Catalyst Distribution Optimization Scheme for Effective Green Hydrogen Production from Biogas Reforming
Abstract
1. Introduction
- Preparation of the numerical simulation considering reforming of model biogas
- Application of the macro-patterning concept for the reactor’s geometry
- Combining the prepared numerical model with a genetic algorithm to find the most optimal catalyst insert design
2. Mathematical Model
2.1. Chemical Reactions
2.2. Heat and Mass Transfer
3. Numerical Model
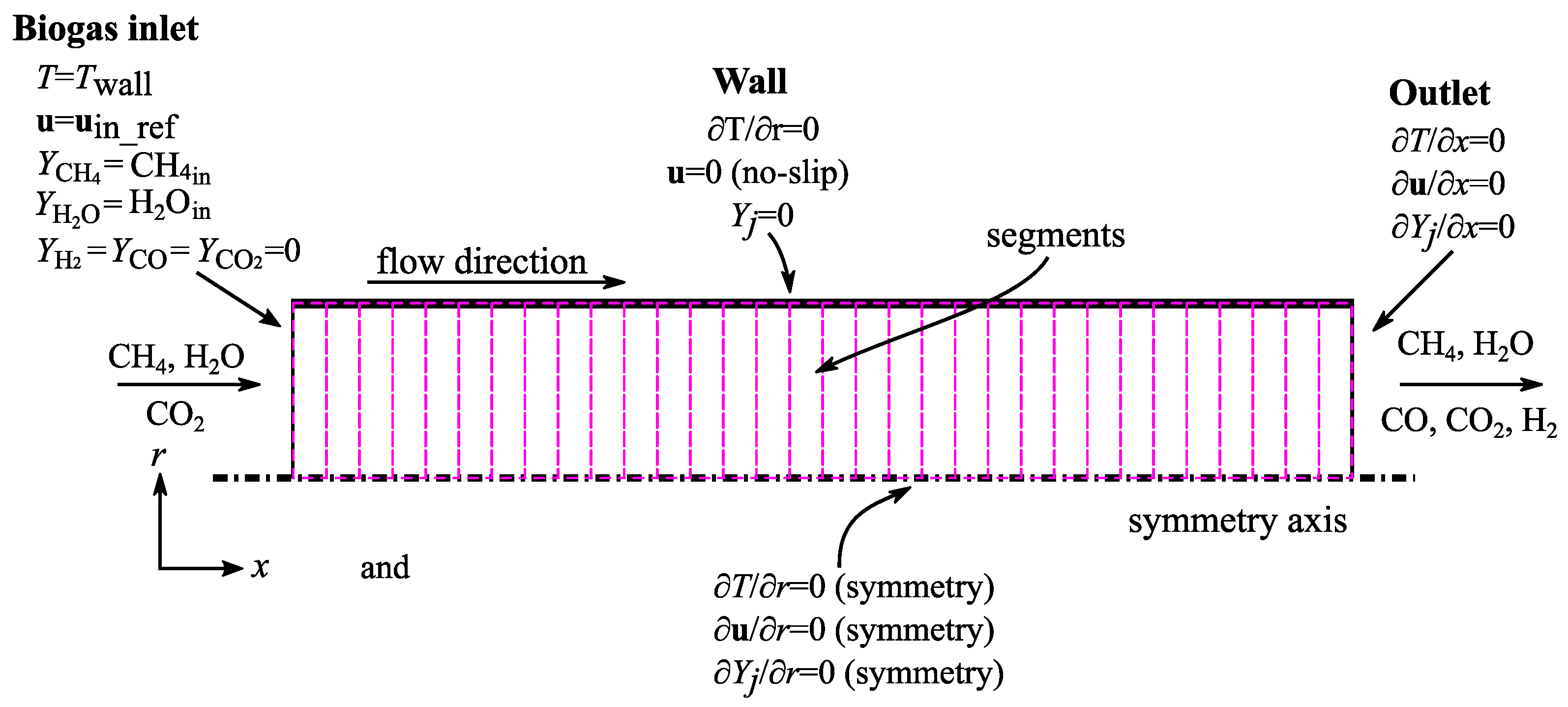
3.1. Boundary Conditions
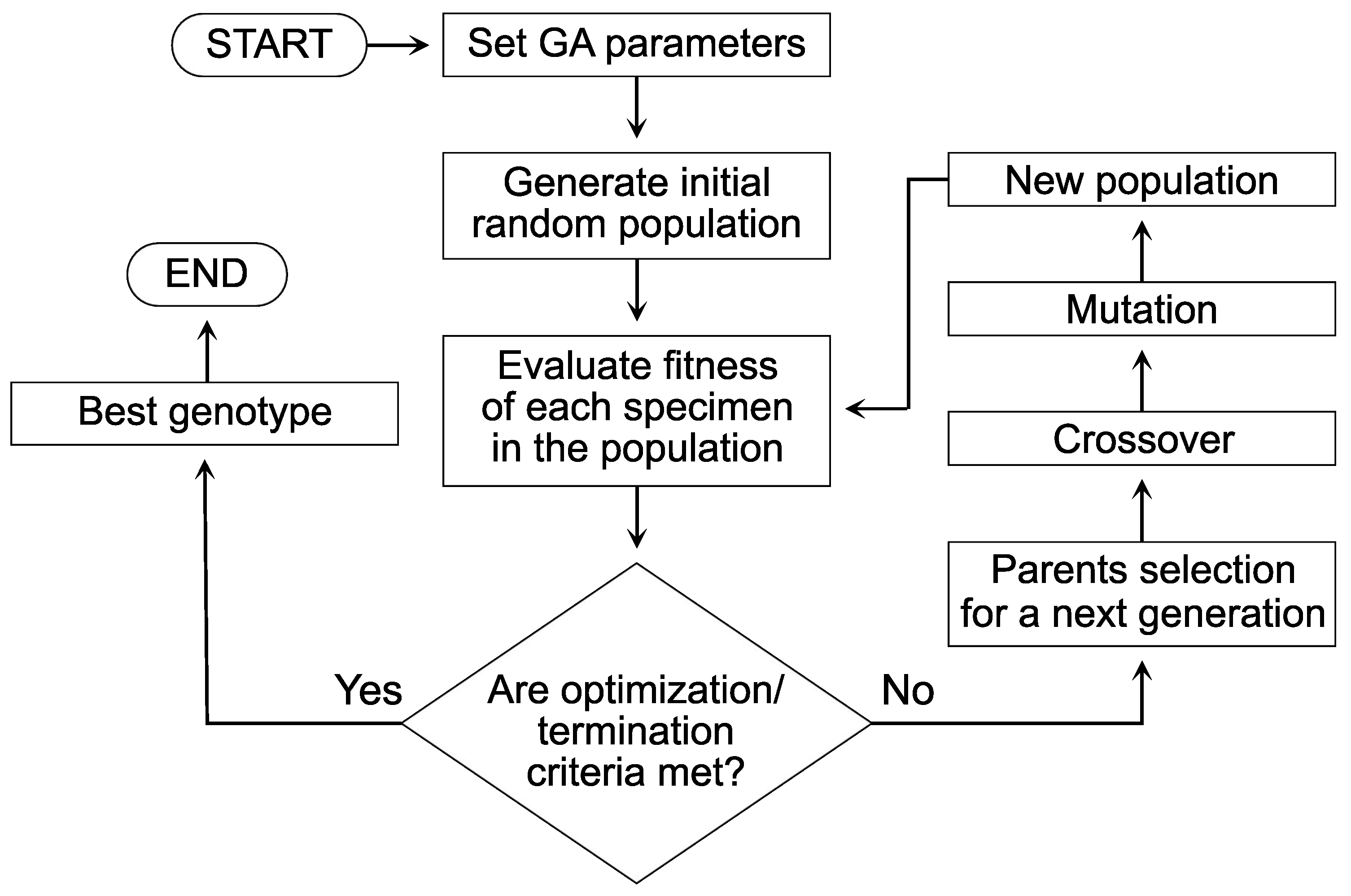
4. Optimization Procedure
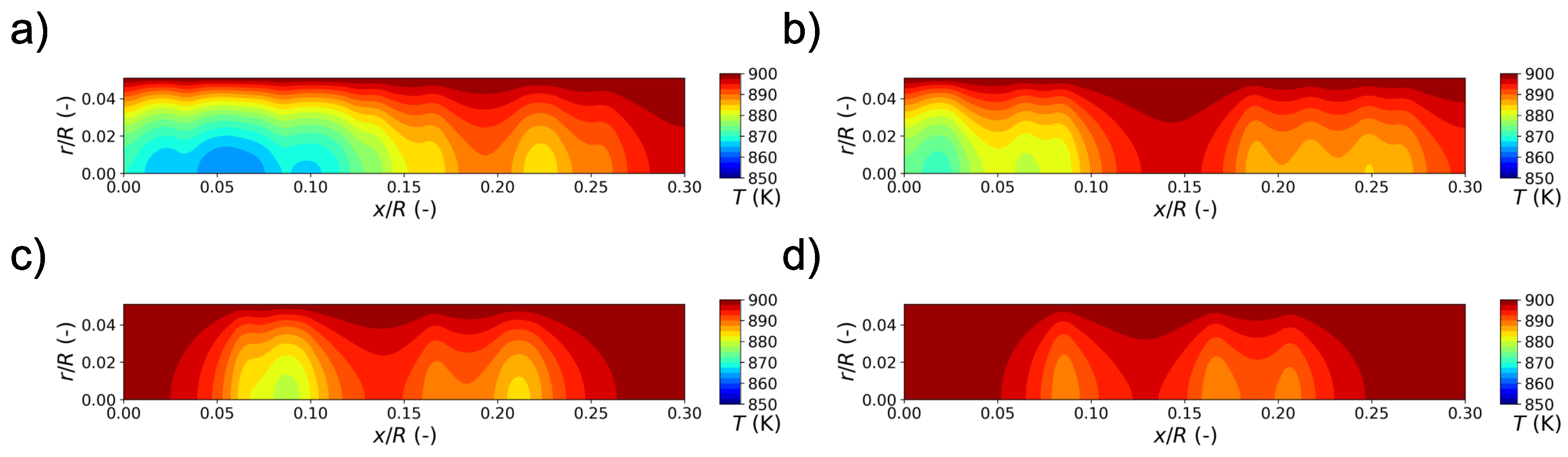
5. Numerical Results
6. Conslusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrog. Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Widera, B. Renewable hydrogen implementations for combined energy storage, transportation and stationary applications. Therm. Sci. Eng. Prog. 2020, 16, 100460. [Google Scholar] [CrossRef]
- Ayodele, F.O.; Mohammad, N.; Mustapa, S.I.; Ayodele, B.V. An overview of economic analysis and environmental impacts of natural gas conversion technologies. Sustainability 2020, 12, 148. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Mutlu, R.N.; Kucukkara, I.; Gizir, A.M. Hydrogen generation by electrolysis under subcritical water condition and the effect of aluminium anode. Int. J. Hydrog. Energy 2020, 45, 12641–12652. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline water electrolysis powered by renewable energy: A review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Baykara, S.Z. Hydrogen: A brief overview on its sources, production and environmental impact. Int. J. Hydrog. Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Karthikeyan, O.P.; Kumar, G.; Dai-Viet, N.V.; Banu, J.R. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Pegram, J.; Falcone, G.; Kolios, A. A review of job role localization in the oil and gas industry. Energies 2018, 11, 2779. [Google Scholar] [CrossRef]
- Tao, S.; Chen, S.; Pan, Z. Current status, challenges, and policy suggestions for coalbed methane industry development in China: A review. Energy Sci. Eng. 2019, 7, 1059–1074. [Google Scholar] [CrossRef]
- Sharma, I.; Friedrich, D.; Golden, T.; Brandani, S. Exploring the opportunities for carbon capture in modular, small-scale steam methane reforming: An energetic perspective. Int. J. Hydrog. Energy 2019, 44, 14732–14743. [Google Scholar] [CrossRef]
- Lee, H.; Jung, I.; Roh, G.; Na, Y.; Kang, H. Comparative analysis of on-board methane and methanol reforming systems combined with HT-PEM fuel cell and CO2 capture/liquefaction system for hydrogen fueled ship application. Energies 2020, 13, 224. [Google Scholar] [CrossRef]
- Alves, H.J.; Bley Junior, C.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Gregorie, E.F.J.; Lamb, J.J.; Lien, K.M.; Pollet, B.G.; Burheim, O.S. Hydrogen and Biogas. In Micro-Optics and Energy: Sensors for Energy Devices; Lamb, J.J., Pollet, B.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 131–155. [Google Scholar] [CrossRef]
- Gonçalves, A.; Puna, J.F.; Guerra, L.; Campos Rodrigues, J.; Gomes, J.F.; Santos, M.T.; Alves, D. Towards the Development of Syngas/Biomethane Electrolytic Production, Using Liquefied Biomass and Heterogeneous Catalyst. Energies 2019, 12, 3787. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Buchireddy, P.R.; Peck, D.; Zappi, M.; Bricka, R.M. Catalytic hot gas cleanup of biomass gasification producer gas via steam reforming using nickel-supported clay minerals. Energies 2021, 14, 1875. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Brus, G.; Sciazko, A.; Komatsu, Y.; Kimijima, S.; Szmyd, J.S. Towards a Thermal Optimization of a Methane/Steam Reforming Reactor. Flow Turbul. Combust. 2016, 97, 171–189. [Google Scholar] [CrossRef]
- Palma, V.; Ricca, A.; Martino, M.; Meloni, E. Innovative structured catalytic systems for methane steam reforming intensification. Chem. Eng. Process. Process Intensif. 2017, 120, 207–215. [Google Scholar] [CrossRef]
- Pajak, M.; Mozdzierz, M.; Chalusiak, M.; Kimijima, S.; Szmyd, J.S.; Brus, G. A numerical analysis of heat and mass transfer processes in a macro-patterned methane/steam reforming reactor. Int. J. Hydrog. Energy 2018, 43, 20474–20487. [Google Scholar] [CrossRef]
- Tomiczek, M.; Kaczmarczyk, R.; Mozdzierz, M.; Brus, G. A numerical analysis of heat and mass transfer during the steam reforming process of ethane. Heat Mass Transf. 2018, 54, 2305–2314. [Google Scholar] [CrossRef]
- Nishino, T.; Szmyd, J.S. Numerical analysis of a cell-based indirect internal reforming tubular SOFC operating with biogas. J. Fuel Cell Sci. Technol. 2010, 7, 0510041–0510048. [Google Scholar] [CrossRef]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; Hemisphere: Washington, DC, USA, 1980. [Google Scholar]
- Xu, J.; Froment, G.F. Methane steam reforming: II. Diffusional limitations and reactor simulation. AIChE J. 1989, 35, 97–103. [Google Scholar] [CrossRef]
- Komatsu, Y.; Kimijima, S.; Szmyd, J.S. A Performance Analysis of a Solid Oxide Fuel Cell—Micro Gas Turbine Hybrid System Using Biogas. ECS Trans. 2009, 25, 1061–1070. [Google Scholar] [CrossRef]
- Sciazko, A.; Komatsu, Y.; Brus, G.; Kimijima, S.; Szmyd, J.S. A novel approach to improve the mathematical modelling of the internal reforming process for solid oxide fuel cells using the orthogonal least squares method. Int. J. Hydrog. Energy 2014, 39, 16372–16389. [Google Scholar] [CrossRef]
- Brus, G.; Nowak, R.; Szmyd, J.S.; Komatsu, Y.; Kimijima, S. An Experimental and Theoretical Approach for the Carbon Deposition Problem during Steam Reforming of Model Biogas. J. Theor. Appl. Mech. 2015, 53, 273–284. [Google Scholar] [CrossRef]
- Mazhar, A.; Khoja, A.H.; Azad, A.K.; Mushtaq, F.; Naqvi, S.R.; Shakir, S.; Hassan, M.; Liaquat, R.; Anwar, M. Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production. Energies 2021, 14, 3347. [Google Scholar] [CrossRef]
- Brus, G.; Komatsu, Y.; Kimijima, S.; Szmyd, J.S. An analysis of biogas reforming process on Ni/YSZ and Ni/SDC catalysts. Int. J. Thermodyn. 2012, 15, 43–51. [Google Scholar] [CrossRef]
- Ahmed, K.; Föger, K. Approach to equilibrium of the water-gas shift reaction on a Ni/zirconia anode under solid oxide fuel-cell conditions. J. Power Sources 2001, 103, 150–153. [Google Scholar] [CrossRef]
- Sciazko, A.; Komatsu, Y.; Brus, G.; Kimijima, S.; Szmyd, J.S. A novel approach to the experimental study on methane/steam reforming kinetics using the Orthogonal Least Squares method. J. Power Sources 2014, 262, 245–254. [Google Scholar] [CrossRef]
- Iwai, H.; Yamamoto, Y.; Saito, M.; Yoshida, H. Numerical simulation of intermediate-temperature direct-internal-reforming planar solid oxide fuel cell. Energy 2011, 36, 2225–2234. [Google Scholar] [CrossRef]
- Brus, G.; Kimijima, S.; Szmyd, J.S. Experimental and numerical analysis of transport phenomena in an internal indirect fuel reforming type Solid Oxide Fuel Cells using Ni/SDC as a catalyst. J. Phys. Conf. Ser. 2012, 395. [Google Scholar] [CrossRef]
- Fanchi, J.R. Reservoir Simulation. In Integrated Reservoir Asset Management; Fanchi, J.R., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2010; pp. 223–241. [Google Scholar]
- Carbonell, R.G.; Whitaker, S. Heat and Mass Transfer in Porous Media. In Fundamentals of Transport Phenomena in Porous Media; Bear, J., Corapcioglu, M.Y., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 121–198. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Calmidi, V.V.; Mahajan, R.L. Thermophysical properties of high porosity metal foams. Int. J. Heat Mass Transf. 2002, 45, 1017–1031. [Google Scholar] [CrossRef]
- Dai, Z.; Nawaz, K.; Park, Y.G.; Bock, J.; Jacobi, A.M. Correcting and extending the Boomsma-Poulikakos effective thermal conductivity model for three-dimensional, fluid-saturated metal foams. Int. Commun. Heat Mass Transf. 2010, 37, 575–580. [Google Scholar] [CrossRef]
- Pajak, M.; Buchaniec, S.; Kimijima, S.; Szmyd, J.S.; Brus, G. A multiobjective optimization of a catalyst distribution in a methane/steam reforming reactor using a genetic algorithm. Int. J. Hydrog. Energy 2021, 46, 20183–20197. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Chalusiak, M.; Kimijima, S.; Szmyd, J.S.; Brus, G. An afterburner-powered methane/steam reformer for a solid oxide fuel cells application. Heat Mass Transf. 2018, 54, 2331–2341. [Google Scholar] [CrossRef]
- Tan, W.C.; Iwai, H.; Kishimoto, M.; Brus, G.; Szmyd, J.S.; Yoshida, H. Numerical analysis on effect of aspect ratio of planar solid oxide fuel cell fueled with decomposed ammonia. J. Power Sources 2018, 384, 367–378. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Brus, G.; Sciazko, A.; Komatsu, Y.; Kimijima, S.; Szmyd, J.S. An attempt to minimize the temperature gradient along a plug-flow methane/steam reforming reactor by adopting locally controlled heating zones. J. Phys. Conf. Ser. 2014, 530. [Google Scholar] [CrossRef]
- Ferziger, J.H.; Peric, M. Computational Methods for Fluid Dynamics; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef][Green Version]
- Chalusiak, M.; Wrobel, M.; Mozdzierz, M.; Berent, K.; Szmyd, J.S.; Brus, G. A numerical analysis of unsteady transport phenomena in a Direct Internal Reforming Solid Oxide Fuel Cell. Int. J. Heat Mass Transf. 2019, 131. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Gao, F.; Zhou, P.; Zhai, Z.J. Literature review on pressure–velocity decoupling algorithms applied to built-environment CFD simulation. Build. Environ. 2018, 143, 671–678. [Google Scholar] [CrossRef]
- MacCormack, R.W.; Candler, G.V. The solution of the Navier-Stokes equations using Gauss-Seidel line relaxation. Comput. Fluids 1989, 17, 135–150. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Brus, G.; Kimijima, S.; Szmyd, J.S. Numerical analysis of helium-heated methane/steam reformer. J. Phys. Conf. Ser. 2016, 745, 032081. [Google Scholar] [CrossRef]
- Powell, R.; Tye, R.; Hickman, M. The thermal conductivity of nickel. Int. J. Heat Mass Transf. 1965, 8, 679–688. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lu, T.J.; Hodson, H.P.; Jackson, J.D. The temperature dependence of effective thermal conductivity of open-celled steel alloy foams. Mater. Sci. Eng. A 2004, 367, 123–131. [Google Scholar] [CrossRef]
- Todd, B.; Young, J.B. Thermodynamic and transport properties of gases for use in solid oxide fuel cell modelling. J. Power Sources 2002, 110, 186–200. [Google Scholar] [CrossRef]
- Golberg, D.E. Genetic Algorithms in Search Optimization & Machine Learning; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1989. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, L.; Shao, Q.; Feng, Y. A Clustering Based GA for Multimodal Optimization in Uneven Search Space. In Proceedings of the 2006 6th World Congress on Intelligent Control and Automation, Dalian, China, 21–23 June 2006; Volume 1, pp. 3134–3138. [Google Scholar]
- Das, S.; Maity, S.; Qu, B.Y.; Suganthan, P. Real-parameter evolutionary multimodal optimization—A survey of the state-of-the-art. Swarm Evol. Comput. 2011, 1, 71–88. [Google Scholar] [CrossRef]
- Zou, P.; Rajora, M.; Liang, S.Y. Multimodal Optimization of Permutation Flow-Shop Scheduling Problems Using a Clustering-Genetic-Algorithm-Based Approach. Appl. Sci. 2021, 11, 3388. [Google Scholar] [CrossRef]
- Rajesh, J.K.; Gupta, S.K.; Rangaiah, G.P.; Ray, A.K. Multiobjective optimization of steam reformer performance using genetic algorithm. Ind. Eng. Chem. Res. 2000, 39, 706–717. [Google Scholar] [CrossRef]
- Azarhoosh, M.J.; Ebrahim, H.A.; Pourtarah, S.H. Simulating and Optimizing Auto-Thermal Reforming of Methane to Synthesis Gas Using a Non-Dominated Sorting Genetic Algorithm II Method. Chem. Eng. Commun. 2016, 203, 53–63. [Google Scholar] [CrossRef]
- Ye, G.Z.; Kang, D.K. Extended Evolutionary Algorithms with Stagnation-Based Extinction Protocol. Appl. Sci. 2021, 11, 3461. [Google Scholar] [CrossRef]
- De Falco, I.; Della Cioppa, A.; Tarantino, E. Mutation-based genetic algorithm: Performance evaluation. Appl. Soft Comput. 2002, 1, 285–299. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Martino, M.; Meloni, E. Innovative catalyst design for methane steam reforming intensification. Fuel 2017, 198, 175–182. [Google Scholar] [CrossRef]
- Settar, A.; Abboudi, S.; Lebaal, N. Effect of inert metal foam matrices on hydrogen production intensification of methane steam reforming process in wall-coated reformer. Int. J. Hydrog. Energy 2018, 43, 12386–12397. [Google Scholar] [CrossRef]




| Species | Mass Generation MSR | Mass Generation WGS | Mass Generation DRY | Summarized Generation |
|---|---|---|---|---|
| + | ||||
| CO | + | |||
| 0 | ||||
| 0 | ||||
| 0 |
| Equation | |
|---|---|
| (10) | |
| (11) | |
| (12) | |
| (16) |
| No. | CH | CO | HO | SC | CC |
|---|---|---|---|---|---|
| (1) | 23% | 30% | 46% | 2.0 | 1.3 |
| (2) | 20% | 40% | 40% | 2.0 | 2.0 |
| (3) | 18% | 50% | 32% | 2.0 | 2.9 |
| Gen. | Composition (1) | Composition (2) | Composition (3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| REF | 0.06 | 0.84 | 0.53 | 0.07 | 0.86 | 0.54 | 0.10 | 0.88 | 0.56 |
| INIT | 0.01 | 0.66 | 0.40 | 0.01 | 0.72 | 0.43 | 0.02 | 0.62 | 0.38 |
| 10th | 0.17 | 0.57 | 0.41 | 0.04 | 0.61 | 0.38 | 0.29 | 0.52 | 0.42 |
| 20th | 0.33 | 0.55 | 0.46 | 0.43 | 0.61 | 0.54 | 0.44 | 0.49 | 0.47 |
| 30th | 0.61 | 0.43 | 0.51 | 0.67 | 0.50 | 0.57 | 0.64 | 0.52 | 0.58 |
| Gen. | Composition (1) | Composition (2) | Composition (3) | |||
|---|---|---|---|---|---|---|
| REF | 100% | 0.40 | 100% | 0.38 | 100% | 0.29 |
| 30th | 17% | 0.94 | 10% | 1.8 | 6% | 2.17 |
| Gen. | Composition (1) | Composition (2) | Composition (3) | |||
|---|---|---|---|---|---|---|
| REF | 84% | 19% | 86% | 21% | 88% | 23% |
| 30th | 43% | 2% | 50% | 4% | 52% | 8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajak, M.; Brus, G.; Szmyd, J.S. Catalyst Distribution Optimization Scheme for Effective Green Hydrogen Production from Biogas Reforming. Energies 2021, 14, 5558. https://doi.org/10.3390/en14175558
Pajak M, Brus G, Szmyd JS. Catalyst Distribution Optimization Scheme for Effective Green Hydrogen Production from Biogas Reforming. Energies. 2021; 14(17):5558. https://doi.org/10.3390/en14175558
Chicago/Turabian StylePajak, Marcin, Grzegorz Brus, and Janusz S. Szmyd. 2021. "Catalyst Distribution Optimization Scheme for Effective Green Hydrogen Production from Biogas Reforming" Energies 14, no. 17: 5558. https://doi.org/10.3390/en14175558
APA StylePajak, M., Brus, G., & Szmyd, J. S. (2021). Catalyst Distribution Optimization Scheme for Effective Green Hydrogen Production from Biogas Reforming. Energies, 14(17), 5558. https://doi.org/10.3390/en14175558






