Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn(dcpa) Metal-Organic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zn(dcpa) Characterization
2.3. Single-Component Adsorption Equilibrium
3. Results and Discussion
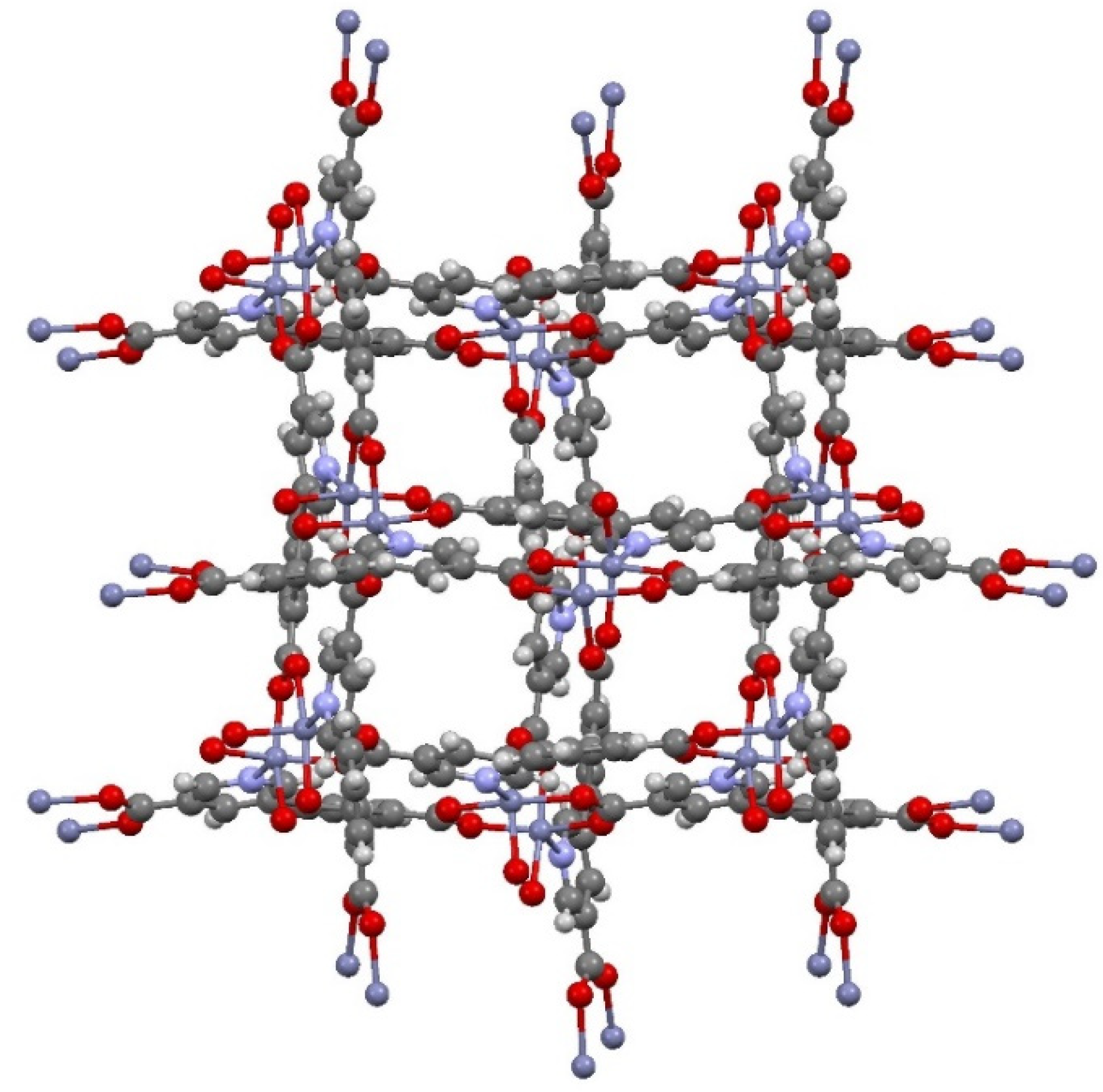
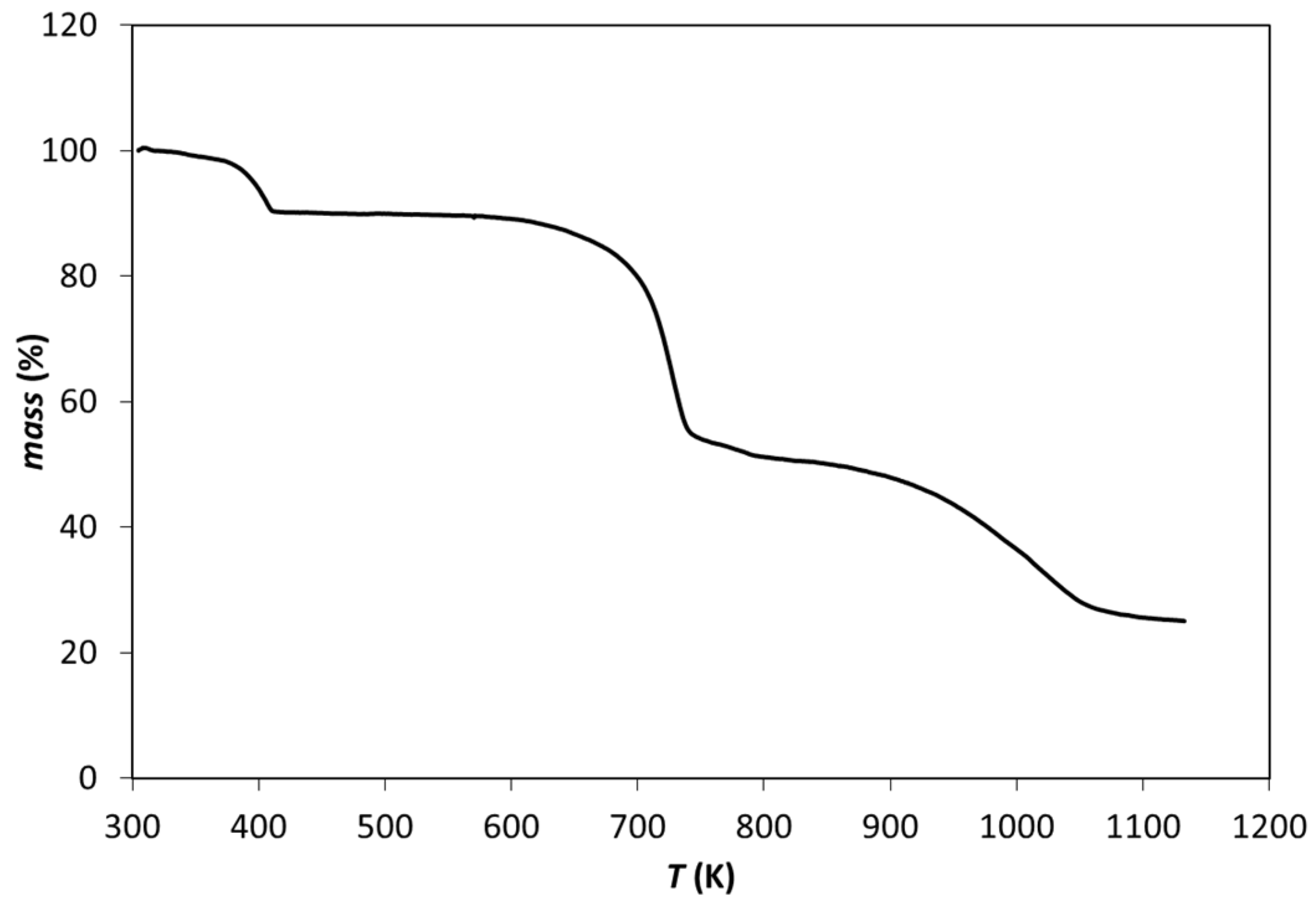
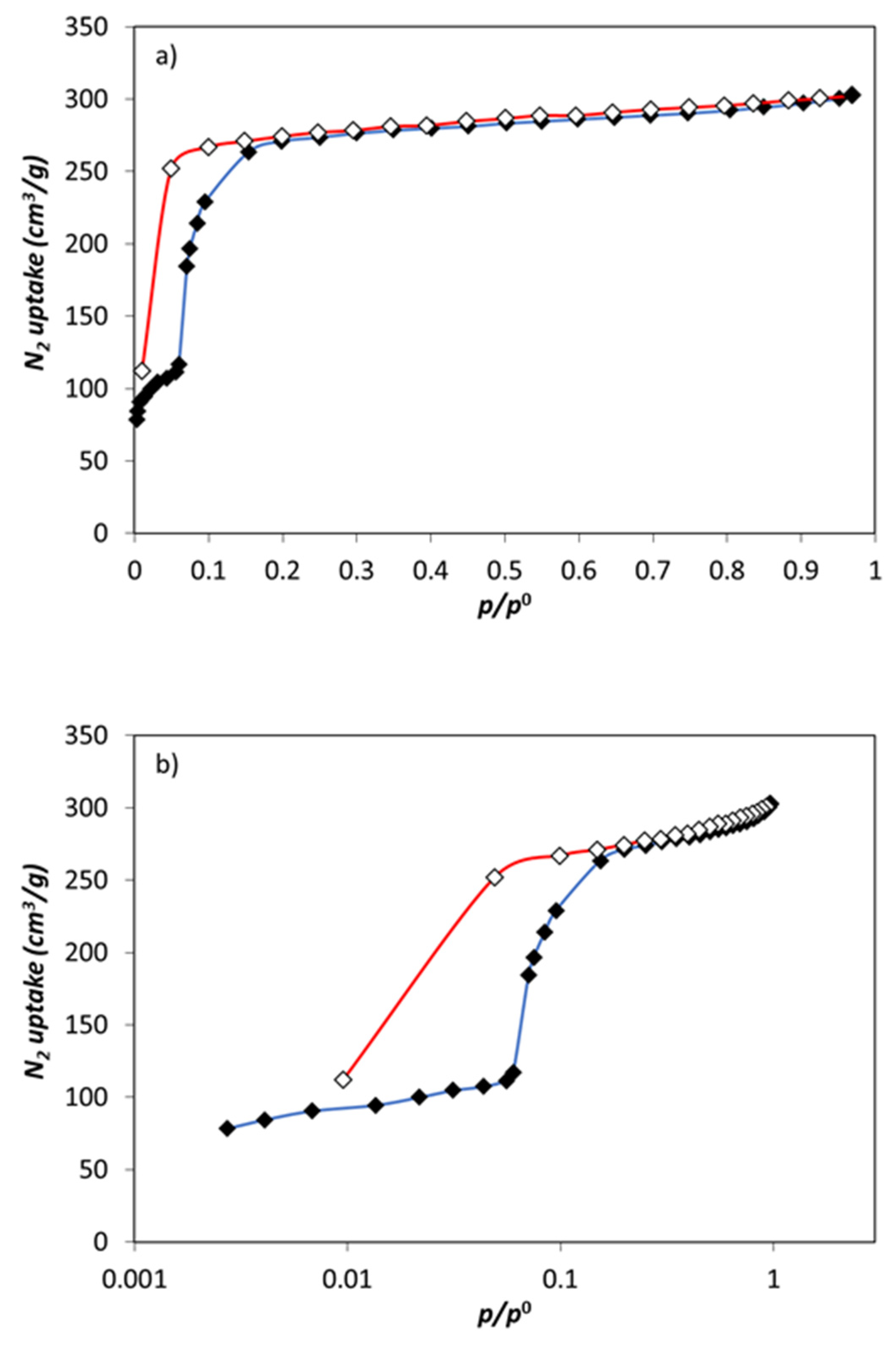
3.1. Zn(dcpa) Characterization
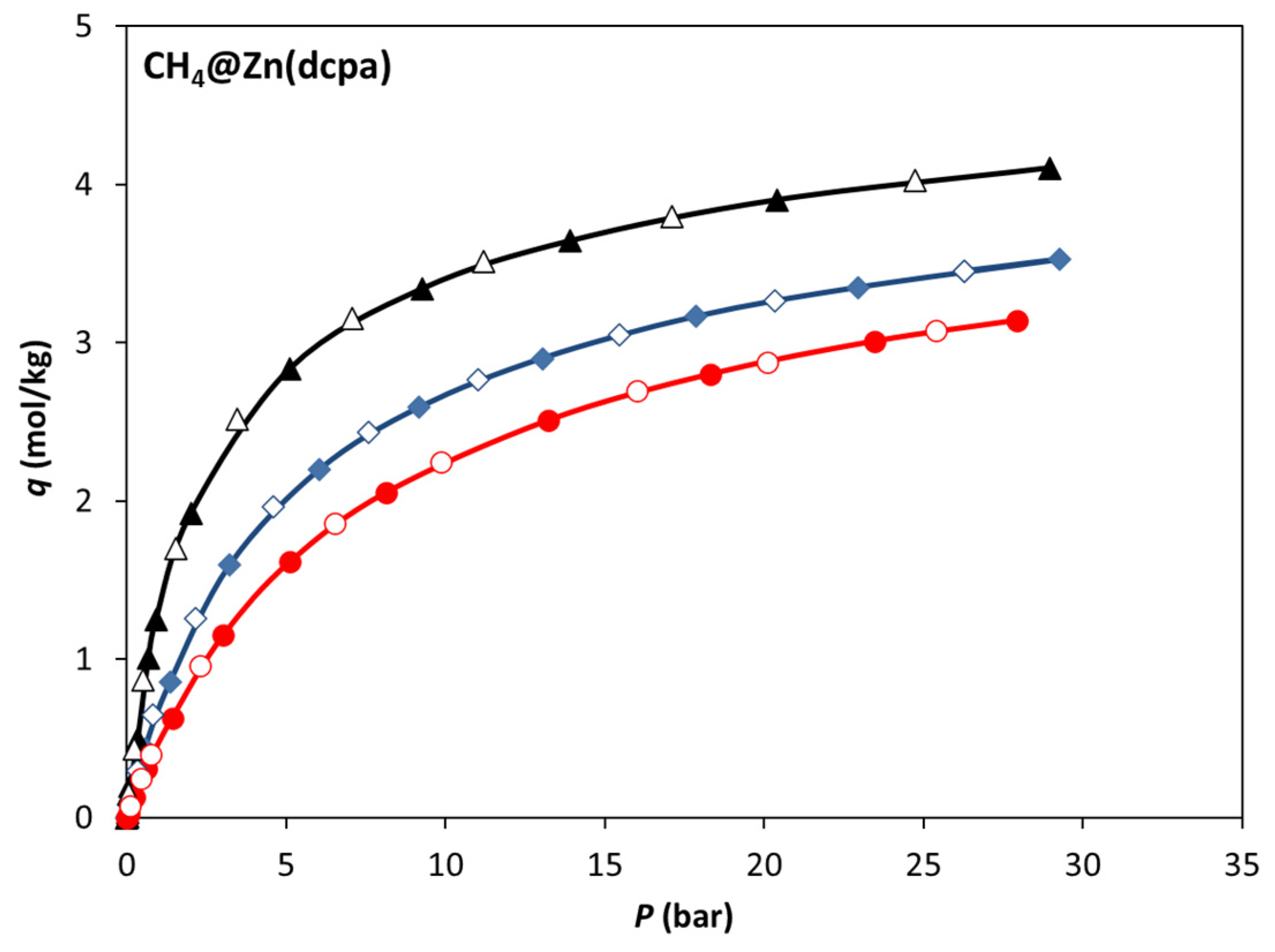
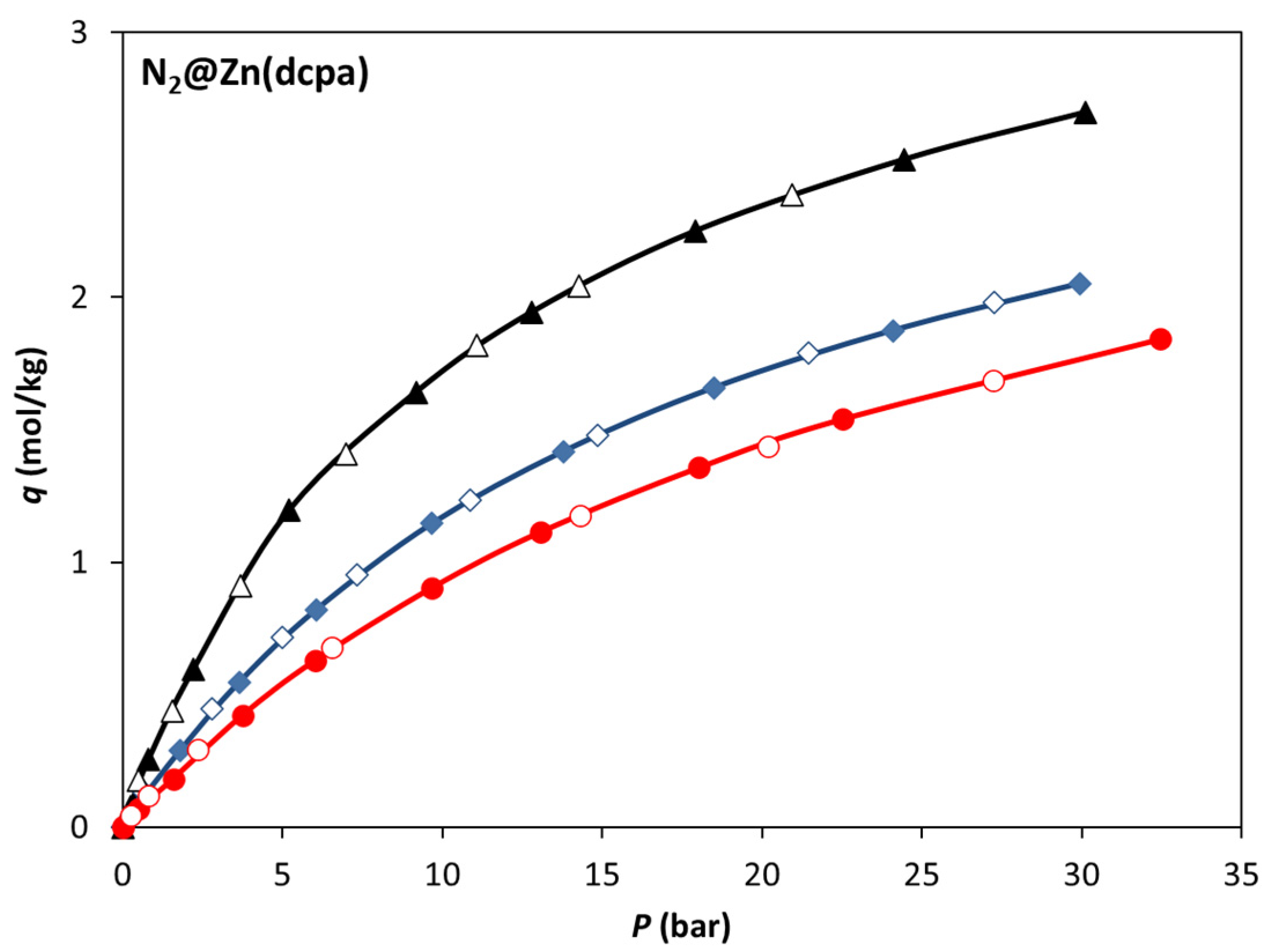
3.2. Single-Component Adsorption Equilibrium
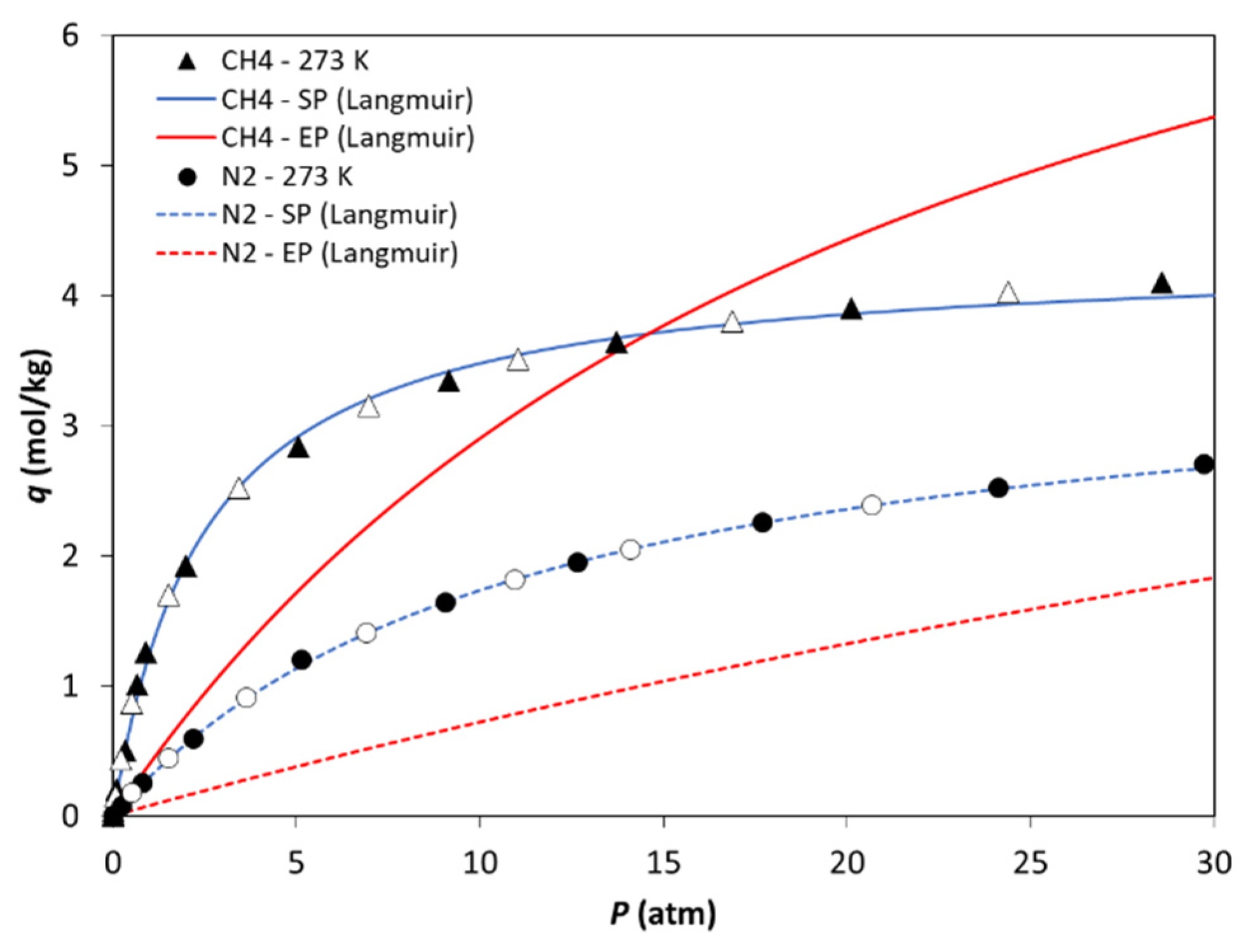
3.3. Osmotic Thermodynamic Theory
3.4. Potential of Zn(dcpa) for CO2/N2 and CO2/CH4 Separation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal-organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.A.; Blom, R.; Andreassen, K.A.; Stensrød, R.E. Experimental results of pressure swing adsorption (PSA) for pre-combustion CO2 capture with metal organic frameworks. Energy Procedia 2017, 114, 2265–2270. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Micropor. Mesopor. Mat. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.U.; Trukhan, N.; Muller, U. Industrial applications of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal-organic frameworks—Pospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Antunes, C.L.; Garate, A.U.; Portela, A.F.; Plaza, M.G.; Mota, J.P.B.; Esteves, I.A.A.C. Binderless shaped metal-organic framework particles: Impact on carbon dioxide adsorption. Microporous Mesoporous Mater. 2019, 275, 111–121. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Li, Y.; Wei, C.; Tao, Y.; Liu, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Heteroatom-doped 3D porous carbon architectures for highly stable aqueous zinc metal batteries and non-aqueous lithium metal batteries. Chem. Eng. J. 2020, 400, 125843. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Li, Y.; Xiong, S.; Zhao, G.; Feng, J.; Qian, Y. Green and tunable fabrication of graphene-like N-doped carbon on a 3D metal substrate as a binder-free anode for high-performance potassium-ion batteries. J. Mater. Chem. A 2019, 7, 21966–21975. [Google Scholar] [CrossRef]
- Alhamami, M.; Doan, H.; Cheng, C.-H. A review on breathing behaviors of metal-organic-frameworks (MOFs) for gas adsorption. Materials 2014, 7, 3198–3250. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [Green Version]
- Coudert, F.-X.; Mellot-Draznieks, C.; Fuchs, A.H.; Boutin, A. Double structural transition in hybrid material MIL-53 upon hydrocarbon adsorption: The termodynamics behind the scenes. J. Am. Chem. Soc. 2009, 131, 3442–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.; Edubilli, S.; Uppara, H.P.; Mandal, B.; Gumma, S. Effect of adsorbent history on adsorption characteristics of MIL-53 (Al) metal organic framework. Langmuir 2013, 29, 12162–12167. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Millange, F.; Thouvenot, C.; Noguès, M.; Marsolier, G.; Louër, D.; Férey, G. Very large breathing effect in the first nanoporous chromium (III)-based solids: MIL-53 or CrIII (OH)·{O₂C−C6H4−CO2}·{HO2C−C6H4−CO2H}x·H2Oy. J. Am. Chem. Soc. 2002, 124, 13519–13526. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Galvelis, R.; Torrisi, A.; Gellan, A.D.; Wharmby, M.T.; Wright, P.A.; Mellot-Draznieks, C.; Duren, T. Flexibility and swing effect on the adsorption of energy-related gases on ZIF-8: Combined experimental and simulation study. Dalton Trans. 2012, 41, 10752–10762. [Google Scholar] [CrossRef] [PubMed]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the gate: Framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Ferey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Férey, G.; Weireld, G.D. Comparative study of hydrogen sulfide adsorption in the MIL-53 (Al, Cr, Fe), MIL-47 (V), MIL-100 (Cr), and MIL-101 (Cr) metal-rganic frameworks at room temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef]
- Chen, L.; Mowat, J.P.S.; Fairen-Jimenez, D.; Morrison, C.A.; Thompson, S.P.; Wright, P.A.; Düren, T. Elucidating the breathing of the metal–organic framework MIL-53 (Sc) with ab initio molecular dynamics simulations and in situ X-ray powder diffraction experiments. J. Am. Chem. Soc. 2013, 135, 15763–15773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutin, A.; Coudert, F.X.; Springuel-Huet, M.A.; Neimark, A.V.; Férey, G.; Fuchs, A.H. The behavior of flexible MIL-53 (Al) upon CH4 and CO2 adsorption. J. Phys. Chem. C 2010, 114, 22237–22244. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Her, J.-H.; Dailly, A.; Ramirez-Cuesta, A.J.; Neumann, D.A.; Brown, C.M. Reversible structural transition in MIL-53 with large temperature hysteresis. J. Am. Chem. Soc. 2008, 130, 11813–11818. [Google Scholar] [CrossRef] [PubMed]
- Beurroies, I.; Boulhout, M.; Llewellyn, P.L.; Kuchta, B.; Férey, G.; Serre, C.; Denoyel, R. Using pressure to provoke the structural transition of metal-rganic frameworks. Angew. Chem. Int. Ed. 2010, 49, 7526–7529. [Google Scholar] [CrossRef] [Green Version]
- Neimark, A.V.; Coudert, F.-X.; Triguero, C.; Boutin, A.; Fuchs, A.H.; Beurroies, I.; Denoyel, R. Structural transitions in MIL-53 (Cr): View from outside and inside. Langmuir 2011, 27, 4734–4741. [Google Scholar] [CrossRef] [Green Version]
- Serre, C.; Bourrelly, S.; Vimont, A.; Ramsahye, N.A.; Maurin, G.; Llewellyn, P.L.; Daturi, M.; Filinchuk, Y.; Leynaud, O.; Barnes, P.; et al. An explanation for the very large breathing effect of a metal–organic framework during CO2 adsorption. Adv. Mater. 2007, 19, 2246–2251. [Google Scholar] [CrossRef]
- Mounfield, W.P., III; Walton, K.S. Effect of synthesis solvent on the breathing behavior of MIL-53 (Al). J. Colloid Interface Sci. 2015, 447, 33–39. [Google Scholar] [CrossRef]
- Heymans, N.; Vaesen, S.; De Weireld, G. A complete procedure for acidic gas separation by adsorption on MIL-53 (Al). Microporous Mesoporous Mater. 2012, 154, 93–99. [Google Scholar] [CrossRef]
- Deniz, E.; Karadas, F.; Patel, H.A.; Aparicio, S.; Yavuz, C.T.; Atilhan, M. A combined computational and experimental study of high pressure and supercritical CO2 adsorption on Basolite MOFs. Microporous Mesoporous Mater. 2013, 175, 34–42. [Google Scholar] [CrossRef]
- Camacho, B.C.R.; Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Mota, J.P.B. Adsorption equilibrium of carbon dioxide and nitrogen on the MIL-53 (Al) metal organic framework. Sep. Purif. Technol. 2015, 141, 150–159. [Google Scholar] [CrossRef]
- Gonzalez-Nelson, A.; Coudert, F.X.; van der Veen, M.A. Rotational dynamics of linkers in metal-organic frameworks. Nanomaterials 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Hefti, M.; Joss, L.; Bjelobrk, Z.; Mazzotti, M. On the potential of phase-change adsorbents for CO2 capture by temperature swing adsorption. Faraday Discuss. 2016, 192, 153–179. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, Y.; Hou, L.; Yang, G.; Wang, Y.Y.; Shi, Q.-Z. Dynamic Zn-based metal-organic framework: Step wise adsorption, hysteretic desorption and selective carbon dioxide uptake. J. Mater. Chem. 2013, 1, 6535–6538. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Barreto, J.; GrossoXavier, M.D.; Martins, D.; Esteves, I.A.A.C.; Branco, M.; Tirolien, T.; Mota, J.P.B.; Bonfait, G. Cryogenic neon adsorption on Co3 (ndc) 3 (dabco) metal-organic framework. Microporousand Mesoporous Mater. 2020, 298, 110055. [Google Scholar] [CrossRef]
- Lyubchyk, A.; Esteves, I.A.A.C.; Cruz, F.J.A.L.; Mota, J.P.B. Experimental and theoretical studies of supercritical methane adsorption in the MIL-53 (Al) metal organic framework. J. Phys. Chem. 2011, 115, 20628–20638. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Camacho, B.C.R.; Lyubchyk, A.; Esteves, I.A.A.C.; Cruz, F.J.A.L.; Mota, J.P.B. Experimental and computational study of ethane and ethylene adsorption in the MIL-53 (Al) metal organic framework. Microporousand Mesoporous Mater. 2016, 230, 154–165. [Google Scholar] [CrossRef]
- Dreisbach, F.; Staudt, R.; Keller, J.U. High pressure adsorption data of methane, nitrogen, carbon dioxide and their binary and ternary mixtures on activated carbon. Adsorption 1999, 5, 215–227. [Google Scholar] [CrossRef]
- Coudert, F.-X.; Jeffroy, M.; Fuchs, A.H.; Boutin, A.; Mellot-Draznieks, C. Thermodynamics of guest-induced structural transitions in hybrid organic−inorganic frameworks. J. Am. Chem. Soc. 2008, 130, 14294–14302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, J.P.B.; Martins, D.; Lopes, D.; Catarino, I.; Bonfait, G. Structural transitions in the MIL-53 (Al) metal–organic framework upon cryogenic hydrogen adsorption. J. Phys. Chem. 2017, 121, 24252–24263. [Google Scholar] [CrossRef]
- Hiraide, S.; Sakanaka, Y.; Kajiro, H.; Kawaguchi, S.; Miyahara, M.T.; Tanaka, H. High-through put gas separation by flexible metal–organic frameworks with fast gating and thermal management capabilities. Nat. Commun. 2020, 11, 3867. [Google Scholar] [CrossRef]
- Ghysels, A.; Vanduyfhuys, L.; Vandichel, M.; Waroquier, M.; Van Speybroeck, V.; Smit, B. On the thermodynamics of framework breathing: A free energy model for gas adsorption in MIL-53. J. Phys. Chem. 2013, 117, 11540–11554. [Google Scholar] [CrossRef] [Green Version]
- Jeffroy, M.; Fuchs, A.H.; Boutin, A. Structural changes in nanoporous solids due to fluid adsorption: Thermodynamic analysis and Monte Carlo simulations. Chem. Commun. 2008, 3275–3277. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.J.; Vera, A.T.; De Moura, B.A.; Esteves, L.M.; Tariq, M.; Esperança, J.M.S.S.; Esteves, I.A.A.C. Paramagnetic ionic liquid/metal organic framework composites for CO2/CH4 and CO2/N2 separations. Front. Chem. 2020, 8, 590191. [Google Scholar] [CrossRef] [PubMed]
- Nabais, A.R.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Alves, V.D.; Esteves, I.A.A.C.; Neves, L.A. CO2/N2 gas separation using Fe (BTC)-based mixed matrix membranes: A view on the adsorptive and filler properties of metal-organic frameworks. Sep. Purif. Technol. 2018, 202, 174–184. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]





| (mol/kg) | (atm −1) | (atm) | (atm) | (J/g) | ||
|---|---|---|---|---|---|---|
| SP | 5.65 | 1.30 | Ads | 23.0 | 1.3 | −4.45 |
| EP | 12.2 | 0.143 | Des | 7.0 | 0.5 | −10.7 |
| (mol/kg) | (atm −1) | (mol/kg) | (atm −1) | |
|---|---|---|---|---|
| 3.681 | 0.089 | 7.948 | 0.010 | |
| 4.328 | 0.411 | 9.346 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Mota, J.P.B. Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn(dcpa) Metal-Organic Framework. Energies 2021, 14, 5598. https://doi.org/10.3390/en14185598
Ribeiro RPPL, Esteves IAAC, Mota JPB. Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn(dcpa) Metal-Organic Framework. Energies. 2021; 14(18):5598. https://doi.org/10.3390/en14185598
Chicago/Turabian StyleRibeiro, Rui P. P. L., Isabel A. A. C. Esteves, and José P. B. Mota. 2021. "Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn(dcpa) Metal-Organic Framework" Energies 14, no. 18: 5598. https://doi.org/10.3390/en14185598
APA StyleRibeiro, R. P. P. L., Esteves, I. A. A. C., & Mota, J. P. B. (2021). Adsorption of Carbon Dioxide, Methane, and Nitrogen on Zn(dcpa) Metal-Organic Framework. Energies, 14(18), 5598. https://doi.org/10.3390/en14185598








