Comparison of Carbonaceous Compounds Emission from the Co-Combustion of Coal and Waste in Boilers Used in Residential Heating in Poland, Central Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Fuel Characteristics
2.3. Sample Collection
2.4. Determination of Particulate Organic Compounds in the Gas and Solid Phase
3. Results
3.1. Phenols
3.2. Alkylphenols
3.3. Phthalates
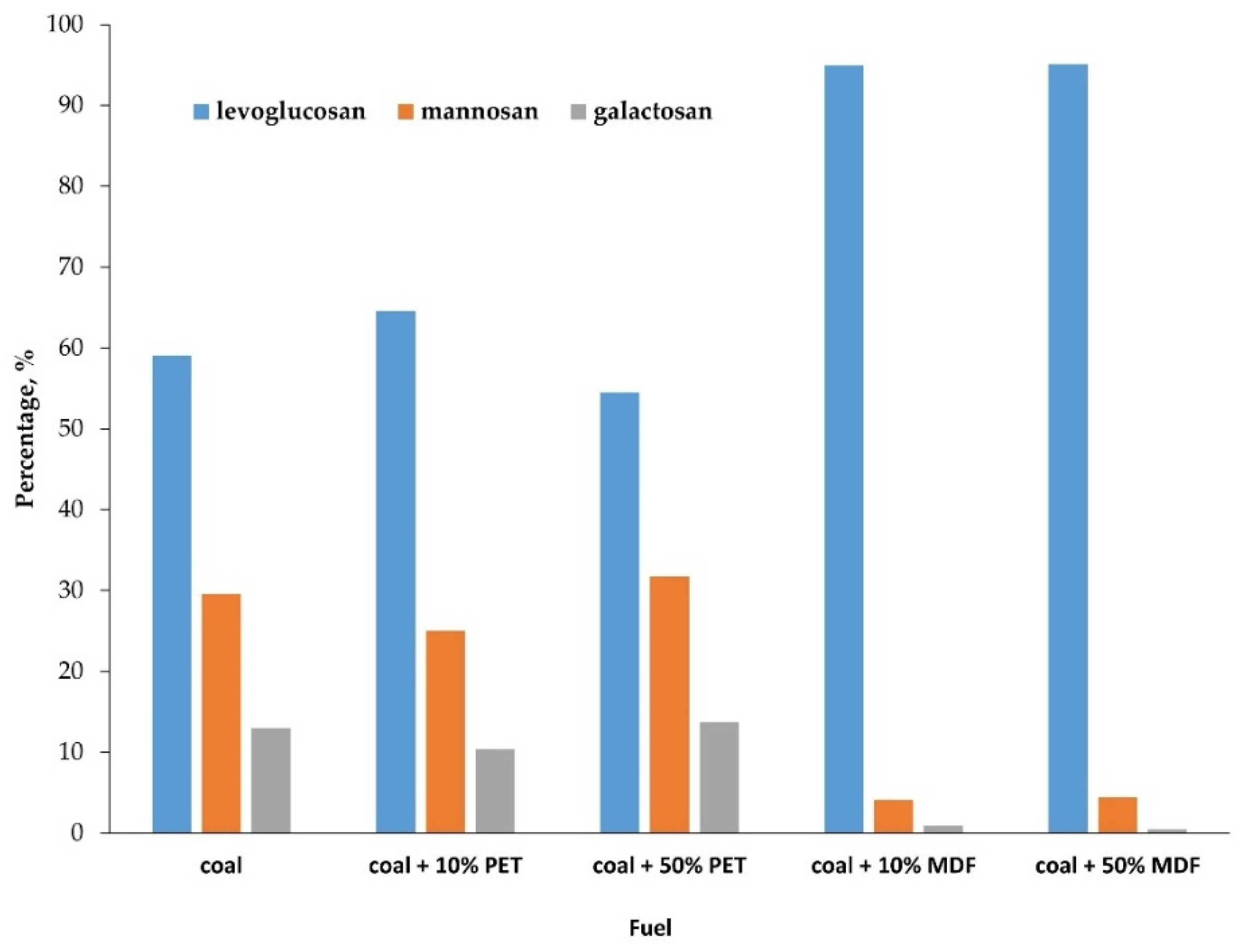
3.4. Biomass Combustion Markers
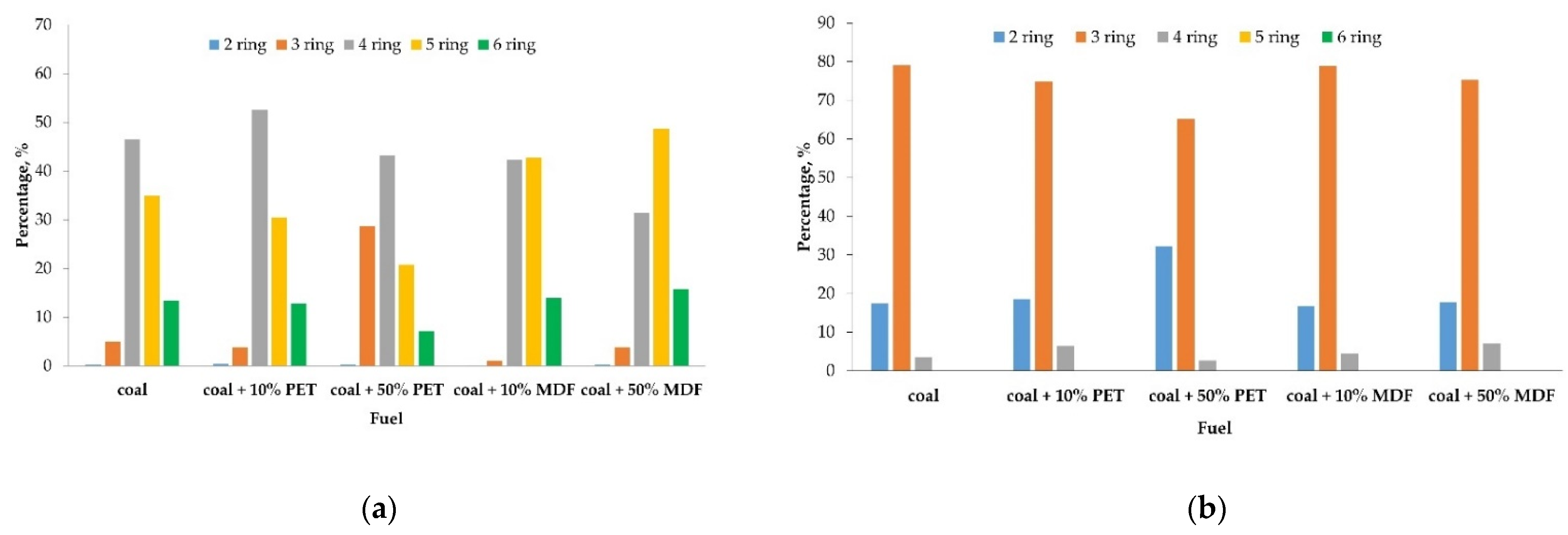
3.5. Polycyclic Aromatic Hydrocarbons
4. Discussion
4.1. Phenols
4.2. Alkylphenols
4.3. Phthalates
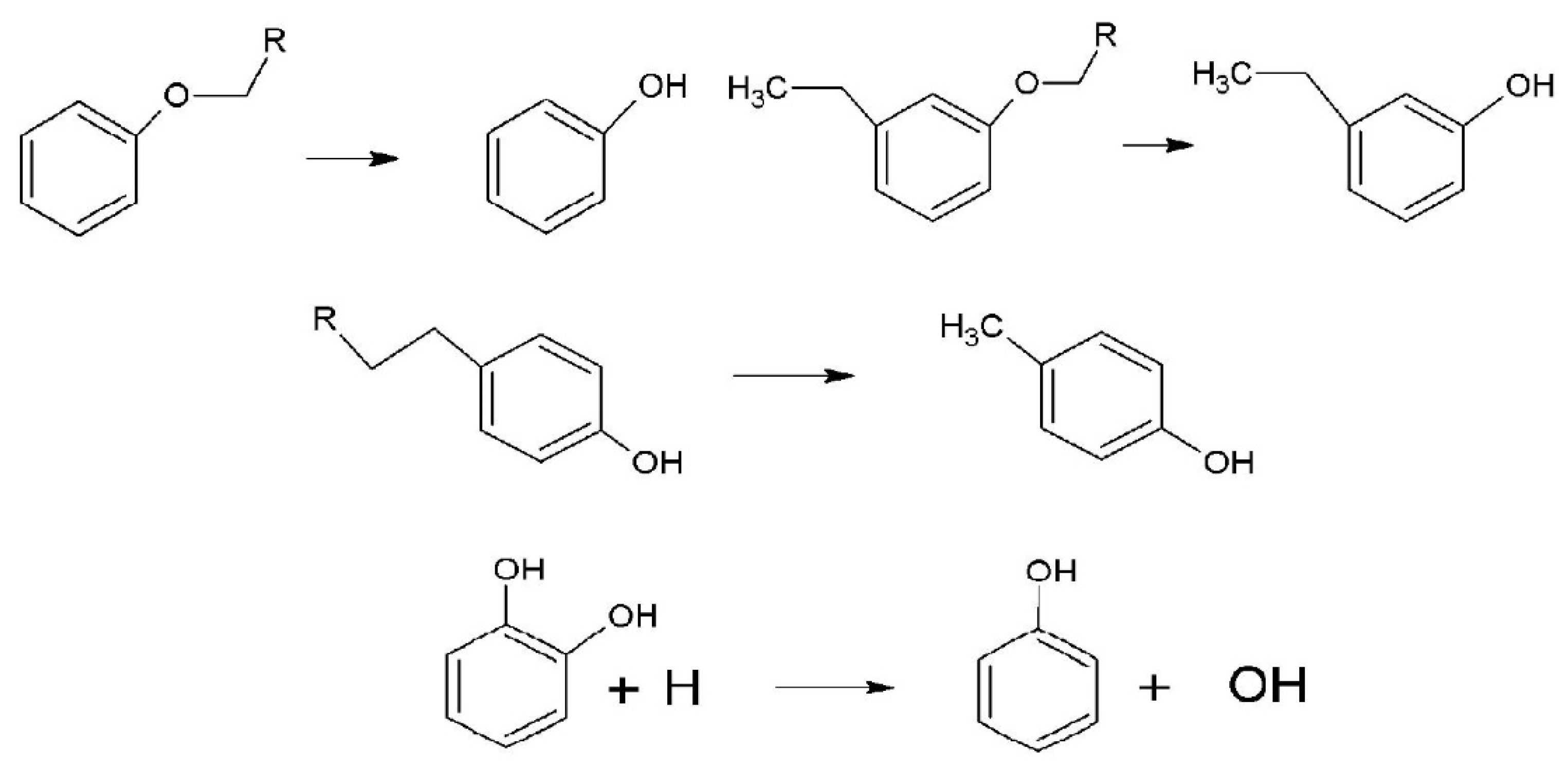
4.4. Biomass Combustion Markers
4.5. Polycyclic Aromatic Hydrocarbons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Ram, L.C.; Masto, R.E.; Verma, S.K. A comparative evaluation of minerals and trace elements in the ashes from lignite coal refuse and biomass fired power plants. Int. J. Coal Geol. 2011, 87, 112–120. [Google Scholar] [CrossRef]
- Tang, H.; Duan, Y.; Zhu, C.; Li, C.; She, M.; Zhou, Q.; Cai, L. Characteristic of a biomass-based sorbent trap and its application to coal-fired flue gas mercury emission monitoring. Int. J. Coal Geol. 2017, 170, 19–27. [Google Scholar] [CrossRef]
- Valentim, B.; Flores, D.; Gueres, A.; Guimarães, R.; Shreya, N.; Paul, B.; Ward, C.R. Notes on the occurrence of phosphate mineral relics and spheres (phosphosperes) in coal and biomass fly ash. Int. J. Coal Geol. 2016, 154–155, 43–56. [Google Scholar] [CrossRef]
- Marangwanda, G.T.; Madyira, D.M.; Babarinde, T.O. Coal combustion models: A review. J. Phys. Conf. Ser. 2019, 1378, 1–11. [Google Scholar] [CrossRef]
- Ma, L.; Jones, J.M.; Pourkashanian, M.; Williams, A. Modelling the combustion of pulverized biomass in an industrial combustion test furnace. Fuel 2007, 86, 1959–1965. [Google Scholar] [CrossRef]
- Tan, P.; Ma, L.; Xia, J.; Fang, Q.; Zhang, C.; Chen, G. Co-firing sludge in a pulverized coal-fired utility boiler: Combustion characteristics and economic impacts. Energy 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Pan, D.-L.; Jiang, W.-T.; Guo, R.-T.; Huang, Y.; Pan, W.-G. Thermogravimetric and kinetic analysis of co-combustion of waste tires and coal blends. ACS Omega 2021, 6, 5479–5484. [Google Scholar] [CrossRef]
- Hower, J.C.; Henke, K.R.; Ward, C.R.; French, D.; Liu, S.; Graham, U.M. Generation and nature of coal fly ash and bottom ash. In Coal Combustion Produccts (CCPs): Characteristics, Utilization and Benefication, 1st ed.; Robl, T., Oberlink, A., Jones, R., Eds.; Woodhead Publishing: Sawston, UK, 2017; Volume 1, pp. 21–60. [Google Scholar]
- Krůmal, K.; Mikuška, P.; Horák, J.; Hopan, F.; Krpec, K. Comparison of emissions of gaseous and particulate pollutants from the combustion of biomass and coal in modern and old-type boilers used for residental heating. Chemosphere 2019, 229, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Johannson, L.S.; Leckner, B.; Gustavsson, L.; Cooper, D.; Tullin, C.; Potter, A. Emissions characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 2004, 38, 4183–4195. [Google Scholar] [CrossRef]
- Krpec, K.; Horák, J.; Laciok, V.; Hopan, F.; Kubesa, P.; Lamberg, H.; Jokiniemi, J.; Tomšejová, S. Impact of boiler type, heat output and combusted fuel on emission factors for gaseous and particulate pollutants. Energy Fuels 2016, 30, 8448–8456. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Bradford, D.; Sharifi, V.; Swithenbank, J. Comparison of emission characteristics of small-scale heating systems using biomass instead of coal. Energy Fuels 2010, 24, 4255–4265. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, C.; Peng, L.; Li, R.; Bai, H. Emission characteristics of volatile organic compounds from coal-, coal gangue-, and biomass-fired power plants in China. Atmos. Environ. 2016, 143, 261–269. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Y.; Wang, T.; Norris, P.; Chen, W.-Y.; Pan, W.-P. Thermogravimetric-Fourier transform infrared spectroscopy-gas chromatography/mass spectrometry study of volatile organic compounds from coal pyrolysis. Energy Fuels 2017, 31, 7042–7051. [Google Scholar] [CrossRef]
- Czaplicka, M.; Cieślik, E.; Komosiński, B.; Rachwał, T. Emission Factors for Biofuels and Coal Combustion in a Domestic Boiler of 18 kW. Atmosphere 2019, 10, 771. [Google Scholar] [CrossRef] [Green Version]
- Andreae, M.O. Emission of trace gases and aerosols from biomass burning—An updated assessment. Atmos. Chem. Phys. 2019, 19, 8523–8546. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.M.; Zhi, G.R.; Tang, W.; Liu, S.J.; Dang, H.Y.; Guo, Z.; Du, J.H.; Du, X.H.; Zhang, W.Q.; Zhang, Y.J.; et al. Air pollutant emission from the underestimated households’ coal consumption source in China. Sci. Total Environ. 2017, 580, 641–650. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Kurkiewicz, S. Biomarkers, aromatic hydrocarbons and polar compound in the neogene lignites and gangue sediments of the Konin and Turoszów Brown Coal Basins (Poland). Int. J. Coal Geol. 2013, 107, 24–44. [Google Scholar] [CrossRef]
- Moroeng, D.M.; Wagner, N.J.; Brandi, D.J.; Roberts, R.J. Magnetic resonance study: Implications for coal formation in the Witbank Coalfield, South Africa. Int. J. Coal Geol. 2018, 188, 145–155. [Google Scholar] [CrossRef]
- Buha-Marković, J.Z.; Marinković, A.D.; Nemoda, S.D.; Savić, J.Z. Distribution of PAHs in coal ashes from the thermal power plant and fluidized bed combustion system estimation of environmental risk of ash disposal. Environ. Pollut. 2020, 266, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.D.; Kautzman, K.E.; Loza, C.L.; Schilling, K.A.; Coggon, M.M.; Chhabra, P.S.; Chan, M.N.; Chan, A.W.H.; Hersey, S.P.; Crounse, J.D.; et al. Secondary organic aerosol formation from biomass burning intermediates: Phenol and methoxyphenols. Atmos. Chem. Phys. 2013, 13, 8019–8043. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Smith, J.; Laskin, A.; Anastasio, C.; Laskin, J.; Zhang, Q. Chemical characterization of SOA formed from aqueous-phase reactions of phenols with the triplet excited state of carbonyl and hydroxyl radical. Atmos. Chem. Phys. 2014, 14, 13801–13816. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.X.; Wei, X.Y.; Shui, H.F.; Wang, Z.C.; Gao, J.; Wei, C. Investigation on the macromolecular network structure of Xianfeng lignite by a new two-step depolymerization. Fuel 2013, 109, 49–53. [Google Scholar] [CrossRef]
- Siskin, M.; Aczel, T. Pyrolysis studies on the structure of ethers and phenols in coal. Fuel 1983, 62, 1321–1326. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, R.; Bai, Y.; Li, G.; Zhang, C.; Li, F. Study on the formation of phenols during coal flash pyrolysis using pyrolysis-GC/MS. Fuel Process. Technol. 2014, 127, 41–46. [Google Scholar] [CrossRef]
- Uğuz, C.; Işcan, M.; Togan, I. Alkylphenols in the environment and their adverse effects on living organisms. Kopcatepe Vet. J. 2009, 2, 49–58. [Google Scholar]
- Zubkova, V.; Czaplicka, M. Changes in the structure of plasticized coals caused by extraction with dichloromethane. Fuel 2012, 96, 298–305. [Google Scholar] [CrossRef]
- Růžičková, J.; Raclavská, H.; Raclavský, K.; Juchelková, D. Phthalates in PM2,5 airborne particles in the Moravian-Silesian Region, Czech Republic. Perspect. Sci. 2016, 7, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhao, Y.; Li, L.; Chen, B.; Zhang, Y. Exposure assessment of phthalates in non-occupational populations in China. Sci. Total Environ. 2012, 428–429, 60–69. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Dong, C. Levoglucosan formation mechanisms during cellulose pyrolysis. J. Anal. Appl. Pyrol. 2013, 104, 19–27. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, W.; Blasiak, W. Formation mechanism of levoglucosan and formaldehyde during cellulose pyrolysis. Energy Fuels 2011, 25, 3739–3746. [Google Scholar] [CrossRef]
- Alves, C.A. Characterisation of solvent extractable organic constituents in atmospheric particulate matter: An overview. An. Acad. Bras. Ciências 2008, 80, 21–82. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, C.; Alves, C.; Pio, C. Inventory of fine particulate organic compound emissions from residential wood combustion in Portugal. Atmos. Environ. 2012, 50, 297–306. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, G.; Feng, Y.; Chongguo, T.; Bi, X.; Li, J.; Zhang, G. Increase in polycyclic aromatic hydrocarbon (PAH) emissions due to briquetting: A challenge to the coal briquetting policy. Environ. Pollut. 2015, 204, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Chen, J.; Yang, X.; Ren, L.; Yin, B.; Liu, X.; Bai, Z. Emission factors of polycyclic aromatic hydrocarbons from domestic coal combustion in China. J. Environ. Sci. 2014, 26, 160–166. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Mu, Y.; Liu, J.; Zhang, Y. Emission of volatile organic compounds from domestic coal stove with the actual alternation of flaming and smoldering combustion processes. Environ. Pollut. 2017, 221, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Dhahak, A.; Grimmer, C.; Neumann, A.; Rüger, C.; Sklorz, M.; Streibel, T.; Zimmermann, R.; Mauviel, G.; Burkle-Vitzthum, V. Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manag. 2020, 106, 226–239. [Google Scholar] [CrossRef]
- Jabłońska, B.; Kiełbasa, P.; Korenko, M.; Dróżdż, T. Physical and chemical properties of waste from PET bottles washing as a component of solid fuels. Energies 2019, 12, 2197. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-Y.; Seo, T.-C. Upgrading biochar via co-pyrolisation of agricultural biomass and polyethylene terephthalate wastes. RCS Adv. 2019, 9, 28284–28290. [Google Scholar]
- Wasilewski, R.; Siudyga, T. Odzysk energetyczny odpadowych tworzyw sztucznych. Chemik 2013, 67, 435–445. [Google Scholar]
- Ishaq, M.; Ahmad, I.; Shakirullah, M.; Arsala Khan, M.; ur Rehman, H.; Bahader, A. Pyrolysis of some whole plastics and plastic coal mixtures. Energy Convers. Manag. 2006, 47, 3216–3223. [Google Scholar] [CrossRef]
- Kojić, I.; Bechtel, A.; Aleksić, N.; Životić, D.; Trifunović, S.; Gajica, G.; Stojanović, K. Study of the synergetic effect of co-pyrolysis of lignite and high-density polyethylene aming to improve utilization of low-rank coal. Polymers 2021, 13, 759. [Google Scholar] [CrossRef]
- Tomsej, T.; Horak, J.; Tomsejova, S.; Krpec, K.; Klanova, J.; Dej, M.; Hopan, F. The impact of co-combustion of polyethylene plastics and wood in a small residential boiler on emissions of gaseous pollutants, particulate matter, PAHs and 1,3,5-triphenylbenzene. Chemosphere. 2018, 196, 18–24. [Google Scholar] [CrossRef]
- Sun, L.; Wang, F.; Xie, Y.; Feng, J.; Wang, Q. The combustion performance of medium density fiberboard treated with fire retardant microspheres. Bioresources 2012, 7, 593–601. [Google Scholar]
- Wang, S.; Wang, W.; Yang, H. Comparison of product carbon footprint protocols: Case study on medium-density fiberboard in China. Int. J. Environ. Res. Public Health 2018, 15, 2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Lu, Q.; Zhou, Y.; Chen, N.; Rao, J.; Fan, M. Circular development of recycled natural fibers from medium density fiberboard wastes. J. Clean. Prod. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Kauneliene, V.; Krugly, E.; Kliucininkas, L.; Stasiulaitiene, L.; Prasauskas, T.; Auzbikaviciute, A.; Bergqvisit, P.A.; Tomsej, T.; Martuzevicius, D. PAHs in indoor and outdoor air from decentralized heating energy production: Comparison of active and passive sampling. Polycycl. Aromat. Comp. 2016, 36, 410–428. [Google Scholar] [CrossRef]
- Hoffer, A.; Jancsek-Turóczi, B.; Tóth, A.; Kiss, G.; Naghiu, A.; Levei, A.E.; Marmureanu, L.; Machon, A.; Gelencsér, A. Emissions factors for PM10 and PAHs from illegal burning of different types of municipal waste in households. Atmos. Chem. Phys. Discuss. 2020, 20, 16135–16144. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Medeiros, P.M.; Dudyk, B.M. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ. Sci. Technol. 2005, 39, 6961–6970. [Google Scholar] [CrossRef]
- Janoszka, K.; Czaplicka, M.; Klejnowski, K. Comparison in biomass burning marker tracer concentration between two winter seasons in Krynica Zdroj for biomas burning markers. Air Qual. Atmos. Health 2020, 13, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Czaplicka, M.; Węglarz, A.; Klejnowski, K. Analysis of organic contaminants from motor vehicles adsorbed on particulate matter for PAHs. Chem. Anal. 2001, 46, 677–689. [Google Scholar]
- Czaplicka, M.; Kaczmarczyk, B. Infrared study of chlorophenols and products of their photodegradation. Talanta 2006, 70, 940–949. [Google Scholar] [CrossRef]
- Jaworek, K.; Czaplicka, M. Determination of phthalates in polymer materials—Comparison of GC/MS and GC/ECD methods. Polímeros 2013, 23, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Li, D.H.; Oh, J.R.; Park, J. Direct extraction of alkylphenols, chlorophenols and bisphenol A from acid-digested sediment suspension for simultaneous gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2003, 1012, 207–214. [Google Scholar] [CrossRef]
- Gil, M.V.; Casal, D.; Pevida, C.; Pis, J.J.; Rubiera, F. Thermal behavior and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef] [Green Version]
- Salapasidou, M.; Samara, C.; Voutsa, D. Endocrine disrupting compounds in the atmosphere of the urban area of Thessaloniki, Greece. Atmos. Environ. 2011, 45, 3720–3729. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.-L.; Guo, Y.; Wu, Q.; Yang, M.; Wu, M.-H.; Kannan, K. Phthalate diesters in airborne PM2,5 and PM10 in a suburban area of Shanghai: Seasonal distribution and risk assessment. Sci. Total Environ. 2014, 497–498, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, S.; Li, Z.; Wang, Y.; Hu, Y.; Xing, Y.; Liu, G.; Fang, R.; Zhu, H. PM2,5 associated phenols, phthalates and water soluble ions from five stationary combustion sources. Aerosol Air Qual. Res. 2020, 20, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Karlovsky, P. Removal of the disrupter butyl benzyl phthalate from the environment. Appl. Microbiol. Biotechnol. 2010, 87, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Simoneit, B.R.T. Biomass burning—A review of organic tracers for smoke from incomplete combustion. Appl. Geochem. 2002, 17, 129–162. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Li, J.; Zhou, B.; Xie, M.; Hu, S.; Kawamura, K.; Chen, Y. Molecular composition and size distribution of sugars, sugar-alcohols and carboxylic acids in airborne particles during a severe urban haze event caused by wheat straw burning. Atmos. Environ. 2011, 45, 2473–2479. [Google Scholar] [CrossRef]
- Fraser, M.P.; Lakshmanan, K. Using levoglucosan as a molecular marker for the long-range transport of biomass combustion aerosols. Environ. Sci. Technol. 2000, 34, 4560–4564. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.X.; Le, H.P.; Wang, F.W.; Guo, Z.G.; Iinuma, Y.; Chen, J.M.; Herrmann, H. Atmospheric outflow of PM2.5 saccharides from megacity Shanghai to East China Sea: Impact of biological and biomass burning sources. Atmos. Environ. 2016, 143, 1–14. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Feng, Y.; Song, W.; Cao, F.; Zhang, Y.; Li, Q.; Yang, X.; Chen, J. Different formation mechanisms of PAH during wood and coal combustion under different temperatures. Atmos. Environ. 2019, 222, 117084. [Google Scholar] [CrossRef]




| Parameter | Unit | Value |
|---|---|---|
| Boiler output | kW | 18 |
| Boiler heating surface | m2 | 1.7 |
| Efficiency | % | 85–85.9 |
| Average steady-state performance | h | 48 |
| Maximum water temperature | °C | 95 |
| Maximum operating pressure | MPa | 0.1 |
| Required minimum flue gas draught | Pa | 16 |
| Tank capacity | m3 | 0.16 |
| Assay | Content/Assigned Value | ||
|---|---|---|---|
| Coal | MDF | PET | |
| (% weight (% w/w)) | |||
| Ash | 3.95 | 0.97 | 14.56 |
| Carbon | 78.39 | 51.42 | 60.11 |
| Hydrogen | 4.19 | 6.12 | 8.02 |
| Sulphur | 0.60 | <0.03 | 0.14 |
| Oxygen (different) | 16,82 | 42.43 | 31.72 |
| (J/g (MJ/kg)) | |||
| Combustion heat | 30.41 | 19.25 | 25.57 |
| Calorific value | 29.51 | 17.92 | 23.85 |
| Compound | Coal | Coal + 10% PET | Coal + 50% PET | Coal + 10% MDF | Coal + 50% MDF |
|---|---|---|---|---|---|
| phenol | 150.5 | 158.6 | 351.5 | 101.6 | 99.9 |
| o-cresol | 77.2 | 79.9 | 104.6 | 52.1 | 51.2 |
| p,m-cresol | 172.1 | 172.2 | 182.4 | 107.8 | 106.0 |
| total | 399.8 | 410.8 | 638.5 | 261.5 | 257.1 |
| Compound | Coal + 10% PET | Coal + 50% PET | Coal + 50% MDF | Coal + 10% MDF | ||||
|---|---|---|---|---|---|---|---|---|
| PM | Gas Phase | PM | Gas Phase | PM | Gas Phase | PM | Gas Phase | |
| 4-tOP | 0.38 | 0.14 | 0.30 | 0.61 | 0.28 | 2.9 | 0.16 | 2.2 |
| 4-nNP | 0.13 | 0.30 | 0.21 | 0.48 | 0.54 | 1.2 | 0.35 | 0.72 |
| BPA | 9.5 | 21.1 | 10.1 | 234.8 | 0.69 | 3.2 | 0.97 | 1.1 |
| total | 10.0 | 21.5 | 10.6 | 235.9 | 1.5 | 7.3 | 1.5 | 4.0 |
| Grand total | 31.5 | 246.5 | 8.8 | 5.5 | ||||
| Compound | Coal | Coal + 10% PET | Coal + 50% PET | Coal + 10% MDF | Coal + 50% MDF |
|---|---|---|---|---|---|
| DMP | 0.76 | 0.66 | 2.21 | 0.96 | 0.98 |
| DEP | <0.05 | <0.05 | 0.10 | 0.10 | <0.05 |
| DBP | 0.25 | 0.22 | 4.5 | 0.44 | 0.52 |
| BBP | 0.23 | <0.05 | <0.05 | 1.4 | 3.0 |
| DEHA | <0.05 | 0.15 | <0.05 | <0.05 | <0.05 |
| DEHP | 0.78 | 4.9 | 9.4 | 0.15 | 0.22 |
| DOP | 0.19 | 0.50 | 2.0 | 0.28 | 0.64 |
| total | 2.2 | 6.4 | 18.1 | 3.3 | 5.4 |
| Compound | Coal | Coal + 10% PET | Coal + 50% PET | Coal + 10% MDF | Coal + 50% MDF |
|---|---|---|---|---|---|
| levoglucosan | 2.6 | 6.2 | 10.3 | 191.8 | 514.8 |
| mannosan | 1.3 | 2.4 | 6.0 | 8.3 | 24.1 |
| galactosan | 0.57 | 1.0 | 2.6 | 1.8 | 2.4 |
| total | 4.4 | 9.6 | 18.9 | 201.9 | 541.3 |
| LG/MN | 2.1 | 2.6 | 1.7 | 23.0 | 21.4 |
| LG/(MN+GA) | 1.4 | 1.9 | 1.2 | 18.9 | 19.5 |
| Compound | Coal | Coal + 10% PET | Coal + 50% PET | Coal +10% MDF | Coal +50% MDF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PM | Gas | PM | Gas | PM | Gas | PM | Gas | PM | Gas | |
| naphthalene | 0.40 | 3.7 | 1.9 | 19.7 | 1.7 | 117.4 | 0.19 | 43.8 | 0.63 | 44.1 |
| acenaphthylene | 0.49 | 7.8 | 0.75 | 31.7 | 1.1 | 123.1 | 0.10 | 52.3 | 0.17 | 34.8 |
| acenaphthene | 0.07 | 0.49 | 0.42 | 4.2 | 0.39 | 5.3 | 0.08 | 9.3 | 0.24 | 11.8 |
| fluorene | 0.48 | 1.9 | 1.6 | 8.0 | 5.0 | 17.3 | 0.43 | 34.8 | 2.6 | 21.0 |
| phenanthrene | 5.4 | 5.2 | 9.4 | 27.2 | 100.4 | 56.9 | 0.73 | 67.0 | 3.8 | 60.4 |
| anthracene | 3.2 | 1.2 | 8.2 | 8.3 | 60.6 | 34.8 | 0.86 | 42.9 | 1.5 | 58.8 |
| fluoranthene | 24.6 | 0.39 | 53.3 | 3.4 | 70.6 | 4.6 | 11.0 | 5.9 | 4.8 | 8.6 |
| pyrene | 24.7 | 0.36 | 77.5 | 3.4 | 97.9 | 4.9 | 17.3 | 5.7 | 8.3 | 8.8 |
| benzo(a)anthracene | 22.7 | 85.1 | 52.5 | 35.5 | 30.3 | |||||
| chrysene | 18.0 | 63.8 | 31.3 | 26.1 | 24.5 | |||||
| benzo(b)fluoranthene | 23.3 | 60.2 | 31,7 | 34.8 | 42.0 | |||||
| benzo(k)fluoranthene | 11.7 | 25.2 | 21.3 | 16.4 | 17.2 | |||||
| benzo(a)pyrene | 19.4 | 49.0 | 45.1 | 27.1 | 32.0 | |||||
| indeno(1,2,3-c,d)pyrene | 19.1 | 42.2 | 22.1 | 20.9 | 23.0 | |||||
| dibenzo(a,h)anthracene | 3.7 | 9.1 | 6.7 | 1.9 | 3.7 | |||||
| benzo(g,h,i)perylene | 6.9 | 25.6 | 19.5 | 8.7 | 11.0 | |||||
| benzo(j)fluoranthene | 10.0 | 18.6 | 16.3 | 10.5 | 10.2 | |||||
| total | 194.1 | 21.0 | 531.7 | 106.0 | 584.0 | 364.3 | 212.5 | 261.7 | 215.8 | 248.2 |
| grand total | 215.1 | 637.7 | 948.3 | 474.2 | 464.0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaplicka, M.; Klyta, J.; Komosiński, B.; Konieczny, T.; Janoszka, K. Comparison of Carbonaceous Compounds Emission from the Co-Combustion of Coal and Waste in Boilers Used in Residential Heating in Poland, Central Europe. Energies 2021, 14, 5326. https://doi.org/10.3390/en14175326
Czaplicka M, Klyta J, Komosiński B, Konieczny T, Janoszka K. Comparison of Carbonaceous Compounds Emission from the Co-Combustion of Coal and Waste in Boilers Used in Residential Heating in Poland, Central Europe. Energies. 2021; 14(17):5326. https://doi.org/10.3390/en14175326
Chicago/Turabian StyleCzaplicka, Marianna, Justyna Klyta, Bogusław Komosiński, Tomasz Konieczny, and Katarzyna Janoszka. 2021. "Comparison of Carbonaceous Compounds Emission from the Co-Combustion of Coal and Waste in Boilers Used in Residential Heating in Poland, Central Europe" Energies 14, no. 17: 5326. https://doi.org/10.3390/en14175326
APA StyleCzaplicka, M., Klyta, J., Komosiński, B., Konieczny, T., & Janoszka, K. (2021). Comparison of Carbonaceous Compounds Emission from the Co-Combustion of Coal and Waste in Boilers Used in Residential Heating in Poland, Central Europe. Energies, 14(17), 5326. https://doi.org/10.3390/en14175326







