Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies
Abstract
1. Introduction
2. Hydrogen as an Energy Carrier
3. Direct Combustion of Hydrogen
4. CHC
4.1. Materials for CHC
4.2. Devices for CHC
5. CHC for Hydrogen Safety Purposes
6. Future Research
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Hoel, M.; Kverndokk, S. Depletion of fossil fuels and the impacts of global warming. Resour. Energy Econ. 1996, 18, 115–136. [Google Scholar] [CrossRef]
- Lonngren, K.E.; Bai, E.-W. On the global warming problem due to carbon dioxide. Energy Policy 2008, 36, 1567–1568. [Google Scholar] [CrossRef]
- Saadi, N.; Miketa, A.; Howells, M. African Clean Energy Corridor: Regional integration to promote renewable energy fueled growth. Energy Res. Soc. Sci. 2015, 5, 130–132. [Google Scholar] [CrossRef]
- Cai, Z.; Dai, S.; Zhao, K.; Yang, J. Future power grid dispatch and control mode with large-scale clean energy integration in China. In Proceedings of the 2016 IEEE PES Asia-Pacific Power and Energy Engineering Conference (APPEEC), Xi’an, China, 25–28 October 2016; pp. 1749–1754. [Google Scholar]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. On-demand hydrogen generation by the hydrolysis of ball-milled aluminum composites: A process overview. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Daramola, M.O.; Mogwase, B.; Engelbrecht, N.; Yoro, K.O.; Petrus du Preez, S.; Mhlongo, S.; Ezeokoli, O.T.; Ghimire, A.; Ayeni, A.O.; et al. Revising the dark fermentative H2 research and development scenario—An overview of the recent advances and emerging technological approaches. Biomass Bioenergy 2020, 140, 105673. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. Int. J. Hydrogen Energy 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Engelbrecht, N.; du Preez, S.P.; Bessarabov, D. Thermophilic biogas upgrading via ex situ addition of H2 and CO2 using codigested feedstocks of cow manure and the organic fraction of solid municipal waste. ACS Omega 2020, 5, 17367–17376. [Google Scholar] [CrossRef]
- Pushkarev, S.A.; Pushkareva, V.I.; Ivanova, A.N.; du Preez, P.S.; Bessarabov, D.; Chumakov, G.R.; Stankevich, G.V.; Fateev, N.V.; Evdokimov, A.A.; Grigoriev, A.S. Pt/C and Pt/SnOx/C catalysts for ethanol electrooxidation: Rotating disk electrode study. Catalysts 2019, 9, 271. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Du Preez, S.P. Hydrogen Generation by the Reaction of Mechanochemically Activated Aluminium and Water. Master’s Thesis, North-West University, Potchefstroom, South Africa, 2019. [Google Scholar]
- Pushkarev, A.; Pushkareva, I.; du Preez, S.; Ivanova, N.; Grigoriev, S.; Slavcheva, E.; Bessarabov, D.; Fateev, V.l.; Aliyev, A. Iridium catalyst supported on conductive titanium oxides for polymer electrolyte membrane electrolysis. Chem. Probl. 2019, 17, 9–15. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Hydrogen generation by the hydrolysis of mechanochemically activated aluminum-tin-indium composites in pure water. Int. J. Hydrogen Energy 2018, 43, 21398–21413. [Google Scholar] [CrossRef]
- Du Preez, S.; Bessarabov, D. Hydrogen generation by means of hydrolysis using activated Al-In-Bi-Sn composites for electrochemical energy applications. Int. J. Electrochem. Sci. 2017, 12, 8663–8682. [Google Scholar] [CrossRef]
- Bessarabov, D.; Human, G.; Kruger, A.J.; Chiuta, S.; Modisha, P.M.; du Preez, S.P.; Oelofse, S.P.; Vincent, I.; Van Der Merwe, J.; Langmi, H.W.; et al. South African hydrogen infrastructure (HySA infrastructure) for fuel cells and energy storage: Overview of a projects portfolio. Int. J. Hydrogen Energy 2017, 42, 13568–13588. [Google Scholar] [CrossRef]
- Trimm, D.L.; Önsan, Z.I. Onboard fuel conversion for hydrogen-fuel-cell-driven vehicles. Catal. Rev. 2001, 43, 31–84. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.; Ashuri, T.; Krohn, J. Hydrogen fuel cell vehicles; current status and future prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Scott, M.; Powells, G. Sensing hydrogen transitions in homes through social practices: Cooking, heating, and the decomposition of demand. Int. J. Hydrogen Energy 2020, 45, 3870–3882. [Google Scholar] [CrossRef]
- Matzen, M.; Alhajji, M.; Demirel, Y. Technoeconomics and sustainability of renewable methanol and ammonia productions using wind power-based hydrogen. J. Adv. Chem. Eng. 2015, 5, 3. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Mosca, L.; Palo, E.; Colozzi, M.; Iaquaniello, G.; Salladini, A.; Taraschi, S. Hydrogen in chemical and petrochemical industry. In Current Trends and Future Developments on (Bio-)Membranes; Iulianelli, A., Basile, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 387–410. [Google Scholar] [CrossRef]
- Bastien, J.; Handler, C. Hydrogen production from renewable energy sources. In Proceedings of the IEEE EIC Climate Change Conference, Ottawa, ON, Canada, 10–12 May 2006; pp. 1–9. [Google Scholar]
- Zsiborács, H.; Baranyai, N.H.; Vincze, A.; Zentkó, L.; Birkner, Z.; Máté, K.; Pintér, G. Intermittent renewable energy sources: The role of energy storage in the european power system of 2040. Electronics 2019, 8, 729. [Google Scholar] [CrossRef]
- Masera, O.R.; Bailis, R.; Drigo, R.; Ghilardi, A.; Ruiz-Mercado, I. Environmental burden of traditional bioenergy use. Annu. Rev. Environ. Resour. 2015, 40, 121–150. [Google Scholar] [CrossRef]
- Bonjour, S.; Adair-Rohani, H.; Wolf, J.; Bruce Nigel, G.; Mehta, S.; Prüss-Ustün, A.; Lahiff, M.; Rehfuess Eva, A.; Mishra, V.; Smith Kirk, R. Solid fuel use for household cooking: Country and regional estimates for 1980–2010. Environ. Health Perspect. 2013, 121, 784–790. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Bailis, R.; Ezzati, M.; Kammen, D.M. Mortality and greenhouse gas impacts of biomass and petroleum energy futures in Africa. Science 2005, 308, 98. [Google Scholar] [CrossRef] [PubMed]
- Rudel, T.K. The national determinants of deforestation in sub-Saharan Africa. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120405. [Google Scholar] [CrossRef]
- Bailis, R.; Drigo, R.; Ghilardi, A.; Masera, O. The carbon footprint of traditional woodfuels. Nat. Clim. Chang. 2015, 5, 266–272. [Google Scholar] [CrossRef]
- Schmidt Rivera, X.C.; Topriska, E.; Kolokotroni, M.; Azapagic, A. Environmental sustainability of renewable hydrogen in comparison with conventional cooking fuels. J. Clean. Prod. 2018, 196, 863–879. [Google Scholar] [CrossRef]
- Büchs, M.; Schnepf, S.V. Who emits most? Associations between socio-economic factors and UK households’ home energy, transport, indirect and total CO2 emissions. Ecol. Econ. 2013, 90, 114–123. [Google Scholar] [CrossRef]
- Gallagher, M.E.; Down, A.; Ackley, R.C.; Zhao, K.; Phillips, N.; Jackson, R.B. Natural gas pipeline replacement programs reduce methane leaks and improve consumer safety. Environ. Sci. Technol. Lett. 2015, 2, 286–291. [Google Scholar] [CrossRef]
- Hydrogen Council. Hydrogen Scaling Up. A Sustainable Pathway for the Global Energy Transition; Hydrogen Council: Brussels, Belgium, November 2017. [Google Scholar]
- Kippers, M.J.; De Laat, J.C.; Hermkens, R.J.M.; Overdiep, J.J.; van der Molen, A.; van Erp, W.C.; van der Meer, A. Pilot project on hydrogen injection in natural gas on Island of Ameland in the Netherlands. In Proceedings of the International Gas Union Research Conference, Seoul, Korea, 19–21 October 2011. [Google Scholar]
- Quarton, C.J.; Samsatli, S. Power-to-gas for injection into the gas grid: What can we learn from real-life projects, economic assessments and systems modelling? Renew. Sustain. Energy Rev. 2018, 98, 302–316. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory NREL: Golden, CO, USA, 2013; p. 73.
- Spazzafumo, G. Power to Fuel. How to Speed Up a Hydrogen Economy, 1st ed.; Elsevier: London, UK, 2021; p. 286. [Google Scholar]
- Wei, T.; Dong, W.; Yan, Q.; Chou, J.; Yang, Z.; Tian, D. Developed and developing world contributions to climate system change based on carbon dioxide, methane and nitrous oxide emissions. Adv. Atmos. Sci. 2016, 33, 632–643. [Google Scholar] [CrossRef]
- Den Elzen, M.; Berk, M.; Schaeffer, M.; Olivier, J.; Hendriks, C.; Metz, B. The Brazilian Proposal and Other Options for International Burden Sharing: An Evaluation of Methodological and Policy Aspects Using the FAIR Model; 728001011; Rijksinstituut voor Volksgezondheid en Milieu RIVM DGM NOP, National Institute of Public Health and The Evironment: Bilthoven, The Netherlands, 1999.
- Ding, Z.; Duan, X.; Ge, Q.; Zhang, Z. Control of atmospheric CO2 concentrations by 2050: A calculation on the emission rights of different countries. Sci. China Earth Sci. 2009, 52, 1447. [Google Scholar] [CrossRef]
- Burck, J.; Hagen, U.; Höhne, N.; Nascimento, L.; Bals, C. Climate Change Perfomance Index (CCPI), Results 2020; Germanwatch, NewClimate Institute, and Climate Action Network: Berlin, Germany, 2020. [Google Scholar]
- Gasworld. LOHC Technology Implemented in China. Available online: https://www.gasworld.com/lohctechnology-implemented-in-china/2014010.article (accessed on 14 June 2021).
- Juelich Forschungszentrum. From the Lab to the Rails: HI-ERN Researchers Plan Hydrogen Trains with LOHC Technology. Available online: www.fz-juelich.de/SharedDocs/Pressemitteilungen/UK/DE/2018/2018-04-19-lohc-zug.html;jsessionid=1C1332AE23754416D20BCC792042618C?nn=721054 (accessed on 14 June 2021).
- Alstom. Alstom’s Hydrogen Fuel Cell Train Wins 2018 GreenTec Mobility Award. Available online: https://www.alstom.com/press-releases-news/2018/5/alstoms-hydrogen-fuel-cell-train-wins-2018-greentec-mobility-award (accessed on 14 June 2021).
- David, R. Solar Power’s Greatest Challenge Was Discovered 10 Years Ago. It Looks Like a Duck. Available online: https://www.vox.com/energy-and-environment/2018/3/20/17128478/solar-duck-curve-nrel-researcher (accessed on 14 June 2021).
- Bird, L.; Milligan, M.; Lew, D. Integrating Variable Renewable Energy: Challenges and Solutions; NREL/TP-6A20-60451; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2013.
- Stram, B.N. Key challenges to expanding renewable energy. Energy Policy 2016, 96, 728–734. [Google Scholar] [CrossRef]
- Byrnes, L.; Brown, C.; Foster, J.; Wagner, L.D. Australian renewable energy policy: Barriers and challenges. Renew. Energy 2013, 60, 711–721. [Google Scholar] [CrossRef]
- Harrison, K.W.; Remick, R.; Hoskin, A.; Martin, G.D. Hydrogen Production: Fundamentals and Case Study Summaries. In Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 16–21 May 2010. [Google Scholar]
- Ball, M.; Wietschel, M. The future of hydrogen—Opportunities and challenges. Int. J. Hydrogen Energy 2009, 34, 615–627. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Justi, E.W. A Solar-Hydrogen Energy System; Plenum Press: New York, NY, USA, 1987. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, K. Review on hydrogen storage materials and methods from an electrochemical viewpoint. J. Energy Storage 2019, 23, 234–249. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Schüth, F. Challenges in hydrogen storage. Eur. Phys. J. Spec. Top. 2009, 176, 155–166. [Google Scholar] [CrossRef]
- Chahine, R.; Bose, T.K. Low-pressure adsorption storage of hydrogen. Int. J. Hydrogen Energy 1994, 19, 161–164. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Green, L. An ammonia energy vector for the hydrogen economy. Int. J. Hydrogen Energy 1982, 7, 355–359. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Schüth, F.; Bogdanović, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 20, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.; Fritz, M.; Ernst, S. Zeolites as media for hydrogen storage. In Proceedings of the Ninth International Zeolite Conference, Montreal, QC, Canada, 5–10 July 1992; pp. 11–19. [Google Scholar]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The prospect of hydrogen storage using liquid organic hydrogen carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767–2773. [Google Scholar] [CrossRef]
- Gamisch, B.; Gaderer, M.; Dawoud, B. On the development of thermochemical hydrogen storage: An experimental study of the kinetics of the redox reactions under different operating conditions. Appl. Sci. 2021, 11, 1623. [Google Scholar] [CrossRef]
- Lorente, E.; Peña, J.A.; Herguido, J. Cycle behaviour of iron ores in the steam-iron process. Int. J. Hydrogen Energy 2011, 36, 7043–7050. [Google Scholar] [CrossRef]
- Langmi, H.W.; Engelbrecht, N.; Modisha, P.M.; Bessarabov, D. Hydrogen storage. In Electrochemical Power Sources: Fundamentals, Systems, and Applications, 1st ed.; Arai, H., Garche, J., Colmenares, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 13; p. 312. [Google Scholar]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen storage technologies for future energy systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Saka, C.; Balbay, A. Influence of process parameters on enhanced hydrogen generation via semi-methanolysis and semi-ethanolysis reactions of sodium borohydride using phosphoric acid. Int. J. Hydrogen Energy 2019, 44, 30119–30126. [Google Scholar] [CrossRef]
- Selvitepe, N.; Balbay, A.; Saka, C. Optimisation of sepiolite clay with phosphoric acid treatment as support material for CoB catalyst and application to produce hydrogen from the NaBH4 hydrolysis. Int. J. Hydrogen Energy 2019, 44, 16387–16399. [Google Scholar] [CrossRef]
- Saka, C. Metal-free catalysts with phosphorus and oxygen doped on carbon-based on Chlorella Vulgaris microalgae for hydrogen generation via sodium borohydride methanolysis reaction. Int. J. Hydrogen Energy 2021, 46, 5150–5157. [Google Scholar] [CrossRef]
- Balbay, A.; Saka, C. The effect of the concentration of hydrochloric acid and acetic acid aqueous solution for fast hydrogen production from methanol solution of NaBH4. Int. J. Hydrogen Energy 2018, 43, 14265–14272. [Google Scholar] [CrossRef]
- Saka, C.; Balbay, A. Fast and effective hydrogen production from ethanolysis and hydrolysis reactions of potassium borohydride using phosphoric acid. Int. J. Hydrogen Energy 2018, 43, 19976–19983. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, Y.; Luo, Y.; Zhu, H. Highly dispersed RuCo bimetallic nanoparticles supported on carbon black: Enhanced catalytic activity for hydrogen generation from NaBH4 methanolysis. J. Mater. Sci. 2018, 53, 6831–6841. [Google Scholar] [CrossRef]
- Wu, Y. Metal Oxides in Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–274. [Google Scholar] [CrossRef]
- Markiewicz, M.; Zhang, Y.Q.; Bösmann, A.; Brückner, N.; Thöming, J.; Wasserscheid, P.; Stolte, S. Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems—Challenges and preliminary results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- Kariya, N.; Fukuoka, A.; Ichikawa, M. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet–dry multiphase conditions”. Appl. Catal. A 2002, 233, 91–102. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, H.; Lu, H.; Zhang, Z.; Chen, Y. Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation. Appl. Surf. Sci. 2017, 422, 905–912. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gosavi, P.V.; Pande, J.V.; Kumar, V.P.; Chary, K.V.R.; Biniwale, R.B. Efficient hydrogen supply through catalytic dehydrogenation of methylcyclohexane over Pt/metal oxide catalysts. Int. J. Hydrogen Energy 2010, 35, 4020–4026. [Google Scholar] [CrossRef]
- Hodoshima, S.; Takaiwa, S.; Shono, A.; Satoh, K.; Saito, Y. Hydrogen storage by decalin/naphthalene pair and hydrogen supply to fuel cells by use of superheated liquid-film-type catalysis. Appl. Catal. A 2005, 283, 235–242. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Sakai, H.; Abe, M.; Rangsunvigit, P.; Horikoshi, S. Microwave effect in the dehydrogenation of tetralin and decalin with a fixed-bed reactor. Int. J. Hydrogen Energy 2012, 37, 3242–3250. [Google Scholar] [CrossRef]
- Gleichweit, C.; Amende, M.; Schernich, S.; Zhao, W.; Lorenz, M.P.A.; Höfert, O.; Brückner, N.; Wasserscheid, P.; Libuda, J.; Steinrück, H.-P.; et al. Dehydrogenation of Dodecahydro-N-ethylcarbazole on Pt(111). Chem. Sus. Chem. 2013, 6, 974–977. [Google Scholar] [CrossRef]
- Peters, W.; Eypasch, M.; Frank, T.; Schwerdtfeger, J.; Körner, C.; Bösmann, A.; Wasserscheid, P. Efficient hydrogen release from perhydro-N-ethylcarbazole using catalyst-coated metallic structures produced by selective electron beam melting. Energy Environ. Sci. 2015, 8, 641–649. [Google Scholar] [CrossRef]
- Leinweber, A.; Müller, K. Hydrogenation of the liquid organic hydrogen carrier compound monobenzyl toluene: Reaction pathway and kinetic effects. Energy Technol. 2018, 6, 513–520. [Google Scholar] [CrossRef]
- Heller, A.; Rausch, M.H.; Schulz, P.S.; Wasserscheid, P.; Fröba, A.P. Binary diffusion coefficients of the liquid organic hydrogen carrier system dibenzyltoluene/perhydrodibenzyltoluene. J. Chem. Eng. Data 2016, 61, 504–511. [Google Scholar] [CrossRef]
- Amende, M.; Kaftan, A.; Bachmann, P.; Brehmer, R.; Preuster, P.; Koch, M.; Wasserscheid, P.; Libuda, J. Regeneration of LOHC dehydrogenation catalysts: In-situ IR spectroscopy on single crystals, model catalysts, and real catalysts from UHV to near ambient pressure. Appl. Surf. Sci. 2016, 360, 671–683. [Google Scholar] [CrossRef]
- Guerra Santin, O. Behavioural patterns and user profiles related to energy consumption for heating. Energy Build. 2011, 43, 2662–2672. [Google Scholar] [CrossRef]
- Nesbakken, R. Energy consumption for space heating: A discrete–continuous approach. Scand. J. Econ. 2001, 103, 165–184. [Google Scholar] [CrossRef]
- Balaras, C.A.; Droutsa, K.; Dascalaki, E.; Kontoyiannidis, S. Heating energy consumption and resulting environmental impact of European apartment buildings. Energy Build. 2005, 37, 429–442. [Google Scholar] [CrossRef]
- Schuler, A.; Weber, C.; Fahl, U. Energy consumption for space heating of West-German households: Empirical evidence, scenario projections and policy implications. Energy Policy 2000, 28, 877–894. [Google Scholar] [CrossRef]
- Jones, D.R.; Al-Masry, W.A.; Dunnill, C.W. Hydrogen-enriched natural gas as a domestic fuel: An analysis based on flash-back and blow-off limits for domestic natural gas appliances within the UK. Sustain. Energy Fuels 2018, 2, 710–723. [Google Scholar] [CrossRef]
- Jovanović, R.Ž.; Sretenović, A.A.; Živković, B.D. Ensemble of various neural networks for prediction of heating energy consumption. Energy Build. 2015, 94, 189–199. [Google Scholar] [CrossRef]
- Park, J.; Hwang, D.J.; Park, J.S.; Kim, J.S.; Keel, S.I.; Cho, H.C.; Noh, D.S.; Kim, T.K. Hydrogen utilization as a fuel: Hydrogen-blending effects in flame structure and NO emission behaviour of CH4–air flame. Int. J. Energy Res. 2007, 31, 472–485. [Google Scholar] [CrossRef]
- Mokheimer, E.M.A.; Sanusi, Y.S.; Habib, M.A. Numerical study of hydrogen-enriched methane–air combustion under ultra-lean conditions. Int. J. Energy Res. 2016, 40, 743–762. [Google Scholar] [CrossRef]
- Nurmukan, D.; Chen, T.J.M.; Hung, Y.M.; Ismadi, M.-Z.; Chong, C.T.; Tran, M.-V. Enhancement of biogas/air combustion by hydrogen addition at elevated temperatures. Int. J. Energy Res. 2020, 44, 1519–1534. [Google Scholar] [CrossRef]
- Di Sarli, V.; Benedetto, A.D. Laminar burning velocity of hydrogen–methane/air premixed flames. Int. J. Hydrogen Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A.; Long, E.J.; Hargrave, G.K. Time-Resolved Particle Image Velocimetry of dynamic interactions between hydrogen-enriched methane/air premixed flames and toroidal vortex structures. Int. J. Hydrogen Energy 2012, 37, 16201–16213. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A. Effects of non-equidiffusion on unsteady propagation of hydrogen-enriched methane/air premixed flames. Int. J. Hydrogen Energy 2013, 38, 7510–7518. [Google Scholar] [CrossRef]
- Das, L.M. Hydrogen-fueled internal combustion engines. In Compendium of Hydrogen Energy; Barbir, F., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Oxford, UK, 2016; pp. 177–217. [Google Scholar] [CrossRef]
- Du Toit, M.H.; Avdeenkov, A.V.; Bessarabov, D. Reviewing H2 combustion: A case study for non-fuel-cell power systems and safety in passive autocatalytic recombiners. Energy Fuels 2018, 32, 6401–6422. [Google Scholar] [CrossRef]
- Merryman, E.L.; Levy, A. Nitrogen oxide formation in flames: The roles of NO2 and fuel nitrogen. Symp. Combust. Proc. 1975, 15, 1073–1083. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Control of Nitrogen Oxides. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 866–945. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Malte, P.C.; Pratt, D.T. Measurement of atomic oxygen and nitrogen oxides in jet-stirred combustion. Symp. Combust. Proc. 1975, 15, 1061–1070. [Google Scholar] [CrossRef]
- Du Toit, M.; Engelbrecht, N.; Oelofse, S.P.; Bessarabov, D. Performance evaluation and emissions reduction of a micro gas turbine via the co-combustion of H2/CH4/CO2 fuel blends. Sustain. Energy Technol. Assess. 2020, 39, 100718. [Google Scholar] [CrossRef]
- Jones, D.R.; Dunnill, C.W. On the initiation of blow-out from cooktop burner jets: A simplified energy-based description for the onset of laminar flame extinction in premixed hydrogen-enriched natural gas (HENG) systems. Fuel 2021, 294, 120527. [Google Scholar] [CrossRef]
- Saint-Just, J.; Etemad, S. Catalytic combustion of hydrogen for heat production. In Compendium of Hydrogen Energy; Barbir, F., Basile, A., Veziroğlu, T.N., Eds.; Elsevier: Woodhead Publishing Limited: Cambridge, UK, 2016; Volume 3, pp. 263–287. [Google Scholar]
- Veziroglu, T.N. Hydrogen Energy System. Production and Utilization of Hydrogen and Future Aspects; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; Volume 295. [Google Scholar]
- Hydrogen Council. Path to Hydrogen Competitiveness. A Cost Perspective; Hydrogen Council: Brussels, Belgium, January 2020. [Google Scholar]
- Ladacki, M.; Houser, T.J.; Roberts, R.W. The catalyzed low-temperature hydrogen-oxygen reaction. J. Catal. 1965, 4, 239–247. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.C.; Kennelly, T.; Waterman, E.M. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Sekizawa, K.; Widjaja, H.; Maeda, S.; Ozawa, Y.; Eguchi, K. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catal. Today 2000, 59, 69–74. [Google Scholar] [CrossRef]
- Eguchi, K.; Arai, H. Recent advances in high temperature catalytic combustion. Catal. Today 1996, 29, 379–386. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic combustion of methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Jones, D.R.; Warwick, M.E.A.; Falch, A.; Sekoai, P.T.; Mota das Neves Quaresma, C.; Bessarabov, D.G.; Dunnill, C.W. Thermally stable Pt/Ti mesh catalyst for catalytic hydrogen combustion. Int. J. Hydrogen Energy 2020, 45, 16851–16864. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Jones, D.R.; Bessarabov, D.G.; Falch, A.; Mota das Neves Quaresma, C.; Dunnill, C.W. Development of a Pt/stainless steel mesh catalyst and its application in catalytic hydrogen combustion. Int. J. Hydrogen Energy 2019, 44, 27094–27106. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Shuro, I.; Bessarabov, D.G. Development of a low purity aluminum alloy (Al6082) anodization process and its application as a platinum-based catalyst in catalytic hydrogen combustion. Surf. Coat. Technol. 2020, 404, 126483. [Google Scholar] [CrossRef]
- Malakhov, A.A.; du Toit, M.H.; du Preez, S.P.; Avdeenkov, A.V.; Bessarabov, D.G. Temperature profile mapping over a catalytic unit of a hydrogen passive autocatalytic recombiner: An experimental and computational fluid dynamics study. Energy Fuels 2020, 34, 11637–11649. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Park, H.-S.; Cho, Y.-H.; Jung, D.-S.; Park, H.-Y.; Sung, Y.-E. Effect of platinum amount in carbon supported platinum catalyst on performance of polymer electrolyte membrane fuel cell. J. Power Sources 2007, 172, 89–93. [Google Scholar] [CrossRef]
- Guha, A.; Lu, W.; Zawodzinski, T.A.; Schiraldi, D.A. Surface-modified carbons as platinum catalyst support for PEM fuel cells. Carbon 2007, 45, 1506–1517. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Modisha, P.; Gqogqa, P.; Garidzirai, R.; Ouma, C.N.M.; Bessarabov, D. Evaluation of catalyst activity for release of hydrogen from liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2019, 44, 21926–21935. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Shtatniy, I.G.; Millet, P.; Porembsky, V.I.; Fateev, V.N. Description and characterization of an electrochemical hydrogen compressor/concentrator based on solid polymer electrolyte technology. Int. J. Hydrogen Energy 2011, 36, 4148–4155. [Google Scholar] [CrossRef]
- Nordio, M.; Rizzi, F.; Manzolini, G.; Mulder, M.; Raymakers, L.; Van Sint Annaland, M.; Gallucci, F. Experimental and modelling study of an electrochemical hydrogen compressor. Chem. Eng. J. 2019, 369, 432–442. [Google Scholar] [CrossRef]
- Zou, J.; Jin, Y.; Wen, Z.; Xing, S.; Han, N.; Yao, K.; Zhao, Z.; Chen, M.; Fan, J.; Li, H.; et al. Insights into electrochemical hydrogen compressor operating parameters and membrane electrode assembly degradation mechanisms. J. Power Sources 2021, 484, 229249. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Malakhov, A.A.; Bessarabov, D.G. A thermally conductive Pt/AAO catalyst for hydrogen passive autocatalytic recombination. Catalysts 2021, 11, 491. [Google Scholar] [CrossRef]
- Schefer, R.W.; Robben, F.; Cheng, R.K. Catalyzed combustion of H2/air mixtures in a flat-plate boundary layer: I. Experimental results. Combust. Flame 1980, 38, 51–63. [Google Scholar] [CrossRef][Green Version]
- Haruta, M.; Souma, Y.; Sano, H. Catalytic combustion of hydrogen—II. An experimental investigation of fundamental conditions for burner design. Int. J. Hydrogen Energy 1982, 7, 729–736. [Google Scholar] [CrossRef]
- Norton, D.G.; Wetzel, E.D.; Vlachos, D.G. Thermal management in catalytic microreactors. Ind. Eng. Chem. Res. 2006, 45, 76–84. [Google Scholar] [CrossRef]
- Saint-Just, J.; der Kinderen, J. Catalytic combustion: From reaction mechanism to commercial applications. Catal. Today 1996, 29, 387–395. [Google Scholar] [CrossRef]
- Haruta, M.; Sano, H. Catalytic combustion of hydrogen—IV. Fabrication of prototype catalytic heaters and their operating properties. Int. J. Hydrogen Energy 1982, 7, 801–807. [Google Scholar] [CrossRef]
- Fumey, B.; Buetler, T.; Vogt, U.F. Ultra-low NOx emissions from catalytic hydrogen combustion. Appl. Energy 2018, 213, 334–342. [Google Scholar] [CrossRef]
- Norton, D.G.; Wetzel, E.D.; Vlachos, D.G. Fabrication of single-channel catalytic microburners: Effect of confinement on the oxidation of hydrogen/air mixtures. Ind. Eng. Chem. Res. 2004, 43, 4833–4840. [Google Scholar] [CrossRef]
- Bamberger, C.E.; Braunstein, J. Hydrogen: A versatile element: Hydrogen generated from water may prove very valuable in extending the world’s dwindling hydrocarbon supply. Am. Sci. 1975, 63, 438–447. [Google Scholar]
- Sharer, J.C.; Pangborn, J.B. Utilization of hydrogen as an appliance fuel. In Proceedings of the Hydrogen Energy Conference, Miami Beach, FL, USA, 18–20 March 1975; pp. 875–887. [Google Scholar]
- Mori, Y.; Miyauchi, T.; Hirano, M. Catalytic combustion of premixed hydrogen-air. Combust. Flame 1977, 30, 193–205. [Google Scholar] [CrossRef]
- Slack, G.A. Platinum as a thermal conductivity standard. J. Appl. Phys. 1964, 35, 339–344. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Fujii, M.; Ago, H.; Takahashi, K.; Ikuta, T.; Abe, H.; Shimizu, T. Thermal and electrical conductivity of a suspended platinum nanofilm. Appl. Phys. Lett. 2005, 86, 171912. [Google Scholar] [CrossRef]
- Powell, R.W.; Tye, R.P.; Woodman, M.J. The thermal conductivity and electrical resistivity of polycrystalline metals of the platinum group and of single crystals of ruthenium. J. Less Common Met. 1967, 12, 1–10. [Google Scholar] [CrossRef]
- Schefer, R.W. Catalyzed combustion of H2/air mixtures in a flat plate boundary layer: II. Numerical model. Combust. Flame 1982, 45, 171–190. [Google Scholar] [CrossRef]
- Haruta, M.; Sano, H. Catalytic combustion of hydrogen I—Its role in hydrogen utilization system and screening of catalyst materials. Int. J. Hydrogen Energy 1981, 6, 601–608. [Google Scholar] [CrossRef]
- Mercea, J.; Grecu, E.; Fodor, T.; Kreibik, S. Catalytic combustor for hydrogen. Int. J. Hydrogen Energy 1982, 7, 483–487. [Google Scholar] [CrossRef]
- Inui, T.; Miyamoto, Y.; Takegami, Y. Low temperature oxidation of hydrogen enhanced by spillover on a nickel-based composite catalyst. In Spillover of Adsorbed Species; Pajonk, G.M., Teichner, S.J., Germain, J.E., Eds.; Elsevier: Studies in Surface Science and Catalysis: Amsterdam, The Netherlands, 1983; Volume 17, pp. 181–190. [Google Scholar]
- Fakheri, A.; Buckius, R.O.; Masel, R.I. Combustion of hydrogen-rich mixtures on a nickel oxide catalyst. Combust. Flame 1988, 72, 259–269. [Google Scholar] [CrossRef]
- Ikeda, H.; Sato, J.; Williams, F.A. Surface kinetics for catalytic combustion of hydrogen-air mixtures on platinum at atmospheric pressure in stagnation flows. Surf. Sci. 1995, 326, 11–26. [Google Scholar] [CrossRef]
- Warnatz, J.; Allendorf, M.D.; Kee, R.J.; Coltrin, M.E. A model of elementary chemistry and fluid mechanics in the combustion of hydrogen on platinum surfaces. Combust. Flame 1994, 96, 393–406. [Google Scholar] [CrossRef]
- Ikeda, H.; Libby, P.A.; Williams, F.A.; Sato, J.i. Catalytic combustion of hydrogen-air mixtures in stagnation flows. Combust. Flame 1993, 93, 138–148. [Google Scholar] [CrossRef]
- Rinnemo, M.; Deutschmann, O.; Behrendt, F.; Kasemo, B. Experimental and numerical investigation of the catalytic ignition of mixtures of hydrogen and oxygen on platinum. Combust. Flame 1997, 111, 312–326. [Google Scholar] [CrossRef]
- Försth, M.; Gudmundson, F.; Persson, J.L.; Rosén, A. The influence of a catalytic surface on the gas-phase combustion of H2 + O2. Combust. Flame 1999, 119, 144–153. [Google Scholar] [CrossRef]
- Das, L.M. Hydrogen-oxygen reaction mechanism and its implication to hydrogen engine combustion. Int. J. Hydrogen Energy 1996, 21, 703–715. [Google Scholar] [CrossRef]
- Kramer, J.F.; Reihani, S.-A.S.; Jackson, G.S. Low-temperature combustion of hydrogen on supported Pd catalysts. Proc. Combust. Inst. 2002, 29, 989–996. [Google Scholar] [CrossRef]
- Seyed-Reihani, S.-A.; Jackson, G.S. Effectiveness in catalytic washcoats with multi-step mechanisms for catalytic combustion of hydrogen. Chem. Eng. Sci. 2004, 59, 5937–5948. [Google Scholar] [CrossRef]
- Choi, W.; Kwon, S.; Dong Shin, H. Combustion characteristics of hydrogen–air premixed gas in a sub-millimeter scale catalytic combustor. Int. J. Hydrogen Energy 2008, 33, 2400–2408. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Yang, W.; Liu, J.; Wang, Z.; Cen, K. Combustion of hydrogen–air in catalytic micro-combustors made of different material. Int. J. Hydrogen Energy 2009, 34, 3535–3545. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Ma, J. Hydrogen catalytic combustion over a Pt/Ce0.6Zr0.4O2/MgAl2O4 mesoporous coating monolithic catalyst. Int. J. Hydrogen Energy 2012, 37, 12941–12946. [Google Scholar] [CrossRef]
- Fernández, A.; Arzac, G.M.; Vogt, U.F.; Hosoglu, F.; Borgschulte, A.; Jiménez de Haro, M.C.; Montes, O.; Züttel, A. Investigation of a Pt containing washcoat on SiC foam for hydrogen combustion applications. Appl. Catal. B Environ. 2016, 180, 336–343. [Google Scholar] [CrossRef]
- Deshpande, P.; Madras, G. Noble metal ionic sites for catalytic hydrogen combustion: Spectroscopic insights. Phys. Chem. Chem. Phys. 2010, 13, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Mercea, J.; Grecu, E.; Fodor, T. Heating of a testing room by use of a hydrogen-fueled catalytic heater. Int. J. Hydrogen Energy 1981, 6, 389–395. [Google Scholar] [CrossRef]
- Haruta, M.; Sano, H. Catalytic combustion of hydrogen—III. Advantages and disadvantages of a catalytic heater with hydrogen fuel. Int. J. Hydrogen Energy 1982, 7, 737–740. [Google Scholar] [CrossRef]
- Dr. Roger Billings Historical Website. 1977—The Hydrogen Homestead. Available online: https://www.rogerebillings.com/1975-the-hydrogen-homestead/ (accessed on 14 June 2021).
- Pyle, W.; Healy, J.; Cortez, R.; Booth, D. Heatin’ with hydrogen. Home Power 1993, 34, 26–29. [Google Scholar]
- Johnson, T.A.; Kanouff, M.P. Development of a hydrogen catalytic heater for heating metal hydride hydrogen storage systems. Int. J. Hydrogen Energy 2012, 37, 2304–2319. [Google Scholar] [CrossRef]
- Vogt, U.; Fumey, B.; Bielmann, M.; Siong, V.; Gallandat, N.; Züttel, A. Catalytic hydrogen combustion on porous SiC ceramics. In Proceedings of the European Fuel Cell Forum 2011, Lucerne, Switzerland, 28 June–1 July 2011; pp. 45–54. [Google Scholar]
- Du Preez, S.P.; Beukes, J.P.; van Zyl, P.G.; Tangstad, M.; Tiedt, L.R. Silicon carbide formation enhanced by in-situ-formed silicon nitride: An approach to capture thermal energy of CO-rich off-gas combustion. Metall. Mater. Trans. B 2018, 49, 3151–3163. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Design Safety Considerations for Water Cooled Small Modular Reactors Incorporating Lessons Learned from the Fukushima Daiichi Accident; IAEA-TECDOC-1785; IAEA: Vienna, Austria, 2016. [Google Scholar]
- Blanchat, T.K.; Malliakos, A. Analysis of hydrogen depletion using a scaled passive autocatalytic recombiner. Nucl. Eng. Des. 1999, 187, 229–239. [Google Scholar] [CrossRef]
- Reinecke, E.-A.; Tragsdorf, I.M.; Gierling, K. Studies on innovative hydrogen recombiners as safety devices in the containments of light water reactors. Nucl. Eng. Des. 2004, 230, 49–59. [Google Scholar] [CrossRef]
- Kelm, S.; Schoppe, L.; Dornseiffer, J.; Hofmann, D.; Reinecke, E.-A.; Leistner, F.; Jühe, S. Ensuring the long-term functionality of passive auto-catalytic recombiners under operational containment atmosphere conditions—An interdisciplinary investigation. Nucl. Eng. Des. 2009, 239, 274–280. [Google Scholar] [CrossRef]
- Anpilov, S.V.; Grigoruk, D.G.; Kondratenko, P.S.; Khristenko, E.B.; Chizhov, M.E. Mathematical modeling of heat and mass transfer in a passive autocatalytic recombiner. Therm. Eng. 2013, 60, 818–822. [Google Scholar] [CrossRef]
- Arnould, F.; Bachellerie, E.; Auglaire, M.; Boeck, B.D.; Braillard, O.; Eckardt, B.; Ferroni, F.; Moffett, R.; Van Goethem, G. State of the art on hydrogen passive auto-catalytic recombiner (european union Parsoar project). In Proceedings of the 9th International Conference on Nuclear Engineering, Nice, France, 8–12 April 2001. [Google Scholar]
- Malakhov, A.A.; Avdeenkov, A.V.; du Toit, M.H.; Bessarabov, D.G. CFD simulation and experimental study of a hydrogen leak in a semi-closed space with the purpose of risk mitigation. Int. J. Hydrogen Energy 2020, 45, 9231–9240. [Google Scholar] [CrossRef]

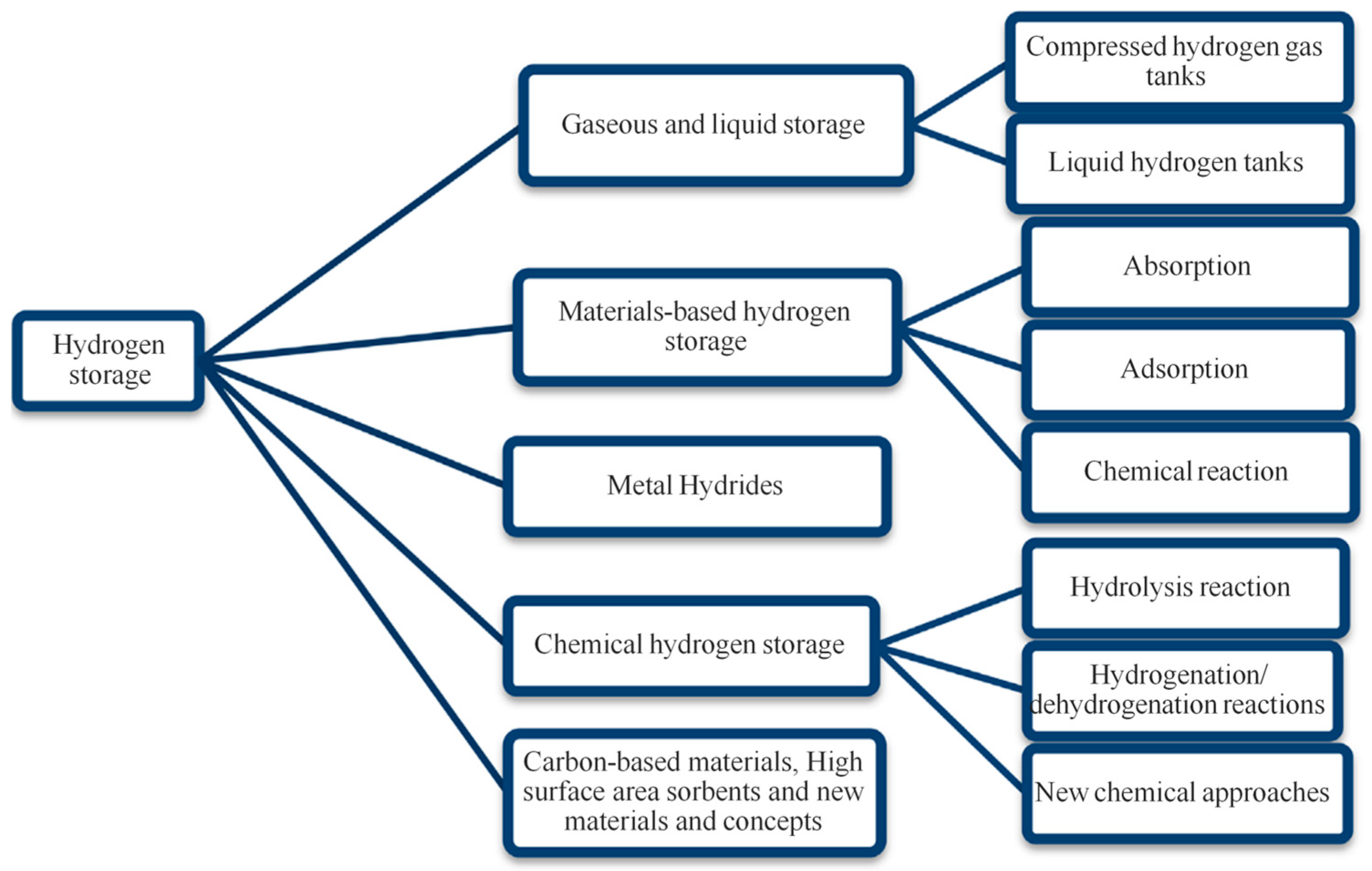


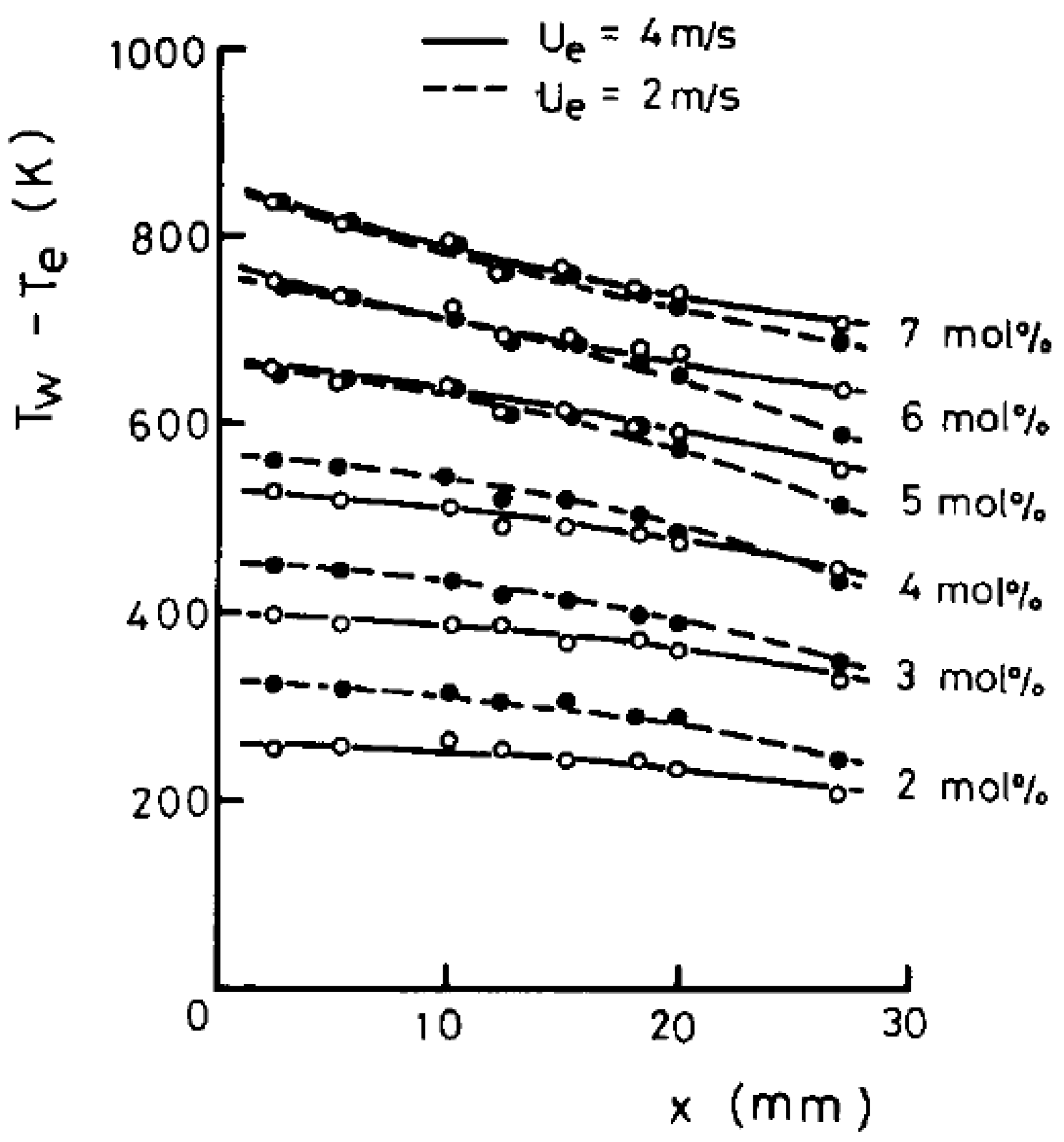


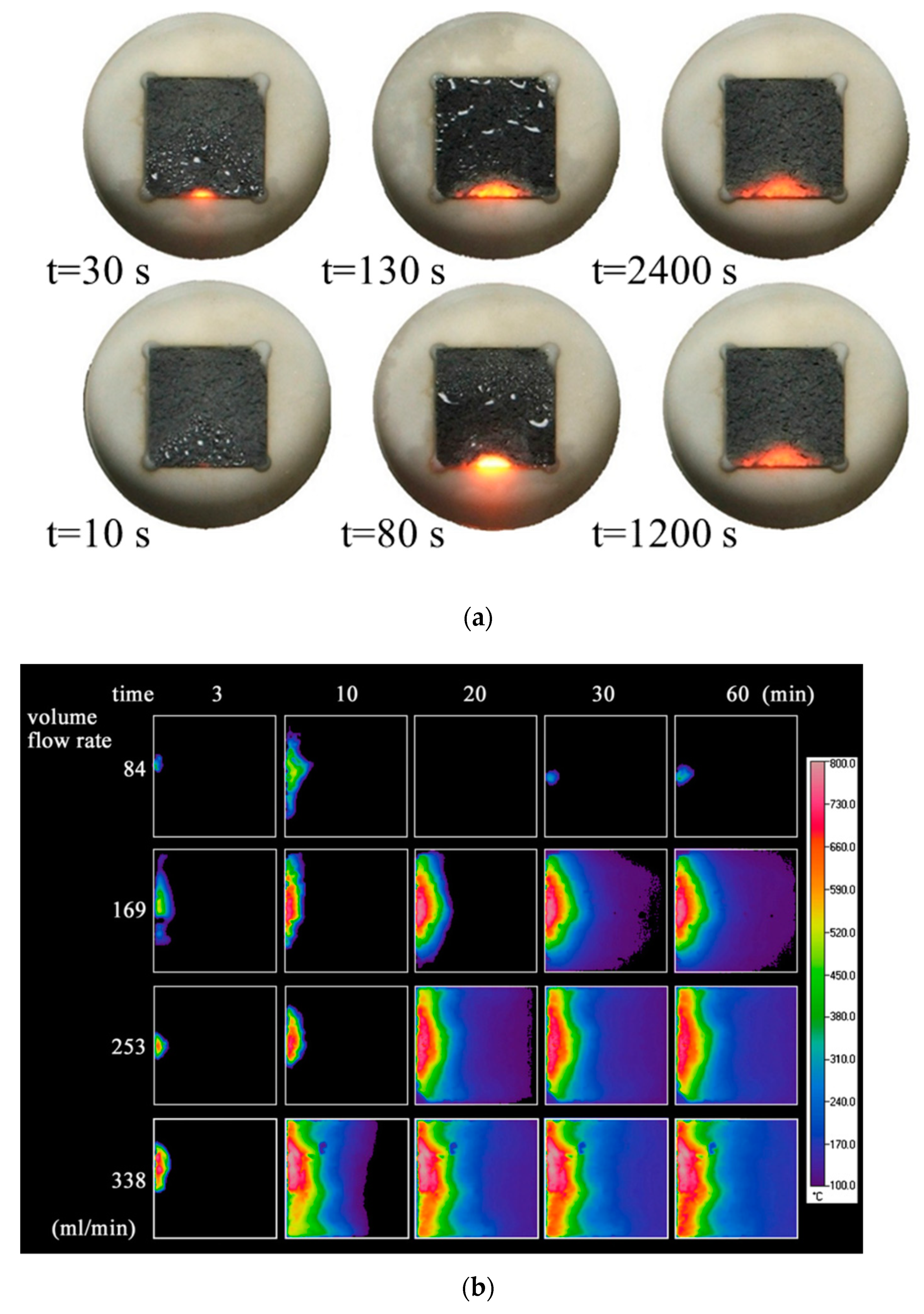
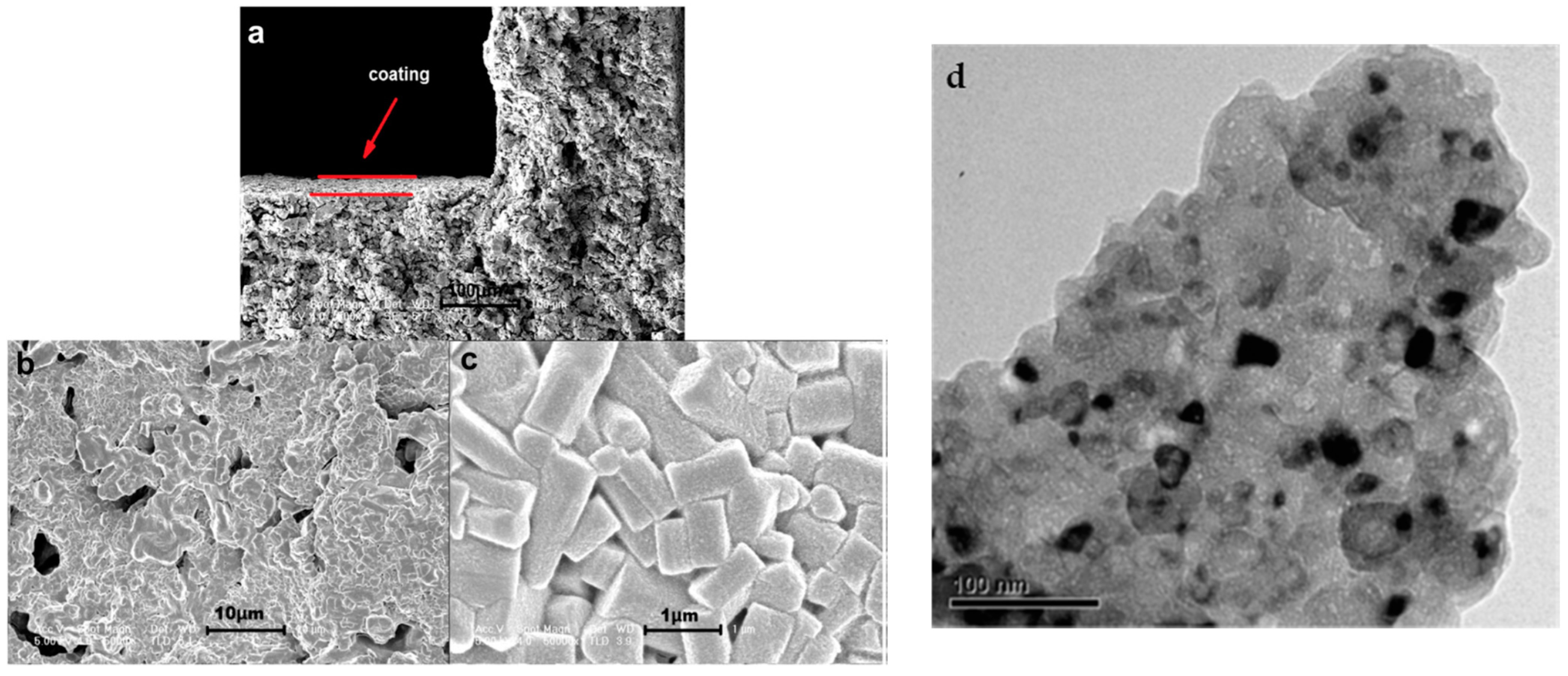
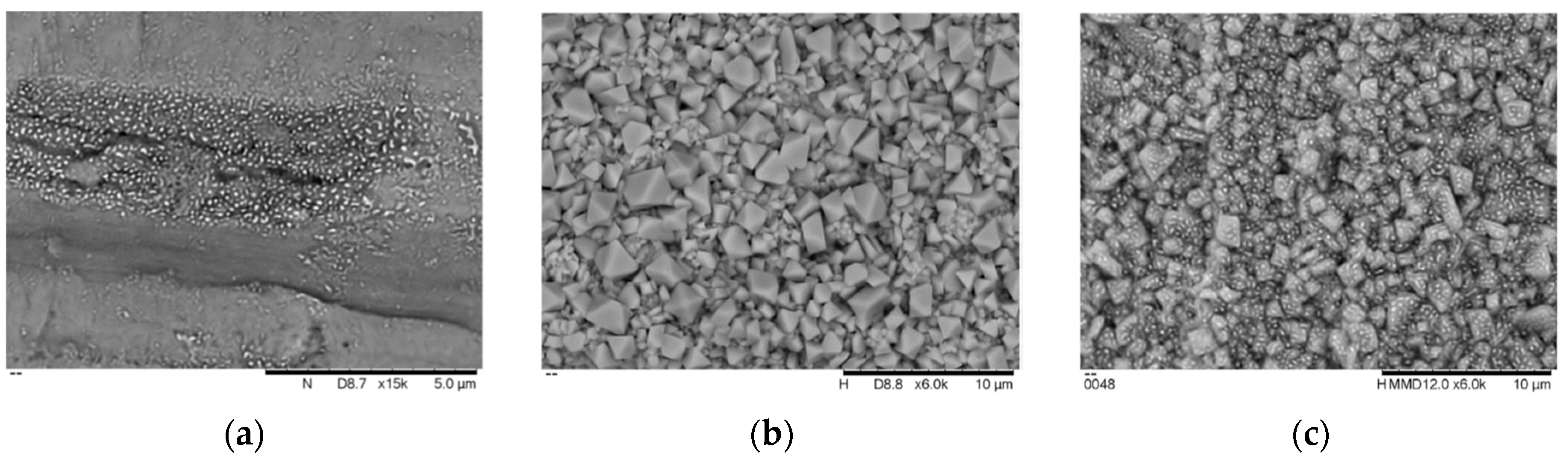
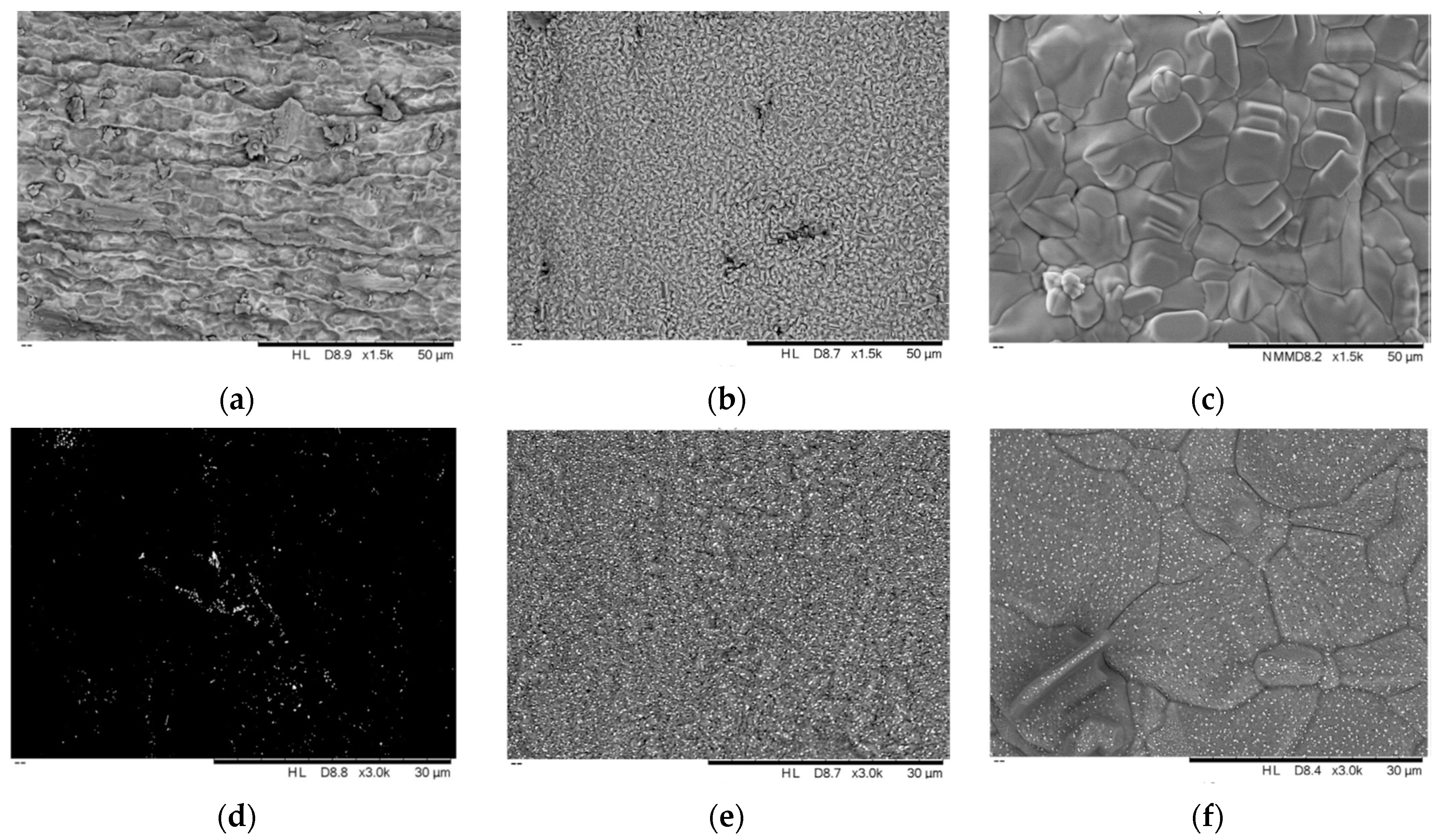

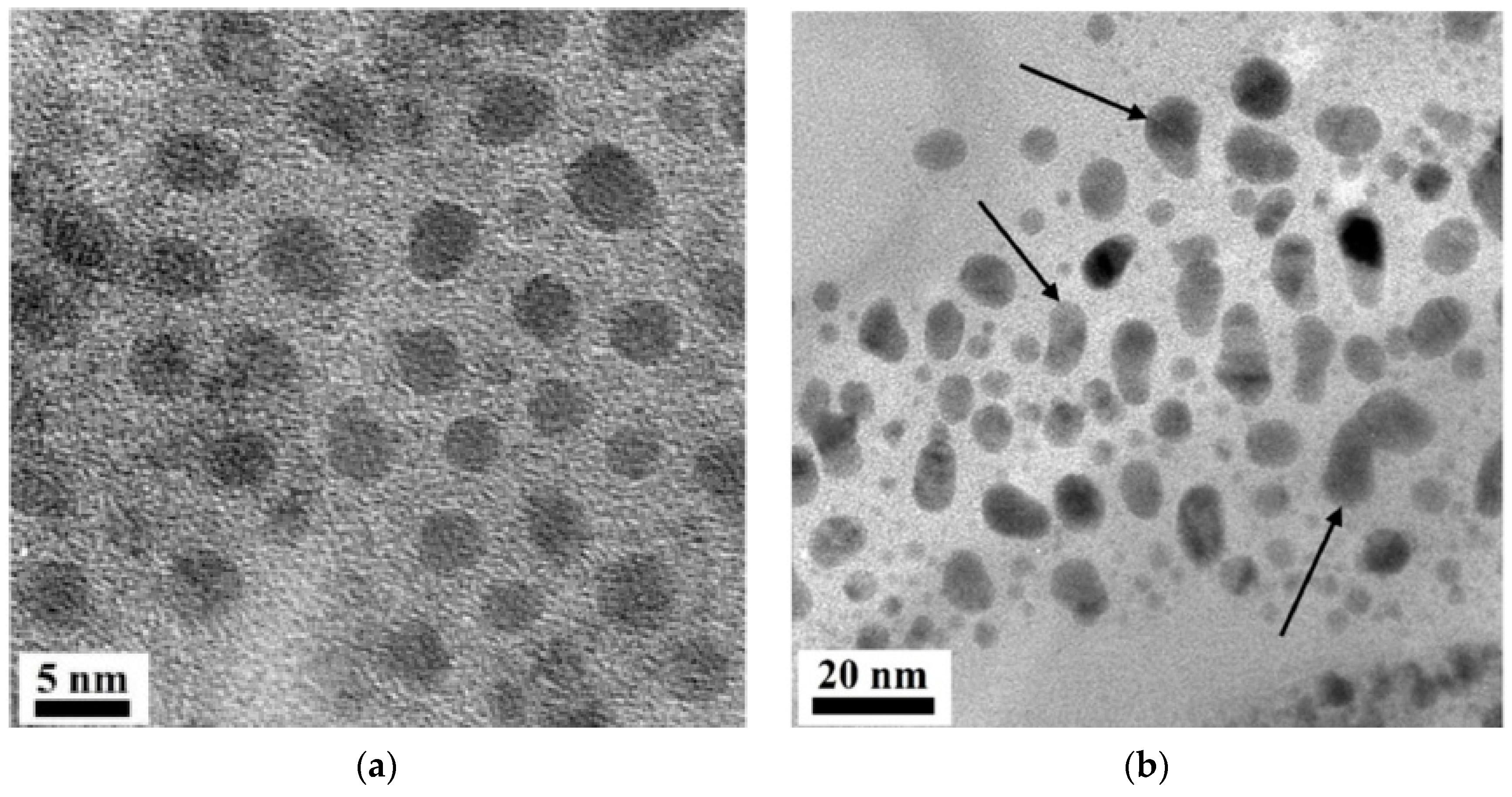
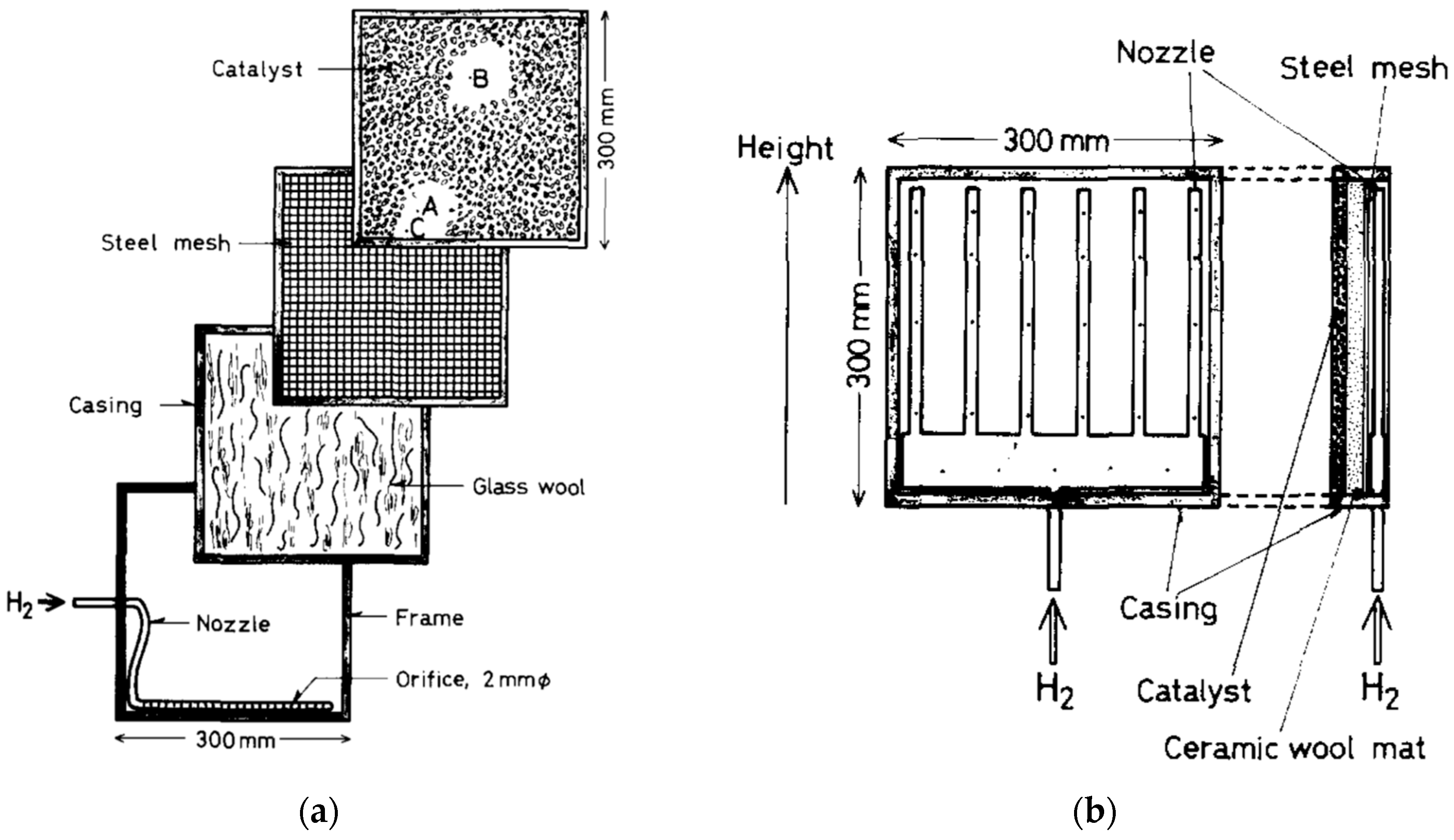
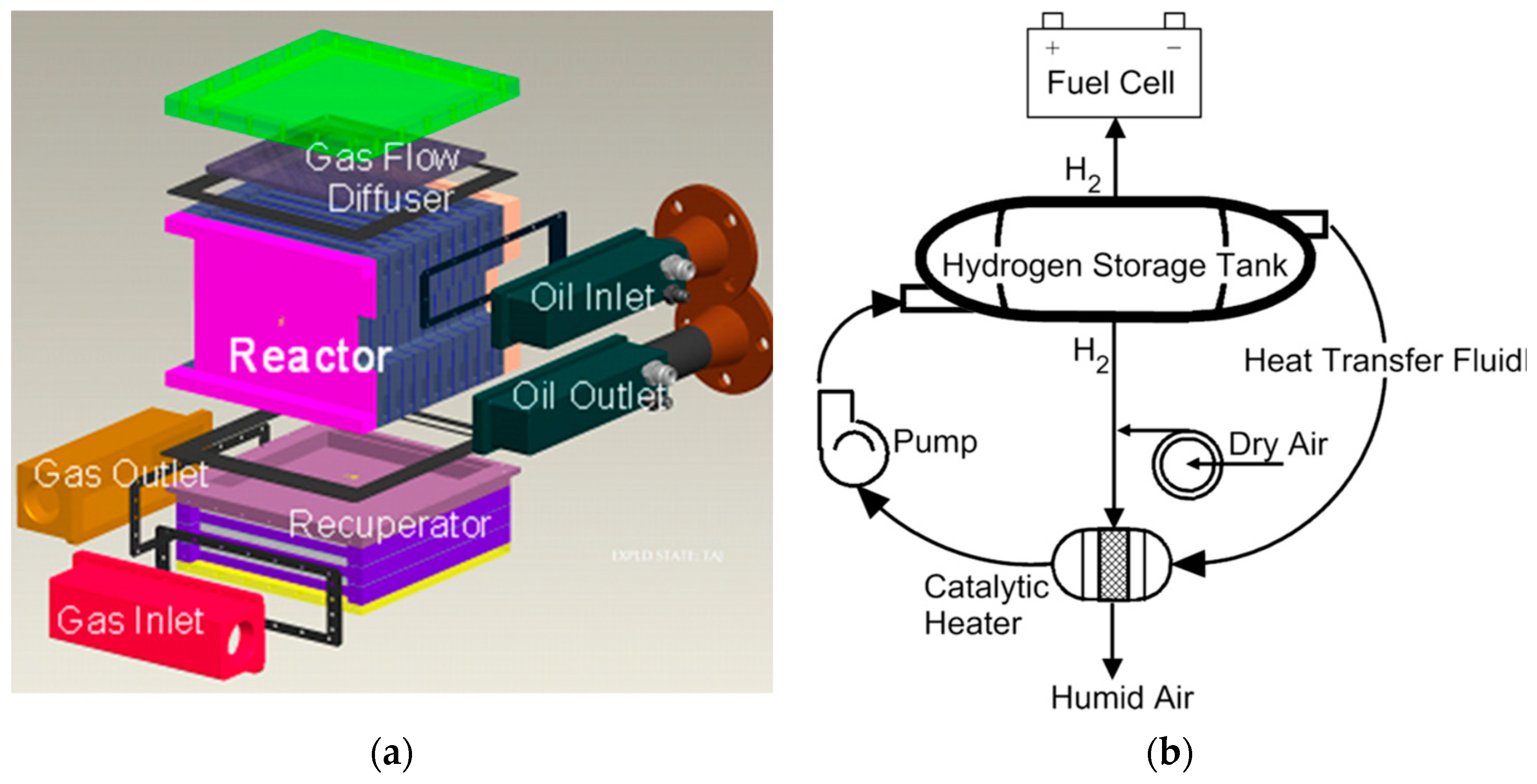
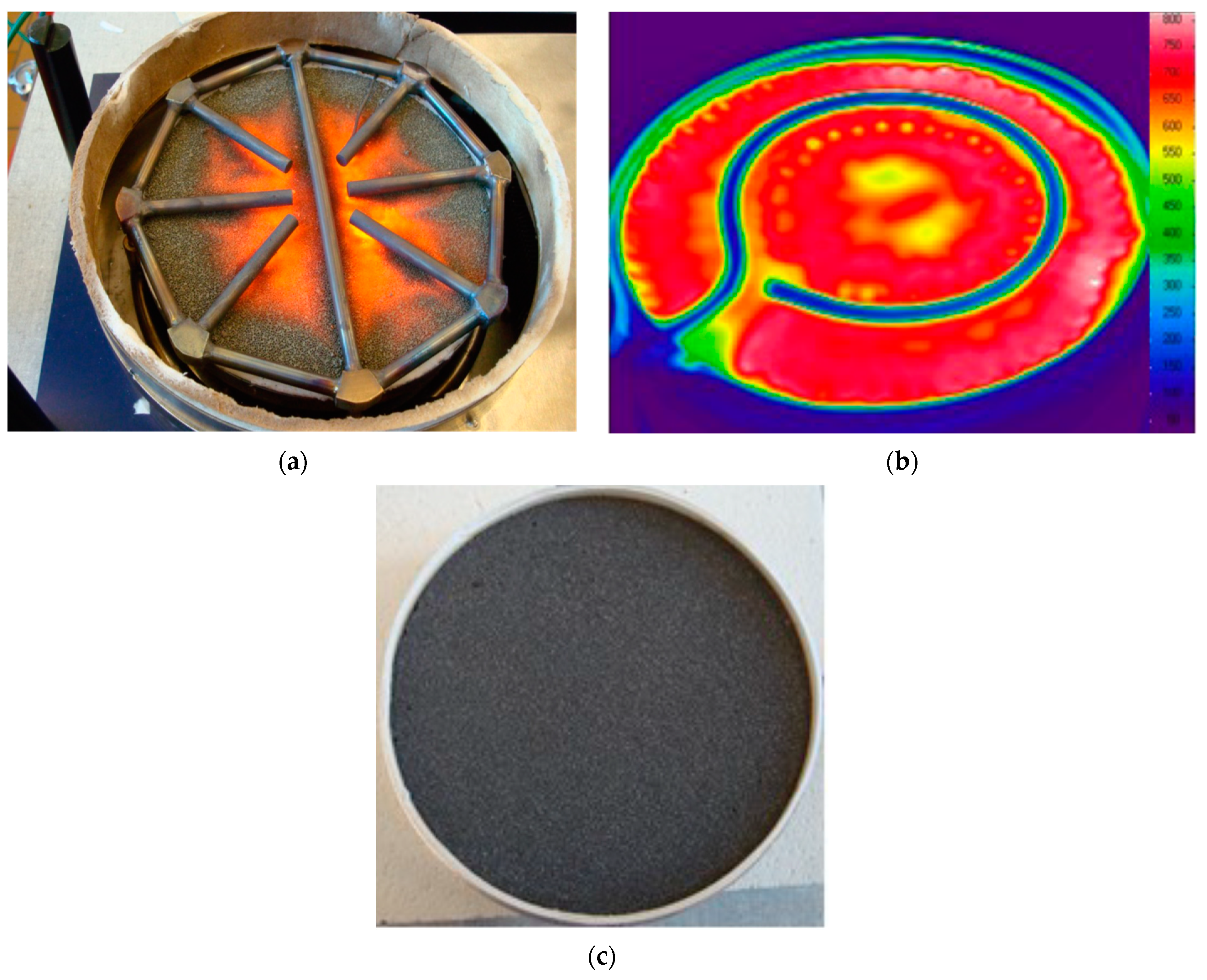
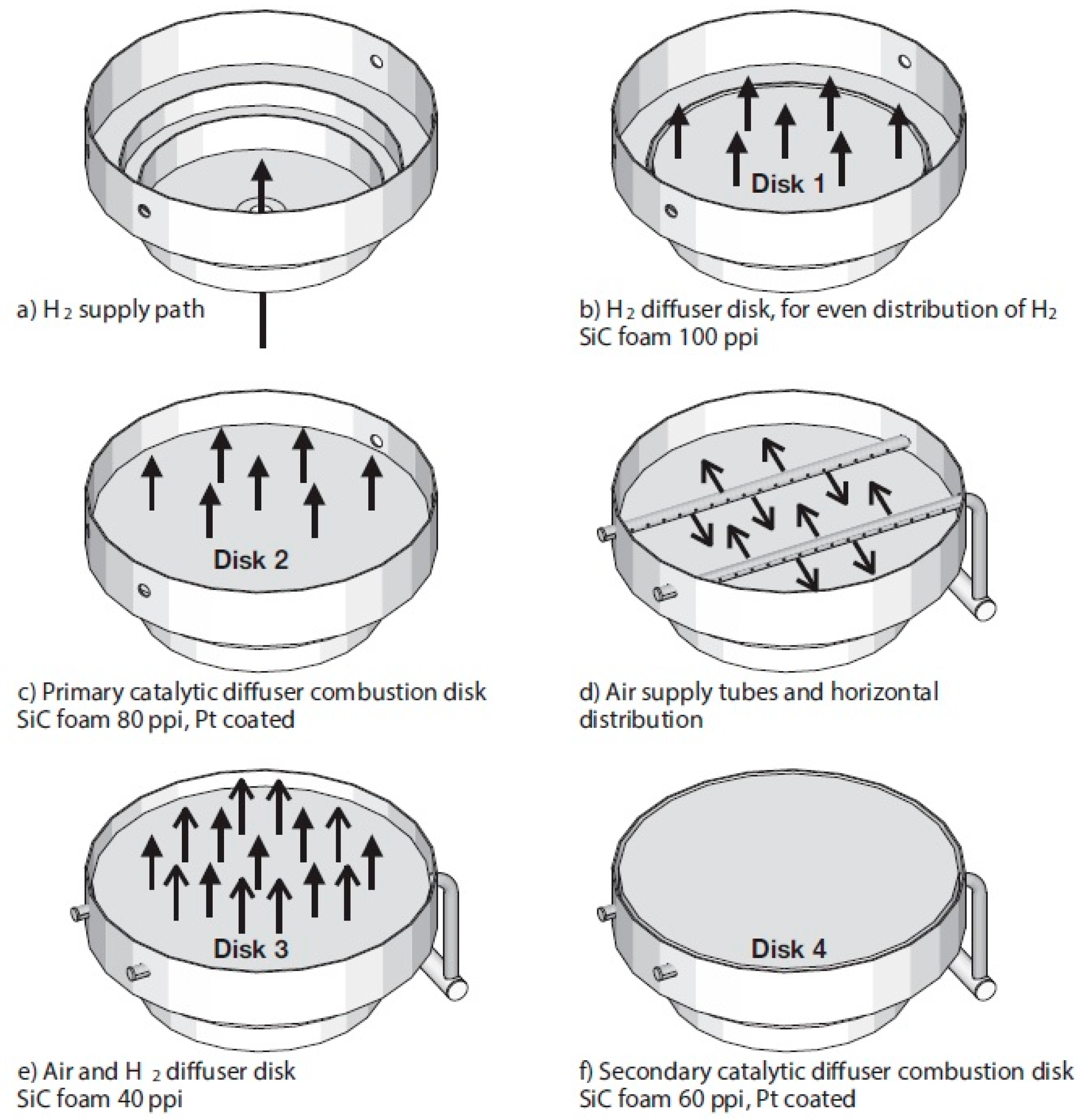

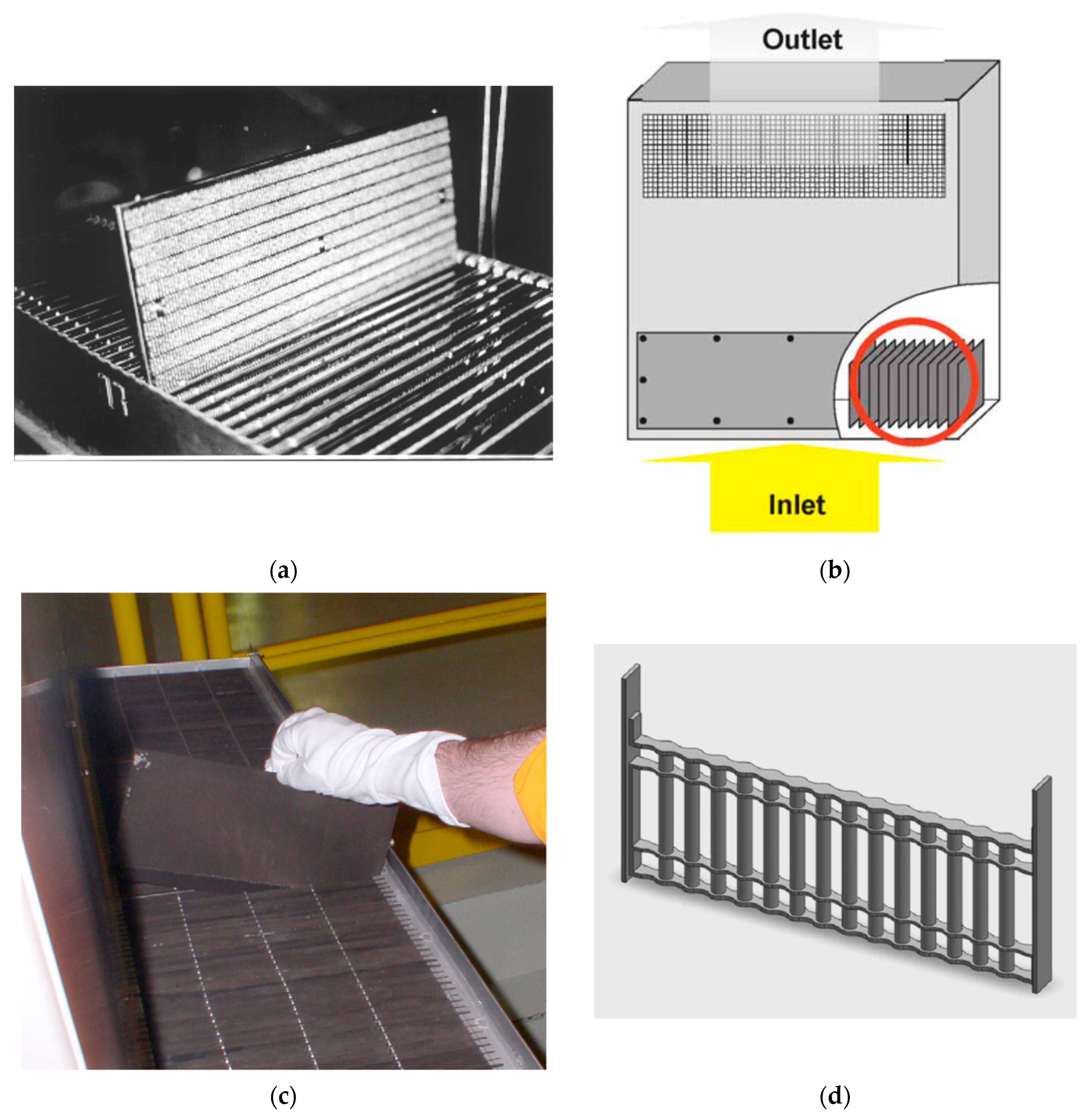

| Properties | Value |
|---|---|
| Flammability range | 4.0–75.0 vol% |
| Minimal ignition energy | 0.02 mJ |
| Autoignition temperature | 585 °C |
| Adiabatic flame temperature | 2045 °C |
| Combustion velocity | 2.65–3.25 m/s |
| Diffusivity in air | 0.63 cm2/s |
| Catalyst | Support | Comment | Reference, Year |
|---|---|---|---|
| Pt plate | – | Nonuniform active site distribution. | [151], 1977 |
| Pt | Quartz plate | Pre-heating required; flame formation. | [142], 1980 |
| Pd | Ni foam | Nonuniform distribution of a surface temperature (ΔT > 200 °C); high combustion efficiency (>90% hydrogen conversion). | [143], 1982 |
| Pt | Ceramic honeycomb | Uniform temperature distribution (ΔT of approximately 50 °C); high efficiency (approximately 90% of hydrogen conversion). | [143], 1982 |
| Co–Mn–Ag oxide (20:4:1 atomic ratio) | Ceramic foam | Uniform temperature distribution (ΔT of approximately 50 °C); high efficiency (93% hydrogen conversion); pre-heating required (50 °C). | [143], 1982 |
| Pt | Fibrous plate | High combustion efficiency (1.0); nonuniform temperature distribution (ΔT up to 120 °C). | [157], 1982 |
| Pt | Ceramic plate | High efficiency (0.95); relatively uniform temperature distribution (ΔT of <60 °C). | [157], 1982 |
| Ni (14.6 wt %) | Ceramic fiber plate (Fiberfrax) | No activity. | [158], 1983 |
| Ce2O3 (4.0 wt %) | Ceramic fiber plate (Fiberfrax) | No activity. | [158], 1983 |
| Pt (0.5 wt %) | Ceramic fiber plate (Fiberfrax) | High combustion efficiency (96.6% of oxygen conversion; the amount of absorbed hydrogen was 0.53 mg/g catalyst). | [158], 1983 |
| Ni (13.9 wt %)–Ce2O3 (3.4 wt %)–Pt (0.4 wt %) | Ceramic fiber plate (Fiberfrax) | High combustion efficiency (100% of O2 conversion and the amount of absorbed hydrogen was 2.83 mg/g catalyst). | [158], 1983 |
| Ni plate | – | Pre-heating required (367 °C); Activation by heating in oxygen at 400 °C for 1 h and reduction in hydrogen at 400 °C for 1 h before every test required. | [159], 1988 |
| Pd | γ-Al2O3 washcoat | Low catalytic activity (hydrogen conversion: <85%); strong dependence on the presence of water. | [166], 2002 |
| Pt (0.4–3.73 wt %) | Isolite B5 | High hydrogen conversion (>95%); nonuniform temperature distribution (ΔT up to 570 °C). | [168], 2008 |
| Pt | Quartz glass | Low thermal conductivity (ΔT up to 1300 °C); hotspot formation. | [169], 2009 |
| Pt | Alumina ceramic | Low thermal conductivity (ΔT up to 1050 °C); hotspot formation. | [169], 2009 |
| Pt | Coper | Relatively uniform temperature distribution (ΔT up to 100 °C). | [169], 2009 |
| Pt Pd | ZrO2 TiO2 | The catalytic activity was higher for Pt/Pd-based TiO2 catalysts; 100% hydrogen conversion was obtained for TiO2 catalysts at 100 °C. | [172], 2010 |
| Pt/Ce0.6Zr0.4O2/MgAl2O4 | Cordierite | High hydrogen conversion (>95%); required input hydrogen concentration of >3 vol%. | [170], 2012 |
| Pt (0.27 wt %) | SiC foam | Low catalyst dispersion (8.5%); high catalyst efficiency (100% of hydrogen conversion); CHC initiated at room temperature. | [171], 2016 |
| Pt (38.3 µg of Pt per 4 cm2 of support side) | SSM | The support required pre-treatment by calcination at 500–1000 °C; pre-heating required (40 °C); Pt aggregation occurred. | [129], 2019 |
| Pt (43.6 µg of Pt per 4 cm2 of the support side | Ti mesh | The support required pre-treatment by calcination at 900–1200 °C; pre-heating required (70 °C); Pt aggregation occurred. | [128], 2020 |
| Pt (1.01 wt %) | Al/AAO | High thermal conductivity (no hotspots formation); CHC initiated at room temperature. | [130], 2020 |
| Pt | Al/AAO | High thermal conductivity (ΔT was 23 °C); CHC initiated at room temperature; high hydrogen conversion (>96%). | [141], 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies. Energies 2021, 14, 4897. https://doi.org/10.3390/en14164897
Kozhukhova AE, du Preez SP, Bessarabov DG. Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies. Energies. 2021; 14(16):4897. https://doi.org/10.3390/en14164897
Chicago/Turabian StyleKozhukhova, Alina E., Stephanus P. du Preez, and Dmitri G. Bessarabov. 2021. "Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies" Energies 14, no. 16: 4897. https://doi.org/10.3390/en14164897
APA StyleKozhukhova, A. E., du Preez, S. P., & Bessarabov, D. G. (2021). Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies. Energies, 14(16), 4897. https://doi.org/10.3390/en14164897








