Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Type of Soils
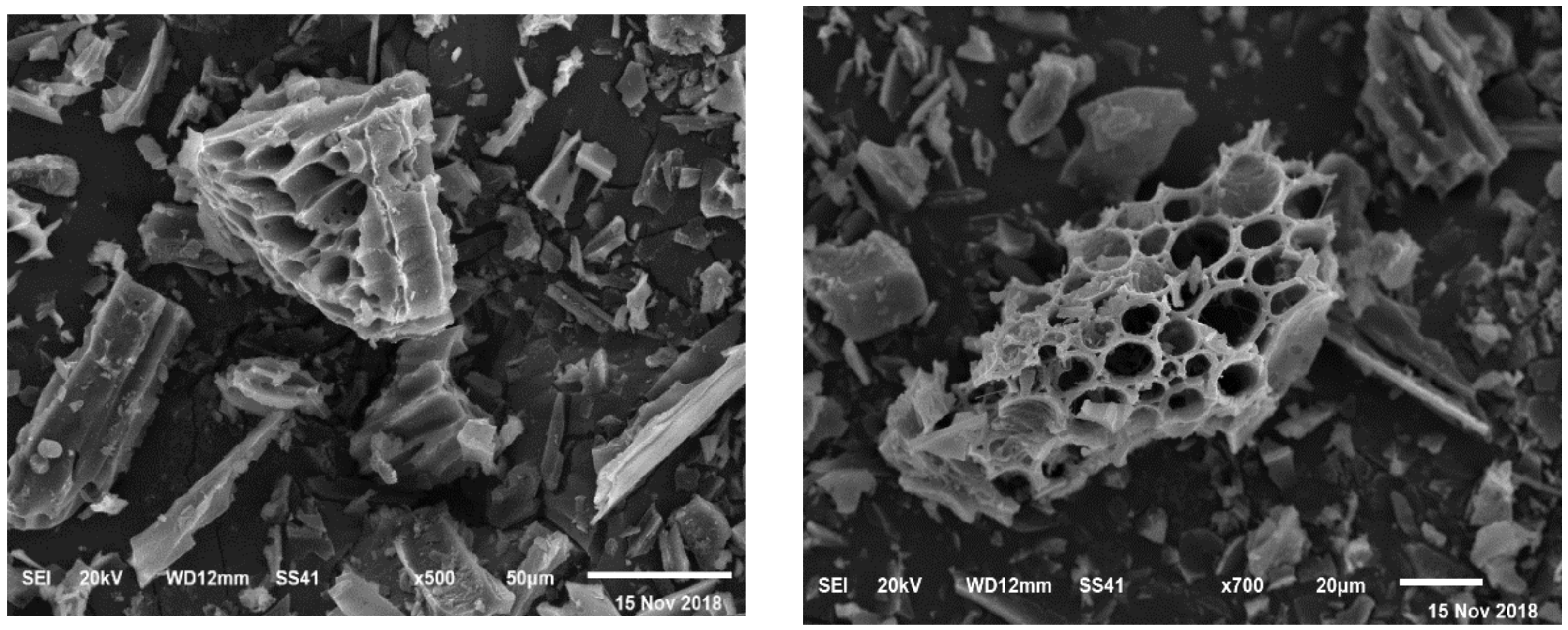
2.2. Source and Type of Biochar
2.3. Heavy Metals
2.4. Greenhouse Experiments
2.5. Total Counts of Bacteria and Fungi
2.6. The Resistance Index (RS)
2.7. Enzymatic Activity
2.8. Media Used
- Nutrient Agar: Beef extract (3 g), yeast extract (2 g), peptone (5 g), agar (15 g), distilled water of 1000 mL and pH (7.0).
- 2.
- Martin’s medium for fungi: Glucose (10 g), peptone (5 g), KH2PO4 (1 g), MgSO4∙7H2O (0.5 g), agar (20 g), distilled water of 1000 mL and rose bengal 1 part in 30,000 parts of medium.
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Selected Heavy Metals on Microbial Activity in Soils Amended with Biochar
3.2. Fungal/Bacterial (F/B) Ratio
3.3. Resistance Index (RS)
3.4. Effect on Nodulation
3.5. Effect of Biochar on Enzyme Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Touceda-González, M.; Prieto-Fernández, Á.; Renella, G.; Giagnoni, L.; Sessitsch, A.; Brader, G.; Kumpiene, J.; Dimitriou, I.; Eriksson, J.; Friesl-Hanl, W.; et al. Microbial community structure and activity in trace element-contaminated soils phytomanaged by Gentle Remediation Options (GRO). Environ. Pollut. 2017, 231, 237–251. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and Enzymatic Soil Properties Influenced by Cropping of Primary Wheat under Organic and Conventional Farming Systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
- Stockdale, E.A.; Brookes, P.C. Detection and quantification of the soil microbial biomass—Impacts on the management of agricultural soils. J. Agric. Sci. 2006, 144, 285–302. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Epelde, L.; Garbisu, C.; Bernal, M.P. Evaluation of the phytostabilisation efficiency in a trace elements contaminated soil using soil health indicators. J. Hazard. Mater. 2014, 268, 68–76. [Google Scholar] [CrossRef]
- Oliveira, A.; Pampulha, M.E. Effects of long-term heavy metal contamination on soil microbial characteristics. J. Biosci. Bioeng. 2006, 102, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Wu, J.; You, A.; Huang, B.; Cao, C. Effects of heavy metals on soil microbial community structure and diversity in the rice (Oryza sativa L. subsp. Japonica, Food Crops Institute of Jiangsu Academy of Agricultural Sciences) rhizosphere. Soil Sci. Plant Nutr. 2016, 63, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Bielińska, E.J.; Kołodziej, B.; Sugier, D. Relationship between organic carbon content and the activity of selected enzymes in urban soils under different anthropogenic influence. J. Geochem. Explor. 2013, 129, 52–56. [Google Scholar] [CrossRef]
- Breza-Boruta, B.; Lemanowicz, J.; Bartkowiak, A. Variation in biological and physicochemical parameters of the soil affected by uncontrolled landfill sites. Environ. Earth Sci. 2016, 75, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.; Hockaday, W.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 2nd ed.; Earthscan: London, UK, 2009; pp. 1–9. [Google Scholar] [CrossRef]
- Jiang, X.; Haddix, M.L.; Cotrufo, M.F. Interactions between biochar and soil organic carbon decomposition: Effects of nitrogen and low molecular weight carbon compound addition. Soil Biol. Biochem. 2016, 100, 92–101. [Google Scholar] [CrossRef]
- Kammann, C.; Ippolito, J.; Hagemann, N.; Borchard, N.; Cayuela, M.L.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Jeffery, S.; Kern, J.; Novak, J.; et al. Biochar as a tool to reduce the agricultural greenhouse-gas burden—Knowns, unknowns and future research needs. J. Environ. Eng. Landsc. Manag. 2017, 25, 114–139. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-Ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan from Routledge: London, UK, 2015; pp. 139–161. ISBN 978-0-415-70415-1. [Google Scholar]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology, 1st ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–29. [Google Scholar]
- Jaafar, N.M.; Clode, P.; Abbott, L. Biochar-Soil Interactions in Four Agricultural Soils. Pedosphere 2015, 25, 729–736. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Hussain, Q.; Khan, K.S.; Akmal, M.; Ijaz, S.S.; Hayat, R.; Khalid, A.; Azeem, M.; Rashid, M. Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: A 2-year field study. Arab. J. Geosci. 2019, 12, 95. [Google Scholar] [CrossRef]
- Lopes, M.G.; Reis, M.M.; Frazão, L.A.; Terra, L.E.D.M.; Lopes, E.F.; dos Santos, M.M.; Fernandes, L.A. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 2021, 21, 101270. [Google Scholar] [CrossRef]
- Polifka, S.; Wiedner, K.; Glaser, B. Increased CO2 fluxes from a sandy Cambisol under agricultural use in the Wendland region, Northern Germany, three years after biochar substrates application. GCB Bioenergy 2018, 10, 432–443. [Google Scholar] [CrossRef]
- Akmal, M.; Maqbool, Z.; Khan, K.S.; Hussain, Q.; Ijaz, S.S.; Iqbal, M.; Aziz, I.; Hussain, A.; Abbas, M.S.; Rafa, H.U. Integrated use of biochar and compost to improve soil microbial activity, nutrient availability, and plant growth in arid soil. Arab. J. Geosci. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Azeem, M.; Hayat, R.; Hussain, Q.; Ahmed, M.; Pan, G.; Tahir, M.I.; Imran, M.; Hassan, M.U. Biochar improves soil quality and N2-fixation and reduces net ecosystem CO2 exchange in a dryland legume-cereal cropping system. Soil Tillage Res. 2018, 186, 172–182. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. (Eds.) Methods of Soil Analysis: Part 2, Chemical and Microbiological Properties; Agronomy Series No 9; American Society of Agricultural and Biological Engineers: Madison, WI, USA, 1982. [Google Scholar]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Horwath, W.R.; Paul, E.A. Microbial biomass. In Methods of Soil Analysis: Part 2, Microbial and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; SSSA Book Series 5; SSSA: Madison, WI, USA, 1994; pp. 753–773. [Google Scholar]
- Akagi, H.; Nishimura, H. Speciation of mercury in the environment. In Advances in Mercury Toxicity; Suzuki, T., Nobumassam, I., Clarkson, W.T., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 53–76. [Google Scholar]
- Haddad, S.; Tabatabai, M.A.; Abdel-Moneim, A.-M.A.; Loynachan, T.E. Inhibition of Nodulation and Nitrogen Nutrition of Leguminous Crops by Selected Heavy Metals. Air Soil Water Res. 2015, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Haddad, S.A.; Lemanowicz, J.; El-Azeim, M.A. Cellulose decomposition in clay and sandy soils contaminated with heavy metals. Int. J. Environ. Sci. Technol. 2018, 16, 3275–3290. [Google Scholar] [CrossRef]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Orwin, K.; Wardle, D. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Alef, K.; Kleiner, D. Applicability of arginine ammonification as indicator of microbial activity in different soils. Biol. Fertil. Soils 1987, 5, 148–151. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2, Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- SAS Institute Inc. BASE SAS 9.3 Procedures Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.; Maddern, T.M.; Murphy, D.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.; Faz, A.; Bååth, E. Microbial growth and community structure in acid mine soils after addition of different amendments for soil reclamation. Geoderma 2016, 272, 64–72. [Google Scholar] [CrossRef]
- de Vries, F.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2013, 65, 28–39. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Kucharski, M.; Borowik, A. Effect of cadmium, copper and zinc on plants, soil microorganisms and soil enzymes. J. Elem. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Wirth, S.J.; Alam, P.; Alyemeni, M.N.; Ahmad, P. Interactive Effects of Nutrients and Bradyrhizobium japonicum on the Growth and Root Architecture of Soybean (Glycine max L.). Front. Microbiol. 2018, 9, 1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beesley, L.; Moreno-Jiménez, E.; Fellet, G.; Carrijo, L.; Sizmur, T. Biochar for Environmental Management: Science and Technology. In Biochar and Heavy Metals; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2015. [Google Scholar]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere 2014, 119, 209–216. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Cui, L.; Yan, J.; Yang, Y.; Li, L.; Quan, G.; Ding, C.; Chen, T.; Fu, Q.; Chang, A. Influence of Biochar on Microbial Activities of Heavy Metals Contaminated Paddy Fields. BioResources 2013, 8, 5536–5548. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Mishra, D.; Khare, P.; Yadav, V.; Deshmukh, Y.; Meena, A. Impact of biochar amendment on enzymatic resilience properties of mine spoils. Sci. Total Environ. 2016, 544, 410–421. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Niemi, R.M.; Heiskanen, I.; Saarnio, S. Weak impacts of biochar amendment on soil enzyme activities in mesocosms in bare or Phleum pratense soil. Boreal Environ. Res. 2015, 20, 324–334. Available online: http://hdl.handle.net/10138/228210 (accessed on 10 September 2021).
- Wyszkowska, J.; Kucharski, J.; Kucharski, M. Activity of β-glucosidase, arylsulfatase and phosphatases in soil contaminated with copper. J. Elem. 2010, 15, 213–226. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Berry, C.M.; Strawn, D.; Novak, J.M.; Levine, J.; Harley, A. Biochars Reduce Mine Land Soil Bioavailable Metals. J. Environ. Qual. 2017, 46, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-T.; Rouland-LeFèvre, C.; Benedetti, M.F.; Li, F.-B.; Pando, A.; Lavelle, P.; Dai, J. Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol. Biochem. 2009, 41, 969–977. [Google Scholar] [CrossRef]


| Texture Grade | Clay Soil | Sandy Soil | ||||
|---|---|---|---|---|---|---|
| (Field capacity) F.C % | 38.26 | 13.96 | ||||
| (Permanent wilting point) PWP % | 15.11 | 3.46 | ||||
| (Water holding capacity) WHC % | 47.66 | 18.22 | ||||
| Available water (F.C–PWP) % | 23.15 | 10.50 | ||||
| Available water (WHC–PWP) % | 32.55 | 14.76 | ||||
| Bulk Density (BD) g cm−3 | 1.18 | 1.63 | ||||
| Particle Density g cm−3 | 2.26 | 2.61 | ||||
| pH (1:2.5 H2O) | 7.7(7.4) a | 8.5(8.3) a | ||||
| CEC (cmol(+) kg−1 soil) | 37.87 | 3.66 | ||||
| EC (dS m−1 at 25 °C) | 1.22 | 2.58 | ||||
| (Soil organic carbon) S.O.C g kg−1 | 16.8 | 4.1 | ||||
| Organic matter g kg−1 | 28.34 b | 7.28 b | ||||
| C/N ratio | 20.99 | 17.75 | ||||
| Total N g kg−1 | 1.41 | 0.41 | ||||
| Total P g kg−1 | 1.16 | 0.29 | ||||
| Total K g kg−1 | 12.88 | 2.7 | ||||
| (Microbial biomass C) Cmic | 61.45 | 34.67 | ||||
| (Microbial biomass N) Nmic | 18.32 | 6.65 | ||||
| Cmic: Nmic | 3.35 | 5.21 | ||||
| Mineral N (mg kg−1) | 78.24 | 22.43 | ||||
| Total heavy metals content (mg kg−1) c | Cd | Ni | Pb | Cd | Ni | Pb |
| 0.55 | 43.7 | 31.4 | 3.9 | 44.3 | 52.7 | |
| Biochar Property | |||
|---|---|---|---|
| Bulk Density g/cm3 | 0.26 | ||
| WHC % | 58.7 | ||
| pH (1: 2.5 H2O) | 6.31 | ||
| EC (dS m−1 at 25 °C) | 0.651 | ||
| CEC (cmol(+) kg−1 soil) | 34.4 | ||
| Ash % | 11.5 | ||
| Total organic carbon g kg−1 | 562 | ||
| Total N g kg−1 | 16.8 | ||
| C/N ratio | 33.2 | ||
| Total P g kg−1 | 3.2 | ||
| N/P ratio | 5.27 | ||
| Total K mg kg−1 | 480 | ||
| Total Ca mg kg−1 | 650 | ||
| Total Mg mg kg−1 | 40.6 | ||
| Total heavy metals (mg kg−1) Dry matter | Cd | Ni | Pb |
| 1.42 | 6.41 | 3.56 | |
| Crop | Soils | |
|---|---|---|
| Clay | Sandy | |
| Treatments | ||
| soybean | Control (clay soil only) | Control (sandy soil only) |
| Cd | Cd | |
| Ni | Ni | |
| Pb | Pb | |
| soybean | Biochar 1 (B1) | Biochar 1 (B1) |
| B1 Cd | B1 Cd | |
| B1 Ni | B1 Ni | |
| B1 Pb | B1 Pb | |
| soybean | Biochar 2 (B2) | Biochar 2 (B2) |
| B2 Cd | B2 Cd | |
| B2 Ni | B2 Ni | |
| B2 Pb | B2 Pb | |
| Treatment | Clay Soil | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after Plant Emergence | ||||||||||||
| 15 | 30 | 60 | 90 | |||||||||
| F | B | F/B | F | B | F/B | F | B | F/B | F | B | F/B | |
| Control | 17 | 26 | 0.65 | 25 | 32.6 | 0.77 | 110 | 125 | 0.88 | 60 | 75 | 0.80 |
| Cd | 7 | 9 | 0.78 | 8 | 10 | 0.80 | 5 | 6 | 0.83 | 3 | 4 | 0.75 |
| Ni | 6 | 7 | 0.86 | 10 | 12 | 0.83 | 6 | 9 | 0.67 | 4 | 8 | 0.50 |
| Pb | 5 | 6 | 0.83 | 18 | 24 | 0.75 | 11 | 17 | 0.65 | 9 | 13 | 0.69 |
| B1 | 30 | 35 | 0.86 | 105 | 61.3 | 1.71 | 180 | 140 | 1.29 | 90 | 90 | 1.00 |
| B1 Cd | 18 | 20 | 0.90 | 39 | 32 | 1.22 | 109 | 95 | 1.15 | 80 | 70 | 1.14 |
| B1 Ni | 20 | 24 | 0.83 | 43 | 38 | 1.13 | 114 | 102 | 1.12 | 86 | 82 | 1.05 |
| B1 Pb | 13 | 18 | 0.72 | 39 | 30 | 1.30 | 89 | 85 | 1.05 | 70 | 68 | 1.03 |
| B2 | 42 | 50 | 0.84 | 160 | 85 | 1.88 | 210 | 186 | 1.13 | 170 | 130 | 1.31 |
| B2 Cd | 30 | 35 | 0.86 | 54 | 49 | 1.10 | 140 | 130 | 1.08 | 103 | 98 | 1.05 |
| B2 Ni | 31 | 37 | 0.84 | 58 | 53 | 1.09 | 145 | 132 | 1.10 | 109 | 89 | 1.22 |
| B2 Pb | 28 | 33 | 0.85 | 49 | 48 | 1.02 | 139 | 120 | 1.16 | 95 | 87 | 1.09 |
| L.S.D at 0.05 | 16.97 | 10.92 | 0.13 | 35.76 | 19.20 | 0.21 | 42.98 | 26.37 | 0.20 | 27.14 | 40.63 | 0.16 |
| L.S.D at 0.01 | 23.08 | 14.40 | 0.18 | 48.47 | 26.02 | 0.29 | 58.24 | 35.74 | 0.27 | 36.78 | 55.07 | 0.21 |
| Treatment | Sandy Soil | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after Plant Emergence | ||||||||||||
| 15 | 30 | 60 | 90 | |||||||||
| F | B | F/B | F | B | F/B | F | B | F/B | F | B | F/B | |
| Control | 12 | 14 | 0.86 | 20 | 25 | 0.80 | 105 | 115 | 0.91 | 52 | 65 | 0.80 |
| Cd | 4 | 6 | 0.67 | 8 | 11 | 0.73 | 3 | 5 | 0.60 | 0 | 3 | - |
| Ni | 5 | 7 | 0.71 | 14 | 15 | 0.93 | 9 | 12 | 0.75 | 3 | 9 | 0.33 |
| Pb | 4 | 5 | 0.80 | 9 | 17 | 0.53 | 8 | 9 | 0.89 | 4 | 5 | 0.80 |
| B1 | 25 | 25 | 1.00 | 63 | 50 | 1.26 | 180 | 133 | 1.35 | 83 | 80 | 1.04 |
| B1 Cd | 18 | 14 | 1.29 | 39 | 22 | 1.77 | 96 | 83 | 1.16 | 72 | 70 | 1.03 |
| B1 Ni | 20 | 16 | 1.25 | 30 | 28 | 1.07 | 98 | 93 | 1.05 | 73 | 74 | 0.99 |
| B1 Pb | 16 | 12 | 1.33 | 24 | 21 | 1.14 | 79 | 72 | 1.10 | 68 | 70 | 0.97 |
| B2 | 38 | 32 | 1.19 | 50 | 70 | 0.71 | 194 | 163 | 1.19 | 112 | 102 | 1.10 |
| B2 Cd | 27 | 23 | 1.17 | 45 | 39 | 1.15 | 109 | 103 | 1.06 | 93 | 90 | 1.03 |
| B2 Ni | 29 | 24 | 1.21 | 48 | 42 | 1.14 | 115 | 111 | 1.04 | 95 | 92 | 1.03 |
| B2 Pb | 24 | 20 | 1.20 | 40 | 39 | 1.03 | 106 | 108 | 0.98 | 87 | 78 | 1.12 |
| L.S.D at 0.05 | 12.61 | 9.89 | 0.50 | 11.05 | 20.08 | 0.15 | 31.12 | 17.47 | 0.19 | 28.83 | 28.82 | 0.22 |
| L.S.D at 0.01 | 17.08 | 13.41 | 0.68 | 14.97 | 27.21 | 0.20 | 42.17 | 23.67 | 0.26 | 38.01 | 39.05 | 0.29 |
| Treatment | Clay Soil | Sandy Soil | ||||||
|---|---|---|---|---|---|---|---|---|
| Days after Plant Emergence | ||||||||
| 15 | 30 | 60 | 90 | 15 | 30 | 60 | 90 | |
| Cd | 0.21 | 0.18 | 0.02 | 0.03 | 0.27 | 0.28 | 0.02 | 0.02 |
| Ni | 0.16 | 0.23 | 0.04 | 0.05 | 0.33 | 0.43 | 0.06 | 0.07 |
| Pb | 0.13 | 0.15 | 0.07 | 0.09 | 0.22 | 0.51 | 0.04 | 0.04 |
| B1 Cd | 0.40 | 0.35 | 0.51 | 0.64 | 0.39 | 0.28 | 0.45 | 0.78 |
| B1 Ni | 0.52 | 0.45 | 0.57 | 0.84 | 0.47 | 0.39 | 0.54 | 0.86 |
| B1 Pb | 0.34 | 0.32 | 0.44 | 0.61 | 0.36 | 0.27 | 0.37 | 0.78 |
| B2 Cd | 0.54 | 0.40 | 0.54 | 0.60 | 0.56 | 0.39 | 0.46 | 0.79 |
| B2 Ni | 0.59 | 0.52 | 0.55 | 0.52 | 0.60 | 0.43 | 0.52 | 0.82 |
| B2 Pb | 0.49 | 0.39 | 0.48 | 0.50 | 0.45 | 0.39 | 0.50 | 0.61 |
| Treatment | Clay Soil | Sandy Soil | ||||||
|---|---|---|---|---|---|---|---|---|
| Days after Plant Emergence | ||||||||
| 15 | 30 | 60 | 90 | 15 | 30 | 60 | 90 | |
| Cd | 0.26 | 0.18 | 0.02 | 0.03 | 0.20 | 0.25 | 0.01 | 0.00 |
| Ni | 0.21 | 0.23 | 0.04 | 0.03 | 0.26 | 0.54 | 0.05 | 0.03 |
| Pb | 0.17 | 0.15 | 0.07 | 0.08 | 0.20 | 0.29 | 0.04 | 0.04 |
| B1 Cd | 0.43 | 0.35 | 0.51 | 0.80 | 0.56 | 0.45 | 0.36 | 0.77 |
| B1 Ni | 0.50 | 0.45 | 0.57 | 0.91 | 0.67 | 0.31 | 0.36 | 0.78 |
| B1 Pb | 0.28 | 0.32 | 0.44 | 0.64 | 0.47 | 0.26 | 0.28 | 0.69 |
| B2 Cd | 0.56 | 0.40 | 0.54 | 0.44 | 0.55 | 0.39 | 0.39 | 0.71 |
| B2 Ni | 0.58 | 0.52 | 0.55 | 0.47 | 0.62 | 0.43 | 0.42 | 0.74 |
| B2 Pb | 0.50 | 0.39 | 0.48 | 0.39 | 0.46 | 0.33 | 0.38 | 0.64 |
| Treatment | Clay Soil | Sandy Soil | ||
|---|---|---|---|---|
| Number of Nodules | ||||
| Flower Stage | Pudding Stage | Flower Stage | Pudding Stage | |
| Control | 19 | 14 | 10 | 8 |
| Cd | 9 | 5 | 0 | 0 |
| Ni | 10 | 4 | 6 | 4 |
| Pb | 7 | 5 | 6.5 | 5 |
| B1 | 32 | 30 | 18 | 19 |
| B1 Cd | 18 | 16 | 8 | 16 |
| B1 Ni | 21 | 18 | 11 | 18 |
| B1 Pb | 17 | 13 | 9 | 14 |
| B2 | 38 | 47 | 26 | 40 |
| B2 Cd | 20 | 27 | 11 | 22 |
| B2 Ni | 26 | 28 | 15 | 24 |
| B2 Pb | 19 | 25 | 12 | 21 |
| L.S.D at 0.05 | 12.38 | 15.12 | 10.93 | 14.45 |
| L.S.D at 0.01 | 16.78 | 20.49 | 14.82 | 19.57 |
| Treatments | Clay Soil | Sandy Soil | ||
|---|---|---|---|---|
| Arginase (μg NH4-N g−1 h−1) | Urease (μmol NH3-N g−1 h−1) | Arginase (μg NH4-N g−1 h−1) | Urase (μmol NH3-N g−1 h−1) | |
| Control | 43 | 7.6 | 35 | 6.8 |
| Cd | 26 | 5.4 | 16 | 3.9 |
| Ni | 28 | 6.3 | 18 | 4.3 |
| Pb | 22 | 4.1 | 12 | 2.4 |
| B1 | 49 | 12.5 | 43 | 10.2 |
| B1 Cd | 38 | 6.3 | 26 | 6.4 |
| B1 Ni | 40 | 8.8 | 30 | 5.9 |
| B1 Pb | 30 | 5.9 | 20 | 4.8 |
| B2 | 54 | 15.6 | 48 | 12.5 |
| B2 Cd | 41 | 11.5 | 31 | 8.4 |
| B2 Ni | 46 | 13.2 | 35 | 9.6 |
| B2 Pb | 39 | 9.8 | 28 | 5.3 |
| L.S.D at 0.05 | 3.57 | 2.66 | 3.95 | 1.68 |
| L.S.D at 0.01 | 4.84 | 3.61 | 5.35 | 2.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, S.A.; Lemanowicz, J. Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals. Energies 2021, 14, 5763. https://doi.org/10.3390/en14185763
Haddad SA, Lemanowicz J. Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals. Energies. 2021; 14(18):5763. https://doi.org/10.3390/en14185763
Chicago/Turabian StyleHaddad, Samir A., and Joanna Lemanowicz. 2021. "Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals" Energies 14, no. 18: 5763. https://doi.org/10.3390/en14185763
APA StyleHaddad, S. A., & Lemanowicz, J. (2021). Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals. Energies, 14(18), 5763. https://doi.org/10.3390/en14185763







