Fuel Cell Electric Vehicle (FCEV) Energy Flow Analysis in Real Driving Conditions (RDC)
Abstract
:1. Introduction
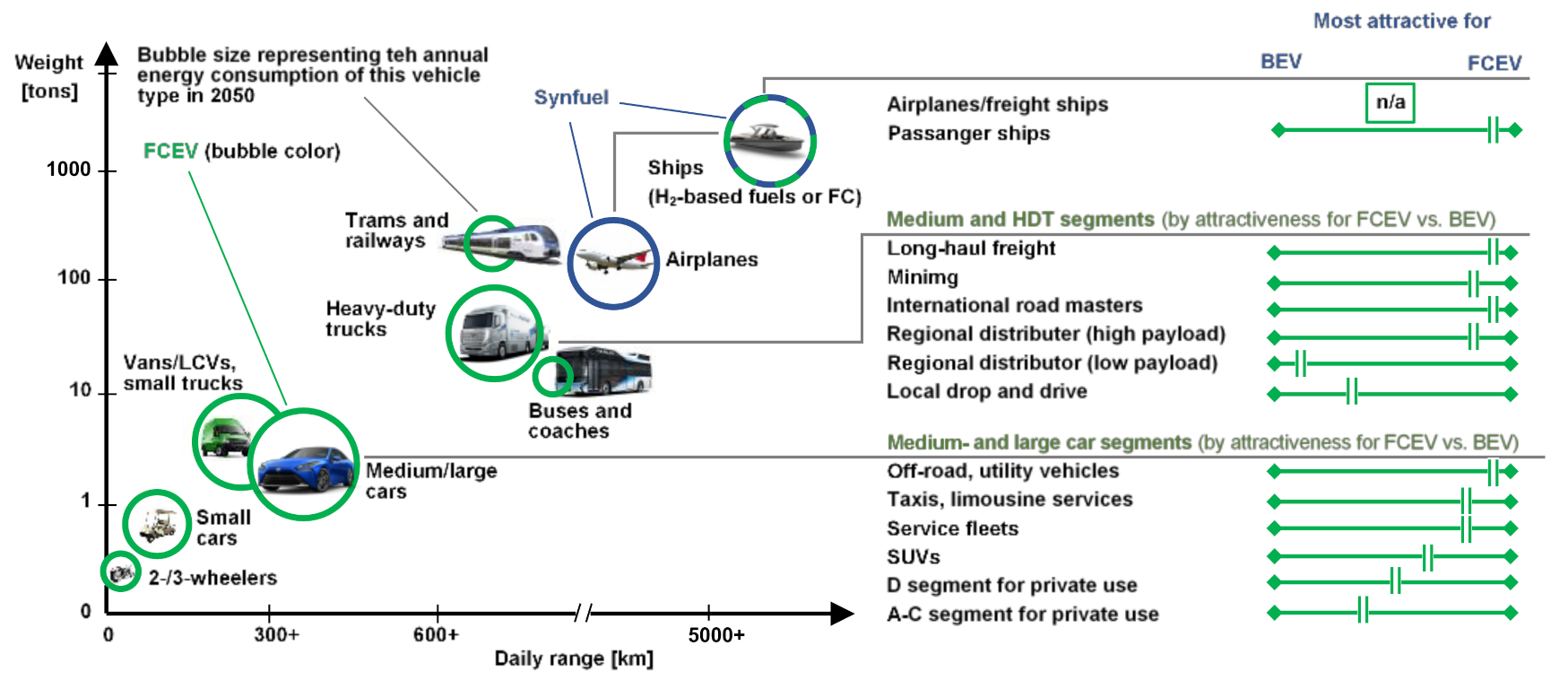
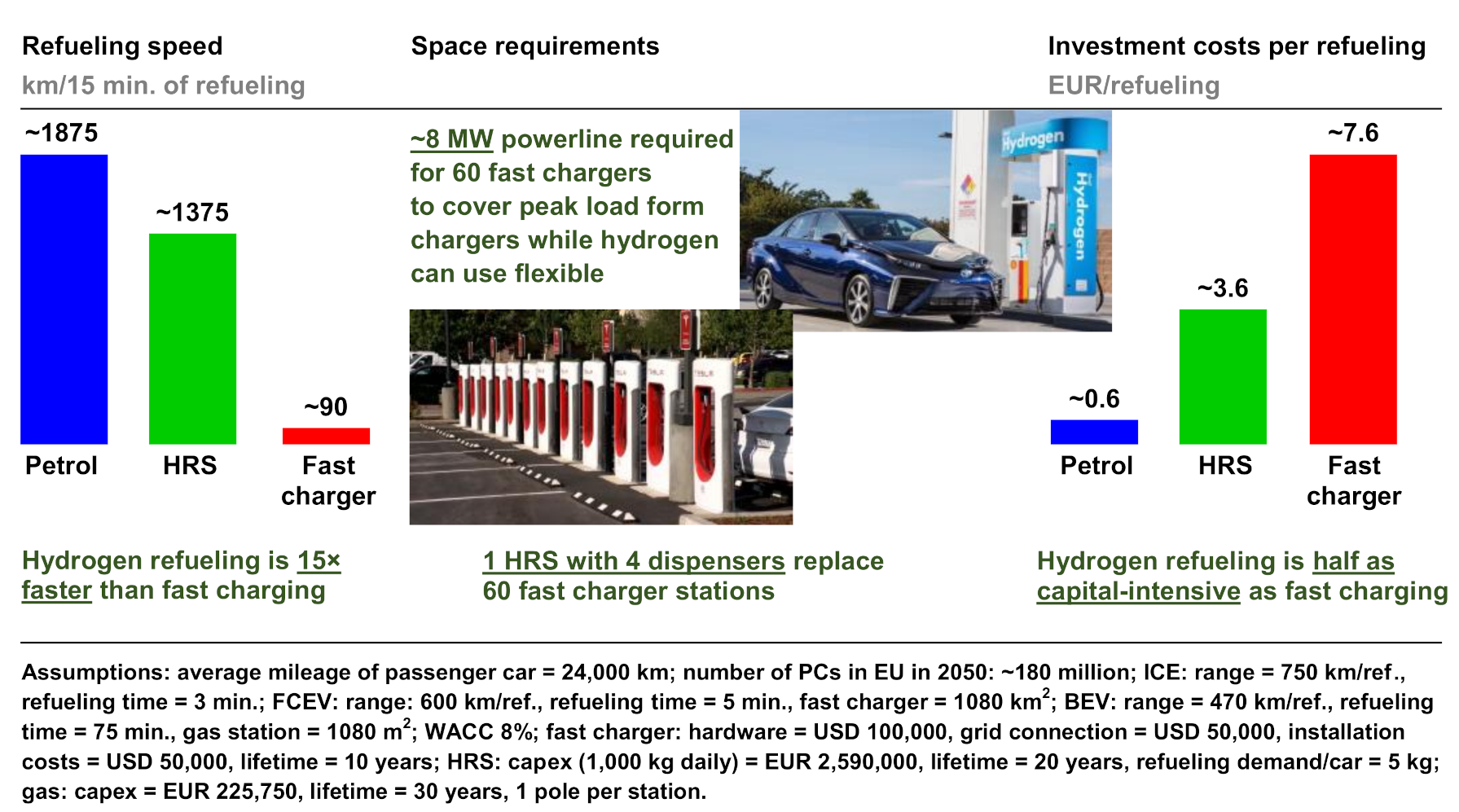
2. Analysis of Hydrogen Usage in Internal Combustion Engines and Fuel Cells
3. Materials and Methods
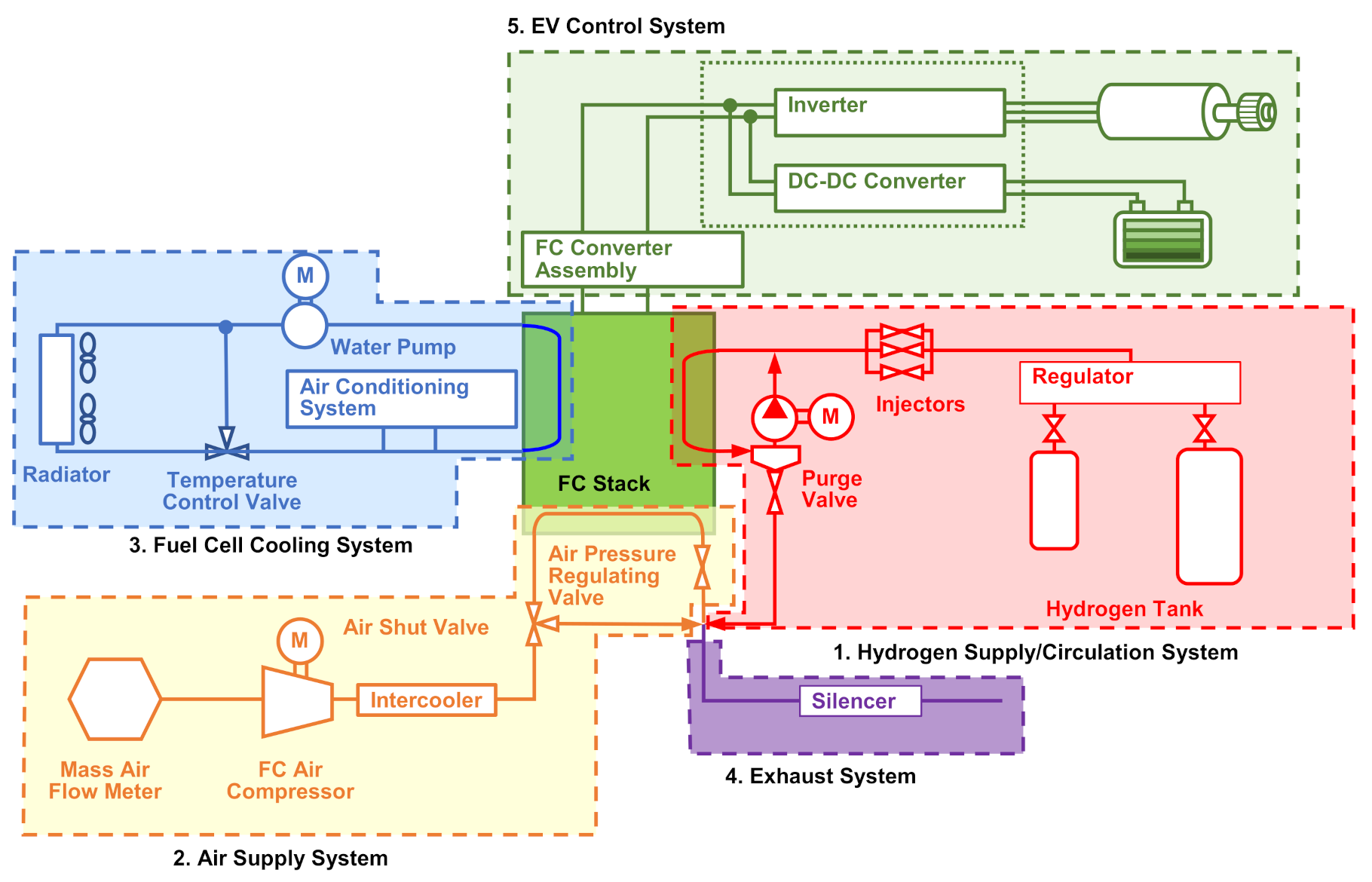
3.1. Research Objects
3.2. Research Equipment and Methodology of Determination of the Energy Flow
- energy flow (urban, rural, motorway):
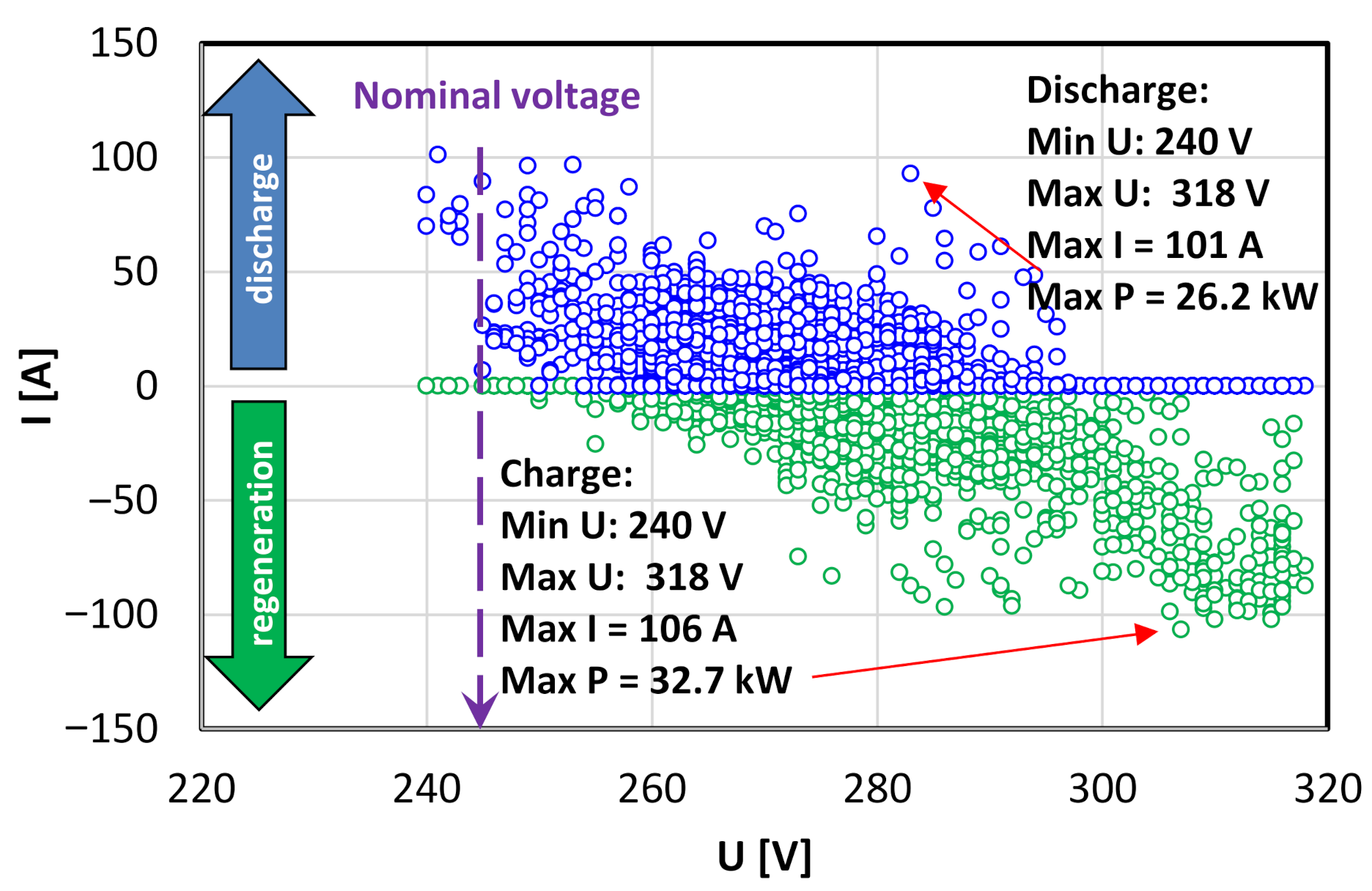
- discharging (urban, rural, motorway):
- charging (urban, rural, motorway):
- regenerative braking (urban, rural, motorway):
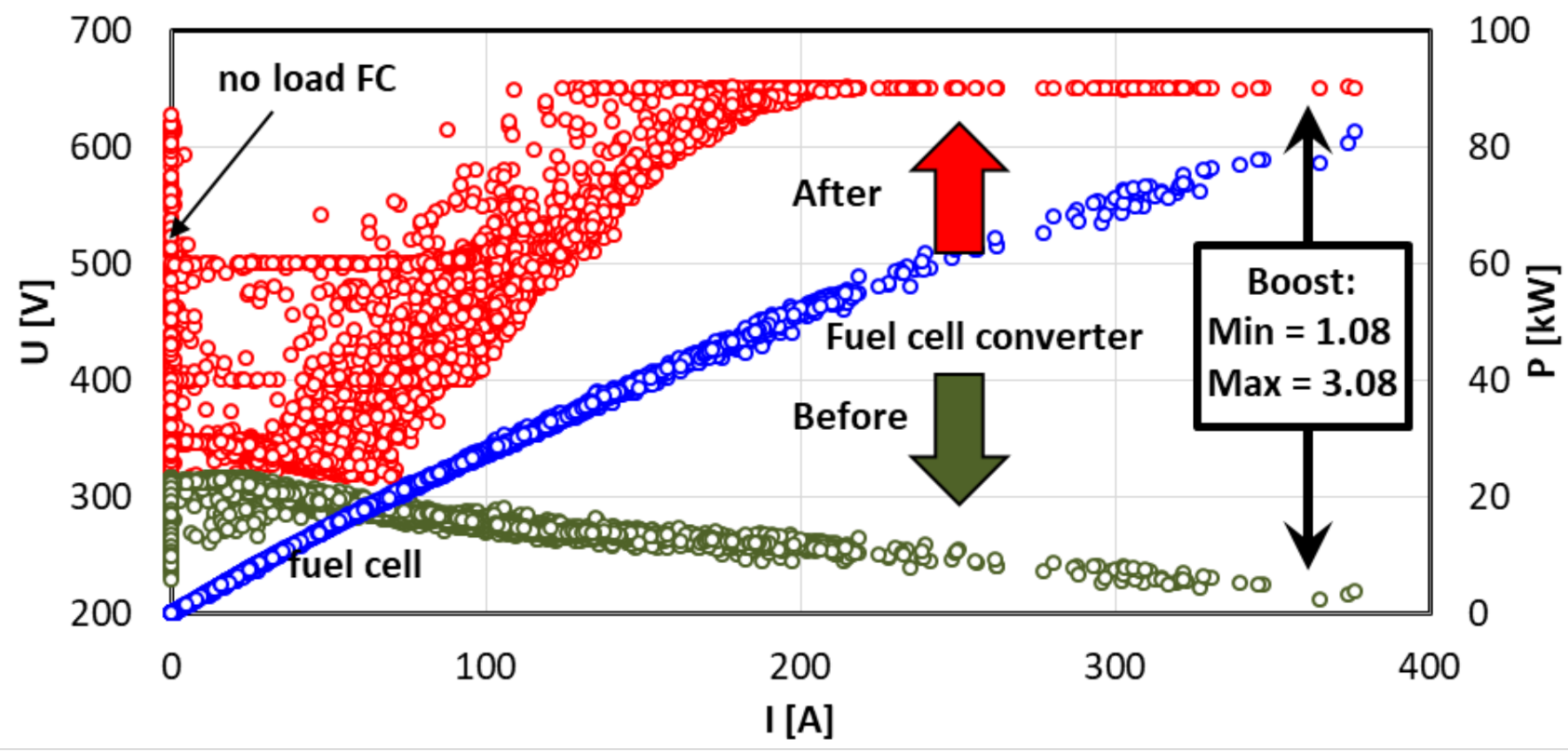
- boost value (urban, rural, motorway):
4. Results
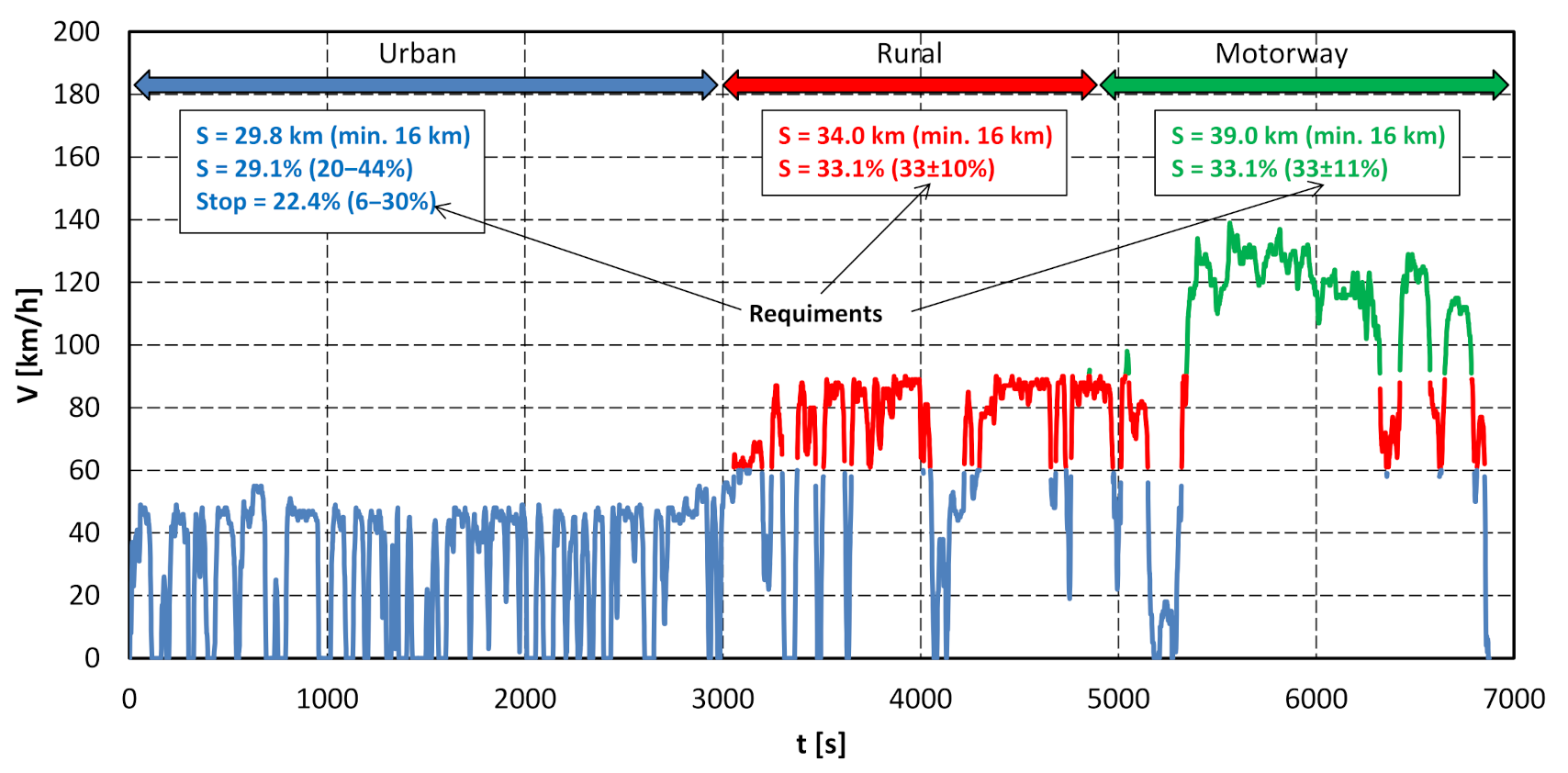
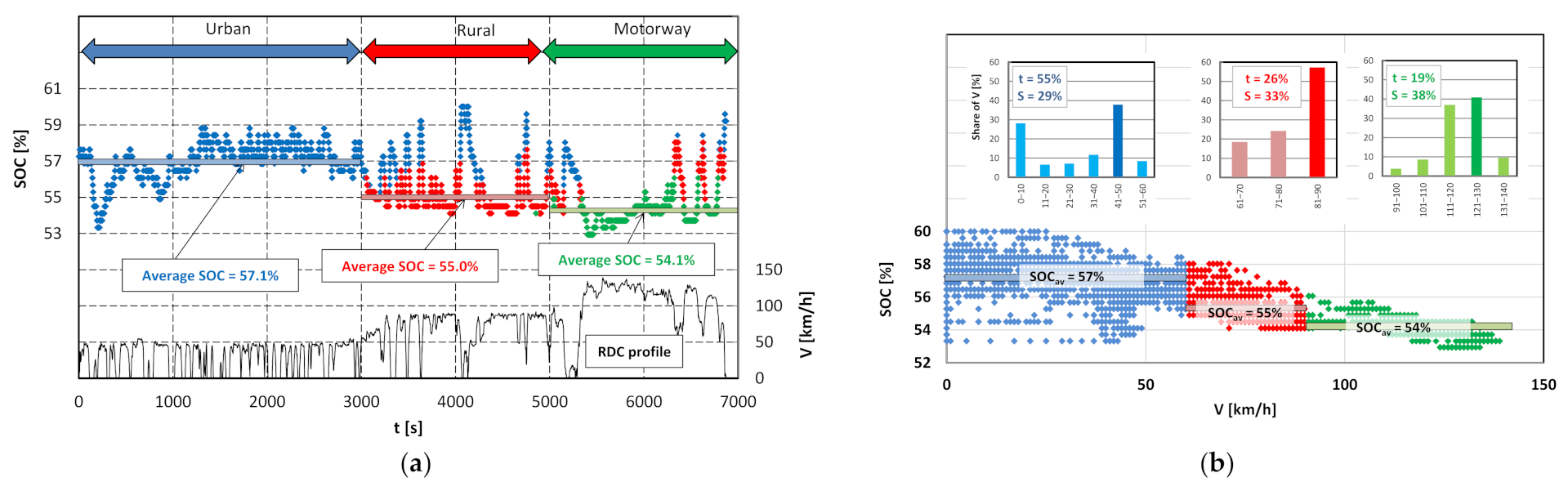
4.1. Driving Test Evaluation
4.2. State of Charge (SOC) Change Analysis
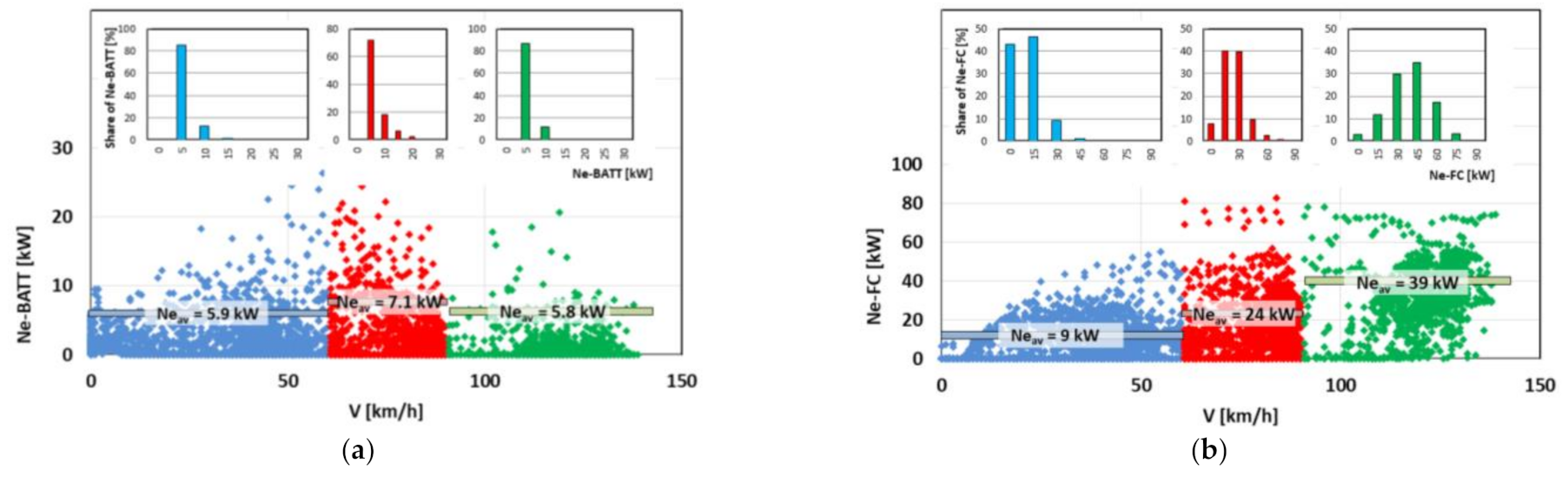
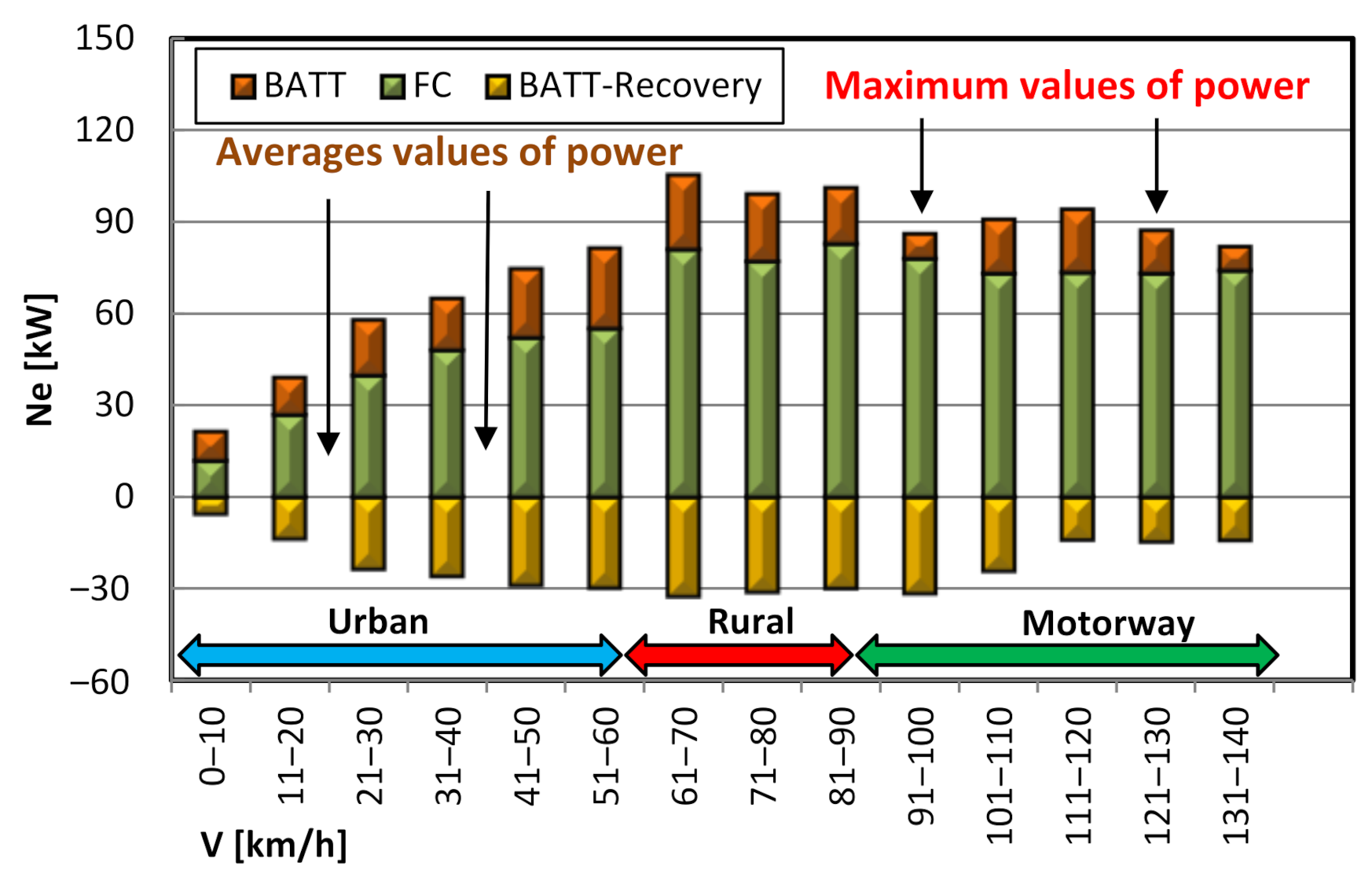
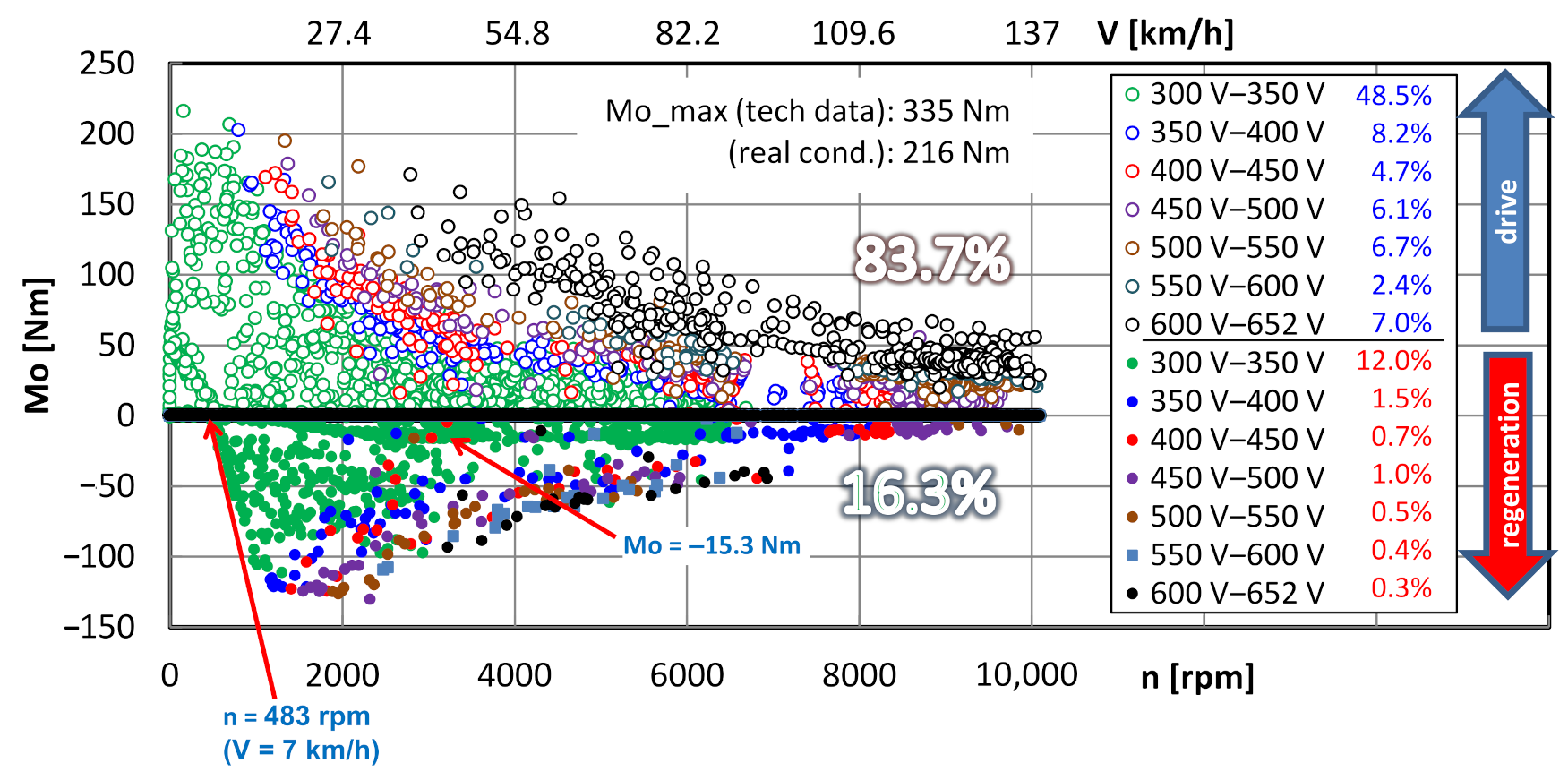
4.3. Powertrain Performance Evaluation
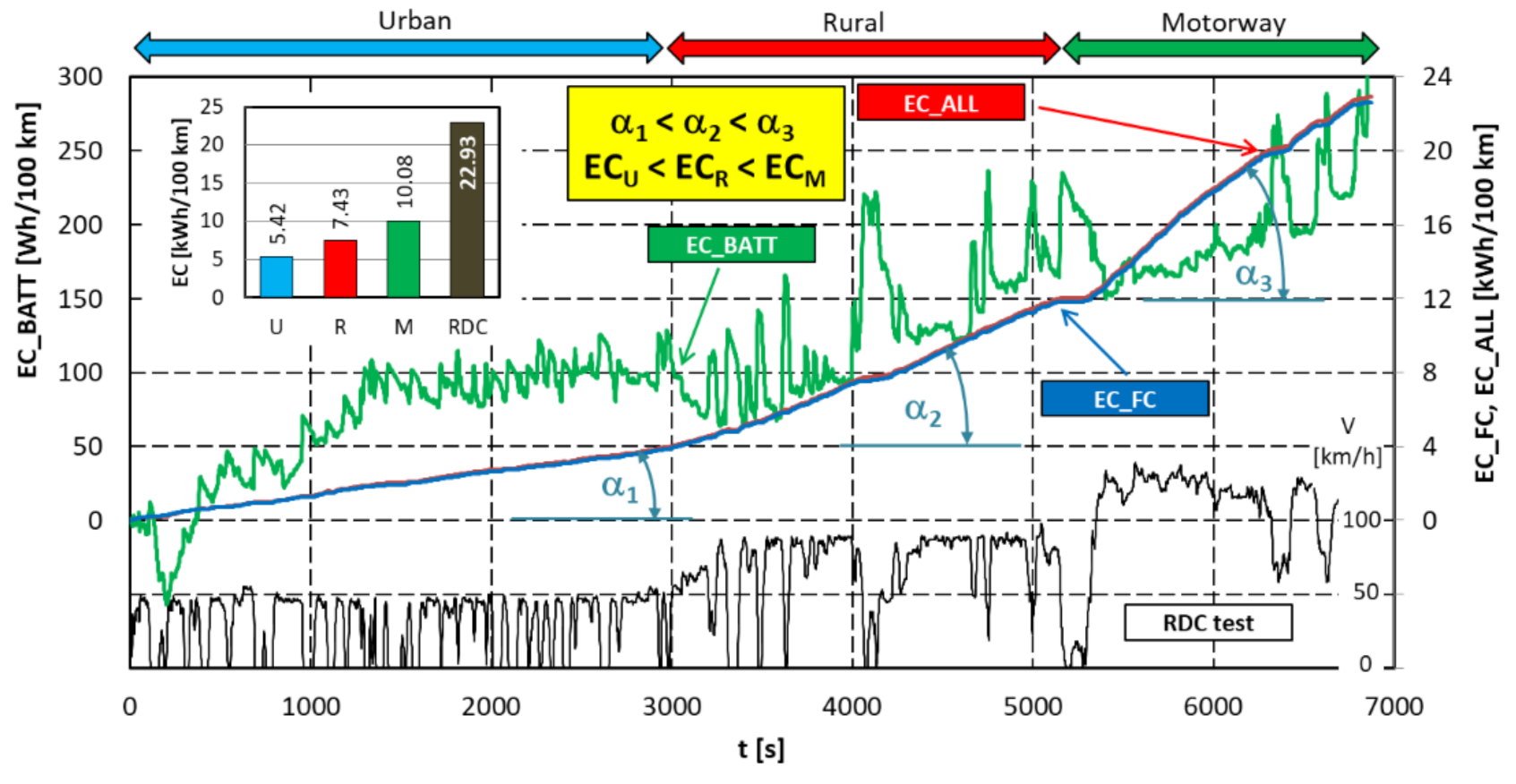
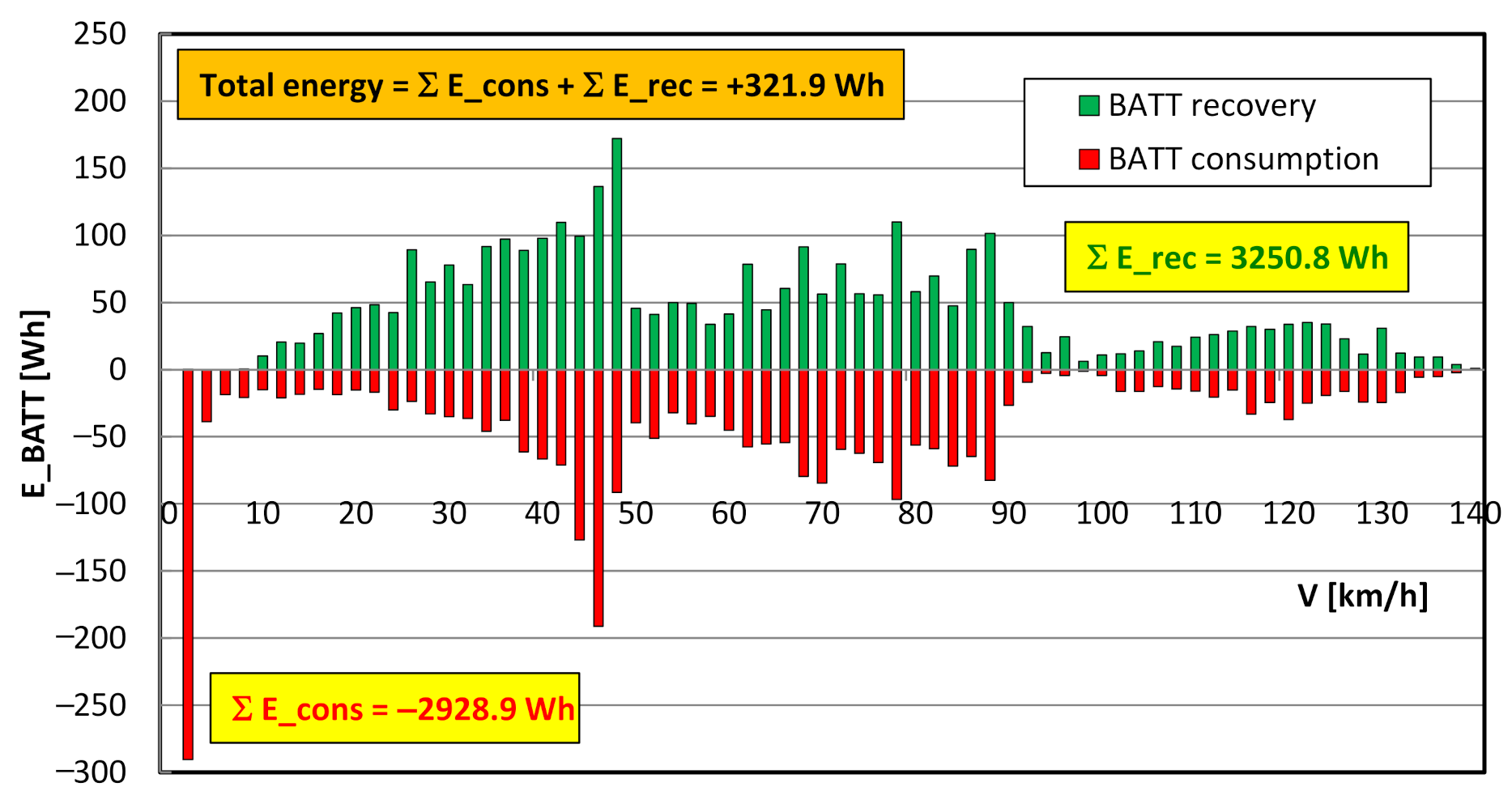
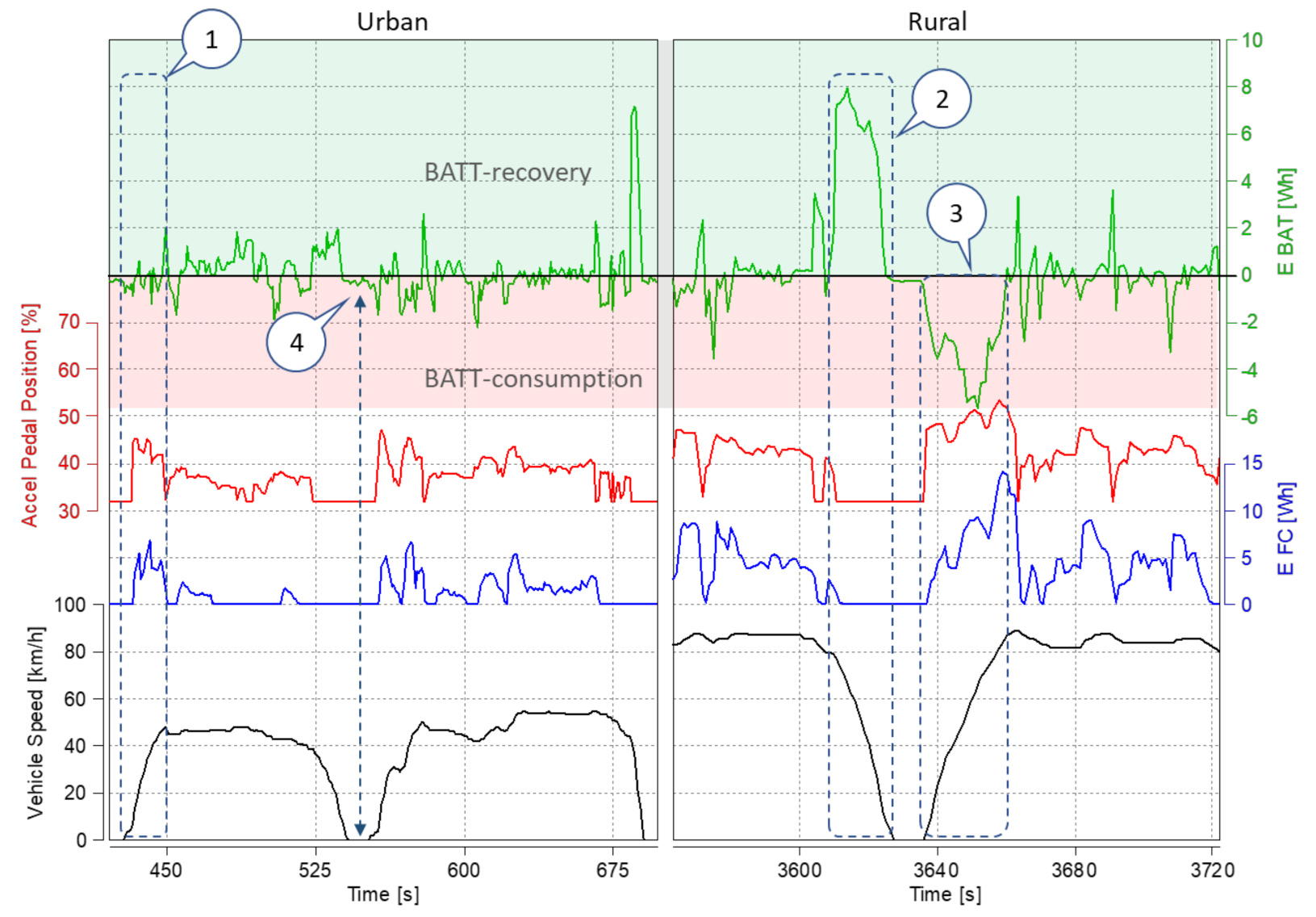
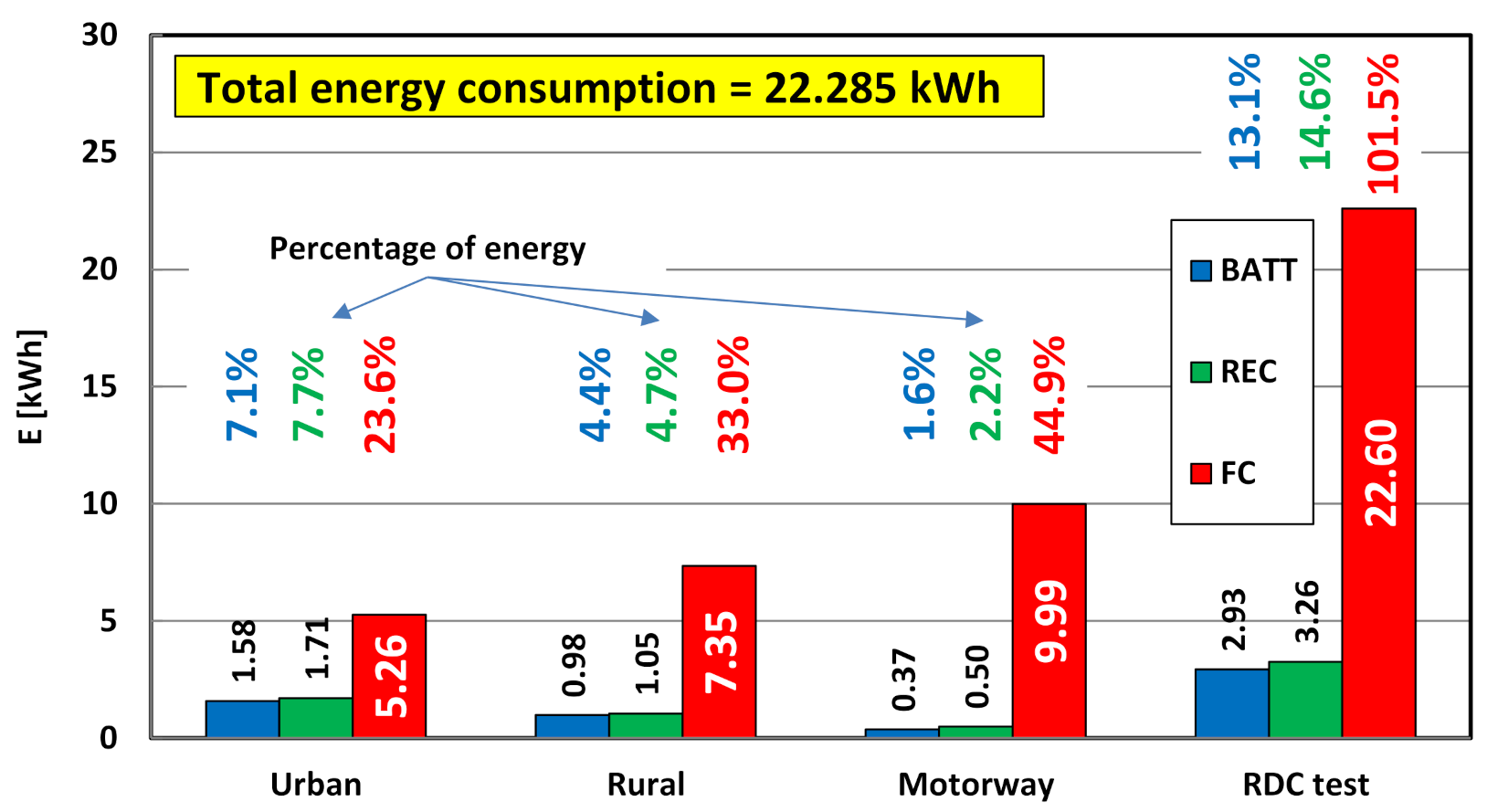
4.4. Evaluation of Electric Drive Operating Conditions and Energy Consumption
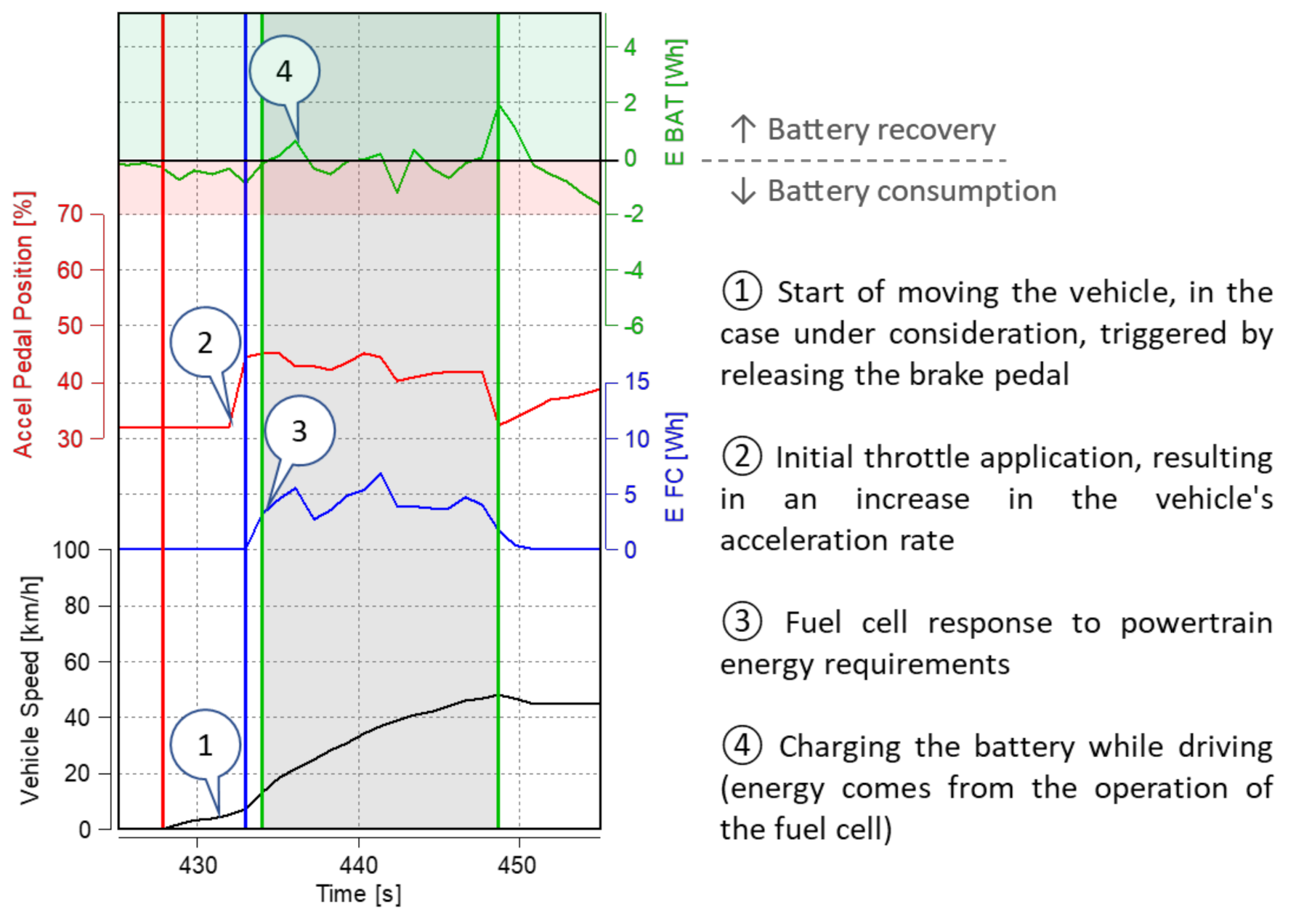
- during vehicle acceleration, the initial energy input from the battery is visible until the fuel cell starts producing energy (for further analysis see Figure 11);
- during braking, the fuel cell operation is shut down and the energy is recovered to the high-voltage battery;
- depending on the vehicle’s acceleration rate, larger amounts of energy are consumed from both the battery and the fuel cell sources;
- when the vehicle is standing still, the energy requirements of the on-board systems (comfort, entertainment) are fulfilled by the high-voltage battery.
5. Conclusions
- in most cases, the high voltage battery is charged only from energy regeneration during braking; however, there are also situations where the battery is charged from the energy generated by the fuel cell (in the test, the battery charge from the fuel cell reached 0.315 kWh—Figure 15);
- as the vehicle speed increases (in other words as the RDC test interval changes), the battery energy consumption decreases and the fuel cell energy consumption increases (Figure 15);
- the vehicle is initially started from standstill by using a high-voltage battery, only after a certain time, depending on the load, is the fuel cell activated (Figure 11).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Technology Roadmap. Hydrogen Fuel Cell. International Energy Agency. Paris, 2015. Available online: www.iea.org (accessed on 21 June 2021).
- Hydrogen Roadmap Europe: A Sustainable Pathway for the European Energy Transition, Fuel Cells and Hydrogen 2 Joint Undertaking; Publications Office of the European Union: Luxembourg, 2019. [CrossRef]
- Path to Hydrogen Competitiveness. A Cost Perspective. Hydrogen Council, 2020. Available online: www.hydrogencouncil.com (accessed on 21 June 2021).
- Hydrogen Scaling Up. A Sustainable Pathway for the Global Energy Transition. Hydrogen Council, November 2017. Available online: https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-scaling-up-Hydrogen-Council.pdf (accessed on 4 August 2021).
- Australia’s National Hydrogen Strategy. COAG Energy Council Hydrogen Working Group. Available online: https://www.industry.gov.au/sites/default/files/2019-11/australias-national-hydrogen-strategy.pdf (accessed on 4 August 2021).
- Bieker, G. A Global Comparison of the Life-Cycle Greenhouse Gas Emissions of Combustion Engine and Electric Passenger Cars. International Council on Clean Transportation Europe. July 2021. Available online: https://theicct.org/sites/default/files/publications/Global-LCA-passenger-cars-jul2021_0.pdf (accessed on 1 August 2021).
- Amant, S.; Meunier, N.; de Cosse Brissac, C. Road Transportation: What Alternative Motorisations are Suitable for the Climate? A Comparison of the Life Cycle Emissions, in France and Europe. Available online: https://www.carbone4.com/wp-content/uploads/2021/02/Road-transportation-what-alternative-motorisations-are-suitable-for-the-climate-Carbone-4.pdf (accessed on 4 August 2021).
- Hydrogen Fuel News. Hydrogen News Daily–Alternative Energy Reports. Available online: https://www.hydrogenfuelnews.com/fuel-cell-passenger-vehicles/8546147 (accessed on 20 June 2021).
- Ludwig-Bölkow-Systemtechnik GmbH. Available online: www.lbst.de (accessed on 21 June 2021).
- Das, L.M. Hydrogen engines: A view of the past and a look into the future. Int. J. Hydrogen Energy 1990, 15, 425–443. [Google Scholar] [CrossRef]
- Yip, H.L.; Srna, A.; Yuen, A.C.Y.; Kook, S.; Taylor, R.A.; Yeoh, G.H.; Medwell, P.R.; Chan, Q.N. A review of hydrogen direct injection for internal combustion engines: Towards carbon-free combustion. Appl. Sci. 2019, 9, 4842. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, D.; Yang, S.; Sun, P.; Guo, Z.; Yang, H.; Li, Y.; Wang, T. Effects of hydrogen direct injection on combustion and emission characteristics of a hydrogen/Acetone-Butanol-Ethanol dual-fuel spark ignition engine under lean-burn conditions. Int. J. Hydrogen Energy 2020, 45, 34193–34203. [Google Scholar] [CrossRef]
- Zareei, J.; Rohani, A.; Mazari, F.; Mikkhailova, M.V. Numerical investigation of the effect of two-step injection (direct and port injection) of hydrogen blending and natural gas on engine performance and exhaust gas emissions. Energy 2021, 231, 120957. [Google Scholar] [CrossRef]
- Younkins, M.; Boyer, B.; Wooldridge, M. Hydrogen DI dual zone combustion system. SAE Int. J. Engines 2013, 6, 45–53. [Google Scholar] [CrossRef]
- Smirnov, N.N.; Nikitin, V.F. Modeling and simulation of hydrogen combustion in engines. Int. J. Hydrogen Energy 2014, 39, 1122–1136. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Periyasamy, M. Impact on the power and performance of an internal combustion engine using hydrogen. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Babayev, R.; Andersson, A.; Dalmau, A.S.; Im, H.G.; Johansson, B. Computational characterization of hydrogen direct injection and nonpremixed combustion in a compression-ignition engine. Int. J. Hydrogen Energy 2021, 46, 18678–18696. [Google Scholar] [CrossRef]
- Zareei, J.; Haseeb, M.; Ghadamkheir, K.; Farkhondeh, S.A.; Yazdani, A.; Ershov, K. The effect of hydrogen addition to compressed natural gas on performance and emissions of a DI diesel engine by a numerical study. Int. J. Hydrogen Energy 2020, 45, 34241–34253. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Ibegbu, A.J.; Aduojo, A.A. Experimental optimization of engine performance of a dual-fuel compression-ignition engine operating on hydrogen-compressed natural gas and Moringa biodiesel. Energy Rep. 2021, 7, 607–619. [Google Scholar] [CrossRef]
- Gong, C.; Li, Z.; Sun, J.; Liu, F. Evaluation on combustion and lean-burn limitof a medium compression ratio hydrogen/methanol dual-injection spark-ignition engine under methanol late-injection. Appl. Energy 2020, 277, 115622. [Google Scholar] [CrossRef]
- Wang, H.; Ji, C.; Shi, C.; Ge, Y.; Wang, S.; Yang, J. Development of cyclic variation prediction model of the gasoline and n-butanol rotary engines with hydrogen enrichment. Fuel 2021, 299, 120891. [Google Scholar] [CrossRef]
- Meng, H.; Ji, C.; Wang, S.; Wang, D.; Yang, J. Optimizing the idle performance of an n-butanol fueled Wankel rotary engine by hydrogen addition. Fuel 2021, 288, 119614. [Google Scholar] [CrossRef]
- Zhen, X.; Li, X.; Wang, Y.; Liu, D.; Tian, Z. Comparative study on combustion and emission characteristics of methanol/hydrogen, ethanol/hydrogen and methane/hydrogen blends in high compression ratio SI engine. Fuel 2020, 267, 117193. [Google Scholar] [CrossRef]
- Chubbock, S.; Clague, R. Comparative analysis of internal combustion engine and fuel cell range extender. SAE Int. J. Alt. Power 2016, 5, 175–182. [Google Scholar] [CrossRef]
- Konno, N.; Mizuno, S.; Nakaji, H.; Ishikawa, Y. Development of compact and high-performance fuel cell stack. SAE Int. J. Alt. Power 2015, 4, 123–129. [Google Scholar] [CrossRef]
- Toyota Motor Europe. Toyota Service and Repair Information. Available online: www.toyota-tech.eu (accessed on 24 June 2021).
- Yoshizumi, T.; Kubo, H.; Okumura, M. Development of high-performance FC stack for the new MIRAI. In SAE Technical; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Kimura, K.; Kawasaki, T.; Ohmura, T.; Atsumi, Y.; Shimizu, K. Development of new fuel cell vehicle Clarity Fuel Cell. Honda RD Tech. Rev. 2016, 28, 1–7. [Google Scholar]
- Matsunaga, M.; Fukushima, T.; Ojima, K. Powertrain system of Honda FCX Clarity fuel cell vehicle. World Electr. Veh. J. 2009, 3, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Tazelaar, E.; Bruinsma, J.; Veenhuizen, B.; Van den Bosch, P. Driving cycle characterization and generation, for design and control of fuel cell buses. World Electr. Veh. J. 2009, 3, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Lohse-Busch, H.; Stutenberg, K.; Duoba, M.; Iliev, S. Technology Assessment of a Fuel Cell Vehicle: 2017 Toyota Mirai, ANL/ESD-18/12, Argonne National Laboratory. 2018. Available online: https://www.anl.gov/argonne-scientific-publications/pub/144774 (accessed on 20 June 2021).
- Outline of the Mirai. Key Specifications, Mirai–Toyota Europe. Available online: www.toyota-europe.com (accessed on 21 June 2021).
- Yumiya, H.; Kizaki, M.; Asai, H. Toyota Fuel Cell System (TFCS). World Electr. Veh. J. 2015, 7, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Imanishi, H.; Nada, M.; Ikogi, Y. Development of the fuel cell system in the Mirai FCV. In SAE Technical; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Pielecha, I.; Cieślik, W.; Szałek, A. Energy recovery potential through regenerative braking for a hybrid electric vehicle in a urban conditions. IOP Conf. Ser. Earth Env. 2019, 214, 012013. [Google Scholar] [CrossRef]
- Pielecha, I.; Cieślik, W.; Szałek, A. The use of electric drive in urban driving conditions using a hydrogen powered vehicle–Toyota Mirai. Comb. Eng. 2018, 172, 51–58. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/631 of the European Parliament and of the Council of 17 April 2019 setting CO2 emission performance standards for new passenger vehicles and for new light commercial vehicles, and repealing Regulations (EC) No 443/2009 and (EU) No 510. Off. J. Eur. Union 2019, 111, 13–534. [Google Scholar]
- Gis, W.; Gis, M.; Pielecha, J.; Skobiej, K. Alternative exhaust emission factors from vehicles in on-road driving tests. Energies 2021, 14, 3487. [Google Scholar] [CrossRef]
- Szałek, A.; Pielecha, I. The Influence of engine downsizing in hybrid powertrains on the energy flow indicators under actual traffic conditions. Energies 2021, 14, 2872. [Google Scholar] [CrossRef]
- Cieslik, W.; Szwajca, F.; Golimowski, W.; Berger, A. Experimental analysis of residential photovoltaic (PV) and electric vehicle (EV) systems in terms of annual energy utilization. Energies 2021, 14, 1085. [Google Scholar] [CrossRef]
| Component | Parameter | Value |
|---|---|---|
| Vehicle | weight | 1850 kg |
| top speed | 179 km/h | |
| acceleration 0 to 60 mph | 9.6 s | |
| range (homologation cycle) | approx. 483 km | |
| Fuel cell | type | PEM (polymer electrolyte) |
| power | 114 kW | |
| power density | 2.0 kW/kg; 3.1 kW/dm3 | |
| number of cells | 370 | |
| humidificiation method | internal circulation system | |
| Electric motor | type | permanent magnet synchronous |
| peak power | 123 kW at 4500 rpm | |
| maximum torque | 335 N·m | |
| total speed reduction ratio | 3.542 | |
| Battery | type | Nickel Metal Hydride (NiMH) |
| capacity | 6.5 Ah | |
| nominal voltage | 244.8 V (7.2 × 34) | |
| hydrogen storage | internal volume | 122.4 dm3 |
| nominal/filling pressure | 70 MPa/87.5 MPa | |
| mass | approx. 5.0 kg | |
| refueling time | 3 min. |
| Component | Parameter | Value |
|---|---|---|
| Compressed hydrogen tanks | Number of tanks | 2 |
| storage density | 5.7 wt% | |
| Front tank | Capacity/weight | 60 dm3/42.8 kg |
| Rear tank | Capacity/weight | 62.4 dm3/44.7 kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szałek, A.; Pielecha, I.; Cieslik, W. Fuel Cell Electric Vehicle (FCEV) Energy Flow Analysis in Real Driving Conditions (RDC). Energies 2021, 14, 5018. https://doi.org/10.3390/en14165018
Szałek A, Pielecha I, Cieslik W. Fuel Cell Electric Vehicle (FCEV) Energy Flow Analysis in Real Driving Conditions (RDC). Energies. 2021; 14(16):5018. https://doi.org/10.3390/en14165018
Chicago/Turabian StyleSzałek, Andrzej, Ireneusz Pielecha, and Wojciech Cieslik. 2021. "Fuel Cell Electric Vehicle (FCEV) Energy Flow Analysis in Real Driving Conditions (RDC)" Energies 14, no. 16: 5018. https://doi.org/10.3390/en14165018
APA StyleSzałek, A., Pielecha, I., & Cieslik, W. (2021). Fuel Cell Electric Vehicle (FCEV) Energy Flow Analysis in Real Driving Conditions (RDC). Energies, 14(16), 5018. https://doi.org/10.3390/en14165018








