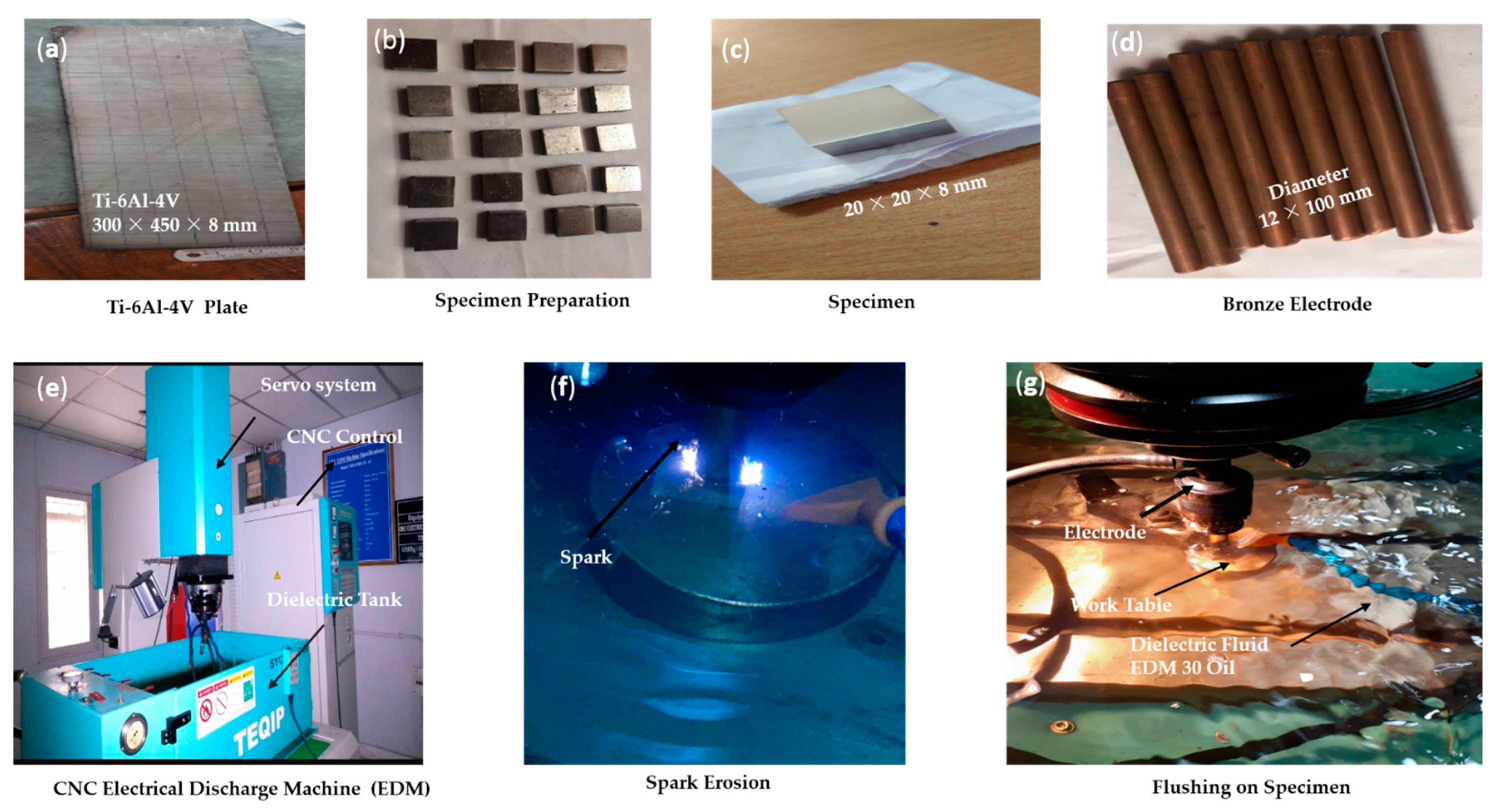
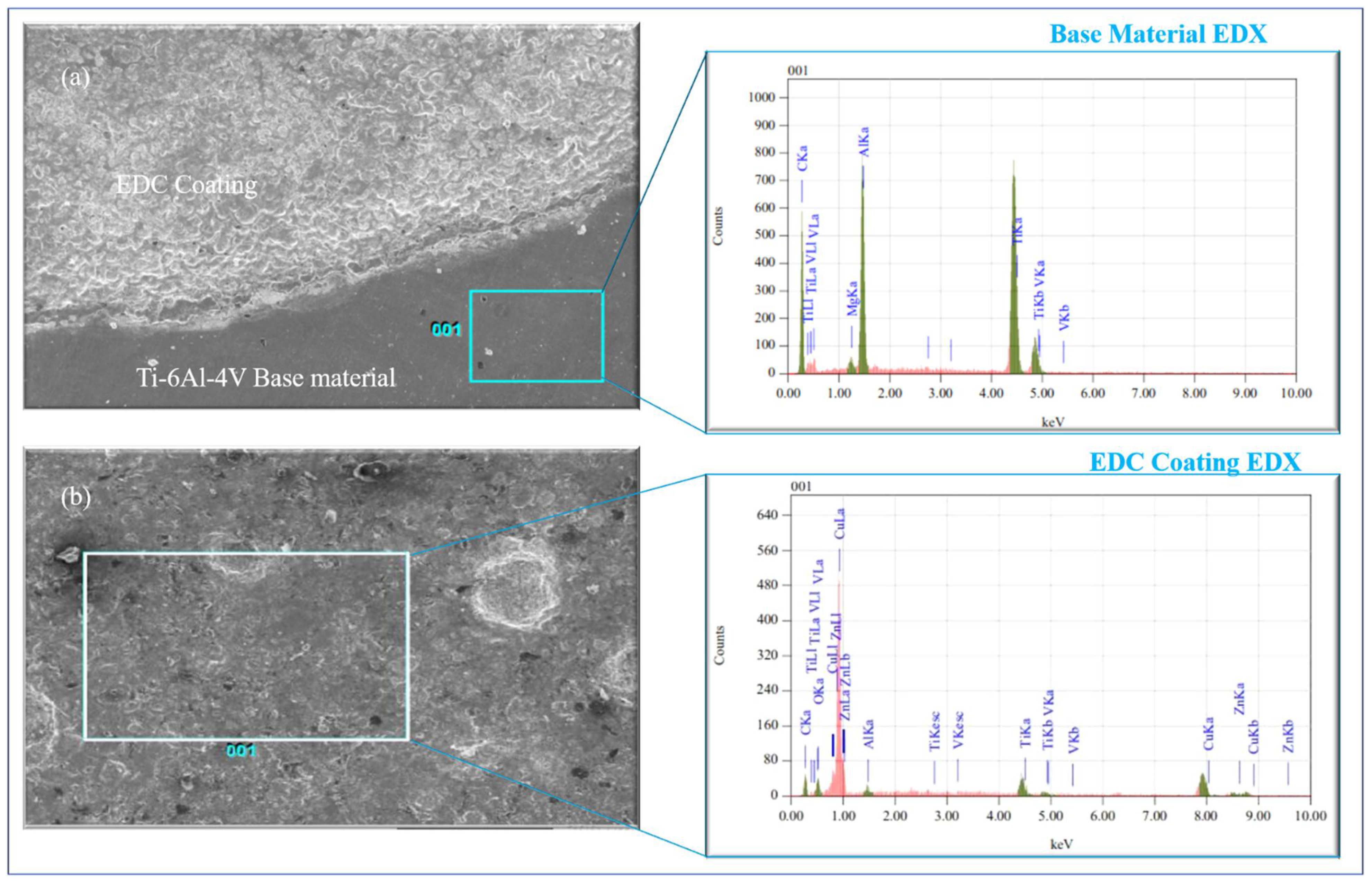
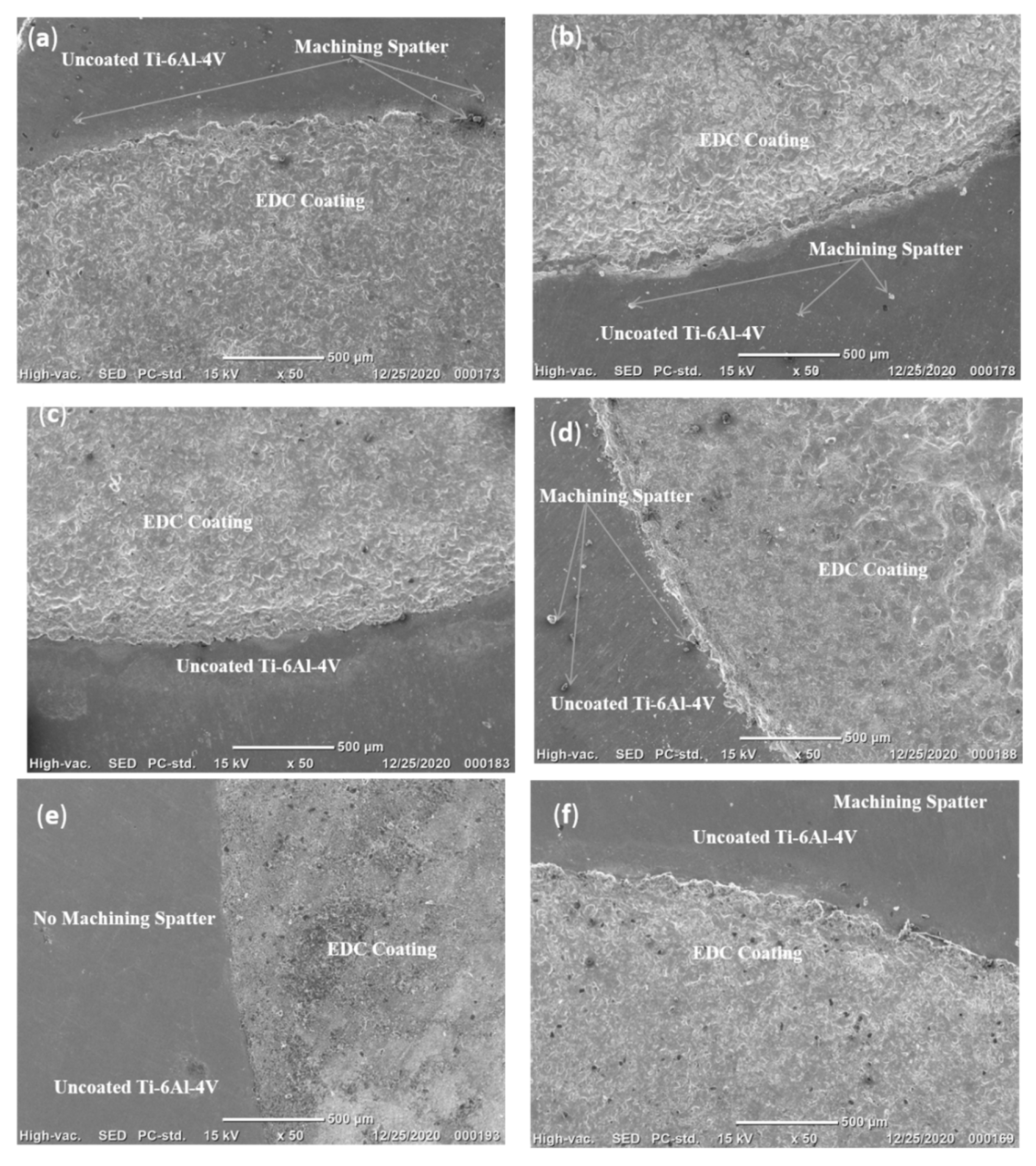
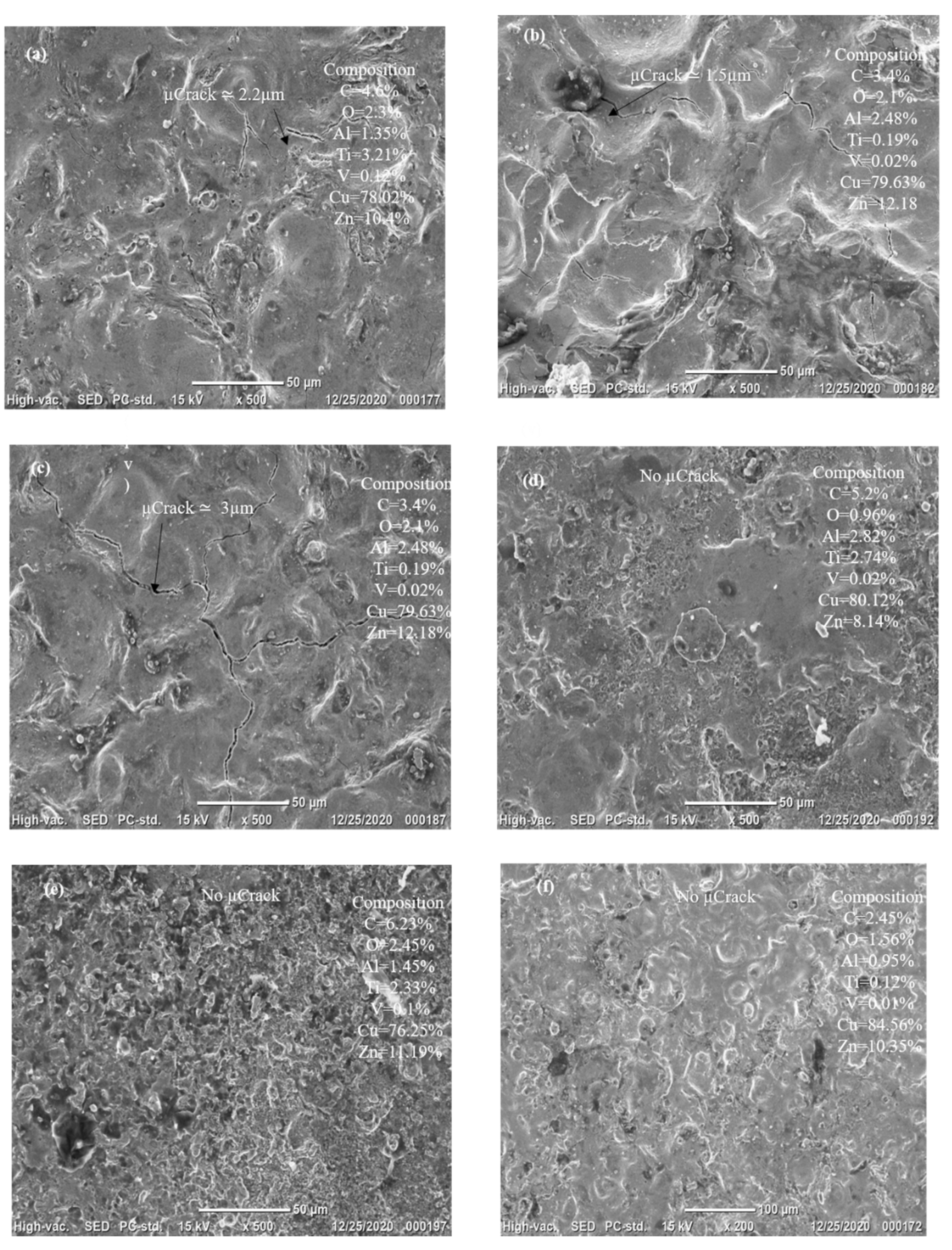
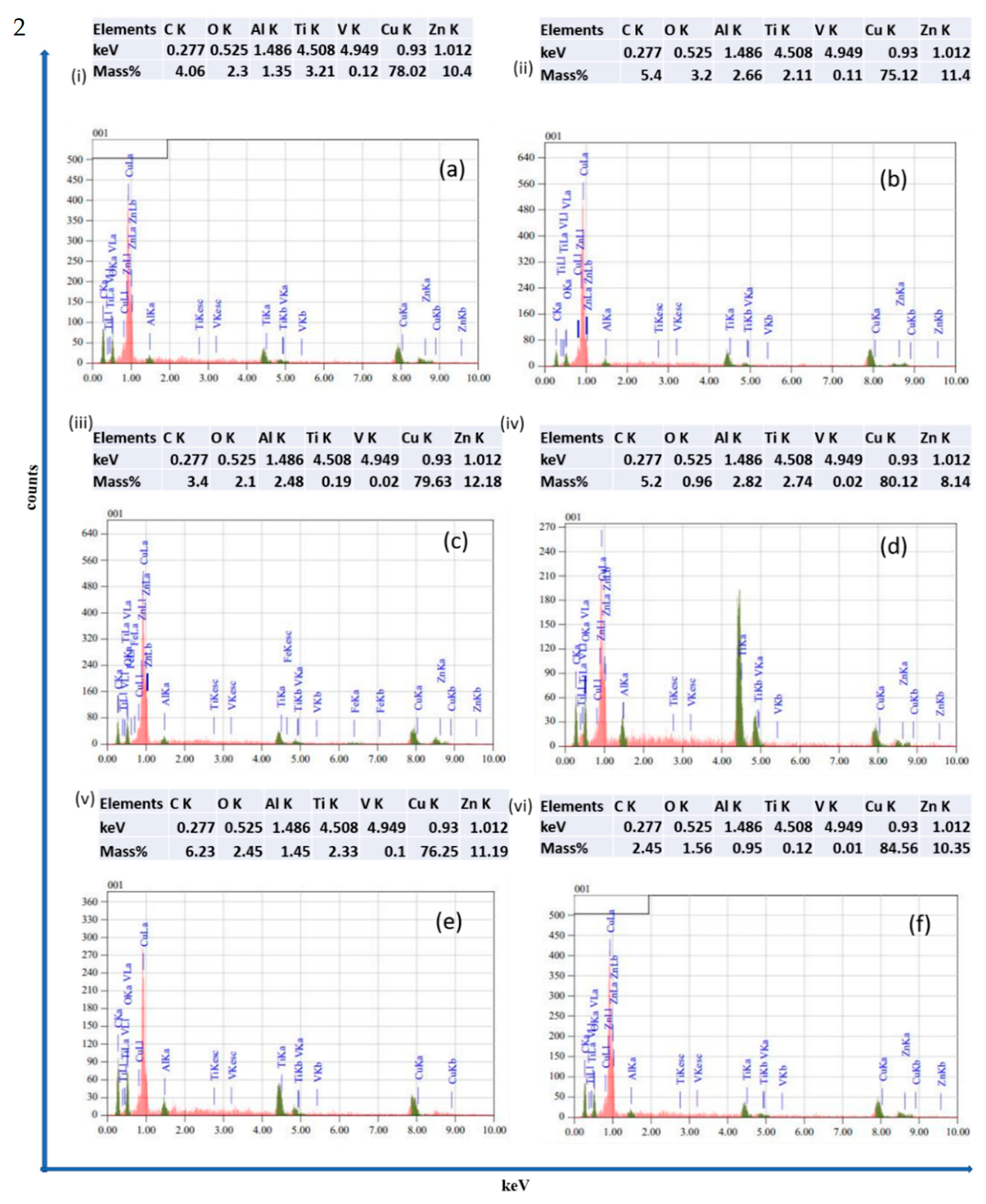
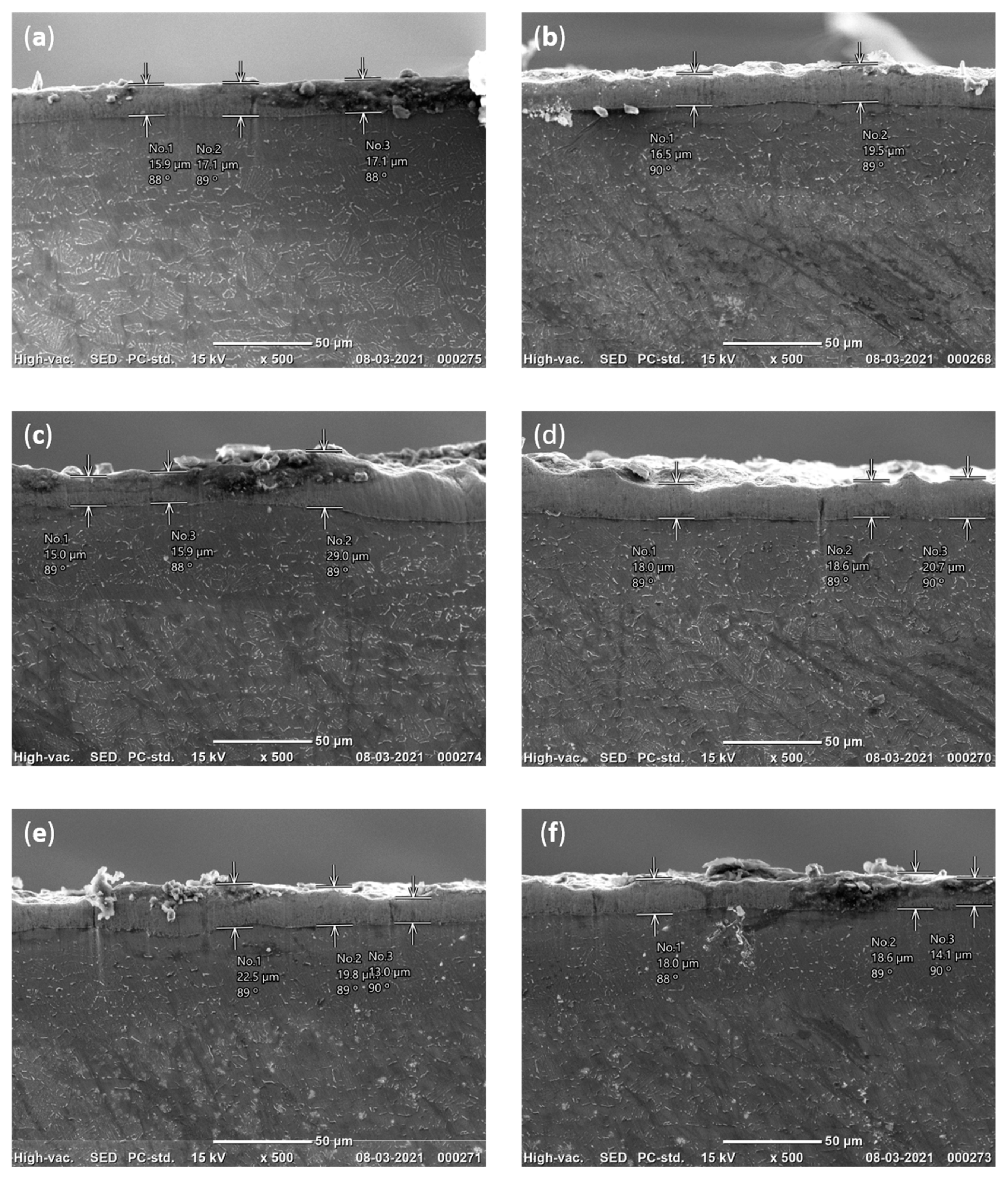
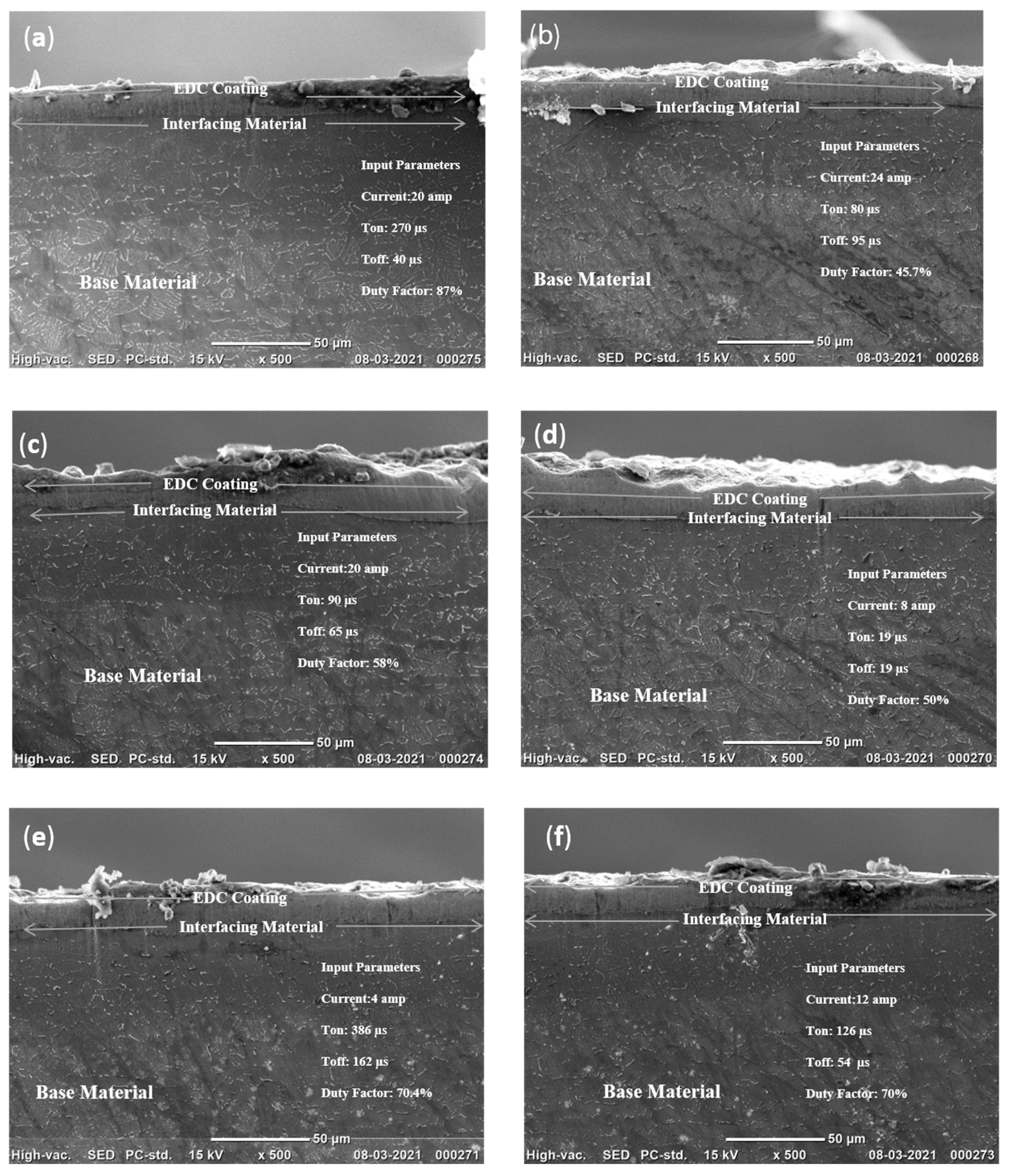
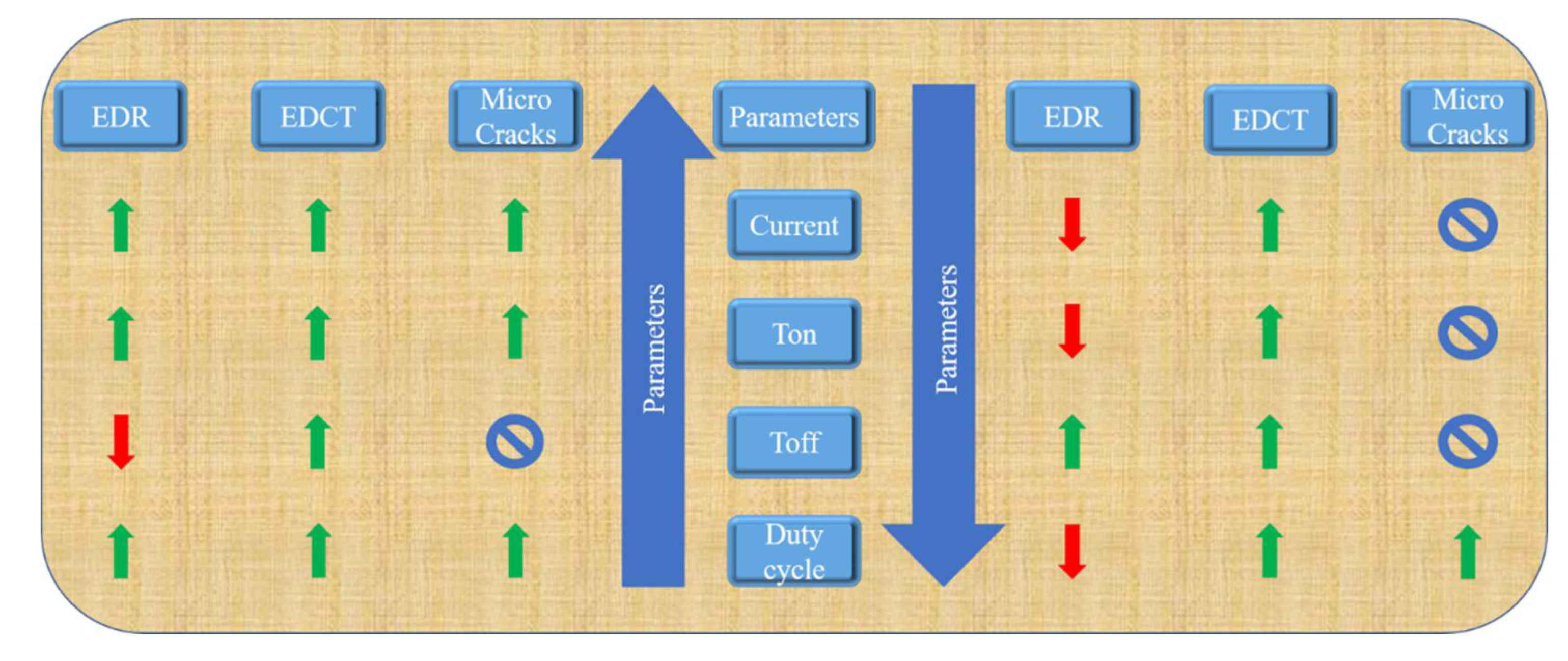
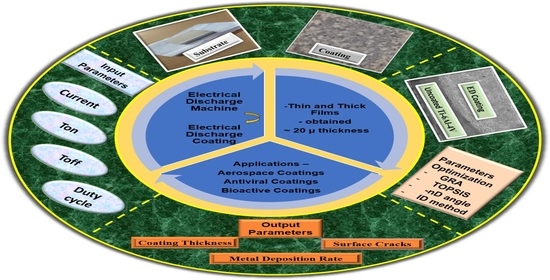
1. Introduction
Electrical Discharge Coating (EDC) has its functional importance in lubrication and anti-wear applications since it has tremendous surface modification capabilities. The EDC process can obtain hard coatings. The coating process is carried out on the conventional Electric Discharge Machine [
1,
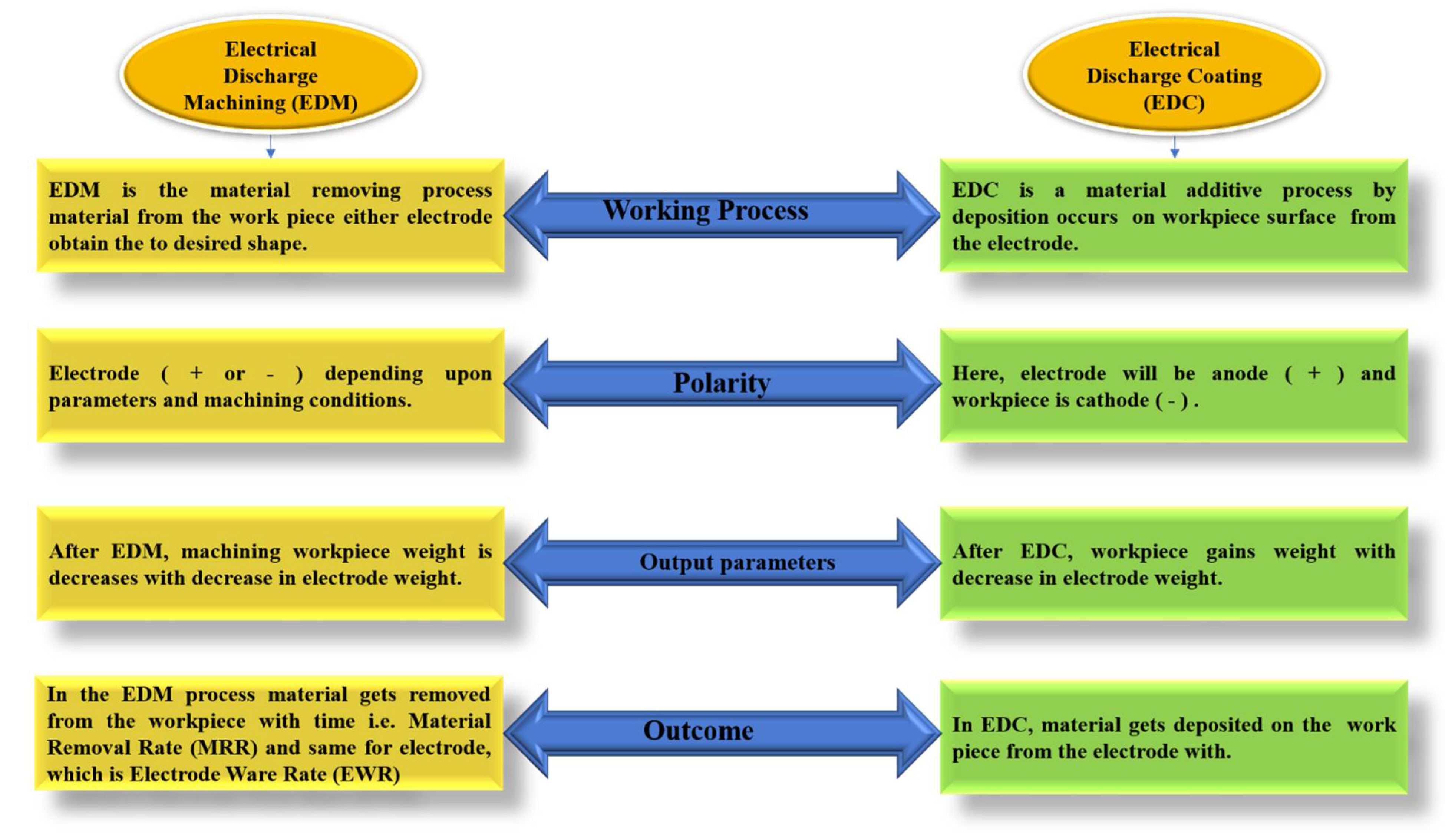
2]. The main differences between EDM and EDC in terms of the working process, polarity, and output parameters are shown in
Figure 1.
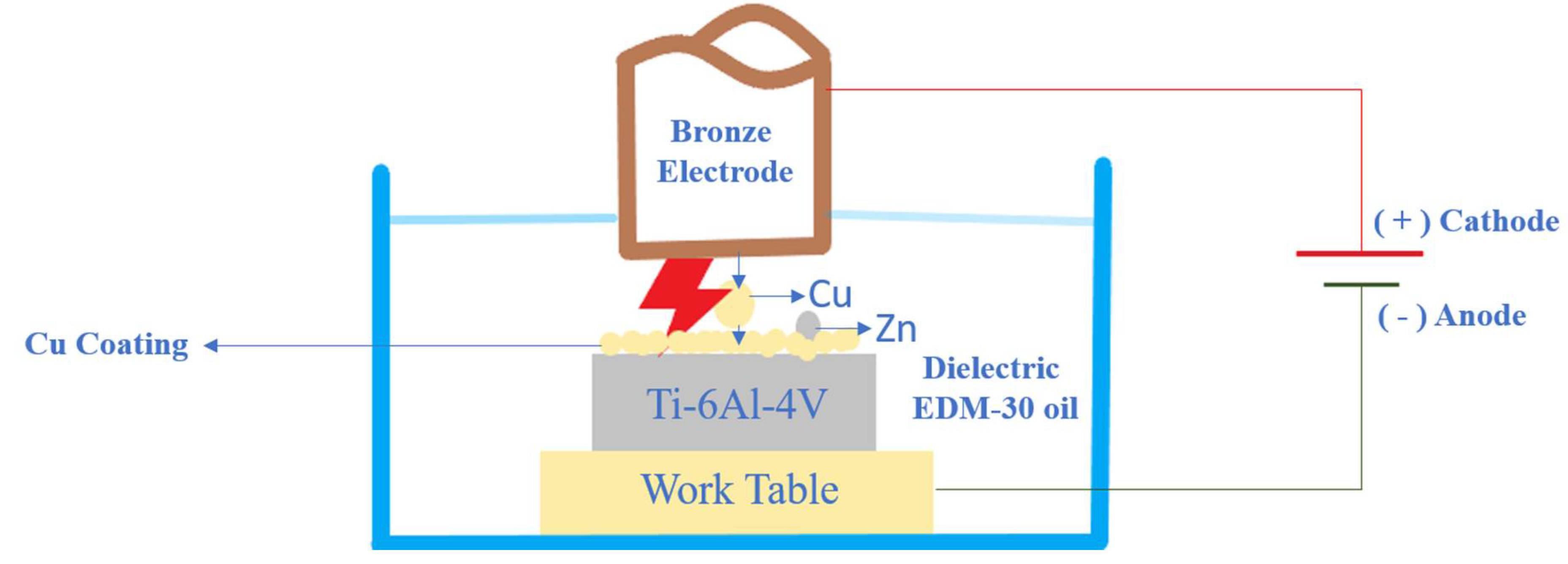
While processing in EDC, the electrode acts as an anode, and the workpiece act as a cathode. There will be an effect of cataphoretic which means under the influence of the electric field, due to the comparatively low melting point of the electrode, the erosion of metal takes place. It gets attracted to the cathode, as shown in
Figure 2. The resistance occurs by forming a conduction bridge in the inter-electrode gap, which is called gap resistance. The involved particles are trapped into the micron-sized molten metal pool and get embedded in the surface of the workpiece [
3].
This process makes it possible to deposit a lot of functional materials onto complex shapes, and EDC can also create uneven surfaces. This process of coating can obtain high hardness. From the literature, in most cases, powder mixed electrode was used to deposit coating using EDC, whereas, in this study, we tried to deposit using a conventional electrode, which is readily available in the market [
4,
5,
6]. The deposition rate depends upon the size of particles in electrode material, workpiece material, and experimental conditions.
This work focuses on coating copper alloy on a workpiece as it has various applications in wastewater treatment plants as antiviral coatings in this COVID pandemic times [
7]. Since virucidal coating has to have Cu/Zn, bronze, an alloy of copper, was considered electrode material in this study. Finally, the copper alloy was coated on the titanium (Ti-6Al-4V) material which has several applications.
Popular coating techniques were studied from the literature review and EDC was selected among the coatings [
8,
9,
10,
11,
12].
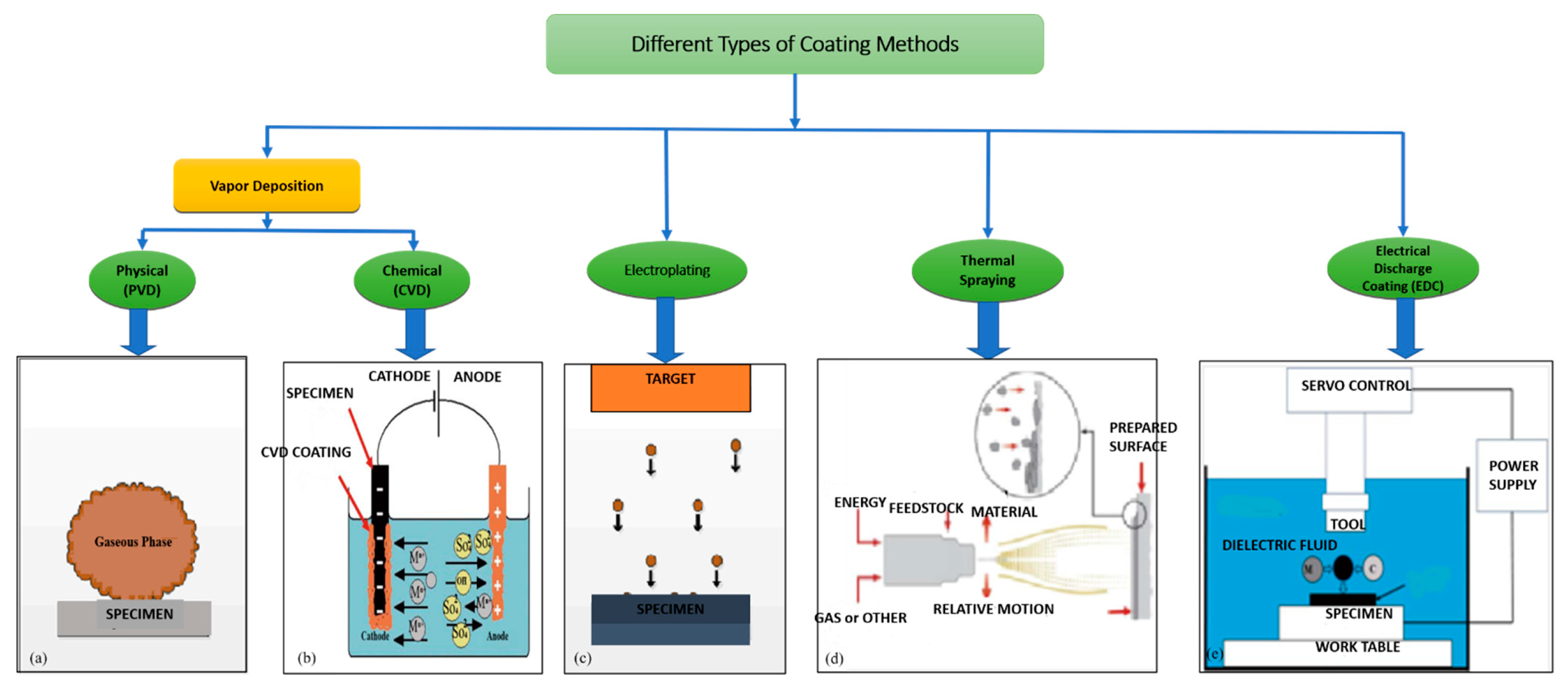
Figure 3 shows the different types of coatings available.
Different types of coating methods are shown here, and few comparisons were made even though every coating method has its own importance with respect to coating applications. Primarily, EDC can coat on complex shapes faster than others [
3] and could be coated on the already available EDM machine. Secondarily, other coating methods such as Physical Vapor Deposition PVD, Chemical Vapor Deposition CVD, electroplating, etc., require complex apparatus and cannot be used for the localized coating process. Thus, coating by EDC is an alternative way to perform localized coating on any workpiece whose melting point is higher than the electrode [
13].
The workpiece selected to coat the copper alloy is titanium (Ti-6Al-4V). High strength, corrosion resistance, and good mechanical properties made this material a more important material in bio-medical [
14], power generation [
15], and aerospace industries [
16].
A study on the related work carried out by other researchers is summarized here. Tijo and Masanta [
8] deposited a composite coating of TiC and TiB
2 with improved hardness (three times) and wear properties (seven times) on the coated surface as compared to that of the substrate (Ti-6Al-4V). As the value of peak current and duty factor increases, the rate of deposition increases, but after that, some values of peak current, deposition rate starts decreasing owing to high discharge energy. The erosion of the workpiece also increases; as such, the combined effect of deposition and erosion of the workpiece results in a decrease in deposition rate. Similarly, WS
2 coating was performed on Ti-6Al-4V by Mohanty et al. [
9] through the micro-EDM process. They used WS
2 powder particles of average size 15 μm, mixed with deionized water. High thermal energy from the electrical sparks was deposited on the tool material and WS
2 on the surface of the workpiece. In the work of Sergey N. Grigoriev et al. [
10], a comprehensive study of two different dielectric fluids and two machines on Al
2O
3 + 30% of TiC was considered for the experiments varying the input parameters and estimation of tool material and dielectric fluid influencing the chemical changes on machining surface. Pay Jun Liew et al. [
3] have authored a review considering the previous research on electrical discharge coating. The parameters and material characteristics of various EDC processes for various materials were discussed and compared. Powder Mixed Electrical Discharge Machine (PMEDM) and Powder Metallurgy (PM) electrode methods are effective solutions for realizing EDC. In the paper authored by Marina A. Volosova et al. [
11], three different approaches using wire EDM processing methods for non-conductive structural and functional ceramics based on ZrO
2, Al
2O
3, and Si
3N
4 were tried and also added powder assistance in a dielectric medium. Muhammad Kashif Bangash et al. [
12] carried out the Ti-6Al-4V workpiece using a micro-wire EDM process to improve the adhesion and joint strength in this case. Machined different shapes V, U, and semi-circle micro slots producing on the surface and then overlapping the two workpieces by applying that commercial epoxy adhesive mixture. Machining slots and heat treatment was carried, and the shear strength and feature analysis were found.
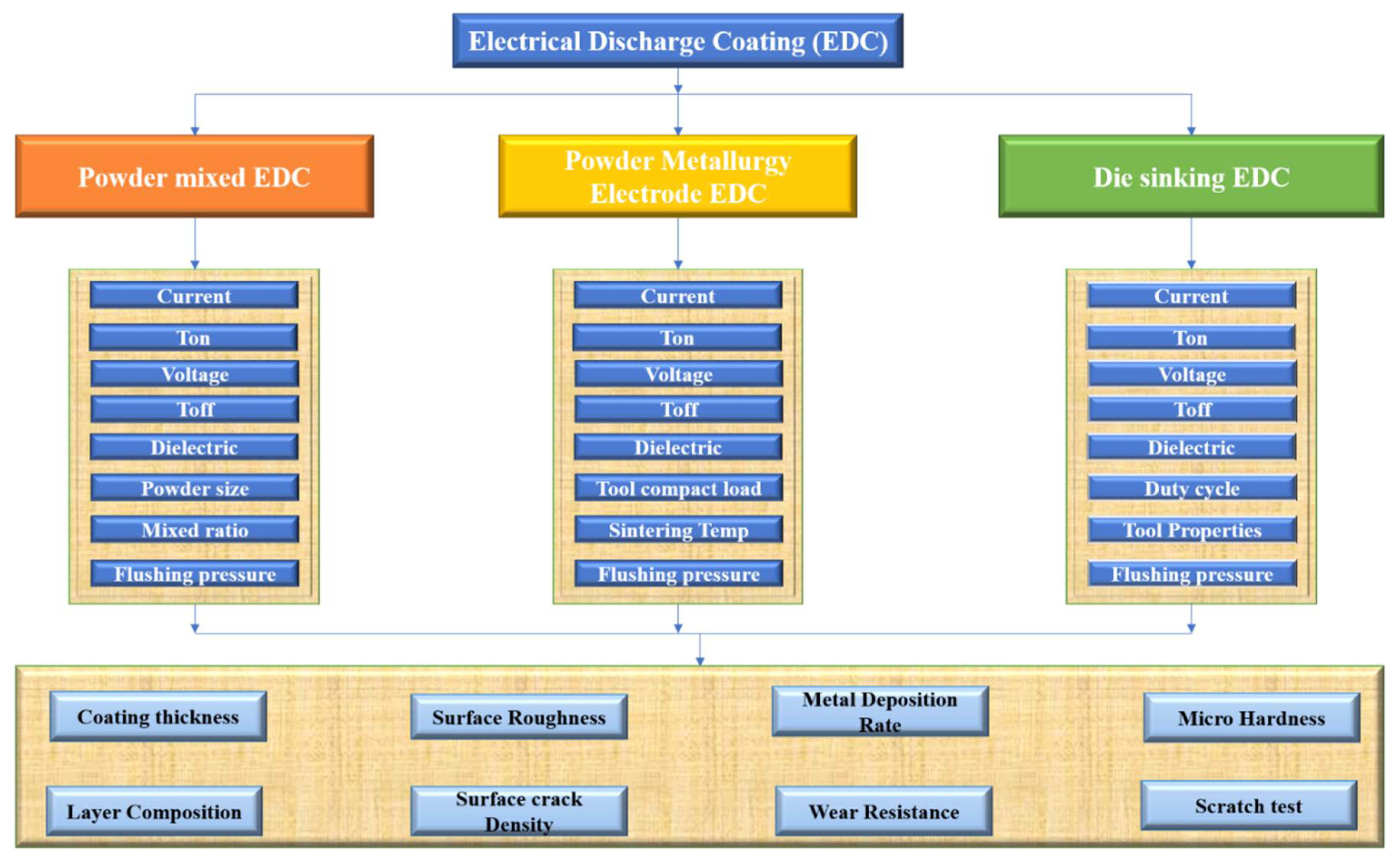
The properties considered in a different procedure carried out in the EDM machine are shown in
Figure 4. In our case, for Die-Sinking EDC, duty cycle, tool properties, and flushing pressure were considered differently than the other procedures (Powder Mixed EDC and Powder Metallurgy Electrode EDC).
Water and wastewater treatment facilities [
17,
18] are difficult to maintain due to ambient exposure. The tanks used for settling treated wastewater in each step, pumping, sludge tanks [
19] clarifying and filtering processes are based on steel and concrete, which is vulnerable to corrosion and sandy. Additionally, corrosive chemicals and aging infrastructure are problematic. In that case, there’s a need for high-performance and durable coating solutions. Coatings used in wastewater treatment facilities must be resistant to corrosive chemicals such as hydrogen sulfide [
20,
21] because these acids and other acidic compounds can cause cracking and corrosion in unprotected concrete in wastewater treatment facilities. Metal coatings such as Cu and Si [
22] for the water and wastewater treatment processes increase durability and are anticorrosive [
23] antiviral, and photoactive. The copper coating would be useful starting from the collection systems, through the clarifiers, digesters, headworks of the treatment process, and secondary containment and storage structures to reduce corrosion [
3] and to protect the surface. Besides these physical and chemical properties, copper coatings would be advantageous from an antiviral application point of view. Cu coatings, i.e., virucidal properties of copper alloy and copper surfaces, have been tested in several studies. It was proven that a small percentage of copper had a significant effect on virucidal properties [
7]. In another study on copper alloys, rapid inactivation of human coronavirus 229E was observed, and Cu/Zn brasses were very effective at lower copper concentrations. The viral genomes got inactivated because of exposure to copper and irreversibly affected virus morphology, including disintegration of envelope and dispersal of surface spikes in Human Coronavirus 229E, used as a surrogate for the more virulent coronaviruses responsible for SARS, MERS, and now COVID-19 [
24].
In this work, a conventional electrode of the copper alloy was coated on titanium alloy (Ti-6Al-4V) using the already available EDM machine for the first time. The parameters required to coat on EDM are found by the trial-and-error method in this work. The parameters influencing the experiments are optimized using different optimization techniques in which two new techniques (−nD angle and information divergence) are introduced.