Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the NM0-TiO2 Nanohybrids
2.3. Characterization of the Nanomaterials
3. Results and Discussion
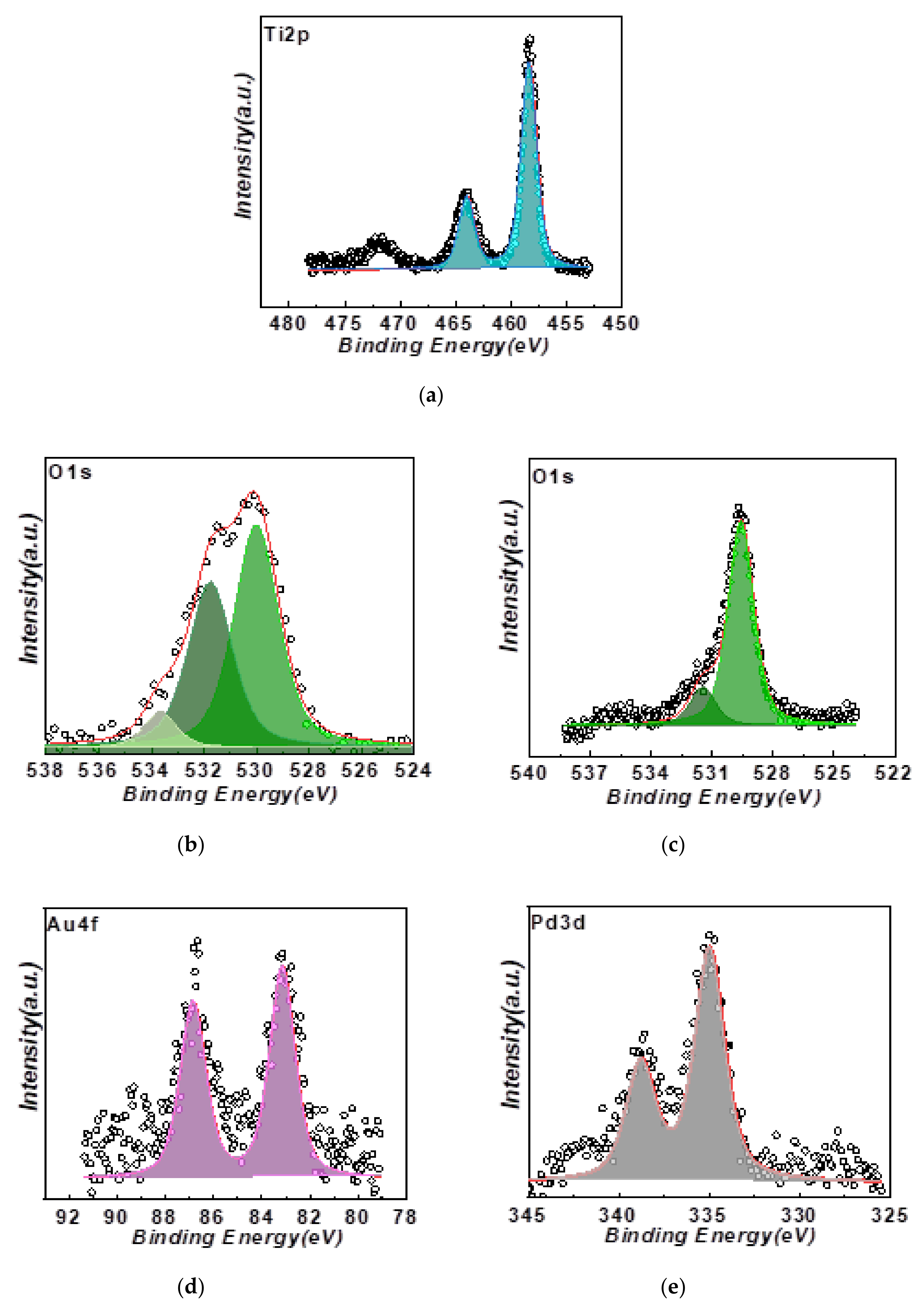
3.1. Characterization of the FSP-Made TiO2-Based Photocatalysts
- (a)
- Eg(Prisitne_TiO2) > Eg(TiO2)ON > Eg(TiO2)DN
- (b)
- Eg(Au/TiO2)ON >Eg(Ag/TiO2)ON > Eg(Pt/TiO2)ON > Eg(Pd/TiO2)ON
- (c)
- Eg(Au/TiO2)DN > Eg(Ag/TiO2)DN > Eg(Pt/TiO2)DN > Eg(Pd/TiO2)DN
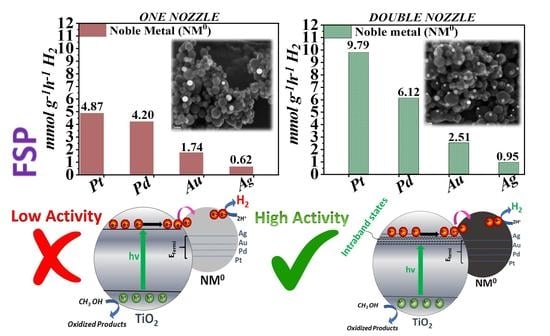
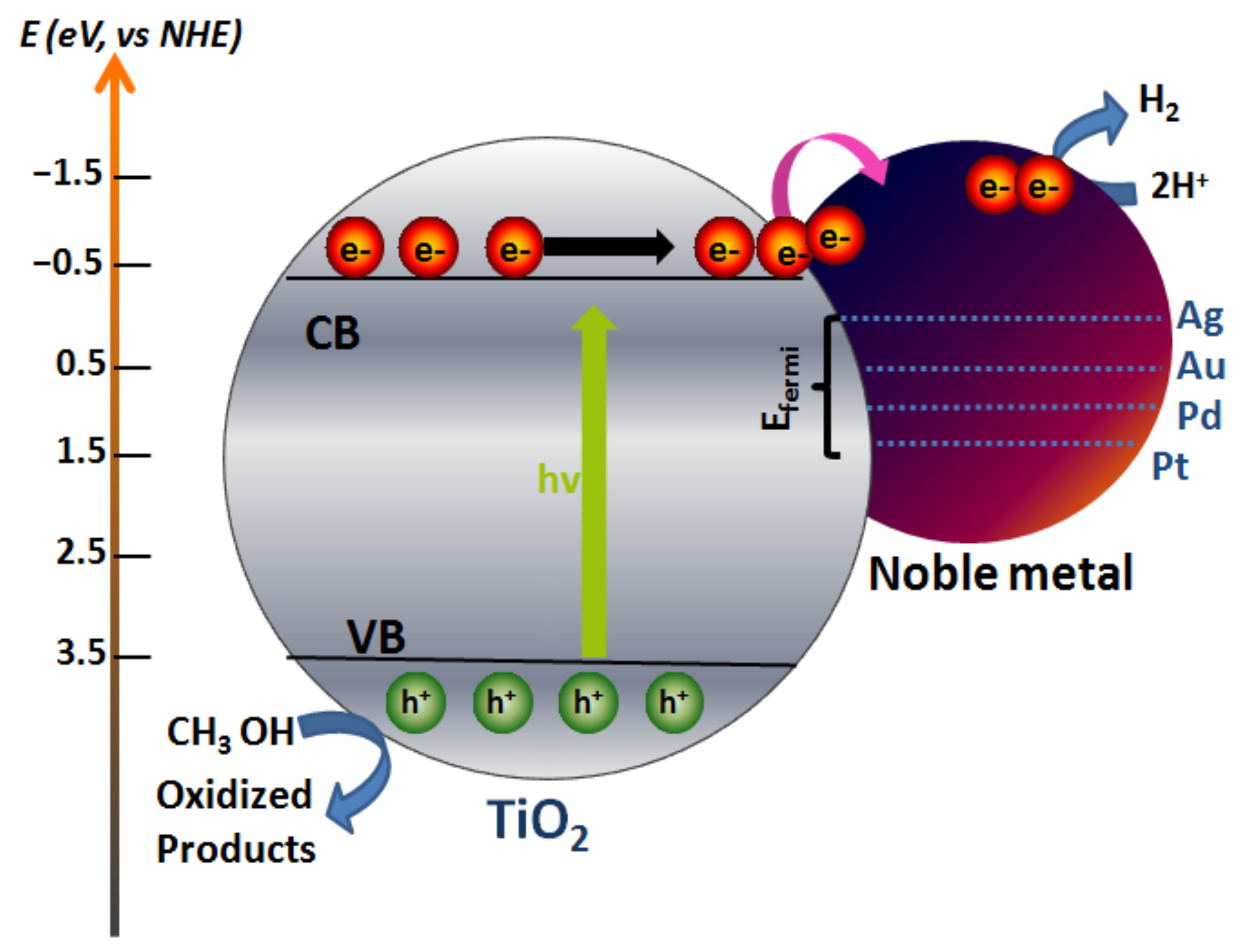
3.2. Photocatalytic Hydrogen Production of NM0-TiO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Li, C.; Domen, K. Recent Developments in Heterogeneous Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Tong, R.; Ng, K.W.; Wang, X.; Wang, S.; Wang, X.; Pan, H. Two-Dimensional Materials as Novel Co-Catalysts for Efficient Solar-Driven Hydrogen Production. J. Mater. Chem. A 2020, 8, 23202–23230. [Google Scholar] [CrossRef]
- Zu, M.; Zhou, X.; Zhang, S.; Qian, S.; Li, D.-S.; Liu, X.; Zhang, S. Sustainable Engineering of TiO2-Based Advanced Oxidation Technologies: From Photocatalyst to Application Devices. J. Mater. Sci. Technol. 2021, 78, 202–222. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A Review on H2 Production through Photocatalytic Reactions Using TiO2/TiO2-Assisted Catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering Charge Kinetics in Photocatalysis: Intersection of Materials Syntheses, Characterization Techniques and Theoretical Simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Halas, S.; Durakiewicz, T. Work Functions of Elements Expressed in Terms of the Fermi Energy and the Density of Free Electrons. J. Phys. Condens. Matter 1998, 10, 10815–10826. [Google Scholar] [CrossRef]
- Sytwu, K.; Vadai, M.; Dionne, J.A. Bimetallic Nanostructures: Combining Plasmonic and Catalytic Metals for Photocatalysis. Adv. Phys. X 2019, 4, 1619480. [Google Scholar] [CrossRef]
- Kamat, P.V.; Hartland, G.V. Plasmons for Energy Conversion. ACS Energy Lett. 2018, 3, 1467–1469. [Google Scholar] [CrossRef]
- Yi, S.-S.; Zhang, X.-B.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Non-Noble Metals Applied to Solar Water Splitting. Energy Environ. Sci. 2018, 11, 3128–3156. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Louloudi, M.; Deligiannakis, Y. From Homogeneous to Heterogenized Molecular Catalysts for H2 Production by Formic Acid Dehydrogenation: Mechanistic Aspects, Role of Additives, and Co-Catalysts. Energies 2020, 13, 733. [Google Scholar] [CrossRef]
- Méndez, F.J.; González-Millán, A.; García-Macedo, J.A. Surface Modification of Titanium Oxide as Efficient Support of Metal Nanoparticles for Hydrogen Production via Water Splitting. Mater. Chem. Phys. 2019, 232, 331–338. [Google Scholar] [CrossRef]
- Rosseler, O.; Shankar, M.V.; Du, M.K.-L.; Schmidlin, L.; Keller, N.; Keller, V. Solar Light Photocatalytic Hydrogen Production from Water over Pt and Au/TiO2(Anatase/Rutile) Photocatalysts: Influence of Noble Metal and Porogen Promotion. J. Catal. 2010, 269, 179–190. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Fernández-González, R.; Díaz, L.; Pulido Melián, E.; Rodríguez, V.D.; Núñez, P. Effect of Reaction Temperature and Sacrificial Agent on the Photocatalytic H2-Production of Pt-TiO2. J. Alloy. Compd. 2017, 721, 405–410. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The Roles of Metal Co-Catalysts and Reaction Media in Photocatalytic Hydrogen Production: Performance Evaluation of M/TiO2 Photocatalysts (M = Pd, Pt, Au) in Different Alcohol–Water Mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Ge, M.-Z.; Cao, C.-Y.; Li, S.-H.; Tang, Y.-X.; Wang, L.-N.; Qi, N.; Huang, J.-Y.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y.-K. In Situ Plasmonic Ag Nanoparticle Anchored TiO2 Nanotube Arrays as Visible-Light-Driven Photocatalysts for Enhanced Water Splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef]
- Pokhrel, S.; Mädler, L. Flame-Made Particles for Sensors, Catalysis, and Energy Storage Applications. Energy Fuels 2020, 34, 13209–13224. [Google Scholar] [CrossRef]
- Pelletier, F.; Thiébaut, B. Improvement of Noble Metal Based Photocatalysts by Spray Pyrolysis Processes. Johns. Matthey Technol. Rev. 2016, 60, 39–54. [Google Scholar] [CrossRef]
- Wang, N.; Niu, F.; Wang, S.; Huang, Y. Catalytic Activity of Flame-Synthesized Pd/TiO2 for the Methane Oxidation Following Hydrogen Pretreatments. Particuology 2018, 41, 58–64. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, Y.; Jiang, H.; Yu, J.; Jiang, R.; Li, C. Engineering TiO2 Supported Pt Sub-Nanoclusters via Introducing Variable Valence Co Ion in High-Temperature Flame for CO Oxidation. Nanoscale 2018, 10, 13384–13392. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Zhao, H. Flame Spray Pyrolysis Made Pt/TiO2 Photocatalysts with Ultralow Platinum Loading and High Hydrogen Production Activity. Proc. Combust. Inst. 2020, in press. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic Hydrogen Production over Flame Spray Pyrolysis-Synthesised TiO2 and Au/TiO2. Appl. Catal. B Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Scavini, M.; Grunwaldt, J.-D.; Selli, E. One Step Flame-Made Fluorinated Pt/TiO2 Photocatalysts for Hydrogen Production. Appl. Catal. B Environ. 2014, 160–161, 144–151. [Google Scholar] [CrossRef]
- Bernareggi, M.; Dozzi, M.; Bettini, L.; Ferretti, A.; Chiarello, G.; Selli, E. Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production. Catalysts 2017, 7, 301. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Tsikourkitoudi, V.; Stathi, P.; Wegner, K.; Papavasiliou, J.; Louloudi, M. PdO/Pd0/TiO2 Nanocatalysts Engineered by Flame Spray Pyrolysis: Study of the Synergy of PdO/Pd0 on H2 Production by HCOOH Dehydrogenation and the Deactivation Mechanism. Energy Fuels 2020, 34, 15026–15038. [Google Scholar] [CrossRef]
- Strobel, R.; Mädler, L.; Piacentini, M.; Maciejewski, M.; Baiker, A.; Pratsinis, S.E. Two-Nozzle Flame Synthesis of Pt/Ba/Al2O3 for NOxStorage. Chem. Mater. 2006, 18, 2532–2537. [Google Scholar] [CrossRef]
- Minnermann, M.; Grossmann, H.K.; Pokhrel, S.; Thiel, K.; Hagelin-Weaver, H.; Bäumer, M.; Mädler, L. Double Flame Spray Pyrolysis as a Novel Technique to Synthesize Alumina-Supported Cobalt Fischer–Tropsch Catalysts. Catal. Today 2013, 214, 90–99. [Google Scholar] [CrossRef]
- Høj, M.; Pham, D.K.; Brorson, M.; Mädler, L.; Jensen, A.D.; Grunwaldt, J.-D. Two-Nozzle Flame Spray Pyrolysis (FSP) Synthesis of CoMo/Al2O3 Hydrotreating Catalysts. Catal. Lett. 2013, 143, 386–394. [Google Scholar] [CrossRef]
- Tepluchin, M.; Pham, D.K.; Casapu, M.; Mädler, L.; Kureti, S.; Grunwaldt, J.-D. Influence of Single- and Double-Flame Spray Pyrolysis on the Structure of MnOx/γ-Al2O3 and FeOx/γ-Al2O3 Catalysts and Their Behaviour in CO Removal under Lean Exhaust Gas Conditions. Catal. Sci. Technol. 2015, 5, 455–464. [Google Scholar] [CrossRef]
- Horlyck, J.; Pokhrel, S.; Lovell, E.; Bedford, N.M.; Mädler, L.; Amal, R.; Scott, J. Unifying Double Flame Spray Pyrolysis with Lanthanum Doping to Restrict Cobalt–Aluminate Formation in Co/Al2O3 Catalysts for the Dry Reforming of Methane. Catal. Sci. Technol. 2019, 9, 4970–4980. [Google Scholar] [CrossRef]
- Lovell, E.C.; Großman, H.; Horlyck, J.; Scott, J.; Mädler, L.; Amal, R. Asymmetrical Double Flame Spray Pyrolysis-Designed SiO2/Ce0.7Zr0.3O2 for the Dry Reforming of Methane. ACS Appl. Mater. Interfaces 2019, 11, 25766–25777. [Google Scholar] [CrossRef]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and Their Evaluation as Non-Noble Metal Catalysts for Efficient Reduction of 4-Nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar] [CrossRef]
- Fragou, F.; Moularas, C.; Adamska, K.; Deligiannakis, Y.; Louloudi, M. Mn(II)-Based Catalysts Supported on Nanocarbon-Coated Silica Nanoparticles for Alkene Epoxidation. ACS Appl. Nano Mater. 2020, 3, 5583–5592. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Schevciw, O.; White, W.B. The Optical Absorption Edge of Rare Earth Sesquisulfides and Alkaline Earth—Rare Earth Sulfides. Mater. Res. Bull. 1983, 18, 1059–1068. [Google Scholar] [CrossRef]
- Moularas, C.; Georgiou, Y.; Adamska, K.; Deligiannakis, Y. Thermoplasmonic Heat Generation Efficiency by Nonmonodisperse Core–Shell Ag0@ SiO2 Nanoparticle Ensemble. J. Phys. Chem. C 2019, 123, 22499–22510. [Google Scholar] [CrossRef]
- Georgiou, Y.; Rapti, S.; Mavrogiorgou, A.; Armatas, G.; Manos, M.J.; Louloudi, M.; Deligiannakis, Y. A Hybrid {Silk@Zirconium MOF} Material as Highly Efficient AsIII-Sponge. Sci. Rep. 2020, 10, 9358. [Google Scholar] [CrossRef] [PubMed]
- Solakidou, M.; Giannakas, A.; Georgiou, Y.; Boukos, N.; Louloudi, M.; Deligiannakis, Y. Efficient Photocatalytic Water-Splitting Performance by Ternary CdS/Pt-N-TiO2 and CdS/Pt-N, F-TiO2: Interplay between CdS Photo Corrosion and TiO2-Dopping. Appl. Catal. B Environ. 2019, 254, 194–205. [Google Scholar] [CrossRef]
- Theodorakopoulos, M.; Solakidou, M.; Deligiannakis, Y.; Louloudi, M. A Use-Store-Reuse (USR) Concept in Catalytic HCOOH Dehydrogenation: Case-Study of a Ru-Based Catalytic System for Long-Term USR under Ambient O2. Energies 2021, 14, 481. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Togawa, Y.; Tsukamoto, D.; Tanaka, S.; Hirai, T. Highly Efficient and Selective Hydrogenation of Nitroaromatics on Photoactivated Rutile Titanium Dioxide. ACS Catal. 2012, 2, 2475–2481. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic Gold Nanocrystals Coupled with Photonic Crystal Seamlessly on TiO2 Nanotube Photoelectrodes for Efficient Visible Light Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 14–20. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Dai, H.; Deng, J.; Zang, H.; Arandiyan, H.; Xie, S.; Yang, H. 3DOM BiVO4 Supported Silver Bromide and Noble Metals: High-Performance Photocatalysts for the Visible-Light-Driven Degradation of 4-Chlorophenol. Appl. Catal. B Environ. 2015, 168–169, 274–282. [Google Scholar] [CrossRef]
- Chala, S.; Wetchakun, K.; Phanichphant, S.; Inceesungvorn, B.; Wetchakun, N. Enhanced Visible-Light-Response Photocatalytic Degradation of Methylene Blue on Fe-Loaded BiVO4 Photocatalyst. J. Alloys Compd. 2014, 597, 129–135. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bottaro, G.; Gross, S.; Gasparotto, A.; Maragno, C.; Tondello, E.; Zattin, A. Introduction to XPS Studies of Metal and Metal-Oxide Nanosystems. Surf. Sci. Spectra 2003, 10, 137–142. [Google Scholar] [CrossRef]
- Lv, K.; Zuo, H.; Sun, J.; Deng, K.; Liu, S.; Li, X.; Wang, D. (Bi, C and N) Codoped TiO2 Nanoparticles. J. Hazard. Mater. 2009, 161, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. Noble Metal Modified TiO2 Microspheres: Surface Properties and Photocatalytic Activity under UV–Vis and Visible Light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Wu, J.C.-S.; Chen, C.-H. A Visible-Light Response Vanadium-Doped Titania Nanocatalyst by Sol–Gel Method. J. Photochem. Photobiol. A Chem. 2004, 163, 509–515. [Google Scholar] [CrossRef]
- Rahulan, K.M.; Ganesan, S.; Aruna, P. Synthesis and Optical Limiting Studies of Au-Doped TiO2 Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 025012. [Google Scholar] [CrossRef]
- Galińska, A.; Walendziewski, J. Photocatalytic Water Splitting over Pt−TiO2 in the Presence of Sacrificial Reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Solakidou, M.; Deligiannakis, Y.; Louloudi, M. Heterogeneous Amino-Functionalized Particles Boost Hydrogen Production from Formic Acid by a Ruthenium Complex. Int. J. Hydrogen Energy 2018, 43, 21386–21397. [Google Scholar] [CrossRef]
- Solakidou, M.; Theodorakopoulos, M.; Deligiannakis, Y.; Louloudi, M. Double-Ligand Fe, Ru Catalysts: A Novel Route for Enhanced H2 Production from Formic Acid. Int. J. Hydrogen Energy 2020, 45, 17367–17377. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic Reforming of Biomass: A Systematic Study of Hydrogen Evolution from Glucose Solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen Production by Photocatalytic Steam Reforming of Methanol on Noble Metal-Modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Kennedy, J.; Morgan, D.J. The Importance of Metal Reducibility for the Photo-Reforming of Methanol on Transition Metal-TiO2 Photocatalysts and the Use of Non-Precious Metals. Int. J. Hydrogen Energy 2015, 40, 1465–1471. [Google Scholar] [CrossRef]
- Al-Mazroai, L.S.; Bowker, M.; Davies, P.; Dickinson, A.; Greaves, J.; James, D.; Millard, L. The Photocatalytic Reforming of Methanol. Catal. Today 2007, 122, 46–50. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, X.; Zhang, L.; Meng, S.; Chen, S. Photocatalytic Reforming of Glycerol for H2 Evolution on Pt/TiO2: Fundamental Understanding the Effect of Co-Catalyst Pt and the Pt Deposition Route. J. Mater. Chem. A 2015, 3, 2271–2282. [Google Scholar] [CrossRef]

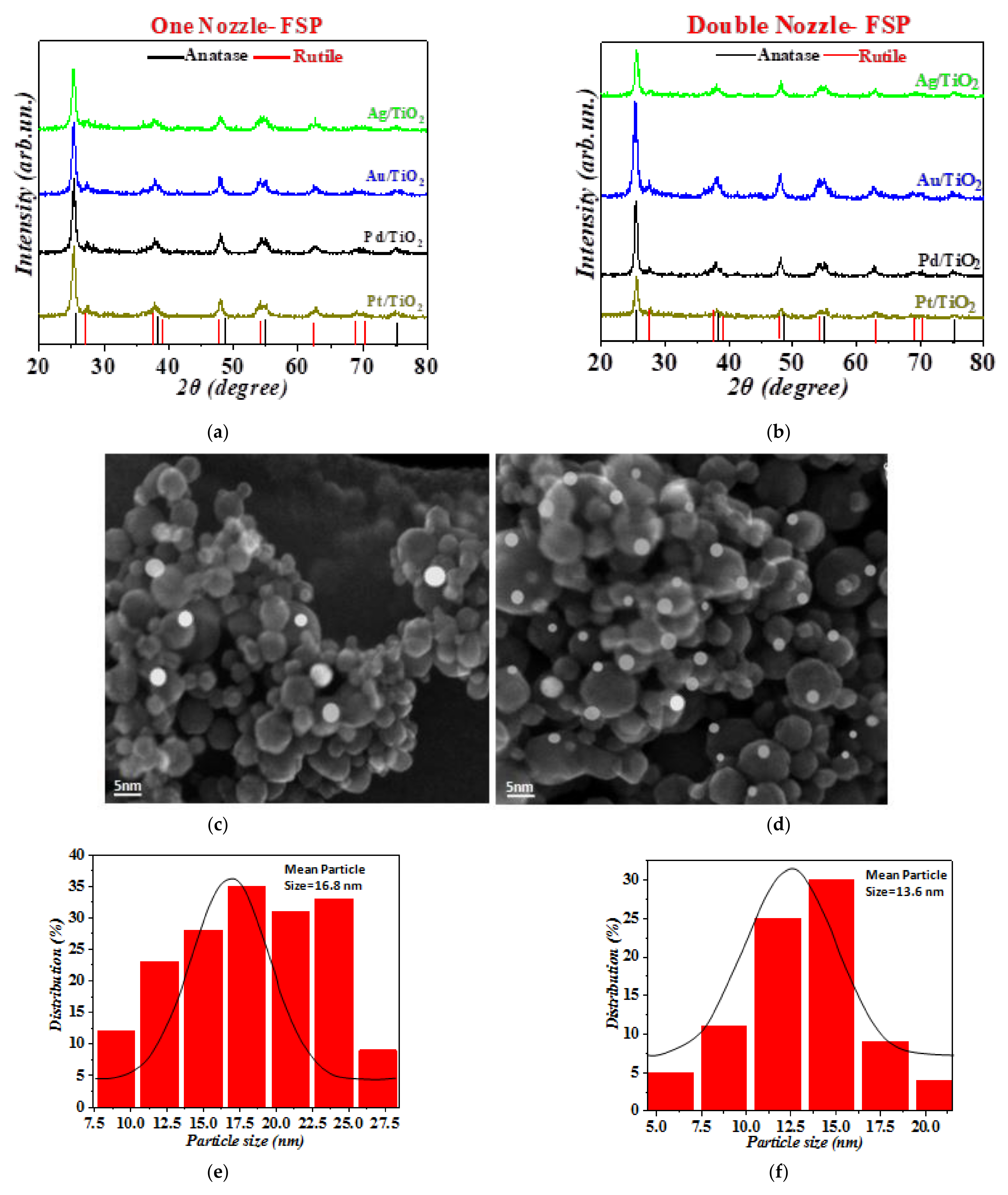
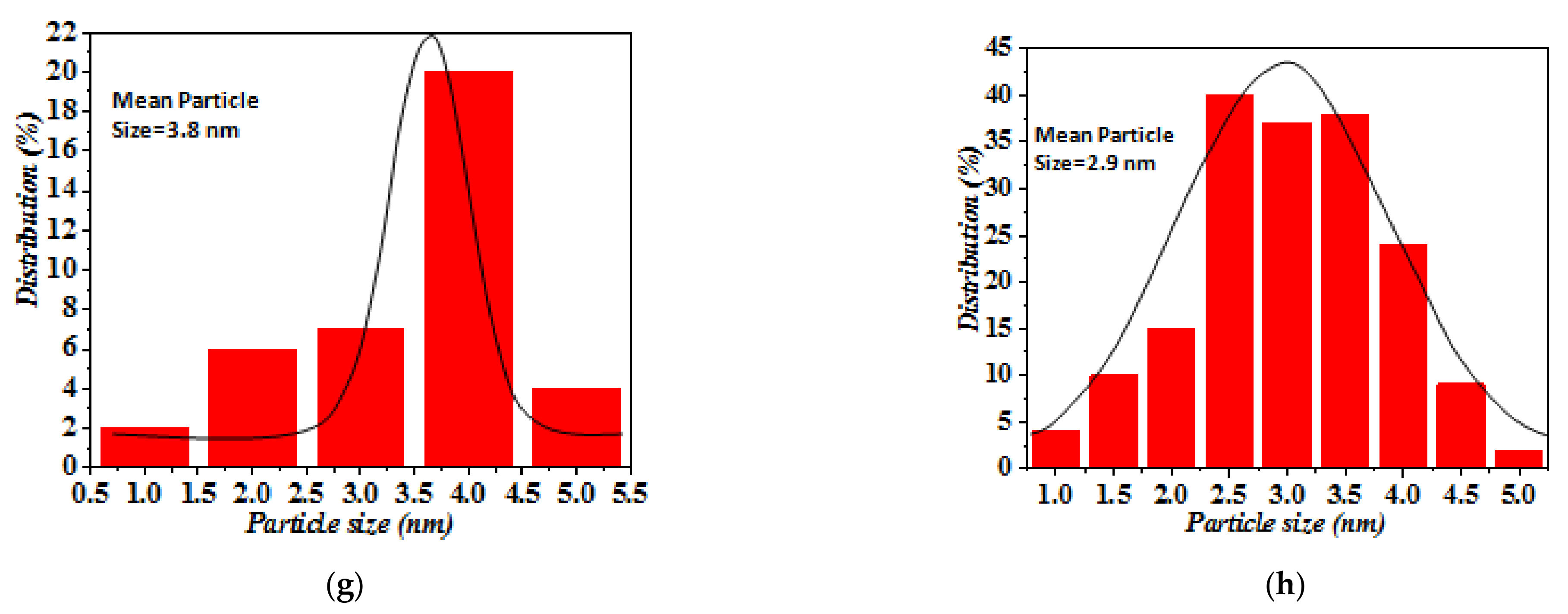


| Material | One-Nozzle FSP | Double-Nozzle FSP | ||||||
|---|---|---|---|---|---|---|---|---|
| dXRD (nm) | dBET (nm) | dTEM (NM0/TiO2) (nm) | SSA (m2 g−1) | dXRD (nm) | dTEM (NM0/TiO2) (nm) | dBET (nm) | SSA (m2 g−1) | |
| TiO2 | 19.7 | 21.4 | -/23.5 | 72.0 | 19.7 | -/21.4 | 21.4 | 72.0 |
| Pt/TiO2 | 14.3 | 15.5 | 3.8/16.8 | 99.2 | 11.2 | 2.9/13.6 | 12.3 | 110.5 |
| Pd/TiO2 | 14.5 | 15.7 | 3.5/17.1 | 97.8 | 12.5 | 3.1/13.5 | 13.2 | 100.1 |
| Au/TiO2 | 13.1 | 14.2 | 4.9/13.8 | 108.3 | 11.1 | 4.4/12.6 | 12.8 | 120.5 |
| Ag/TiO2 | 16.5 | 17.9 | 3.1/14.5 | 86.0 | 10.2 | 2.5/11.7 | 12.5 | 199.8 |
| Material | One-Nozzle FSP | Double-Nozzle FSP |
|---|---|---|
| TiO2 | 2.90 | 2.83 |
| Pt-TiO2 | 2.78 | 2.53 |
| Pd-TiO2 | 2.73 | 2.16 |
| Au-TiO2 | 2.94 | 2.68 |
| Ag-TiO2 | 2.83 | 2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solakidou, M.; Georgiou, Y.; Deligiannakis, Y. Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production. Energies 2021, 14, 817. https://doi.org/10.3390/en14040817
Solakidou M, Georgiou Y, Deligiannakis Y. Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production. Energies. 2021; 14(4):817. https://doi.org/10.3390/en14040817
Chicago/Turabian StyleSolakidou, Maria, Yiannis Georgiou, and Yiannis Deligiannakis. 2021. "Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production" Energies 14, no. 4: 817. https://doi.org/10.3390/en14040817
APA StyleSolakidou, M., Georgiou, Y., & Deligiannakis, Y. (2021). Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production. Energies, 14(4), 817. https://doi.org/10.3390/en14040817