Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flame Spray Pyrolysis (FSP) Synthesis of Nanocatalysts
2.2. Characterization of the Nanomaterials
2.2.1. Material Morphology
2.2.2. Optical Spectroscopy
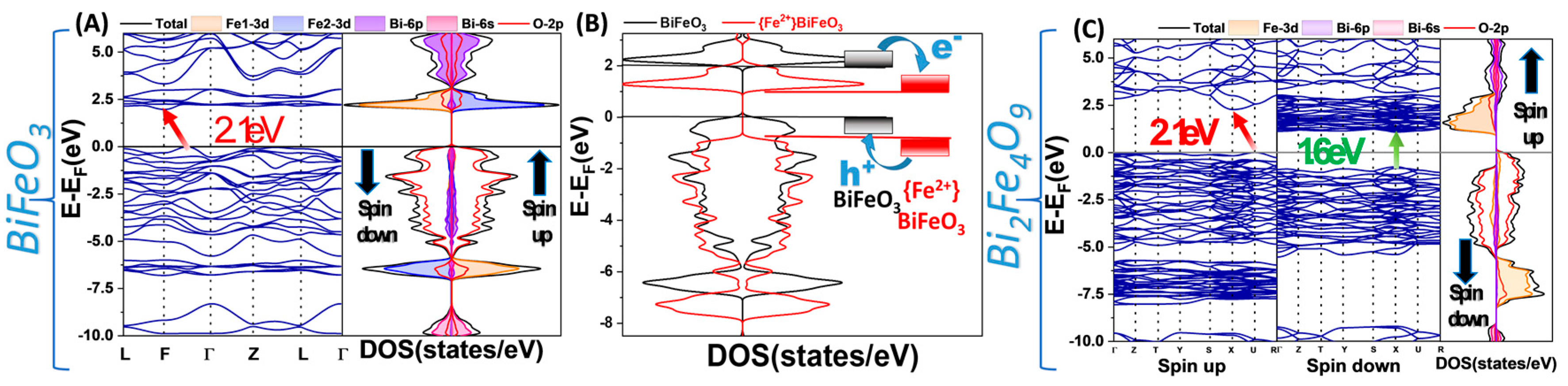
2.2.3. Theoretical Modeling (DFT) Calculations
2.3. Photocatalytic O2 and •OH Generation
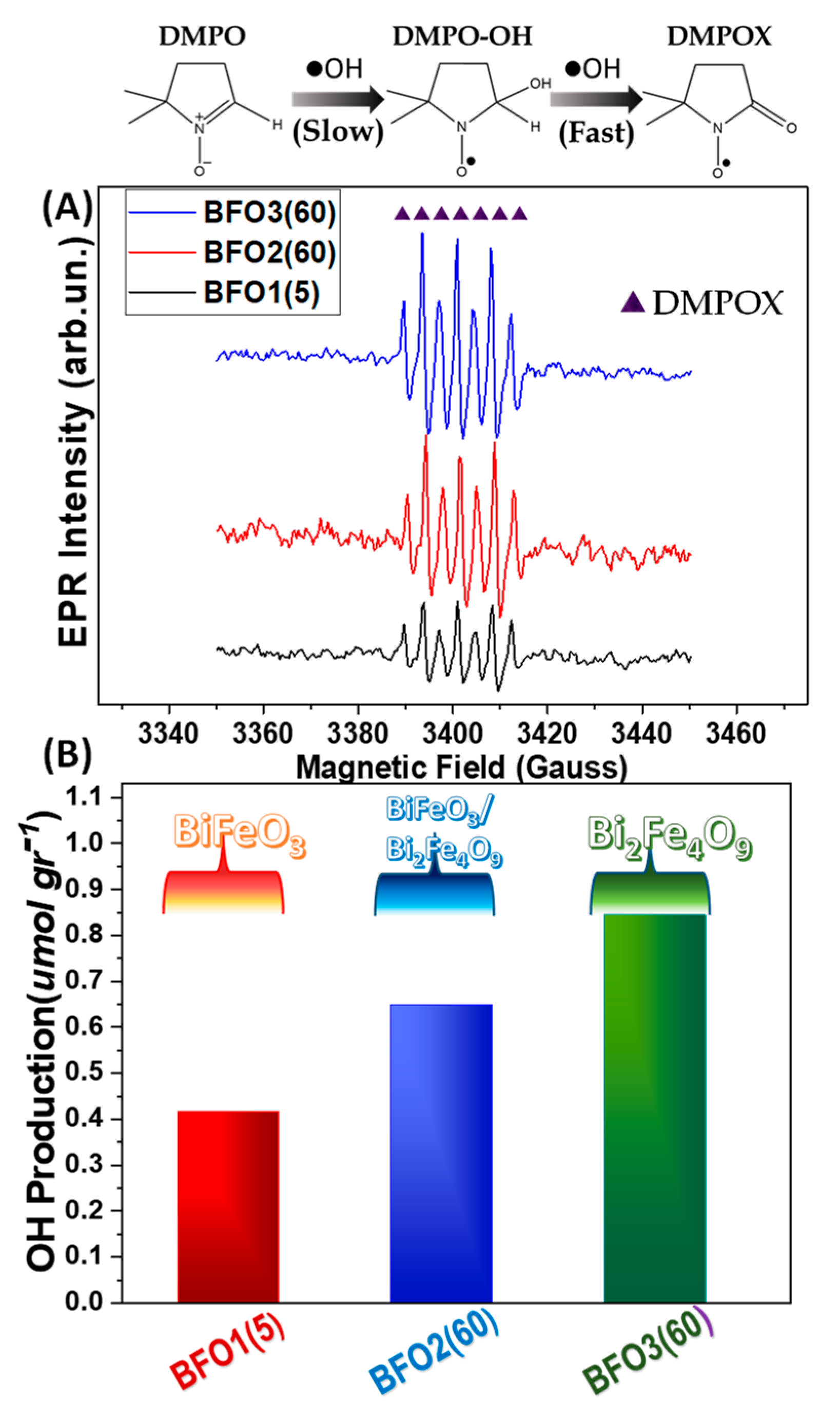
2.3.1. Electron Paramagnetic Resonance (EPR) Spectroscopy (•OH Generation)
2.3.2. Photocatalytic O2 Evolution Measurements
3. Results
3.1. Structure and Morphology
3.1.1. XRD and TEM Structures
3.1.2. Diffuse Reflectance Uv-Vis Spectroscopy: Band-Gap Estimates
3.1.3. FT-IR, Raman Spectroscopy
3.1.4. X-ray Photoelectron Spectroscopy
3.2. Photocatalysis
3.2.1. Photocatalytic O2 Production from H2O
- Photocatalytic O2-evolution is improved significantly on going from BiFeO3 towards Bi2Fe4O9
- For materials of the same phase, the annealing protocol parameters, Tanneal, tanneal, allow modulation of the O2 evolution trend. In all cases, longer tanneal is detrimental
- Bi2Fe4O9 is sensitive to prolonged tanneal, i.e., severe crystal quality deterioration occurs in BFO3(120) and this causes severe deterioration of its photocatalytic activity. In contrast, BiFeO3 shows less-pronounced effects upon prolonged annealing.
- The XPS data reveal a correlation trend between the Fe2+ centers in BiFeO3 and O2 production.
3.2.2. Photogenerated Holes of the Photocatalytic Mechanism
3.2.3. Insight into the Role of Fe2+ Centers in BiFeO3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.-Z.; Ji, M.; Yan, C.; Gu, K.; Xia, J.; Li, H.; Li, S.; Liu, Z. Defect Engineering in Atomically-Thin Bismuth Oxychloride Towards Photocatalytic Oxygen Evolution. J. Mater. Chem. A 2017, 5, 14144–14151. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Zhou, X.; Li, C. A Theoretical Study on the Mechanism of Photocatalytic Oxygen Evolution on BiVO4in Aqueous Solution. Chem. Eur. J. 2013, 19, 1320–1326. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Ji, M.; Xia, J.; Yan, C.; Hao, W.; Li, S.; Li, H.; Liu, Z. Bismuth Vacancy Mediated Single Unit Cell Bi2WO6 Nanosheets for Boosting Photocatalytic Oxygen Evolution. Appl. Catal. B Environ. 2018, 238, 119–125. [Google Scholar] [CrossRef]
- Papadas, I.; Christodoulides, J.A.; Kioseoglou, G.; Armatas, G.S. A High Surface Area Ordered Mesoporous Bifeo3 Semiconductor with Efficient Water Oxidation Activity. J. Mater. Chem. A 2015, 3, 1587–1593. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Kibria, M.G.; Mi, Z.; Chaker, M.; Ma, D.; Nechache, R.; Rosei, F. Remarkably Enhanced Photocatalytic Activity ff Laser Ablated au Nanoparticle Decorated Bifeo3 Nanowires Under Visible-Light. Chem. Commun. 2013, 49, 5856–5858. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Quiñonez, J.L.; Díaz, D.; Zumeta-Dubé, I.; Arriola-Santamaría, H.; Betancourt, I.; Santiago-Jacinto, P.; Nava-Etzana, N. Easy Synthesis of High-Purity BiFeO3 Nanoparticles: New Insights Derived from the Structural, Optical, and Magnetic Characterization. Inorg. Chem. 2013, 52, 10306–10317. [Google Scholar] [CrossRef]
- Kirsch, A.; Murshed, M.M.; Schowalter, M.; Rosenauer, A.; Gesing, T.M. Nanoparticle Precursor into Polycrystalline Bi2Fe4O9: An Evolutionary Investigation of Structural, Morphological, Optical, and Vibrational Properties. J. Phys. Chem. C 2016, 120, 18831–18840. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Liu, J.-M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.-C.; Mohamed, A.R. A Newly Emerging Visible Light-Responsive BiFeO3 Perovskite for Photocatalytic Applications: A Mini Review. Mater. Res. Bull. 2017, 90, 15–30. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, T.; Chen, J.; Cheng, J. Synthesis and Visible Light Photocatalytic Properties of Bi2Fe4O9 Particles via EDTA-Assisted Sol–Gel Route. J. Sol-Gel Sci. Technol. 2016, 78, 135–143. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Lei, M.; She, Y.; Cao, M.; Tang, H. Ligand-Induced Drastic Enhancement of Catalytic Activity of Nano-BiFeO3 for Oxidative Degradation of Bisphenol A. ACS Catal. 2011, 1, 1193–1202. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Xu, G.; Wei, X.; Ren, Z.; Shen, G.; Han, G. Phase and Morphology Evolution of Bismuth Ferrites via Hydrothermal Reaction Route. Mater. Res. Bull. 2013, 48, 1694–1699. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Y.; Qiu, Y.; Liu, Y.; Xiong, R.; Shi, J.; Wei, J. Facial Synthesis and Photoreaction Mechanism of BiFeO3/Bi2Fe4O9 Heterojunction Nanofibers. ACS Sustain. Chem. Eng. 2017, 5, 4630–4636. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced Visible-Light Activities of Porous Bifeo3 by Coupling with Nanocrystalline Tio2 and Mechanism. Appl. Catal. B Environ. 2016, 180, 219–226. [Google Scholar] [CrossRef]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and Their Evaluation as Non-Noble Metal Catalysts for Efficient Reduction of 4-Nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar] [CrossRef]
- Madler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled Synthesis of Nanostructured Particles by Ame Spray Pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Palai, R.; Katiyar, R.S.; Schmid, H.; Tissot, P.; Clark, S.J.; Robertson, J.; Redfern, S.A.T.; Catalan, G.; Scott, J.F. βphase Andγ−Βmetal-Insulator Transition in Multiferroicbifeo3. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef] [Green Version]
- Palewicz, A.; Przenioslo, R.; Sosnowska, I.; Hewat, A.W. Atomic Displacements in Bifeo3 as a Function of Temperature: Neutron Diffraction Study. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 537–544. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21. [Google Scholar] [CrossRef] [PubMed]
- Cococcioni, M.; de Gironcoli, S. Linear Response Approach to the Calculation of the Effective Interaction Parameters in Thelda+Umethod. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71. [Google Scholar] [CrossRef] [Green Version]
- Kanigaridou, Y.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Solakidou, M.; Konstantinou, I.; Deligiannakis, Y.; Mantzavinos, D.; Kondarides, D.I. Solar Photocatalytic Degradation of Bisphenol a with CuOx/BiVO4: Insights into the Unexpectedly Favorable Effect of Bicarbonates. Chem. Eng. J. 2017, 318, 39–49. [Google Scholar] [CrossRef]
- Solakidou, M.; Georgiou, Y.; Deligiannakis, Y. Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production. Energies 2021, 14, 817. [Google Scholar] [CrossRef]
- Moreau, J.M.; Michel, C.; Gerson, R.; James, W.J. Ferroelectric BiFeO3 X-ray and Neutron Diffraction Study. J. Phys. Chem. Solids 1971, 32, 1315–1320. [Google Scholar] [CrossRef]
- Moularas, C.; Georgiou, Y.; Adamska, K.; Deligiannakis, Y. Thermoplasmonic Heat Generation Efficiency by Nonmonodisperse Core–Shell Ag0@SiO2 Nanoparticle Ensemble. J. Phys. Chem. C 2019, 123, 22499–22510. [Google Scholar] [CrossRef]
- Shamir, N.; Gurewitz, E.; Shaked, H. The magnetic structure of Bi2Fe4O9– analysis of neutron diffraction measurements. Acta Crystallogr. Sect. 1978, 34, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Sowmya, N.S.; Srinivas, A.; Suresh, P.; Shukla, A.; Kamat, S.V. Synthesis and Study of Structural, Magnetic and Microwave Absorption Properties in Multiferroic Bifeo3 Electroceramic. J. Mater. Sci. Mater. Electron. 2015, 26, 5368–5372. [Google Scholar] [CrossRef]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Mädler, L.; Kudo, A.; Amal, R. Flame Preparation of Visible-Light-Responsive BiVO4 Oxygen Evolution Photocatalysts with Subsequent Activation via Aqueous Route. ACS Appl. Mater. Interfaces 2011, 3, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Deligiannakis, Y. Nanomaterials for Environmental Solar Energy Technologies: Applications & Limitations. KONA Powder Part. J. 2018, 35, 14–31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Gong, W.; Wang, J.; Ning, X.; Wang, Z.; Zhao, X.; Ren, W.; Zhang, Z. Size-Dependent Magnetic, Photoabsorbing, and Photocatalytic Properties of Single-Crystalline Bi2Fe4O9 Semiconductor Nanocrystals. J. Phys. Chem. C 2011, 115, 25241–25246. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Shang, M. Visible Light-Induced Photocatalytic Oxidation of Phenol and Aqueous Ammonia in Flowerlike Bi2Fe4O9 Suspensions. J. Phys. Chem. C 2009, 113, 12826–12831. [Google Scholar] [CrossRef]
- Srivastav, S.K.; Gajbhiye, N.S. Low Temperature Synthesis, Structural, Optical and Magnetic Properties of Bismuth Ferrite Nanoparticles. J. Am. Ceram. Soc. 2012, 95, 3678–3682. [Google Scholar] [CrossRef]
- Kaczmarek, W.; Graja, A. Lattice Dynamics Study of the Solid Solution (Bi1−xLax) FeO3 by i.r. Spectroscopy. Solid State Commun. 1975, 17, 851–853. [Google Scholar] [CrossRef]
- Voll, D.; Beran, A.; Schneider, H. Variation of Infrared Absorption Spectra in the System Bi2Al4−xFexO9 (x = 0–4), Structurally Related to Mullite. Phys. Chem. Miner. 2006, 33, 623–628. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Xu, Y.; He, Y.; Chen, W. Synthesis and Characterization of Bi2Fe4O9 Powders. Mater. Chem. Phys. 2011, 128, 388–391. [Google Scholar] [CrossRef]
- Fujiwara, K.; Deligiannakis, Y.; Skoutelis, C.G.; Pratsinis, S.E. Visible-Light Active Black TiO2-Ag/TiOx Particles. Appl. Catal. B Environ. 2014, 154–155, 9–15. [Google Scholar] [CrossRef]
- Arora, M.; Sati, P.C.; Chauhan, S.; Chhoker, S.; Panwar, A.K.; Kumar, M. Structural, Optical and Multiferroic Properties of BiFeO3 Nanoparticles Synthesized by Soft Chemical Route. J. Supercond. Nov. Magn. 2013, 26, 443–448. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.Y.; Zhu, K.; Liu, Y.L.; Chen, J.; Xing, X.R. Raman Study of BiFeO3 with Different Excitation Wavelengths. Phys. B Condens. Matter 2009, 404, 171–174. [Google Scholar] [CrossRef]
- Iliev, M.N.; Litvinchuk, A.P.; Hadjiev, V.G.; Gospodinov, M.M.; Skumryev, V.; Ressouche, E. Phonon and Magnon Scattering of Antiferromagnetic Bi2Fe4O9. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef] [Green Version]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Dharmadhikari, V.S.; Sainkar, S.R.; Badrinarayan, S.; Goswami, A. Characterisation of Thin Films of Bismuth Oxide by X-ray Photoelectron Spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1982, 25, 181–189. [Google Scholar] [CrossRef]
- Wang, P.W.; Guttag, M.; Tu, C.-S. Surface Modification of Multiferroic BiFeO3 Ceramic by Argon Sputtering. J. Surf. Eng. Mater. Adv. Technol. 2014, 04, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Baraneedharan, P.; Hussain, S.I.; Dinesh, V.P.; Siva, C.; Biji, P.; Sivakumar, M. Lattice Soped Zn–SnO2 Nanospheres: A Systematic Exploration of Dopant Ion Effects on Structural, Optical, and Enhanced Gas Sensing Properties. Appl. Surf. Sci. 2015, 357, 1511–1521. [Google Scholar] [CrossRef]
- He, J.; Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Characterization and Visible Light Photocatalytic Mechanism of Size-Controlled BiFeO3 Nanoparticles. Mater. Res. Bull. 2013, 48, 3017–3024. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Tian, P.; Chin, W.S.; Zhang, C.M. A Facile Approach to Pure-Phase Bi2Fe4O9 Nanoparticles Sensitive to Visible Light. Appl. Surf. Sci. 2014, 321, 144–149. [Google Scholar] [CrossRef]
- Primo, A.; Marino, T.; Corma, A.; Molinari, R.; García, H. Efficient Visible-Light Photocatalytic Water Splitting by Minute Amounts of Gold Supported on Nanoparticulate CeO2Obtained by a Biopolymer Templating Method. J. Am. Chem. Soc. 2011, 133, 6930–6933. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial Separation of Photogenerated Electrons and Holes Among {010} And {110} Crystal Facets of BiVO4. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezova, V.; Dvoranova, D.; Stasko, A. Characterization of Titanium Dioxide Photoactivity Following the Formation of Radicals by EPR Spectroscopy. Res. Chem. Intermed. 2007, 33, 251–268. [Google Scholar] [CrossRef]
- Rosen, G.M.; Rauckman, E.J. Spin Trapping of the Primary Radical Involved in the Activation of the Carcinogen N-Hydroxy-2-Acetylaminofluorene by Cumene Hydroperoxide-Hematin. Mol. Pharmacol. 1980, 17, 233–238. [Google Scholar] [PubMed]
- Hölzl, J.; Schulte, F.K. Work Function of Metals. In Solid Surface Physics; Hölzl, J., Schulte, F.K., Wagner, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 1–150. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, R.; Pal, B. Influence of Coinage and Platinum Group Metal Co-Catalysis for the Photocatalytic Reduction of M-Dinitrobenzene by P25 and Rutile TiO2. J. Mol. Catal. A: Chem. 2015, 397, 99–105. [Google Scholar] [CrossRef]
- H’mŏk, H.L.; Aguilar, E.M.; García, J.A.; Ariño, J.R.; Mestres, L.; Alemany, P.; Galván, D.H.; Beltrones, J.M.S.; Herrera, O.R. Theoretical Justification of Stable Ferromagnetism in Ferroelectric BiFeO3 by First-Principles. Comput. Mater. Sci. 2019, 164, 66–73. [Google Scholar] [CrossRef]
- Clark, S.J.; Robertson, J. Energy Levels of Oxygen Vacancies in BiFeO3 by Screened Exchange. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef] [Green Version]
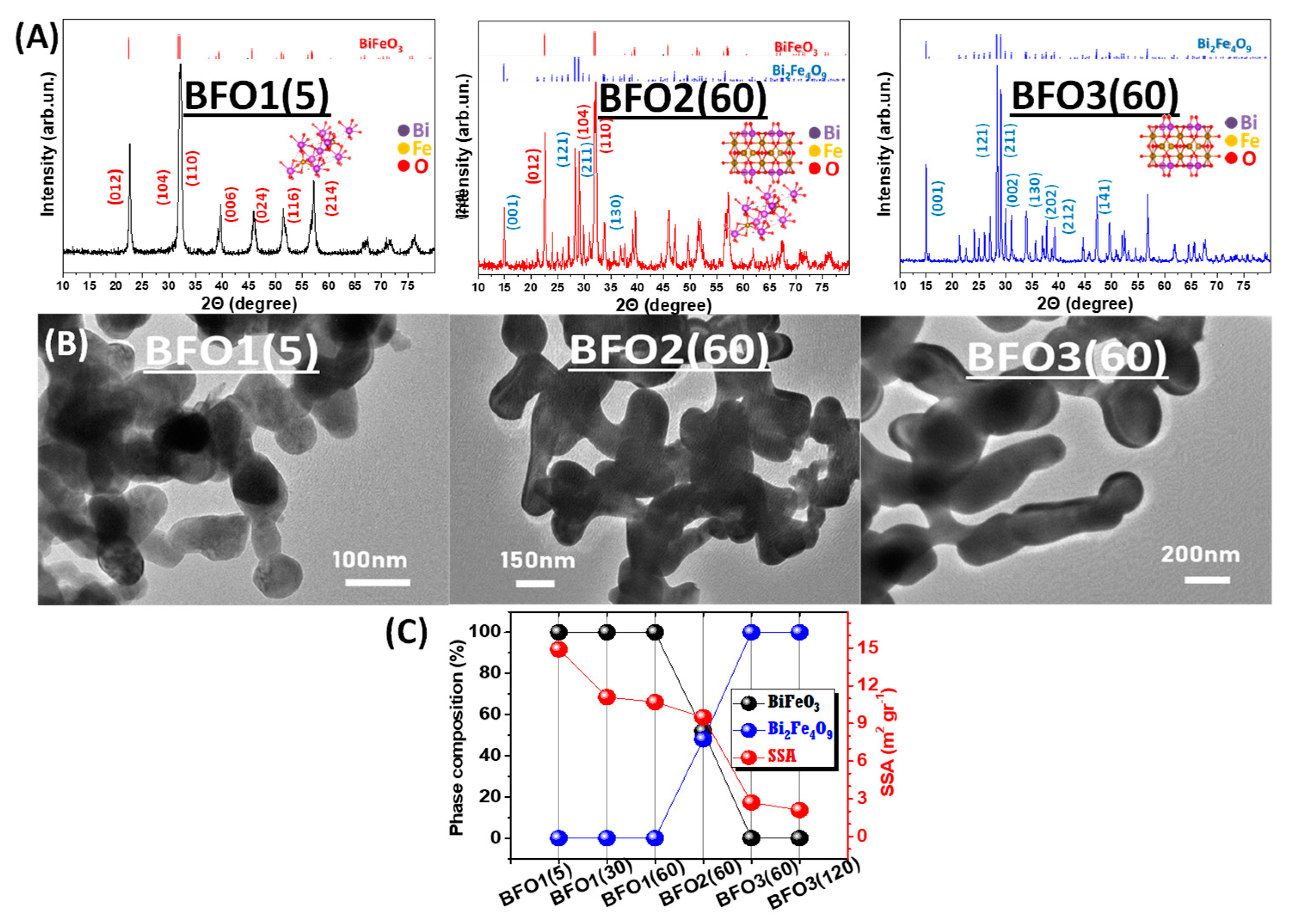
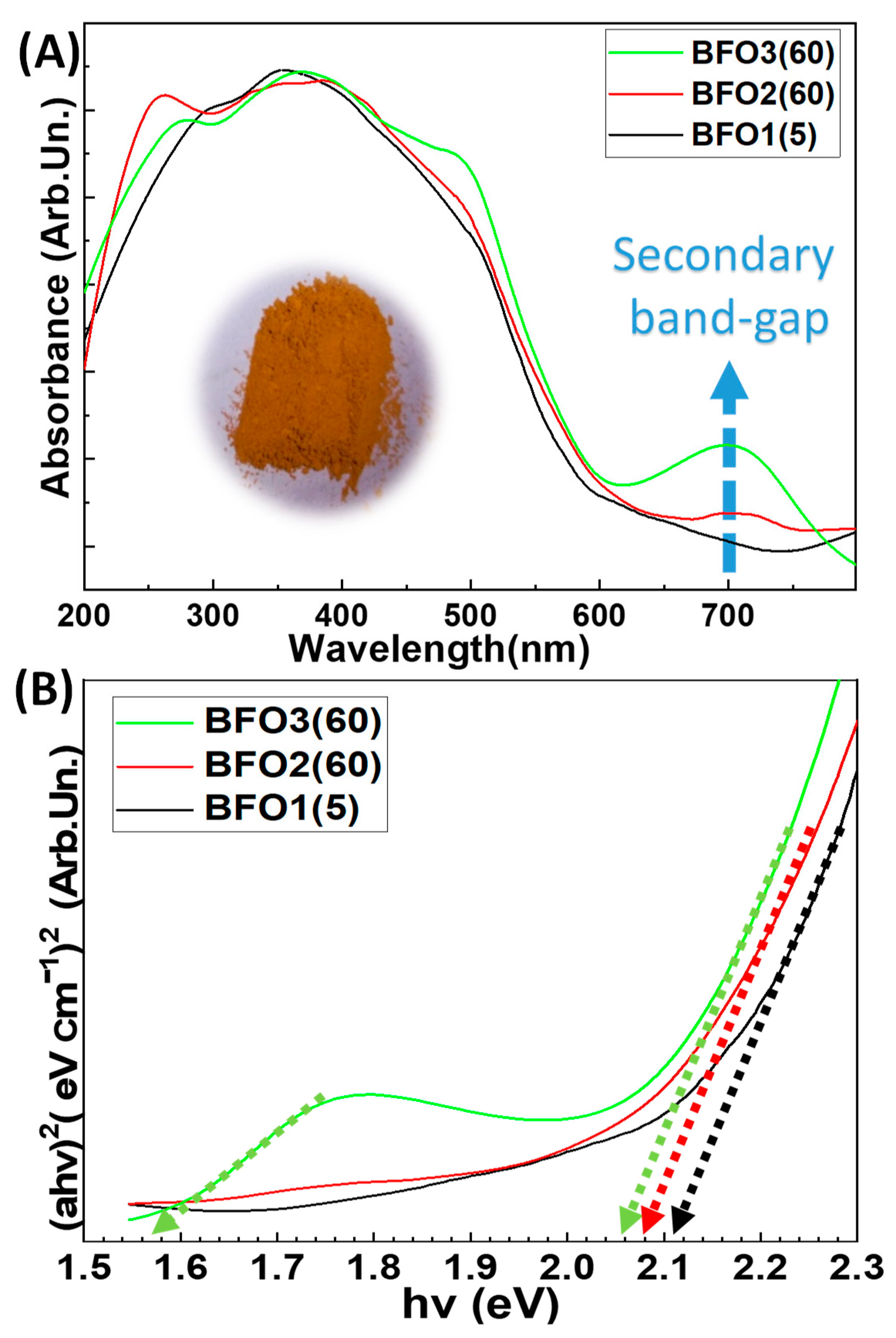

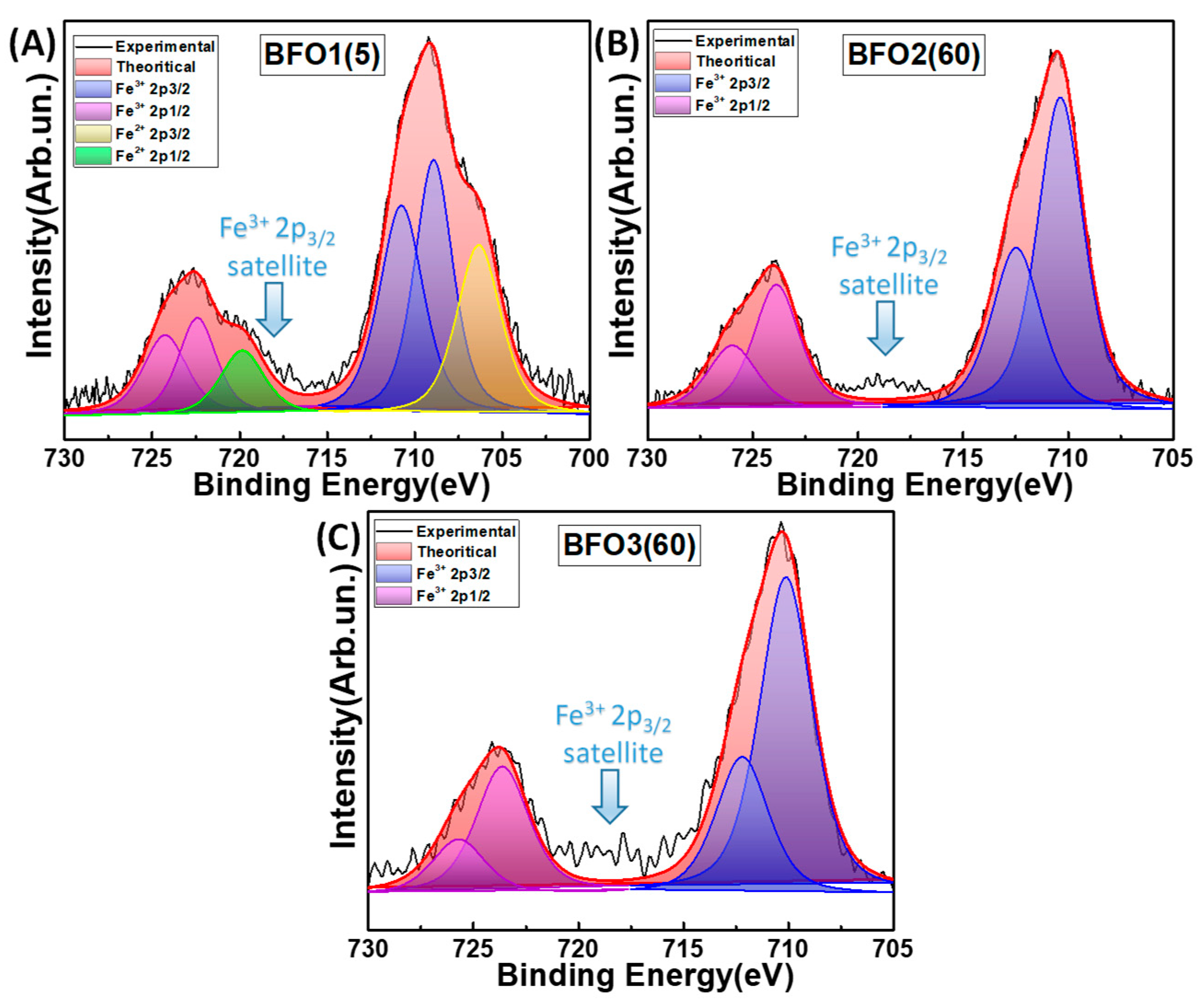
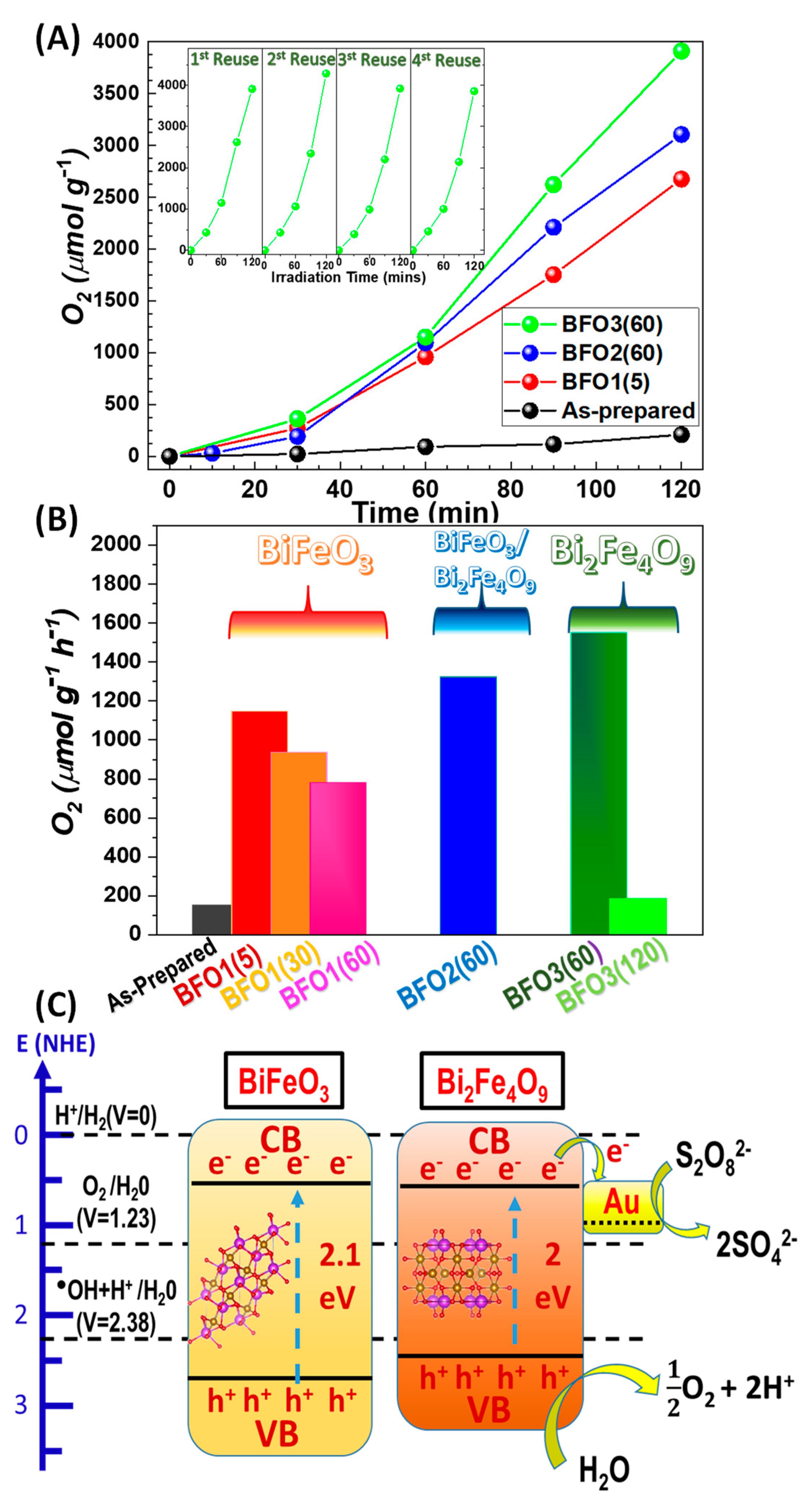
| Photocatalyst | Annealing T/Time (℃)/(Min) | %Crystalline Phase Fraction | Crystallite Size (nm) | SSA (m2 g−1) | Total Pore Volume (cm3 g−1) | Band Gap Eg (eV) | ||
|---|---|---|---|---|---|---|---|---|
| BiFeO3 | Bi2Fe4O9 | dBET | dTEM | |||||
| BFO1(5) | 550/5 | 100 | 0 | 48 | 55 | 14.1 | 4.01 × 10−2 | 2.1 |
| BFO1(30) | 550/30 | 100 | 0 | 64 | - | 12.1 | 2.83 × 10−2 | 2.1 |
| BFO1(60) | 550/60 | 100 | 0 | 67 | - | 10.7 | 2.81 × 10−2 | 2.1 |
| BFO2(60) | 625/60 | 52 | 48 | 75 | 130 | 9.5 | 2.11 × 10−2 | 2.08 |
| BFO3(60) | 700/60 | 0 | 100 | 268 | 240 | 2.7 | 0.56 × 10−2 | 2.05/1.55 |
| BFO3(120) | 700/120 | 0 | 100 | 336 | - | 2.1 | 0.46 × 10−2 | 2.05/1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psathas, P.; Solakidou, M.; Mantzanis, A.; Deligiannakis, Y. Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting. Energies 2021, 14, 5235. https://doi.org/10.3390/en14175235
Psathas P, Solakidou M, Mantzanis A, Deligiannakis Y. Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting. Energies. 2021; 14(17):5235. https://doi.org/10.3390/en14175235
Chicago/Turabian StylePsathas, Pavlos, Maria Solakidou, Asterios Mantzanis, and Yiannis Deligiannakis. 2021. "Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting" Energies 14, no. 17: 5235. https://doi.org/10.3390/en14175235
APA StylePsathas, P., Solakidou, M., Mantzanis, A., & Deligiannakis, Y. (2021). Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting. Energies, 14(17), 5235. https://doi.org/10.3390/en14175235







