Wear Resistance of Spark Ignition Engine Piston Rings in Hydrogen-Containing Environments
Abstract
:1. Introduction, State of the Art, and Problem Formulation
2. Interaction of External and Internal Hydrogen in “Cylinder–Piston Ring” Friction Couple
3. Hydrogen Wear of Piston Rings of Engine Cylinders and Ways to Reduce It
- -
- The maximum ratio of the concentration of internal hydrogen is achieved for the porous chromium ring material and the minimum for the molybdenum material, and the bottom ring was tinned;
- -
- The ratio of the concentration of internal hydrogen for rings made of porous chromium used in various engines is questionable.
- -
- The maximum ratio of the concentration of internal hydrogen is achieved for the materials of rings with different coatings for the Nippon manufacturer rings, and the minimum for the Teikoku rings;
- -
- A significant decrease in the concentration of hydrogen is achieved due to various types of coatings of piston rings, compared to rings without coatings;
- -
- In comparison to the maximum hydrogen absorption (different for different structural materials), in the case of low hydrogen absorption, the wear resistance of the rings increases by 20–25%.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balyts’kyi, O.I.; Chmiel, J.; Krause, P.; Niekrasz, J.; Maciag, M. Role of hydrogen in the cavitation fracture of 45 steel in lubricating media. Mater. Sci. 2009, 45, 651–654. [Google Scholar] [CrossRef]
- Balyts’kyi, O.I.; Abramek, K.F.; Mruzik, M.; Shtoeck, T.; Osipowicz, T. Evaluation of the losses of hydrogen-containing gases in the process of wear of pistons of an internal-combustion engine. Mater. Sci. 2017, 53, 289–294. [Google Scholar] [CrossRef]
- Kindrachuk, M.V.; Vol’chenko, D.A.; Vol’chenko, N.A.; Stebeletskaya, N.M.; Voznyi, A.V. Influence of hydrogen on the wear resistance of materials in the friction couples of braking units. Mater. Sci. 2017, 53, 282–288. [Google Scholar] [CrossRef]
- Dmytrakh, I. Corrosion fracture of structural metallic materials: Effect of electrochemical conditions in crack. Strain Int. J. Exp. Mech. 2011, 47, 427–435. [Google Scholar] [CrossRef]
- Suranov, G.I. Hydrogen: Fracture, Wear, Lubrications of Machine Parts; UTU: Uchta, Russia, 2015; 224p. [Google Scholar]
- Rovin, L.E.; Zayac, T.M.; Valickaya, O.M. Recycling of ferrous metal shavings. Cast. Metall. 2017, 89, 94–101. [Google Scholar] [CrossRef]
- Myshkin, M.I.; Petrokovets, N.K. Friction, lubrication and wear. In Physical Foundations and Technical Applications of Tribology; Fiz-matlit: Moscow, Russia, 2007; 368p. [Google Scholar]
- Balyts’kyi, O.I.; Abramek, K.F.; Shtoeck, T.; Osipowicz, T. Diagnostics of degradation of the lock of a sealing ring according to the loss of working gases of an internal combustion engine. Mater. Sci. 2014, 50, 156–159. [Google Scholar] [CrossRef]
- Brunhart, M.; Soteriou, C.; Daveau, C.; Gavaises, M. Cavitation erosion risk indicators for a thin gap within a diesel fuel pump. Wear 2020, 203024. [Google Scholar] [CrossRef]
- Yudin, V.M.; Lukashev, E.A.; Stavrovsky, M.E. Tribochemistry of Hydrogen Wear; MGUPS: Moscow, Russia, 2004; 282p. [Google Scholar]
- Liu, D.; Bai, B.; Fang, H.; Zhang, W.; Gu, J.; Chang, K. Effect of temperature and carbide free bainite on the mechanical characteristics of a high strength-low alloy steel. Mater. Sci. Eng. A 2004, 371, 40–44. [Google Scholar] [CrossRef]
- Deulin, E.A.; Michailov, Y.V.; Panfilov, R.A. Mechanics and Physics of Precise Vacuum Mechanisms; Springer: New York, NY, USA, 2012; 234p. [Google Scholar]
- Pashechko, M.; Jozwik, J.; Dziedzic, K.; Usydus, I. Surface hardening of HS6-5-2 quick-cutting steel in the course of chemical thermal treatment. Mater. Sci. 2017, 52, 834–840. [Google Scholar] [CrossRef]
- Simon, L.; Moraes, C.A.M.; Modolo, R.C.E.; Vargas, M.; Calheiro, D.; Brehm, F.A. Recycling of contaminated metallic chip based on eco-efficiency and eco-effectiveness approaches. J. Clean. Prod. 2017, 153, 417–424. [Google Scholar] [CrossRef]
- Weber, U.; Deimel, P.; Saraev, D.; Sattler, E.; Schmauder, S.; Soppa, E. Influence of hydrogen on the deformation behavior of a ferritic fine-grained low alloy steel. Comput. Mater. Sci. 2005, 32, 577–587. [Google Scholar] [CrossRef]
- Holubets, V.M.; Pashechko, M.I.; Dzedzic, K.; Borc, J.; Tisov, A.V. Frictional strength of electric spark coatings from powder wires under friction without lubrication. J. Frict. Wear 2020, 41, 443–446. [Google Scholar] [CrossRef]
- Pashechko, M.; Montusiewicz, J.; Dziedzic, K.; Jozwik, J. Multicriterion assessment of wear resistance of Fe–Mn–C–B eutectic coatings alloyed with Si, Ni, and Cr. Powder Metall. Met. Ceram. 2017, 56, 316–322. [Google Scholar] [CrossRef]
- Jozwik, J.; Dziedzic, K.; Barszcz, M.; Pashechko, M. Analysis and comparative assessment of basic tribological properties of selected polymer composites. Materials 2020, 13, 75. [Google Scholar] [CrossRef] [Green Version]
- Balitskii, O.A.; Kolesnikov, V.O.; Balitskii, A.I.; Eliasz, J.J.; Havrylyuk, M.R. Hydrogen effect on the high-nickel surface steel properties during machining and wear with lubricants. Arch. Mater. Sci. Eng. 2020, 104, 49–57. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Kolesnikov, V.O.; Balitskii, A.I. Wear resistance of hydrogenated high nitrogen steel at dry and solid state lubricants assistant friction. Arch. Mater. Sci. Eng. 2019, 98, 57–67. [Google Scholar] [CrossRef]
- Balyts’kyi, O.I.; Krokhmal’nyi, O.O. Pitting corrosion of 12Kh18AG18Sh steel in chloride solutions. Mater. Sci. 1999, 35, 389–394. [Google Scholar] [CrossRef]
- Akid, R.; Dmytrakh, I.M.; Gonzalez-Sanchez, J. Fatigue damage accumulation: The role of corrosion on the early stages of crack development. Corros. Eng. Sci. Technol. 2006, 41, 328–335. [Google Scholar] [CrossRef]
- Michelich, J.L.; Troiano, A.R. Delayed failure in hydrogenated facecentred cubic alloy in nickel. Nature 1963, 197, 996–997. [Google Scholar] [CrossRef]
- Bandurin, D.A.; Tyurnina, A.V.; Geliang, L.Y.; Mishchenko, A.; Zólyomi, V.; Morozov, S.V.; Kumar, R.K.; Gorbachev, R.V.; Kudrynskyi, Z.R.; Pezzini, S.; et al. High electron mobility, quantum Hall effect and anomalous optical response in atomically thin InSe. Nat. Nanotechnol. 2017, 12, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.C.; Voïtchovsky, K.; Dotan, H.; Leroy, C.M.; Cornuz, M.; Stellacci, F.; Hébert, C.; Rothschild, A.; Grätzel, M. Identifying champion nanostructures for solar water-splitting. Nat. Mater. 2013, 12, 842–849. [Google Scholar] [CrossRef] [Green Version]
- Felton, J.; Blundo, E.; Ling, S.; Glover, J.; Kudrynskyi, Z.R.; Makarovsky, O.; Kovalyuk, Z.D.; Besley, E.; Walker, G.; Polimeni, A.; et al. The interaction of hydrogen with the Van der Waals crystal γ-InSe. Molecules 2020, 25, 2526. [Google Scholar] [CrossRef] [PubMed]
- Mudd, G.W.; Svatek, S.A.; Hague, L.; Makarovsky, O.; Kudrynskyi, Z.R.; Mellor, C.J.; Beton, P.H.; Eaves, L.; Novoselov, K.S.; Kovalyuk, Z.D.; et al. High broad-band photoresponsivity of mechanically formed InSe-graphene Van der Waals heterostructures. Adv. Mater. 2015, 27, 3760–3766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhirko, Y.I.; Kovalyuk, Z.D.; Trachevsky, V.V.; Melnik, V.V. NMR investigations of hydrogen intercalates in GaSe layered crystals. NATO Sci. Ser. Environ. Secur. 2012, 2, 443–458. [Google Scholar] [CrossRef]
- Kaminskii, V.M.; Kovalyuk, Z.D.; Pyrlya, M.N.; Gavrilyuk, S.V. Properties of hydrogenated GaSe crystals. Inorg. Mater. 2005, 41, 793–795. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Leshchak, R.L.; Syrotyuk, A.M.; Barna, R.A. Effect of hydrogen concentration on fatigue crack growth behaviour in pipeline steel. Int. J. Hydrog. Energy 2017, 42, 6401–6408. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Ma, L.; Zhao, J.; Wang, B.; Wang, G. Structures, electronic properties, and hydrogen-storage capacity of single-walled TiO2 nanotubes. Phys. E Low Dimens. Syst. Nanostructures 2009, 41, 838–842. [Google Scholar] [CrossRef]
- Ubbelohde, A.R. Anisotropy of synthetic metals. Nature 1971, 232, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Matsuo, T.; Ikeda, T. The effect of graphite size on hydrogen absorption and tensile properties of ferritic ductile cast iron. Procedia Struct. Integr. 2019, 14, 18–25. [Google Scholar] [CrossRef]
- Balyts’kyi, O.O. Elastic characteristics of laminated gallium and indium chalcogenides. Mater. Sci. 2004, 40, 706–709. [Google Scholar] [CrossRef]
- Prajwowski, K.; Golebiewski, W.; Lisowski, M.; Abramek, K.F.; Galdynski, D. Modeling of working machines synergy in the process of the hybrid electric vehicle acceleration. Energies 2020, 13, 5818. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Grab-Rogalski, K. Combusting stability, performance and emission characteristics of a CI engine fueled with diesel/n-butanol blends. Energies 2021, 14, 2817. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Romanov, R.I.; Fominski, D.V.; Novikov, S.M.; Chesnokov, A.V. Features of sliding friction on thin-film Mo–S–C coatings prepared by pulsed laser deposition. J. Frict. Wear 2020, 41, 18–24. [Google Scholar] [CrossRef]
- Osenin, Y.I.; Antoshkina, L.I.; Bugaenko, V.V.; Krivosheya, Y.V.; Chesnokov, A.V. The noise-generating mechanism during the application of disc brakes on rolling stock. J. Frict. Wear 2020, 41, 178–182. [Google Scholar] [CrossRef]

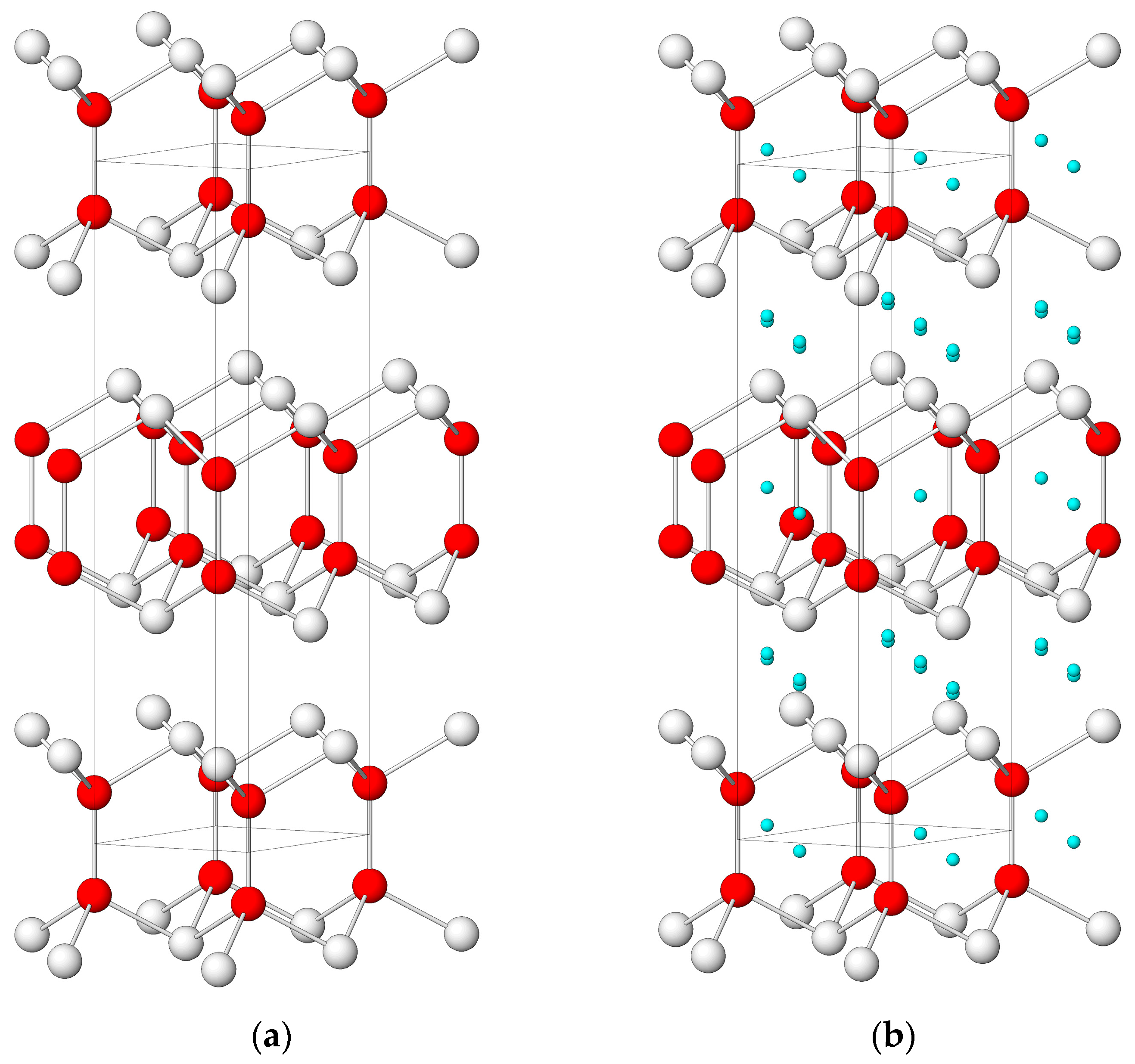



| Diameter, nm | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
|---|---|---|---|---|---|---|
| ηH, % | 3.4 | 4.4 | 5.4 | 6.5 | 7.6 | 8.6 |
| Stages | Processes, Phenomena, and Effects Arising from the Action | |
|---|---|---|
| Alternating Loading in Friction Couple | Result | |
| 1 | Intensive release of hydrogen in the friction zone from decomposed lubricant. | Tribochemical reactions are intensified: When the rings slide against the cylinder, an electromagnetic field is created, which captures atomic hydrogen, directing it into microcracks. |
| 2 | Desorption of water from the cylinder surface. | The temperature of the friction surface rises. |
| 3 | Hydrogen adsorption by the working surface of the cylinder and piston ring materials. | Intensive diffusion of external hydrogen. |
| 4 | Diffusion of atomic hydrogen into the crystal lattice of the piston rings and the surface layers of the cylinder; their intensity is determined by the gradients of the surface and volumetric temperatures and equivalent stresses. | Combination of external hydrogen with carbon (C), which is present in the crystal lattice of the metal (Fe3C); temperature gradients and mechanical-temperature stresses increase. |
| 5 | The hydrogen concentration in the subsurface layer with maximum temperature. | An increase in temperature gradients in the subsurface layer. |
| 6 | A. Low-temperature brittle fracture of the surface layer of metallic friction elements saturated with hydrogen as a result of the formation of a large number of microcracks on the cylinder surface. | The periodic occurrence of a traveling stress wave (tension–compression) enhances the saturation, with the hydrogen of the parts deformed by the friction of the near-surface volumes of the metal; equivalent stresses increase, hydrogen mobilization occurs. |
| B. High-temperature viscous destruction of the rubbing layer of the rings in the form of spreading it on the surface of the cylinder mirror as a result of metal flow. | At temperatures of about 1073–1273 K, the metal surfaces of the rings are oversaturated with hydrogen. | |
| Ring Position | Engine and Coating Type | |||
| PD-10UD | D-50 | D-240 | ZMZ-24 | |
| Chromium | Molybdenum | |||
| Porous | Hard | |||
| Hydrogen Concentration CH, ppm | ||||
| Top | ||||
| Bottom, tin-coated | ||||
| No | Manufacturer | Ring | CH, ppm | Coating |
|---|---|---|---|---|
| 1 | “Riken” | Top | Hard chromium | |
| (Japan) | Bottom | Phosphating | ||
| 2 | “Nippon” | Top | Hard chromium | |
| (Japan) | Bottom | Ferrox | ||
| 3 | “Teikoku” | Top | Phosphating | |
| (Japan) | Bottom | Porous chromium | ||
| 4 | “Getz” | Top | Phosphating | |
| (Germany) | Bottom | Porous chromium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kindrachuk, M.; Volchenko, D.; Balitskii, A.; Abramek, K.F.; Volchenko, M.; Balitskii, O.; Skrypnyk, V.; Zhuravlev, D.; Yurchuk, A.; Kolesnikov, V. Wear Resistance of Spark Ignition Engine Piston Rings in Hydrogen-Containing Environments. Energies 2021, 14, 4801. https://doi.org/10.3390/en14164801
Kindrachuk M, Volchenko D, Balitskii A, Abramek KF, Volchenko M, Balitskii O, Skrypnyk V, Zhuravlev D, Yurchuk A, Kolesnikov V. Wear Resistance of Spark Ignition Engine Piston Rings in Hydrogen-Containing Environments. Energies. 2021; 14(16):4801. https://doi.org/10.3390/en14164801
Chicago/Turabian StyleKindrachuk, Myroslav, Dmytro Volchenko, Alexander Balitskii, Karol F. Abramek, Mykola Volchenko, Olexiy Balitskii, Vasyl Skrypnyk, Dmytro Zhuravlev, Alina Yurchuk, and Valerii Kolesnikov. 2021. "Wear Resistance of Spark Ignition Engine Piston Rings in Hydrogen-Containing Environments" Energies 14, no. 16: 4801. https://doi.org/10.3390/en14164801
APA StyleKindrachuk, M., Volchenko, D., Balitskii, A., Abramek, K. F., Volchenko, M., Balitskii, O., Skrypnyk, V., Zhuravlev, D., Yurchuk, A., & Kolesnikov, V. (2021). Wear Resistance of Spark Ignition Engine Piston Rings in Hydrogen-Containing Environments. Energies, 14(16), 4801. https://doi.org/10.3390/en14164801








