Solar-Driven Desalination Using Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles and Fluids
2.2. Experimental System
3. Results and Discussion
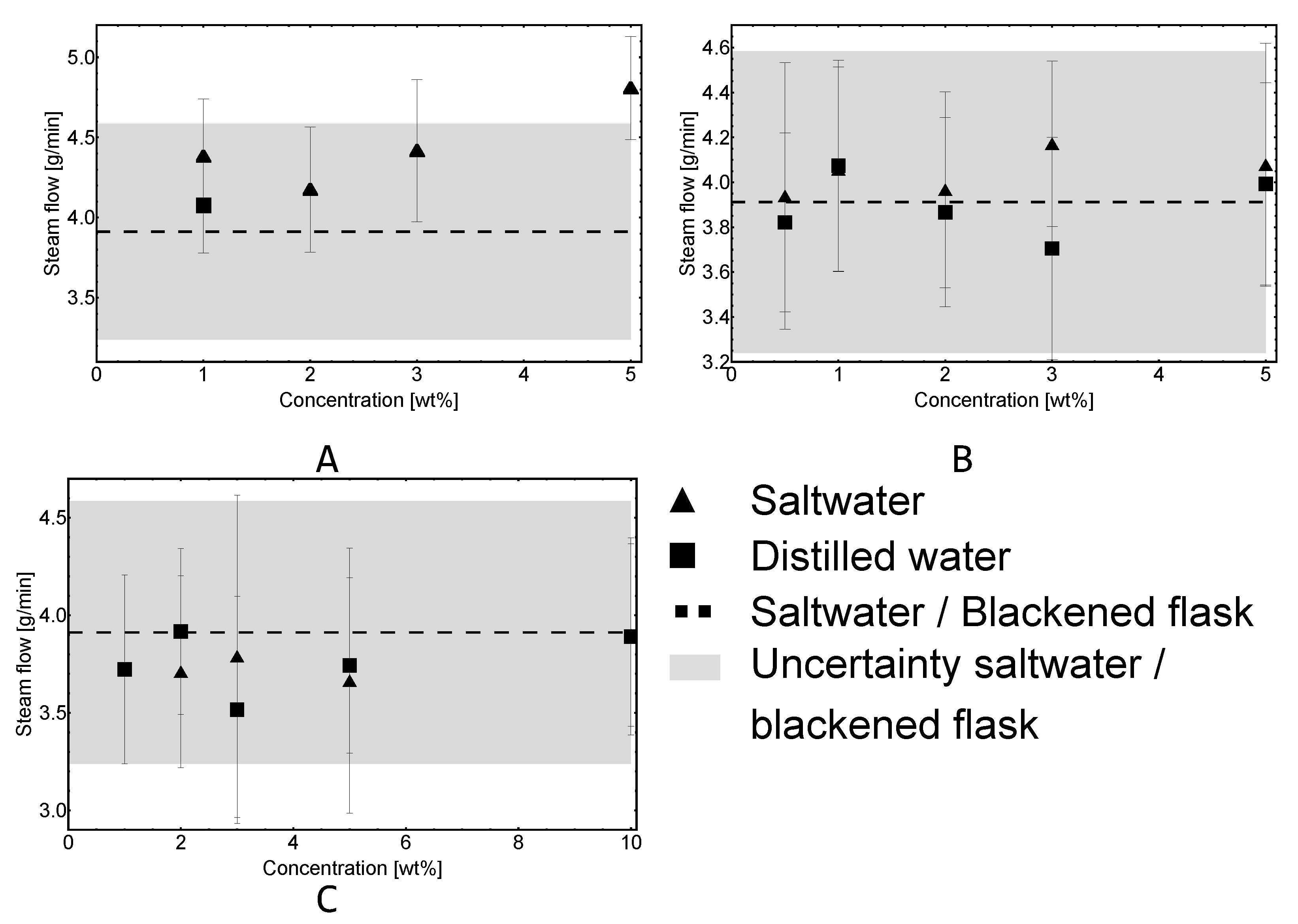
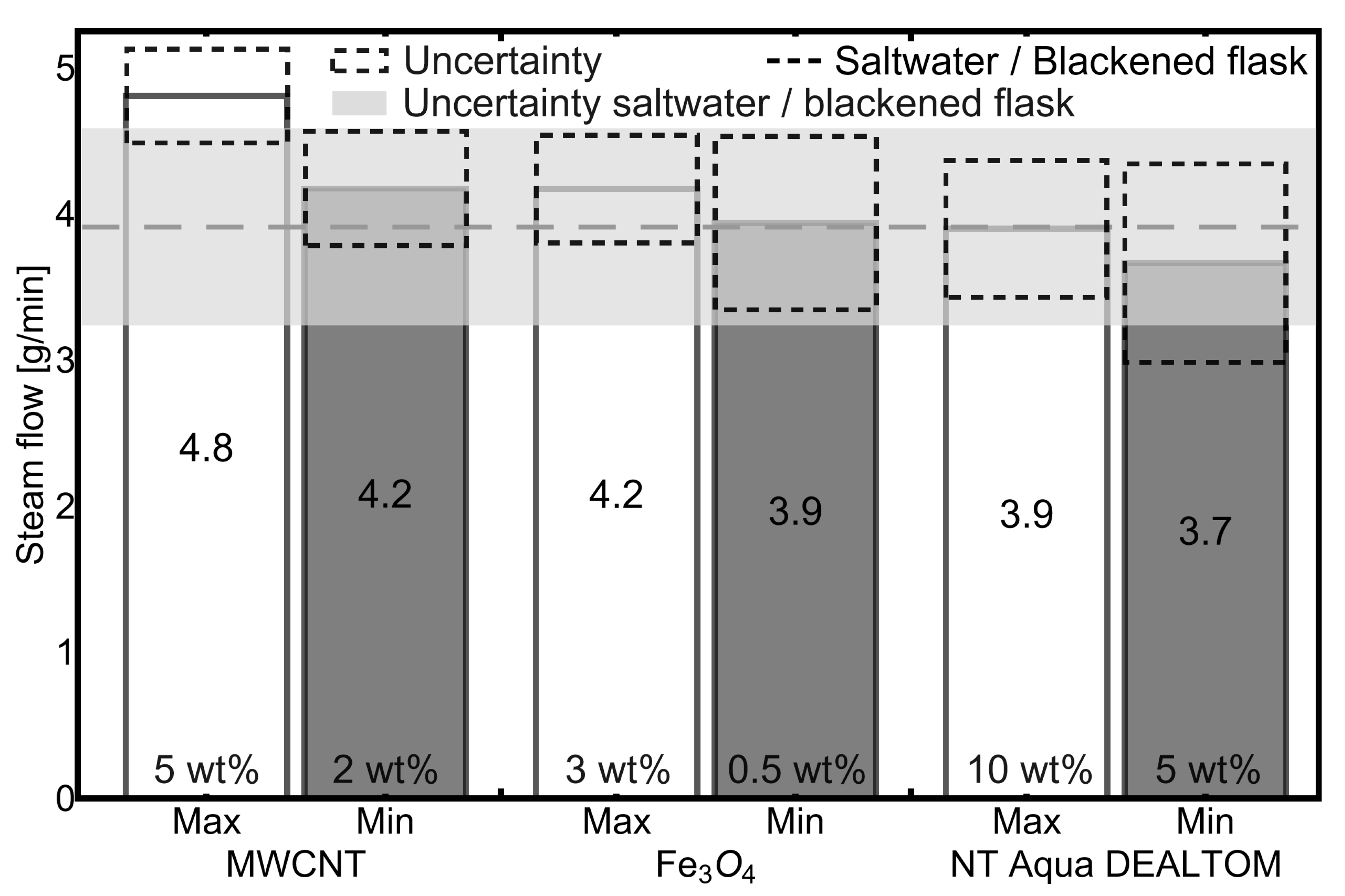
3.1. Experiments
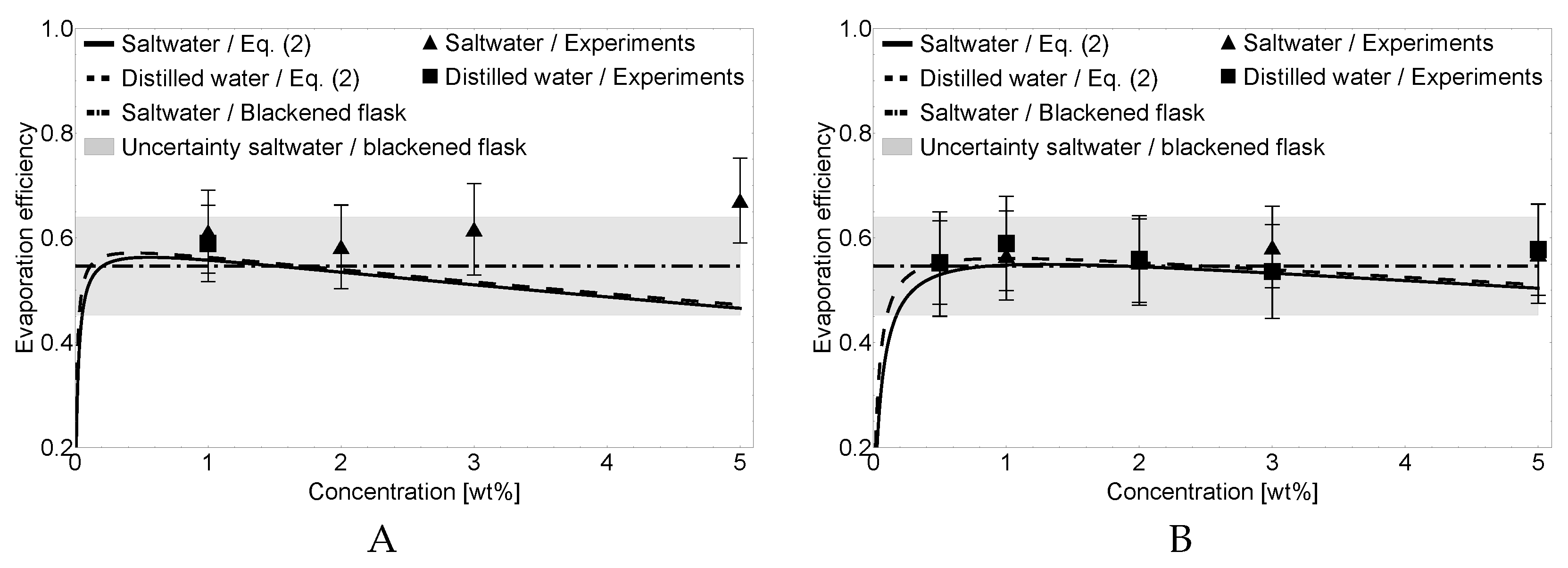
3.2. Evaporation Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO World Water Assessment Programme. The United Nations World Water Development Report 2019: Leaving No One behind; UNESCO: Paris, France, 2019. [Google Scholar]
- Ercin, A.; Hoekstra, A. Water footprint scenarios for 2050: A global analysis. Environ. Int. 2014, 64, 71–82. [Google Scholar] [CrossRef]
- Ahmed, F.; Hashaikeh, R.; Hilal, N. Solar powered desalination–Technology, energy and future outlook. Desalination 2019, 453, 54–76. [Google Scholar] [CrossRef] [Green Version]
- Panagopoulos, A. Water-energy nexus: Desalination technologies and renewable energy sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Mahavar, S.; Goyal, A.; Balakin, B.V. Investigation of a Solar Concentrator for Water Distillation. In Advances in Thermal Engineering, Manufacturing, and Production Management; Springer: Singapore, 2020; pp. 209–217. [Google Scholar]
- Ihsanullah, I.; Atieh, M.; Sajid, M.; Nazal, M. Desalination and environment: A critical analysis of impacts, mitigation strategies, and greener desalination technologies. Sci. Total Environ. 2021, 780, 146585. [Google Scholar] [CrossRef]
- Ahmed, F.; Khalil, A.; Hilal, N. Emerging desalination technologies: Current status, challenges and future trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- Sattar, A.; Farooq, M.; Amjad, M.; Saeed, M.; Nawaz, S.; Mujtaba, M.; Anwar, S.; El-Sherbeeny, A.; Soudagar, M.; Bandarra Filho, E.; et al. Performance evaluation of a direct absorption collector for solar thermal energy conversion. Energies 2020, 13, 4956. [Google Scholar] [CrossRef]
- Kasaeian, A.; Daneshazarian, R.; Pourfayaz, F. Comparative study of different nanofluids applied in a trough collector with glass-glass absorber tube. J. Mol. Liq. 2017, 234, 315–323. [Google Scholar] [CrossRef]
- Boldoo, T.; Ham, J.; Kim, E.; Cho, H. Review of the photothermal energy conversion performance of nanofluids, their applications, and recent advances. Energies 2020, 13, 5748. [Google Scholar] [CrossRef]
- Boldoo, T.; Ham, J.; Cho, H. Comprehensive experimental study on the thermophysical characteristics of di water based co0.5zn0.5fe2o4 nanofluid for solar thermal harvesting. Energies 2020, 13, 6218. [Google Scholar] [CrossRef]
- Lucas, M.; Kosinski, P.; Balakin, B. Eulerian–Eulerian model for photothermal energy conversion in nanofluids. AIP Conf. Proc. 2019, 2116, 030011. [Google Scholar] [CrossRef]
- Sani, E.; Papi, N.; Mercatelli, L.; Żyła, G. Graphite/diamond ethylene glycol-nanofluids for solar energy applications. Renew. Energy 2018, 126, 692–698. [Google Scholar] [CrossRef]
- Wang, Y.; Zaytsev, M.; The, H.; Eijkel, J.; Zandvliet, H.; Zhang, X.; Lohse, D. Vapor and Gas-Bubble Growth Dynamics around Laser-Irradiated, Water-Immersed Plasmonic Nanoparticles. ACS Nano 2017, 11, 2045–2051. [Google Scholar] [CrossRef]
- Neumann, O.; Urban, A.; Day, J.; Lal, S.; Nordlander, P.; Halas, N. Solar vapor generation enabled by nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lin, G.; Bai, L.; Zeiny, A.; Wen, D. Steam generation in a nanoparticle-based solar receiver. Nano Energy 2016, 28, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Ulset, E.; Kosinski, P.; Zabednova, Y.; Zhdaneev, O.; Struchalin, P.; Balakin, B. Photothermal boiling in aqueous nanofluids. Nano Energy 2018, 50, 339–346. [Google Scholar] [CrossRef]
- Technology Company “Nanopowders”. 2021. Available online: http://www.technopark-mordovia.ru/ (accessed on 13 July 2021).
- DEALTOM. 2021. Available online: http://dealtom.ru/ (accessed on 8 June 2021).
- Doctor Sea. 2021. Available online: https://www.doctor-sea.com/ (accessed on 13 July 2021).
- Struchalin, P.; Thon, H.; Kuzmenkov, D.; Kutsenko, K.; Kosinski, P.; Balakin, B. Solar steam generation enabled by iron oxide nanoparticles: Prototype experiments and theoretical model. Int. J. Heat Mass Transf. 2020, 158, 119987. [Google Scholar] [CrossRef]
- Kuzmenkov, D.; Delov, M.; Zeynalyan, K.; Struchalin, P.; Alyaev, S.; He, Y.; Kutsenko, K.; Balakin, B.V. Solar steam generation in fine dispersions of graphite particles. Renew. Energy 2020, 161, 265–277. [Google Scholar] [CrossRef]
- OSRAM. 2021. Available online: https://www.osram.com/ (accessed on 8 June 2021).
- Sukhatme, S.; Nayak, J. Solar Energy: Principles of Thermal Collection and Storage; Tata McGraw Hill: New Delhi, India, 2011. [Google Scholar]
- Ulset, E. Utilizing Solar Vapour Energy by Use of Nanofluids in a Direct Absorption Solar Collector. Master’s Thesis, University of Bergen, Bergen, Norway, 2018. [Google Scholar]
- Camozzi Automation. 2021. Available online: https://www.camozzi.ru/ (accessed on 13 July 2021).
- Prist. 2021. Available online: https://prist.ru/ (accessed on 13 July 2021).
- Aqua Computer. 2021. Available online: https://shop.aquacomputer.de/ (accessed on 13 July 2021).
- Prosperetti, A. Vapor Bubbles. Annu. Rev. Fluid Mech. 2017, 49, 221–248. [Google Scholar] [CrossRef]
- Ulset, E.; Kosinski, P.; Balakin, B. Solar steam in an aqueous carbon black nanofluid. Appl. Therm. Eng. 2018, 137, 62–65. [Google Scholar] [CrossRef]
- Ni, G.; Miljkovic, N.; Ghasemi, H.; Huang, X.; Boriskina, S.; Lin, C.T.; Wang, J.; Xu, Y.; Rahman, M.; Zhang, T.; et al. Volumetric solar heating of nanofluids for direct vapor generation. Nano Energy 2015, 17, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Sharqawy, M.; Lienhard V, J.; Zubair, S. Thermophysical properties of seawater: A review of existing correlations and data. Desalin. Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzmenkov, D.M.; Struchalin, P.G.; Olkhovskii, A.V.; Yunin, V.S.; Kutsenko, K.V.; Balakin, B.V. Solar-Driven Desalination Using Nanoparticles. Energies 2021, 14, 5743. https://doi.org/10.3390/en14185743
Kuzmenkov DM, Struchalin PG, Olkhovskii AV, Yunin VS, Kutsenko KV, Balakin BV. Solar-Driven Desalination Using Nanoparticles. Energies. 2021; 14(18):5743. https://doi.org/10.3390/en14185743
Chicago/Turabian StyleKuzmenkov, Dmitrii M., Pavel G. Struchalin, Andrey V. Olkhovskii, Vladimir S. Yunin, Kirill V. Kutsenko, and Boris V. Balakin. 2021. "Solar-Driven Desalination Using Nanoparticles" Energies 14, no. 18: 5743. https://doi.org/10.3390/en14185743
APA StyleKuzmenkov, D. M., Struchalin, P. G., Olkhovskii, A. V., Yunin, V. S., Kutsenko, K. V., & Balakin, B. V. (2021). Solar-Driven Desalination Using Nanoparticles. Energies, 14(18), 5743. https://doi.org/10.3390/en14185743





