolysis of biomass was studied [42]. The reaction was carried out in two stages: first, the thermal liquefaction of biochar was carried out in tetralin (solvent/biochar ratio = 1/3) at 425 °C. Then, the resulting mixture was hydrogenated by adding hydrogen (at a pressure of 5 MPa). The total biochar conversion was 84% and the bio-oil yield was 72%. It was found that, already at the stage of thermal dissolution, a very rapid liquefaction of biochar occurs and oil products and gas are directly formed. The part of the mixture that is barely reactive, reacts in the second stage with the participation of hydrogen, forming intermediate and then final reaction products. The thermal dissolution rate constant is two orders of magnitude higher than the hydrogenation rate constant, due to the hypercrosslinked molecular network of the part of the biochar that is barely reactive. A detailed study of the stage of the thermal dissolution of the tetralin of biochar obtained by the liquid-phase pyrolysis of biomass showed that the yield and composition of its products depend on temperature [37]. At 370 °C, the yield of liquid products was only 10%. It was found that, at this temperature, the conversion of tetralin to naphthalene with the release of hydrogen reaches 8%. An increase in temperature to 425 °C led to an increase in the conversion of tetralin to 24%, as well as to an increase in the conversion of biochar up to 37%, and the yield of bio-oil up to 24%.
The production of biochar from “pure” cellulose and lignin, biomass components, by the method of hydrothermal carbonization and the thermal dissolution of the obtained biochar in tetralin were studied [38]. It was found that with an increase in the carbonization temperature, the C/O ratio in biochar increases. It was observed that biochar obtained from cellulose at 230 °C, with the highest C/O = 1.1, showed the highest conversion (90 wt.%). The low C/O ratio and the presence of water soluble organic substances formed in the biochar production stage significantly improved the thermal dissolution of the biochar. It has been suggested that these compounds initiate the dissolution reaction by interacting with biochar through the cleavage of its ether bonds and the formation of phenoxy radicals. These radicals were stabilized with hydrogen released from the tetralin to form lower molecular weight products.
The kinetics of the direct liquefaction of biochar obtained by hydrothermal carbonization of cellulose was studied [43]. During the study of the dissolution reaction, it was revealed that through the process of biochar heating from 200–400 °C its maximum conversion occurs. The isothermal stage (400 ° C) in comparison with the heating stage is not accompanied by remarkable changes in conversion. It was also concluded that the effect of unreacted cellulose after hydrothermal carbonization on the number of reaction steps was made. It was found that the thermal dissolution of biochar obtained at lower temperatures and usually possessing cellulose in the composition have two stages. The first stage is associated with the destruction of the remaining cellulose (200–325 °C), the second stage is connected with the destruction of the biochar itself with the formation of products (325–400 °C). For biochar obtained at higher temperatures and characterized by a greater degree of transformation, as well as high C/O and the absence of unprocessed cellulose, one stage is observed.
The purpose of this work was to study the features of obtaining the liquid products of the thermal dissolution of biochar, obtained from sawdust, by the method of hydrothermal carbonization.
2. Materials and Methods
For this research, sawdust of coniferous trees (Siberian region, Russia) was selected as a raw material. The hydrogen donor solvent used in this study was tetralin (1,2,3,4-tetrahydronaphthalene) supplied by Alfa Aesar. The solvents used for product extraction, namely, hexane (ACS grade), toluene (ACS grade) and tetrahydrofuran (HPLC grade), were obtained from Rushim.
The experiments were carried out in reactors developed by the design bureau of the TIHS RAS and manufactured in a mechanical workshop.
To carry out the process of hydrothermal carbonization, a steel autoclave reactor with a volume of 0.5 L was used. The reactor is equipped with a mechanical stirrer, thermocouple, pressure gauge, tube furnace, and an isothermal controller. Hydrothermal carbonization was conducted at temperatures of 190 °C. Raw material (sawdust) with weight = 50 g was mixed with water in ratio 1/3 and then placed into the reactor. The reactor was heated to a required temperature and maintained isothermal for 4 h. Then, the reactor was cooled to room temperature for further unloading of the resulting suspension. The resulting suspension was separated on filter paper (with a pore size of 3–5 μm) installed in a glass funnel into a solid residue and liquid. The filtration occurred naturally without additional influences. Solid residue dried at 105 °C for 24 h.
Thermal dissolution of biochar was carried out in a steel batch reactor. The volume of the reactor was 60 mL. The reactor is equipped with a magnetic stirrer, thermocouple, pressure gauge, tube furnace, and an isothermal controller.
Biochar weighing 2 g was loaded into the reactor and tetralin was poured in a ratio of 1/3 and 1/4. The reactor was then equipped with a magnetic stirrer, sealed and purged with hydrogen to extract oxygen. The reactor was heated to a certain temperature (350 to 450 °C) and held for 1 h. After the reactor cooled down, the resulting liquid/solid mixture was dumped onto filter paper (20–25 µm pore diameter) for further studies.
Experiments on thermal dissolution for each temperature and ratio of raw materials: tetralin were repeated several times to ensure the reproducibility of the obtained data. The error in measuring the data corresponds to the error in measuring of the instruments with which they were obtained.
ASTM E1755-01 method was used to determine ash (A). BCS2H combustion calorimeter (relative measurement error of 0.2%) was used for the higher heating value (MJ/kg) determination. The elemental composition (C, H, N, S) of biochar and raw materials measurements were conducted using an Thermo Flash 2000 elemental analyzer (relative measurement error—less than 0.3%). Oxygen (O, wt.%) for dry state was calculated from material balance:
where C, H, N, S, A are carbon, hydrogen, nitrogen, sulfur and ash, calculated on dry basis.
Thermal gravimetric analysis was performed with the use of TGA/DSC 1 instrument from MettlerToledo (relative measurement error of specific heat measurements ± 1.5%). SEM-images of sawdust and biochar were obtained using a scanning electron microscope Phenom XL G2. Gaseous products were analyzed by gas chromatography (GC) on a Krystallux-4000 (relative measurement error of ±5%). The Raman spectra of the initial sawdust and biochar obtained by hydrothermal carbonization were recorded on a Senterra II confocal Raman microscope. The spectra were processed using the OPUS 8.5 software package. Chromato-mass spectrometry of liquids obtained after thermal dissolution of biochar in tetralin was carried out on mass spectrometer MALDI Bruker at autoflex speed.
The mass yield describes a percentage of raw material remaining in biochar and it is calculated as the ratio of carbonized product in weight () to raw biomass weight ().
The energy yield, , indicates that a percentage of feedstock caloricity remains in the solid residue [44].
The index is calculated as:
where and are the highest heating values of product and feedstock respectively.
The liquefaction products were separated into oil (oil from filtration + hexane soluble), asphaltenes (hexane insoluble but toluene soluble), pre-asphaltenes (toluene insoluble but THF soluble) and residue (THF insoluble).
To analyze liquid products, the obtained liquid/solid mixture from the reactor was dumped on filter paper. After the oil had drained off, the direct oil fraction was collected for further analysis. Then the filter with the sample was washed with hexane. The oil dissolved in hexane (hexane fraction) was collected for further studies, and the sample was left for a day at room temperature to dry, after which the residue was weighed. Then, the sample was washed with toluene and left for a day at room temperature to dry, after which the residue was weighed. At the last stage, the sample was washed with THF and left to dry for a day at room temperature, after which the residue was weighed.
Parameters such as gas (G), oil (O), asphaltenes (A), pre-asphaltenes (PA) residue (R) and conversion were calculated according to following formulas [37]
3. Results
3.1. Biochar and Its Characteristics
Biochar is a dark colored carbon like product. The biochar yield was 72.43% of the initial feedstock load. The energy yield was 79.78%. Similar dependencies are observed in the literature [45].
Technical analysis (Table 1) indicates that the proportion of fixed carbon in the original sawdust was relatively small (27%), and the yield of volatile substances, on the contrary, was very significant (72%). Hydrothermal treatment of this material at 190 °C for 4 h led to an increase in the proportion of the fixed material almost twofold (up to 43%) and a decrease in the yield of volatiles (up to 55%). The ash content in both materials was low and did not exceed 3%.

Table 1.
Properties of raw sawdust, biochar produced and brown coal.
According to the elemental analysis data (Table 1), the organic part of the biomass consisted mainly of carbon (64%), oxygen (29%), and hydrogen (6%). After the HTC, the proportion of carbon in the material increased to 73%, while the proportion of oxygen and hydrogen decreased to 21% and 5%, respectively. The proportion of heteroatoms in the organic part of biomass and biochar did not exceed 1%.
Thus, in the course of heat treatment, as expected, deoxygenation and an increase in carbon in the material took place. One of the advantages of the hydrothermal carbonization process is the ability to obtain a carbon product of the required quality. According to literary sources [47] low quality coals, in particular brown coal, achieved the highest conversion during thermal dissolution in a hydrogen donor solvent, in comparison with high quality coals. From Table 1, it follows that the obtained biochar has very similar characteristics with brown coals, which also can be seen in the van Krevelen diagram (Figure 1). The van Krevelen diagram can be viewed as a schematic representation of the evolution as biochar after hydrothermal carbonization based on the main components of the fuel (C, O, H) [48]. It should be noted that the hydrothermal treatment of sawdust led to the displacement of the biochar to the area of coals, which indicates the significant dehydration of the material. Considering the similarity of the properties of biochar and brown coals, biochar can potentially be considered as a carbon material with high reactivity during thermal dissolution.
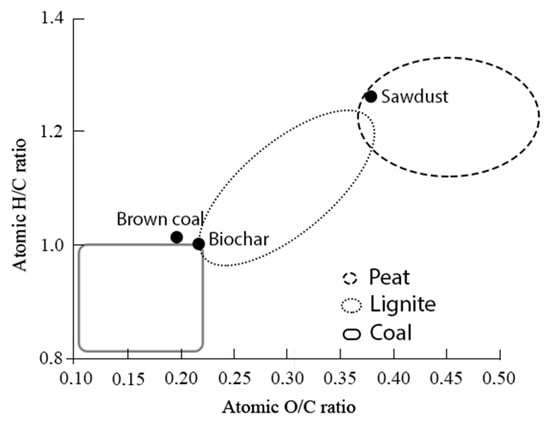
Figure 1.
Van Krevelen diagram.
It should be noted that the small amount of reacted hydrogen, in comparison with oxygen (ΔC/ΔH~11), suggests that, in the process of HTC, the removal of oxygen from the biomass occurs mainly with the participation of water hydrogen.
3.2. Texture
Figure 2 shows micrographs of the initial sawdust (a) and biochar (b) obtained by hydrothermal carbonization. At low resolution (Figure 2a), the micrograph of the original sawdust shows the fibrous structure of wood with a relatively smooth surface. Increasing the resolution allows the lignin sealed cellulose microfibrils to be seen.
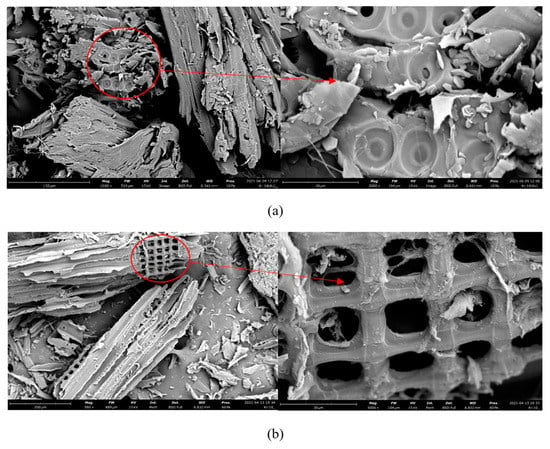
Figure 2.
Micrographs of initial sawdust (a) and biochar (b) obtained by hydrothermal carbonization.
Biochar retains the fibrous structure of the original sawdust (Figure 2b). However, the treatment led to the partial destruction and decomposition of cellulose components in the sawdust, which led to the formation of a rougher surface on the material, with pieces of nondegraded fiber. Similar relationships are observed for other types of lignocellulosic biomass [49]. At a higher resolution, a row of hollow square tubes can be seen. It is assumed that such tubes can be formed as a result of the decomposition of cellulose sawdust micelles inside the microfibril they form. The boundaries of microfibrils are indistinct, apparently due to the partial melting of lignin, which is part of the original sawdust. It can be assumed that lignin prevents the decomposition of a part of the cellulose that forms the outer layer of the microfibril, and it remains in the biochar composition. Studies also show that part of the cellulose, and sometimes a very small part of the hemicellulose, is retained in biochar after hydrothermal treatment [50,51].
3.3. TGA–DTG–DSC Data
Figure 3 shows a biochar derivatogram. As follows from the TGA–DTG data, biochar weight loss is observed in two stages. At the first stage (below 100 °C), water is lost. The weight loss in this area is small and was only 1.6%, since a dry sample was used. Above 150 °C, the main change in the weight of the sample begins, which ends at a temperature of about 900 °C. The total weight loss was 49%. At the same time, in the temperature range of 200–300 °C, characteristic of the thermal decomposition of hemicellulose and cellulose [52], the weight loss was relatively small (about 10%), which indicates a significant decomposition of these components of the biomass in the process of hydrothermal carbonization. Nevertheless, there are still some of them in the biochar. It should be noted that no thermal effects are observed in this area. In the literature, there are similar dependences for biochars also obtained by the method of the hydrothermal carbonization of sawdust [53].
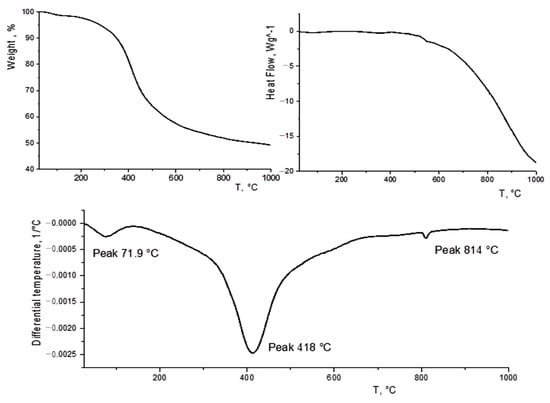
Figure 3.
TGA–DTG–DSC data.
The main weight loss of biochar occurs in the region of 350–500 °C (with a maximum at 418 °C), which is responsible for the decomposition of lignin, which is part of the original biomass and biochar [54]. There are also no noticeable thermal effects in this area. However, above 400 °C, a gradually increasing endothermic effect can be observed on the DSC curve, which is probably due to the compaction of the formed carbon material.
3.4. Raman Spectroscopy of Sawdust and Biochar
The Raman spectrum of the wood pulp is shown in Figure 4. The Raman spectrum of the sample contains bands related to the stretching and deformation vibrations of functional groups of cellulose, hemicellulose, and lignin [55]. A high intensity band at 1598 cm−1 corresponds to the vibrations of aromatic rings; at 1657 cm−1, to stretching vibrations of C=O groups conjugated to an aromatic ring.
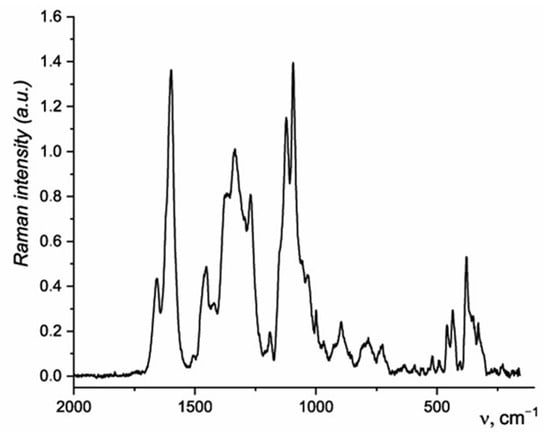
Figure 4.
Raman spectrum of sawdust.
The Raman spectrum of the sample after hydrothermal carbonization (Figure 5) contains a high intensity G-band at 1595 cm−1 and a wide D band with a pronounced maximum at 1400 cm−1, which is typical for the Raman spectra of coals [56]. The G band corresponds to the stretching vibrations of sp2-hybridized carbon atoms in aromatic rings and carbon chains. The D band corresponds to defects in the graphite structure (for example, nonhexagonal rings, sp3-hybridized carbon atoms). The data obtained indicate the appearance of amorphous carbon in biochar, as well as a denser carbon packing. No bands related to the vibrations of C=O groups were found in the Raman spectrum of biochar, which correlates with the significant deoxidation of the material.
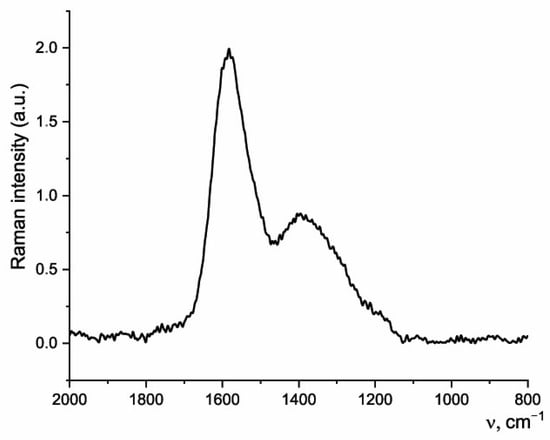
Figure 5.
Raman spectrum of biochar.
3.5. Thermal Dissolution of Biochar
At a biochar/tetralin ratio of 1/4 (wt.%), an increase in temperature from 350 °C to 425 °C led to a greater thermal dissolution of the biochar: the biomass conversion increased from 72 to 93% (Table 2). In this case, a thick dark liquid was formed, the composition of which changed depending on the temperature. Below 400 °C, the products were mainly represented by asphaltenes and pre-asphaltenes, the total share of which, at 350 °C, was 72%. In this case, the PA/A ratio was 1.1. With an increase in temperature, the content of heavy products in the mixture decreased by more than three times (up to 19% at 450 °C). At the same time, the content of liquid products increased in the products of thermal dissolution (up to 80%). The proportion of gases at all temperatures was small and did not exceed 0.3%

Table 2.
The main characteristics of the thermal dissolution of biochar in tetralin.
At a biochar/tetralin ratio of 1/3 (wt.), an increase in temperature from 350 to 400 °C also led to a slightly lower thermal dissolution of biochar, in comparison with a more dilute mixture (Table 2). The biomass conversion increased with temperature from 68% to 79%. A further increase in temperature, to 450 °C, had practically no effect on this indicator. At all temperatures, the formation of a thick dark liquid was observed, the composition of which varied with temperature. Below 400 °C, the products were mainly represented by asphaltenes and pre-asphaltenes. Their total content at 350 °C reached 72%, and the PA/A ratio was slightly higher than at stronger dilution. That is, a decrease in the proportion of solvent in the system led to the formation of denser carbon containing synthesis products. With an increase in temperature, the yield of heavy products decreased 2.5 times (up to 30% at 450 °C). At the same time, the content of liquid products increased up to 70%. This figure was 10% lower than in the experiment with high dilution. The gas yield in the studied temperature range was very low and did not exceed 0.3%.
3.6. Composition of the Liquid Products of the Thermal Dissolution of Biochar
Liquid products are the target products of the thermal dissolution of biomass. During the experiment, these products were found in the composition of two fractions: (a) liquid products obtained directly by thermal dissolution (“direct fraction”); and (b) liquid products obtained by extraction with hexane from the precipitate separated from the liquid product (“extract”).
Table 3 shows the effect of dilution and reaction temperature on the composition of the products of both liquid fractions. All direct fractions and extracts contain a variety of organic compounds. They include liquid hydrocarbons (alkanes, benzenes, alkenes, bicycles, etc.), liquid oxygen containing compounds (phenols, benzodiols, etc.), as well as solid hydrocarbon dissolved in organic liquids (biphenyls, fluorenes, polyaromatics, etc.) and oxygen containing products (abietates, naphthols, etc.).

Table 3.
Effect of dilution and reaction temperature on the composition of liquid products obtained by thermal dissolution of sawdust.
Liquid alkanes and benzenes are of the greatest interest in terms of fuel use. The amount of alkanes in the obtained direct liquid fractions was relatively small and did not exceed 5% (Table 3). However, in hexane extracts, the proportion of these compounds was quite high. Thus, the extract obtained at 350 °C and dilution ¼ (wt.) contained 42% alkanes. An increase in temperature to 400 °C led to a decrease in alkanes by almost an order of magnitude. A further increase in temperature, to 450 °C, made it possible to slightly increase alkanes (up to 12%). The same patterns were observed for the effect of temperature on the change in alkanes obtained at a dilution of 1/3 (wt.). However, in this case, the extract obtained at 350 °C contained half the amount of alkanes; the maximum content of alkanes (24%) was observed at 450 °C.
The proportion of benzenes in all the liquid fractions increased uniformly with temperature (Table 3). The maximum content of these products (60%) was obtained in the hexane extract at 450 °C and dilution 1/3 (wt.). In other fractions, this figure was ~50%.
The significant increase in benzenes at 450 °C can be explained by the fact that tetralin can convert not only to naphthalene and methylindane, but also to substituted benzene [57].
The main liquid oxygen containing products of the thermal dissolution of sawdust were phenols (Table 3). In all fractions, their maximum amount (up to 32%) was recorded at 400–425 °C. In this case, the extracts contained slightly more phenols than the direct fractions. They also contained a slightly larger amount of other oxygenates (abietates, naphthols, etc.).
Abietic or sylvic acid (C19H29COOH) is one of the natural resin acids of plant origin [58]. It belongs to the diterpene tricyclic group of natural compounds (compounds obtained from four isoprene fragments). Abietic acid is present in the resin of coniferous trees. It is used as a natural emulsifier and thickener. Esters or salts of abietic acid are called abietates. Abietates, in significant quantities (10–27%), were found in all the liquid fractions of the thermal dissolution of biochar at temperatures below 400 °C. Obviously, these compounds were present in the biochar obtained by hydrothermal carbonization from the sawdust of coniferous trees under mild conditions (190 °C). Their appearance in the products of the thermal dissolution of biochar can be explained by extraction with tetralin. In the liquid products obtained above 400 °C, the concentration of abietates did not exceed 5%, apparently due to their inclusion in thermochemical transformations. However, the very presence of abietates at the initial stages of thermal liquefaction at all temperatures could have a positive effect on the efficiency of the process, since abietates are natural emulsifiers and contribute to the homogenization of the reaction mixture.
3.7. Composition of Gases Obtained after the Thermal Dissolution of Biochar
The results of the chromatography of the gas phase after the thermal dissolution of biochar (Table 4) showed that the temperature of the process has a greater effect on the gas composition than the feed/tetralin ratio. The gas mixture was mainly composed of methane and COx gas. For COx gas, the distribution was characterized as CO2 > CO for all temperature reactions. Similar dependences are observed in the case of the direct liquefaction of coal in tetralin under hydrogen pressure [57]. For temperatures up to 375 °C, the gas content was ordered as follows: CO2 > CO > CH4 > C2-an > C2-en > C3 > C4. After 375 °C, the percentage of gases in the composition changed towards an increase in methane and was as follows: CH4 > CO2 > CO > C2-an > C2-en > C3 > C4. The highest CH4 value was observed at temperatures of 400–450 °C. It is assumed that this is due to the thermal destruction of lignin, the peak of which falls at 418 °C (Figure 3). At these temperatures, in the course of the thermal destruction of lignin, the homolytic cleavage of the O–CH3 bond attached to the aromatic rings of lignin occurs, associated with an ipso rearrangement starting from the phenoxy radical of guaiacol [59]. The CH3 ion in a hydrogen donor medium is reduced to CH4.

Table 4.
Influence of dilution and reaction temperature on the composition of gaseous products obtained by thermal dissolution of sawdust.
4. Discussion
For thermal dissolution, biochar obtained from sawdust by the method of hydrothermal carbonization was used. Carbonization was carried out under mild conditions (190 °C, 4 h) in order to increase the carbon efficiency of the process (first of all, to reduce gas formation). The resulting biochar was a black powder, which differed from the original sawdust by exhibiting almost twice the higher content of fixed carbon (27% and 43%, respectively), a lower yield of volatile substances (72% and 55%, respectively), and a lower oxygen content (29% and 21%, respectively). The C/O ratio in biochar was 3.4.
The role of water in hydrothermal reactions is complex. It can act as a solvent, catalyst, reagent, and as a medium for the transfer of matter and energy. It should be noted that the physical and chemical properties of water are highly dependent on environmental conditions [60]. Above 200 °C, they differ greatly from its properties under normal conditions [61,62]: (1) water has a lower dielectric constant, (2) the number of hydrogen bonds in it is fewer and they are less pronounced, (3) its isothermal compressibility is higher, and (4) the dissociation constant is three orders of magnitude higher. These changes determine the new properties of water as a solvent: it dissolves organic compounds better, and the solubility of its inorganic salts decreases. At 250–350 °C, the properties of water as a solvent approach the properties of organic solvents at room temperature. In the subcritical region (100–374 °C), the ionization constant of water increases with increasing temperature. In this case, water “autoionization” occurs with the formation of an acidic hydronium ion (H3O+), which acts as a hydrolysis catalyst, and a basic hydroxyl ion (OH–). As a result, the pH of the reaction medium drops to 5 and below. This process can be accelerated by adding an acid to the reactor, usually acetic or citric [63].
According to modern concepts, the first step of the HTC reaction is hydrolysis: water reacts with hemicellulose or cellulose and breaks the bonds of ethers and esters [64]. It is known that “pure” hemicellulose begins to hydrolyze above 180 °C, while hydrolysis of “pure” cellulose proceeds above 230 °C [65]. Under conditions of hydrothermal carbonization, lignin remains mainly unchanged [25]. A very small fraction of the “pure” lignin reacts at a higher temperature (260 °C), with the release of phenol and phenolic derivatives [66]. Acids or bases catalyze the hydrolysis process and can affect the yield and composition of the resulting products.
When biochar is produced by hydrothermal carbonization, rather low temperatures are used. As a result, biochar has a fairly high C content, which leads to a greater number of surface oxygen containing functional groups, in comparison with other methods of biochar production [67].
The study of the texture of the obtained material (Figure 2) suggests that during the hydrothermal treatment of sawdust there is a complete destruction of hemicellulose and only partial transformation of cellulose. The micrographs clearly show the walls of the microfibrils. However, the boundaries of the microfibrils are indistinct, apparently due to the partial melting of lignin, which is part of the original sawdust. It can be assumed that it is lignin that prevents the decomposition of a part of the cellulose that forms the outer layer of the microfibril, and it remains in the biochar composition. These assumptions are supported by the TGA–DTG–DSC data. In the temperature range of 200–300 °C, characteristic of the thermal decomposition of hemicellulose and cellulose, a relatively small weight loss (about 10%) was observed, which indicates a significant conversion of these components of the biomass in the process of hydrothermal carbonization. The main weight loss of biochar occurred in the region of 350–500 °C (with a maximum at 418 °C), which is responsible for the decomposition of lignin, which is part of the initial biomass and biochar.
The thermal dissolution of biochar in tetralin was carried out in the temperature range 350–450 °C. In this case, the conversion of biochar significantly depended on the amount of the donor solvent: the more solvent, the higher the conversion (Figure 6). At 350 °C and a ratio of biochar/tetralin = 1/4, the conversion of biochar was 3.5 times higher than at a ratio of 1/3, and reached 72%. This difference noticeably decreased with an increase in the dissolution temperature, decreasing to 1.2 at 450 °C. The data obtained can be compared with the thermal dissolution of biochar prepared from cellulose and lignin by the method of hydrothermal carbonization [51]. Despite the fact that, in this case, a 1/10 dilution was used and, it would seem, the total amount of hydrogen in the system was higher, the conversion at 400 °C of biochar with a close C/O ratio (3.7 and 3.1 for cellulose and lignin biochar, respectively) was noticeably lower (below 80%) than in our experiments (about 90%). The resulting discrepancy can be explained as follows. According to [51], the liquid organic part obtained by the HTC of cellulose is mainly THF and furfural, and by the HTC of lignin, guaiacol, creosol and catechol (pyrocatechin). The presence of oxygenates in biochar during thermal dissolution has a beneficial effect on the course of the process. It can be assumed that both types of the oxygenates formed from cellulose and lignin are present in our experiments, which enhances the efficiency of the thermal dissolution of sawdust biochar.
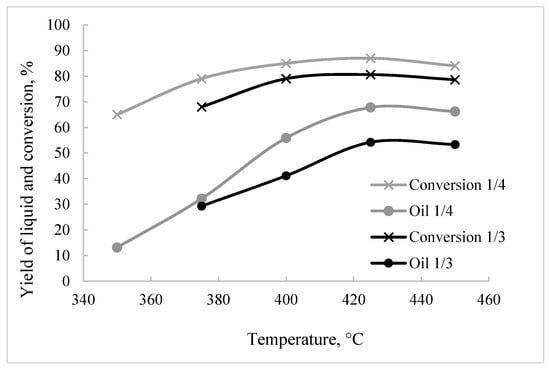
Figure 6.
Effect of temperature and dilution with tetralin on biochar conversion and yield of liquid products.
The product of the thermal dissolution of biochar was a thick dark liquid, the composition of which changed depending on temperature. Below 400 °C, the products were mainly represented by asphaltenes and pre-asphaltenes, the share of which was 60–70%. An increase in the temperature of the thermal dissolution, to 425 °C, led to a noticeable increase in liquid products (Figure 6). In this case, the temperature curve of liquid products for the ratio biochar/tetralin = 1/4 was located noticeably higher than the analogous curve for the ratio biochar/tetralin = 1/3. The maximum yield of liquid products (70% and 81% at a biochar/tetralin ratio of 1/3 and 1/4, respectively) was observed at 425 °C. This fact, most likely, can be explained by the large amount of hydrogen released above 400 °C. According to [68], it is in this region that tetralin dehydrogenation, with the formation of decalin and naphthalene, is observed.
The liquid products obtained by the thermal dissolution of biochar were a complex mixture including liquid hydrocarbons (alkanes, benzenes, alkenes, bicycles, etc.), liquid oxygen containing compounds (phenols, benzodiols, etc.), as well as solid hydrocarbons dissolved in organic liquids (biphenyls, fluorenes, polyaromatics, etc.) and oxygen containing products (abietates, naphthols, etc.).
Liquid hydrocarbon products are the target products of the thermal dissolution of biomass. They were found in the composition of two fractions: (a) the “direct fraction” obtained by separation from the precipitate during filtration of the thermal dissolution products; and (b) the “extract” obtained by extraction with hexane from the isolated precipitate. Figure 7 illustrates the effect of temperature and dilution with tetralin on the content of liquid hydrocarbons (alkanes and benzenes) and liquid oxygen containing compounds (in the example of phenols) in the products of thermal dissolution of biochar. The amount of alkanes in the obtained direct liquid fractions was relatively small and did not exceed 5% (Figure 7a,b). However, in hexane extracts, the proportion of these compounds was quite high. For example, the extract obtained at 350 °C and dilution 1/4 (wt.) contained 42% alkanes. As expected, an increase in tetralin in the initial mixture led to an increase in the yield of alkanes.
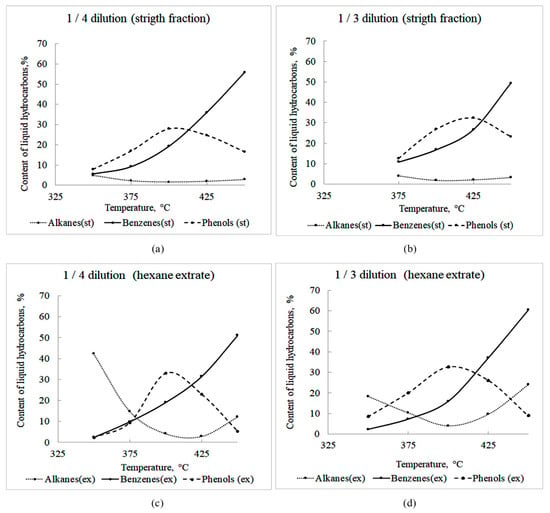
Figure 7.
Effect of temperature on the content of alkanes, benzenes and phenols in liquid products of thermal dissolution of biochar in: (a) strait fraction fractions of liquid products in dilution with tetralin 1/4, (b) strait fraction fractions of liquid products in dilution with tetralin 1/3, (c) hexane extracts fractions of liquid products in dilution with tetralin 1/4, and (d) hexane extracts fractions of liquid products in dilution with tetralin 1/3.
Benzenes were found in the direct fractions of liquid products and in hexane extracts (Figure 7). Their proportion in all liquid fractions increased uniformly with temperature. Their maximum amount (60%) was obtained in a hexane extract at 450 °C and dilution 1/3 (wt.). In other fractions, this figure was ~50%.
The main liquid oxygen containing products of the thermal dissolution of sawdust were phenols (Figure 7). In all fractions, their maximum amount (up to 32%) was recorded at 400–425 °C. In this case, the extracts contained slightly more phenols than the direct fractions.
A significant number of abietates (up to 40%) were recorded in the products. Their presence in tetralin–coal mixtures during thermal dissolution can contribute to the homogenization of the suspension, since abietates are natural emulsifiers [69]. The homogenization of the reaction mixture can serve as one of the reasons for the higher conversion of biochar and a higher yield of liquid synthesis products, as compared to the thermal dissolution of biochar prepared from “pure” cellulose or lignin.
To assess the effect of temperature and dilution on the ratio of liquid hydrocarbons to oxygenates, the Alk + Ben/Ph ratio was used, which characterizes the ratio of the sum of alkanes and benzenes to phenols (Table 3). It can be seen that, in direct fractions and depending on temperature, it ranged from 0.7 to 3.6, and in extracts from 0.7 to 9.9 it even reached 19 (at 350 °C and dilution ¼). Thus, a greater dilution of sawdust with tetralin during thermal dissolution led to an increase in the yield of hydrocarbon fractions for fuel purposes.
5. Conclusions
The processes of obtaining hydrocarbons from biomass for fuel purposes are currently of high interest among the scientific community. These processes are characterized by a high degree of environmental friendliness compared to similar processes, while still using fossil fuels.
In this work, the thermal dissolution of biochar obtained by the method of the hydrothermal carbonization of sawdust, at a temperature of 190 °C and for 4 h, was studied. In terms of its physical and chemical characteristics, biochar was close to brown coals, which are considered the most reactive in direct liquefaction processes. The dissolution of biochar in tetralin (hydrogen donor solvent) was studied at different temperatures (350–450 °C), in the absence of any catalyst and with two types of dilution of the mixture with tetralin: 1/3 and 1/4.
It was found that the highest conversion and yield of liquid biochar for thermal dissolution were observed at 425 °C. It is known that, for natural brown coals, the optimal temperature is also 425 °C [70]. However, the yield of liquids and conversion of biochar was higher than that of subbituminous coals at the same temperature and dilution, which may indicate a higher reactivity of biochar [71]. This fact is possibly related to the difference in the morphology and degree of metamorphism of freshly synthesized biochar in comparison with coal formed over many years under natural conditions.
It was found that the degree of dilution significantly affects the yield and composition of the resulting liquid products. The highest conversion and yield of liquid was observed at a ratio of 1/4 biocoal/tetralin. As with native coals, an increase in the amount of potential hydrogen donor (tetralin) in the system provokes reducing the contribution of the side reactions of gassing and intensifies the liquefaction process [57].
Author Contributions
A.K. (Alla Krylova) was responsible for the conceptualization, investigation, methodology, project administration, resources, supervision, writing of the original draft, reviewing, and editing; K.K. for data curation, writing the original draft, formal analysis, investigation, visualization, methodology, reviewing, and editing; M.K. for data curation, project administration, resources, investigation, writing the original draft, reviewing and editing; A.K. (Albert Kulikov) for project administration, resources, writing the original draft, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the State Program of TIPS RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Studies were performed using equipment of the Shared Research Center Analytical Center of the Problems of Deep Petroleum Processing and Petrochemistry, Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yılmaz, S.; Selim, H. A review on the methods for biomass to energy conversion systems design. Renew. Sustain. Energy Rev. 2013, 25, 420–430. [Google Scholar] [CrossRef]
- Stranges, A.N. Friedrich bergius and the rise of the german synthetic fuel industry. Isis 1984, 75, 643–667. Available online: www.jstor.org/stable/232411 (accessed on 5 August 2021). [CrossRef]
- Alagidede, P.; Adu, G.; Frimpong, P.B. The effect of climate change on economic growth: Evidence from Sub-Saharan Africa. Environ. Econ. Pol. Stud. 2016, 183, 417–436. [Google Scholar] [CrossRef]
- Angulo-Mosquera, L.S.; Alvarado-Alvarado, A.A.; Rivas-Arrieta, M.J.; Cattaneo, C.R.; Rene, E.R.; García-Depraect, O. Production of solid biofuels from organic waste in developing countries: A review from sustainability and economic feasibility perspectives. Sci. Total Environ. 2021, 795, 148816. [Google Scholar] [CrossRef]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct liquefaction of biomass. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Krysanova, K.O.; Krylova, A.Y.; Zaichenko, V.M. Application of hydrothermal carbonization to improve the energy properties of peat. Solid Fuel Chem. 2021, 55, 123–128. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Nimz, H.H.; Schmitt, U.; Schwab, E.; Wittmann, O.; Wolf, F. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000. [Google Scholar]
- Baruya, P. World Forest and Agricultural Crop Residue Resources for Cofiring; IEA Clean Coal Centre: London, UK, 2015. [Google Scholar]
- Koopmans, A.; Koppejan, J. Agricultural and forest residues: Generation, utilization and availability. In Proceedings of the Regional Consultation on Modern Applications of Biomass Energy, Kuala Lumpur, Malaysia, 6–10 January 1997. [Google Scholar]
- Cocchi, M. Global Wood Pellet Industry Market and Trade Study. IEA Bioenergy Task 40: Sustainable International Bioenergy Trade; International Energy Agency: Paris, France, 2011. [Google Scholar]
- Bergius, F. Die Anwendung Hoher Drücke Bei Chemischen Vorgängen und Eine Nachbildung des Entstehungsprozesses der Steinkohle; W. Knapp: Berlin, Germany, 1913; pp. 2–58. [Google Scholar]
- Leibniz, E.; Simon, A. Zum fünfundsechzigsten Geburtstage. Prakt. Chem. 1958, 5, 209–211. [Google Scholar] [CrossRef]
- Method of Obtaining Coal from Biowaste. Available online: http://www.ecotoc.ru/traditional/ugol/d742/ (accessed on 5 August 2021).
- Lu, X.; Yamauchi, K.; Phaiboonsilpa, N. Two-step hydrolysis of Japanese beech as treated by semi-flow hot-compressed water. J. Wood Sci. 2009, 55, 367–375. [Google Scholar] [CrossRef]
- Brown, R.C. Biorenewable Resources: Engineering New Products from Agriculture; Iowa State Press: Ames, IA, USA, 2003; pp. 30–37. [Google Scholar]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Schwaiger, N.; Feiner, R.; Pucher, H.; Ellmaier, L.; Ritzberger, J.; Treusch, K.; Pucher, P.; Siebenhofer, M. Biomass pyrolysis refinery. Chem. Ing. Technol. 2015, 87, 803–809. [Google Scholar] [CrossRef]
- Li, C.; Ashida, S.; Iino, M.; Takanohashi, T. Coal dissolution by heat treatments in nmethyl-2-pyrrolidinone, 1, 4, 5, 8, 9, 10-hexahydroanthracene, and their mixed solvents: A large synergistic effect of the mixed solvents. Energy Fuels 2000, 14, 190–196. [Google Scholar] [CrossRef]
- Guin, J.; Tarrer, A.; Taylor, L.; Prather, J.; Green, S. Mechanisms of coal particle dissolution. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 490–494. [Google Scholar] [CrossRef]
- Shalabi, M.A.; Baldwin, R.M.; Bain, R.L.; Gary, J.H.; Golden, J.O. Noncatalytic coal liquefaction in a donor solvent. Rate of formation of oil, asphaltenes, and preasphaltenes. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 474–479. [Google Scholar] [CrossRef]
- Connors, W.; Johanson, L.; Sarkanen, K.; Winslow, P. Thermal degradation of kraft lignin in tetralin. Holzforschung 1980, 34, 29–37. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kuznetsova, L.I.; Buryukin, F.A.; Marakushina, E.N.; Frizorger, V.K. Methods for the preparation of coal-tar pitch. Solid Fuel Chem. 2015, 49, 213–225. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Marakushina, E.N.; Kazbanova, A.V.; Kolesnikova, S.M.; Kuznetsova, L.I.; Buryukin, F.A.; Kositcyna, S.S. Getting an alternative pitch binder by thermal dissolution of coal. Am. J. Appl. Sci. 2016, 13, 7–13. [Google Scholar] [CrossRef]
- Weller, S.; Pelipetz, M.G.; Friedman, S. Kinetics of coal hydrogenation conversion of asphalt. Ind. Eng. Chem. 1951, 43, 1572–1575. [Google Scholar] [CrossRef]
- Weller, S.; Clark, E.L.; Pelipetz, M.G. Mechanism of coal hydrogenation. Ind. Eng. Chem. 1950, 42, 334–336. [Google Scholar] [CrossRef]
- Weller, S.; Pelipetz, M.G. Coal hydrogenation catalysts studies of catalyst distribution. Ind. Eng. Chem. 1951, 43, 1243–1246. [Google Scholar] [CrossRef]
- Ancheyta, J.; Trejo, F.; Rana, M.S. Asphaltenes: Chemical Transformation during Hydroprocessing of Heavy Oils, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 213–231. [Google Scholar]
- Kaneko, T.; Derbyshire, F.; Makino, E.; Gray, D.; Tamura, M. Coal Liquefaction. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: New York, NY, USA, 2001; pp. 1–83. [Google Scholar]
- Vasireddy, S.; Morreale, B.; Cugini, A. Clean liquid fuels from direct coal liquefaction: Chemistry, catalysis, technological status and challenges. Energy Environ. Sci. 2011, 4, 311–345. [Google Scholar] [CrossRef]
- Haenel, M.W. Catalysis in direct coal liquefaction. In Handbook of Heterogeneous Catalysis; Wiley: New York, NY, USA, 2008; pp. 3023–3036. [Google Scholar]
- Feiner, R.; Schwaiger, N.; Pucher, H.; Ellmaier, L.; Pucher, P.; Siebenhofer, M. Liquefaction of pyrolysis derived biochar: A new step towards biofuel from renewable resources. RSC Adv. 2013, 3, 17898–17903. [Google Scholar] [CrossRef]
- Kundu, R.; Ramsurn, H. Non-catalytic dissolution of biochar in hydrogen donor solvent. J. Anal. Appl. Pyrolysis 2019, 140, 227–238. [Google Scholar] [CrossRef]
- Trautmann, M.; Lang, S.; Traa, Y. Direct liquefaction of lower-rank coals and biocoals with magnetically separable catalysts as a sustainable route to fuels. Fuel 2015, 151, 102–109. [Google Scholar] [CrossRef]
- Wang, C.; Pan, J.; Li, J.; Yang, Z. Comparative studies of products produced from four different biomass samples via deoxy-liquefaction. Bioresour. Technol. 2009, 99, 2778–2786. [Google Scholar] [CrossRef]
- Trautmann, M.; Löwe, A.; Traa, Y. An alternative method for the production of second-generation biofuels. Green Chem. 2014, 16, 3710–3714. [Google Scholar] [CrossRef]
- Feiner, R.; Schwaiger, N.; Pucher, H.; Ellmaier, L.; Reiter, A.; Derntl, M.; Glatz, T.; Pucher, P.; Siebenhofer, M. Kinetics of biochar liquefaction. BioEnergy Res. 2014, 7, 1343–1350. [Google Scholar] [CrossRef]
- Kundu, R.; Ramsurn, H. Kinetic study of noncatalytic dissolution of cellulose biochar in hydrogen donor solvent. ACS Sustain. Chem. Eng. 2020, 8, 11606–11617. [Google Scholar] [CrossRef]
- Poudel, S.; Oh, S.J. Effect of torrefaction on the properties of corn stalk to enhance solid fuel qualities. Energies 2014, 7, 5586–5600. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–555. [Google Scholar] [CrossRef]
- Huang, Y.; Rolfe, A.; Rezvani, S.; Hidalgo, H.; José, M.; Franco, F.; Pinto, F.; Snape, C.; Hewitt, N. Converting brown coal to synthetic liquid fuels through direct coal liquefaction technology: Techno-economic evaluation. Int. J. Energy Res. 2020, 44, 11827–11839. [Google Scholar] [CrossRef]
- Koriakin, A.; Moon, S.; Kim, D.W.; Lee, C.H. Liquefaction of oil palm empty fruit bunch using sub- and supercritical tetralin, n-dodecane, and their mixture. Fuel 2017, 208, 184–192. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Liang, M.; Lu, W.; Lei, P.; Wang, L.; Wang, B.; Li, B.; Shen, Y.; Zhang, K. Physical and combustion properties of binder-assisted hydrochar pellets from hydrothermal carbonization of tobacco stem. Waste Biomass Valorization 2020, 11, 6369–6382. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, Q.; Zhu, M.; Sun, G.; Zhang, T.; Kang, K. Comparative evaluation of hydrothermal carbonization and low temperature pyrolysis of eucommia ulmoides oliver for the production of solid biofuel. Sci. Rep. 2019, 9, 5535. [Google Scholar] [CrossRef]
- Kambo, H.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass—Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Maryandyshev, P.A.; Popova, E.I.; Chernov, A.A.; Popov, M.S.; Lyubov, V.K.; Trouve, G.; Kehrli, D.; Brillard, A.; Brilhac, J.-F. Thermal decomposition and combustion of biofuels. Solid Fuel Chem. 2017, 51, 370–378. [Google Scholar] [CrossRef]
- Edwards, G.M.; de Olivieira, L.F.C.; Nesbitt, M. Fourier-transform Raman characterization of brazilwood trees and substitutes. Analyst 2003, 128, 82–87. [Google Scholar] [CrossRef]
- Quirico, E.; Bonal, L.; Montagnas, G.; Beck, P.; Reynard, B. New insights into the structure and formation of coals, terrestrial and extraterrestrial kerogens from resonant UV Raman spectroscopy. Geochim. Cosmoscim. Acta 2020, 282, 156–176. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Du, S.; Shen, K.; Yang, Q.; Yi, H.; Zhang, J. Transformation Characteristics of Hydrogen-Donor Solvent Tetralin in the Process of Direct Coal Liquefaction. Front. Chem. 2019, 7, 737. [Google Scholar] [CrossRef]
- Norlin, L.-H. Tall Oil. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Dorrestijn, E.; Mulder, P. The radical-induced decomposition of 2-methoxyphenol. J. Chem. Soc. Perkin Trans. 1999, 2, 777–780. [Google Scholar] [CrossRef]
- Hatate, Y. Decomposition Behavior of Plant Biomass in Hot-Compressed Water. Ind. Eng. Chem. Res. 2009, 39, 3688–3693. [Google Scholar]
- Yu, Y.; Lou, X.; Wu, H. Some recent advances in hydrolysis of biomass in hot-compressed water and its comparisons with other hydrolysis methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Loppinet-Serani, A.; Aymonier, C.; Cansell, F. Supercritical water for environmental technologies. J. Chem. Technol. Biotechnol. 2010, 85, 583–589. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, H.J.; Ramaswamy, S. Reaction kinetics of the hydrothermal treatment of lignin. Appl. Biochem. Biotechnol. 2008, 147, 119–131. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Robert, J.H.; Hendrik, A.J.B.; David, G.E. Thermal dissociation of tetralin between 300 and 450 °C. Fuel 1979, 58, 132–138. [Google Scholar]
- Harris, G.C.; Sanderson, T.F. Resin acids; the position of the ring double bond in dextropimaric acid and the structure of isodextropimaric acid. J. Am. Chem. Soc. 1948, 70, 2081–2085. [Google Scholar] [CrossRef]
- Shui, H.; Cai, Z.; Xu, C. Recent Advances in Direct Coal Liquefaction. Energies 2010, 3, 155–170. [Google Scholar] [CrossRef]
- De Marco Rodriguez, I.; Chomón, M.J.; Caballero, B.; Arias, P.L.; Legarreta, J.A. Liquefaction behavior of a Spanish subbituminous coal under different conditions of hydrogen availability. Fuel Proc. Technol. 1998, 58, 17–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).