Water-Driven Structural Transformation in Cobalt Trimesate Metal-Organic Frameworks
Abstract
:1. Introduction
2. Materials and Methods
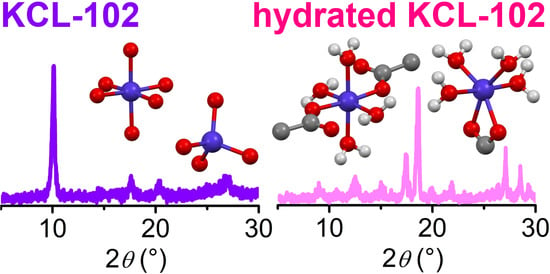
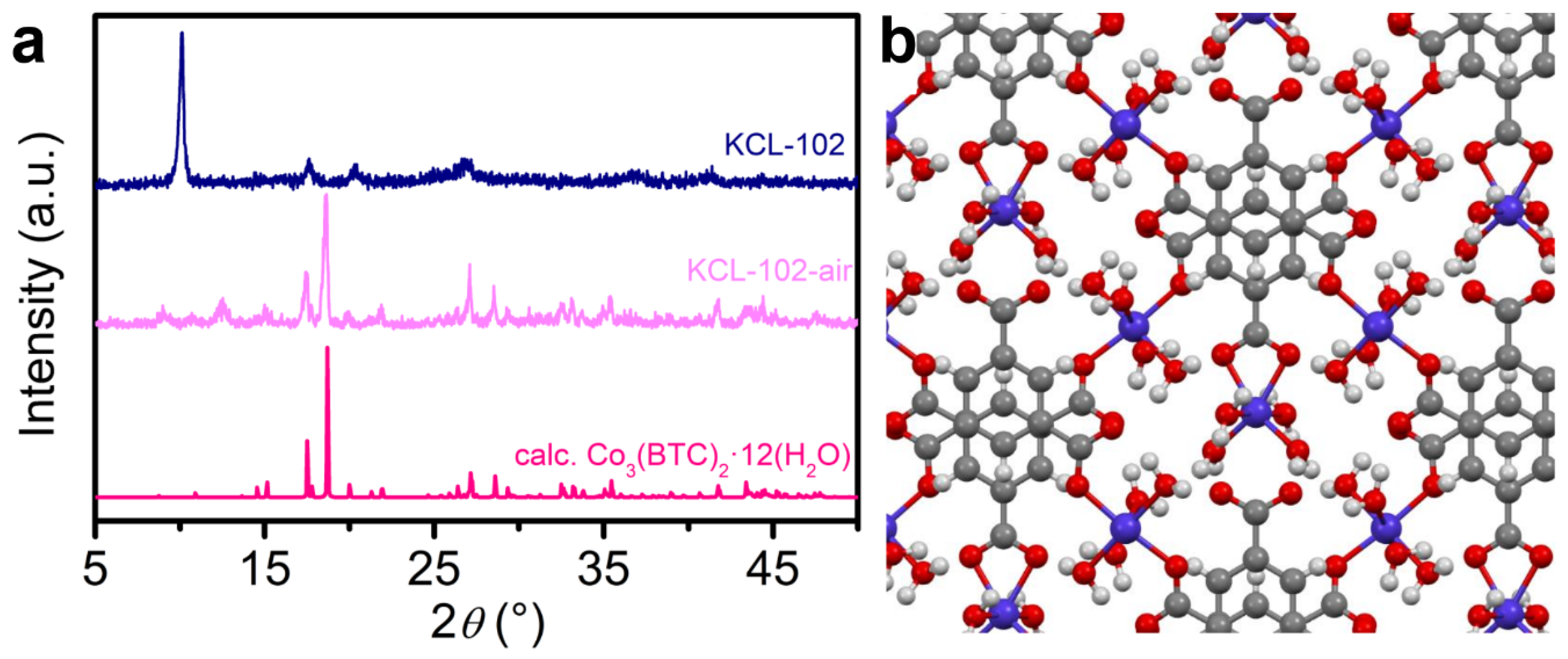
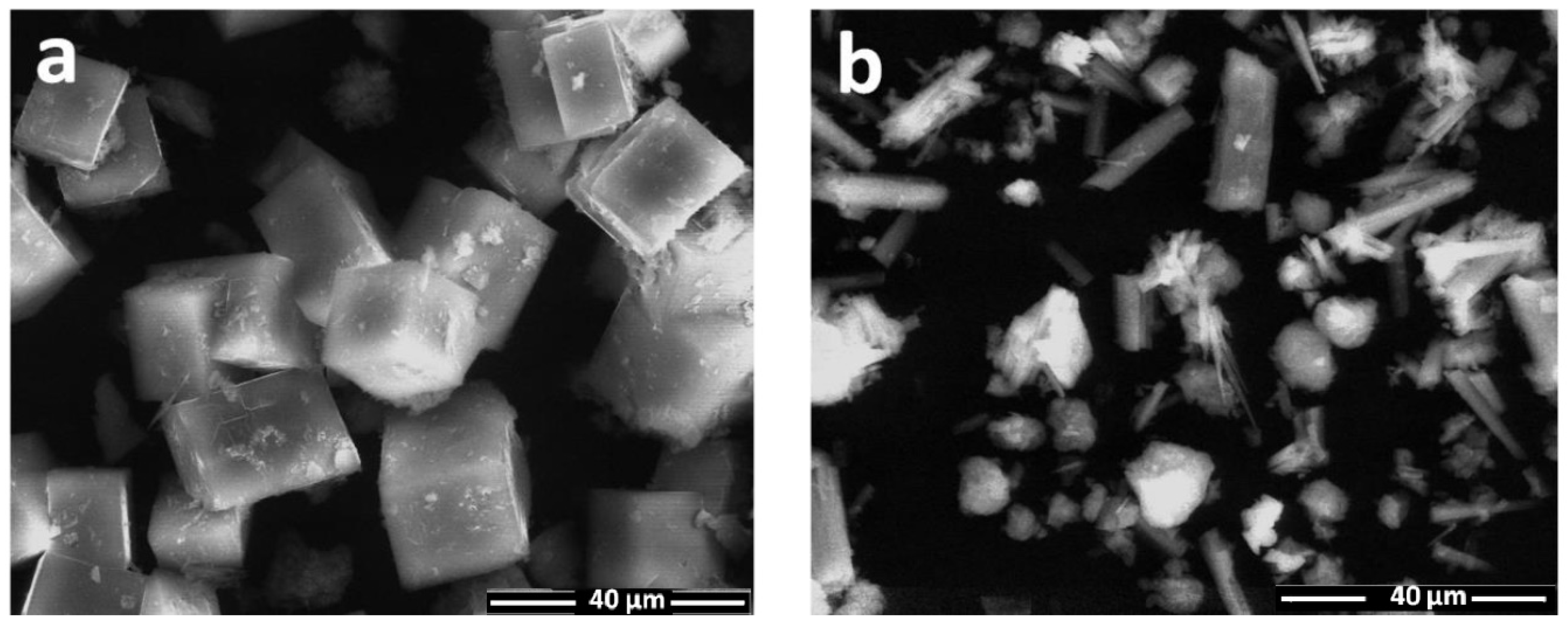
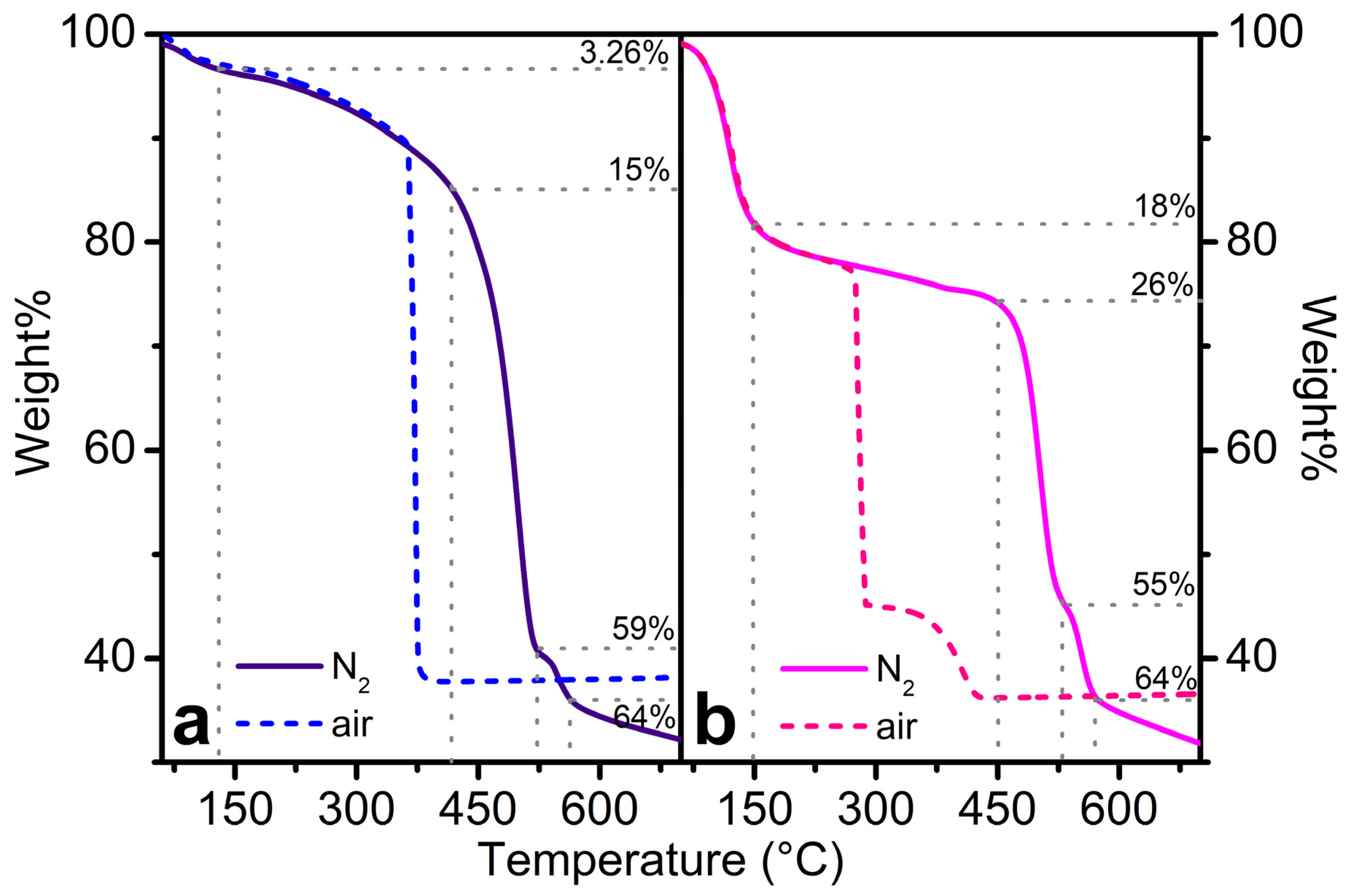
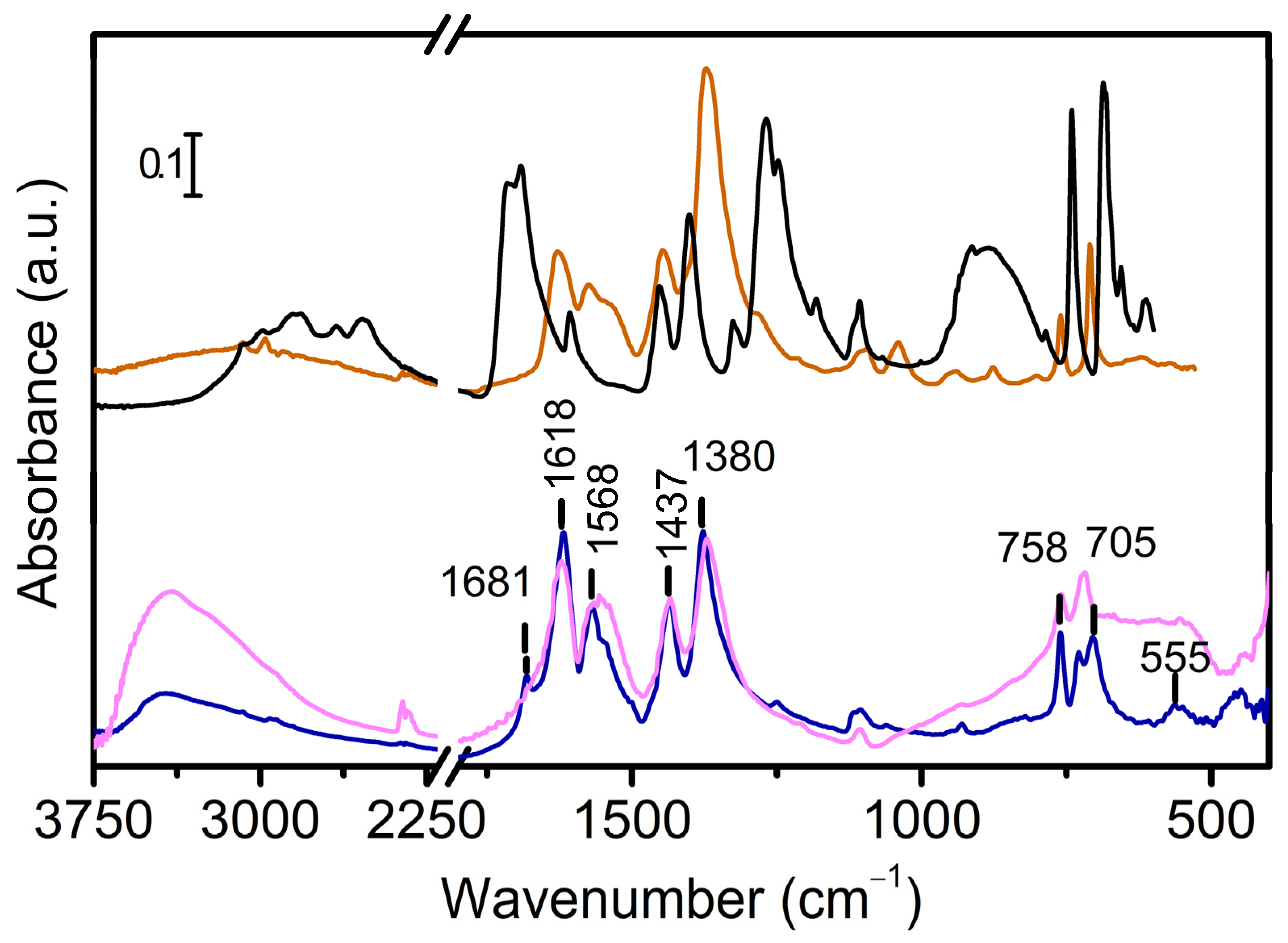
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ralph, F.; Stefano, C.; Seth, M.C.; Wei, Y.; Hexiang, D.; Vincent, G.; Mohamed, E.; David, G.M.; David, F.-J.; Hao, L.; et al. 25 years of Reticular Chemistry. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Alhamami, M.; Doan, H.; Cheng, C.-H. A Review on Breathing Behaviors of Metal-Organic-Frameworks (MOFs) for Gas Adsorption. Materials 2014, 7, 3198–3250. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Wanigarathna, D.K.J.A.; Gao, J.; Liu, B. Metal organic frameworks for adsorption-based separation of fluorocompounds: A review. Mater. Adv. 2020, 1, 310–320. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014. [Google Scholar] [CrossRef] [Green Version]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Pascanu, V.; Miera, G.G.; Inge, A.K.; Martin-Matute, B. Metal-Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Tian, H. Metal-Organic Framework (MOF)-Based Drug Delivery. Curr. Med. Chem. 2020, 27, 5949–5969. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Punde, N.S.; Rawool, C.R.; Rajpurohit, A.S.; Karna, S.P.; Srivastava, A.K. Hybrid Composite Based on Porous Cobalt-Benzenetricarboxylic Acid Metal Organic Framework and Graphene Nanosheets as High Performance Supercapacitor Electrode. ChemistrySelect 2018, 3, 11368–11380. [Google Scholar] [CrossRef]
- Ryder, M.R.; Dona, L.; Vitillo, J.G.; Civalleri, B. Understanding and Controlling the Dielectric Response of Metal-Organic Frameworks. ChemPlusChem 2018, 83, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Shooto, N.D.; Ayawei, N.; Wankasi, D.; Sikhwivhilu, L.; Dikio, E.D. Study on cobalt metal organic framework materials as adsorbent for lead ions removal in aqueous solution. Chem. Asian J. 2016, 28, 277–281. [Google Scholar] [CrossRef]
- Danaci, D.; Bui, M.; Mac Dowell, N.; Petit, C. Exploring the limits of adsorption-based CO2 capture using MOFs with PVSA—from molecular design to process economics. Mol. Syst. Des. Eng. 2020, 5, 212–231. [Google Scholar] [CrossRef]
- Dong, J.-L.; Xie, F.; Du, J.-Q.; Lan, H.-M.; Yang, R.-X.; Wang, D.-Z. Cobalt MOFs base on benzimidazol and varied carboxylate ligands with higher capacitance for supercapacitors and magnetic properties. J. Solid State Chem. 2019, 279, 120917. [Google Scholar] [CrossRef]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Lavrov, A.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. 3D Metal–Organic Frameworks Based on Co(II) and Bithiophendicarboxylate: Synthesis, Crystal Structures, Gas Adsorption, and Magnetic Properties. Molecules 2021, 26, 1269. [Google Scholar] [CrossRef] [PubMed]
- Rieth, A.J.; Dincă, M. Controlled Gas Uptake in Metal–Organic Frameworks with Record Ammonia Sorption. J. Am. Chem. Soc. 2018, 140, 3461–3466. [Google Scholar] [CrossRef]
- Ethiraj, J.; Palla, S.; Reinsch, H. Insights into high pressure gas adsorption properties of ZIF-67: Experimental and theoretical studies. Microporous Microporous Mater. 2020, 294. [Google Scholar] [CrossRef]
- Marri, S.R.; Chauhan, N.; Tiwari, R.K.; Kumar, J.; Behera, J.N. Two novel 3D-MOFs (Ca-TATB and Co-HKUST): Synthesis, structure and characterization. Inorg. Chim. Acta 2018, 478, 8–14. [Google Scholar] [CrossRef]
- Ghosh, S.; Bloom, B.P.; Lu, Y.; Lamont, D.; Waldeck, D.H. Increasing the Efficiency of Water Splitting through Spin Polarization Using Cobalt Oxide Thin Film Catalysts. J. Phys. Chem. C 2020, 124, 22610–22618. [Google Scholar] [CrossRef]
- Han, W.; Li, M.; Ma, Y.; Yang, J. Cobalt-Based Metal-Organic Frameworks and Their Derivatives for Hydrogen Evolution Reaction. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, R.K.; Samantara, A.K.; Behera, J.N. A cobalt metal-organic framework (Co-MOF): A bi-functional electro active material for the oxygen evolution and reduction reaction. Dalton Trans. 2019, 48, 10557–10564. [Google Scholar] [CrossRef]
- Pang, H.; Gao, F.; Chen, Q.; Liu, R.; Lu, Q. Dendrite-like Co3O4 nanostructure and its applications in sensors, supercapacitors and catalysis. Dalton Trans. 2012, 41, 5862–5868. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-Organic Framework as a Template for Porous Carbon Synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Liu, X.; Shi, C.; Zhai, C.; Cheng, M.; Liu, Q.; Wang, G. Cobalt-Based Layered Metal–Organic Framework as an Ultrahigh Capacity Supercapacitor Electrode Material. ACS Appl. Mater. Interfaces 2016, 8, 4585–4591. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, Z.; Hofmann, J.P.; Lutz, M.; Mazaj, M.; Logar, N.Z.; Weckhuysen, B.M. Controlled Synthesis of Phase-Pure Zeolitic Imidazolate Framework Co-ZIF-9. Eur. J. Inorg. Chem. 2015, 1625–1630. [Google Scholar] [CrossRef]
- Tu, T.N.; Nguyen, K.D.; Nguyen, T.N.; Thanh, T.; Phan, N.T.S. New topological Co-2(BDC)(2)(DABCO)as a highly active heterogeneous catalyst for the amination of oxazoles via oxidative C-H/N-H couplings. Catal. Sci. Technol. 2016, 6, 1384–1392. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Sillar, K.; Sauer, J. Heats of Adsorption of CO and CO2 in Metal-Organic Frameworks: Quantum Mechanical Study of CPO-27-M (M = Mg, Ni, Zn). J. Phys. Chem. C 2011, 115, 21777–21784. [Google Scholar] [CrossRef]
- Masala, A.; Vitillo, J.G.; Bonino, F.; Manzoli, M.; Grande, C.A.; Bordiga, S. New insights into UTSA-16. Phys. Chem. Chem. Phys. 2016, 18, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Lawson, S.; Al-Naddaf, Q.; Krishnamurthy, A.; Amour, M.S.; Griffin, C.; Rownaghi, A.A.; Knox, J.C.; Rezaei, F. UTSA-16 Growth within 3D-Printed Co-Kaolin Monoliths with High Selectivity for CO2/CH4, CO2/N-2, and CO2/H-2 Separation. ACS Appl. Mater. Interfaces 2018, 10, 19076–19086. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Runcevski, T.; Oktawiec, J.; Gonzalez, M.I.; Siegelman, R.L.; Mason, J.A.; Ye, J.; Brown, C.M.; Long, J.R. Tuning the Adsorption-Induced Phase Change in the Flexible Metal Organic Framework Co(bdp). J. Am. Chem. Soc. 2016, 138, 15019–15026. [Google Scholar] [CrossRef]
- Cambridge Structural Database. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 10 June 2021).
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Hamidipour, L.; Farzaneh, F. Cobalt metal organic framework as an efficient heterogeneous catalyst for the oxidation of alkanes and alkenes. React. Kinet. Mech. Catal. 2013, 109, 67–75. [Google Scholar] [CrossRef]
- Crane, J.L.; Anderson, K.E.; Conway, S.G. Hydrothermal Synthesis and Characterization of a Metal–Organic Framework by Thermogravimetric Analysis, Powder X-ray Diffraction, and Infrared Spectroscopy: An Integrative Inorganic Chemistry Experiment. J. Chem. Educ. 2015, 92, 373–377. [Google Scholar] [CrossRef]
- Ejegbavwo, O.A.; Berseneva, A.A.; Martin, C.R.; Leith, G.A.; Pandey, S.; Brandt, A.J.; Park, K.C.; Mathur, A.; Farzandh, S.; Klepov, V.V.; et al. Heterometallic multinuclear nodes directing MOF electronic behavior. Chem. Sci. 2020, 11, 7379–7389. [Google Scholar] [CrossRef]
- Zhou, K.; Jiang, F.-L.; Chen, L.; Wu, M.-Y.; Zhang, S.-Q.; Ma, J.; Hong, M.-C. Unprecedented three-level hierarchical entanglement in a coordination polymer. Chem. Commun. 2012, 48, 12168–12170. [Google Scholar] [CrossRef]
- Israr, F.; Chun, D.; Kim, Y.; Kim, D.K. High yield synthesis of Ni-BTC metal–organic framework with ultrasonic irradiation: Role of polar aprotic DMF solvent. Ultrason. Sonochem. 2016, 31, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Nowacka, A.; Briantais, P.; Prestipino, C.; Llabrés i Xamena, F.X. Facile “Green” Aqueous Synthesis of Mono- and Bimetallic Trimesate Metal–Organic Frameworks. Cryst. Growth Des. 2019, 19, 4981–4989. [Google Scholar] [CrossRef]
- Steenhaut, T.; Hermans, S.; Filinchuk, Y. Green synthesis of a large series of bimetallic MIL-100(Fe,M) MOFs. New J. Chem. 2020, 44, 3847–3855. [Google Scholar] [CrossRef]
- Faustini, M.; Kim, J.; Jeong, G.Y.; Kim, J.Y.; Moon, H.R.; Ahn, W.S.; Kim, D.P. Microfluidic Approach toward Continuous and Ultrafast Synthesis of Metal-Organic Framework Crystals and Hetero Structures in Confined Microdroplets. J. Am. Chem. Soc. 2013, 135, 14619–14626. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Tan, H.; Liu, C.; Yin, Y.; Wu, J. Simple Preparation of Crystal Co-3(BTC)(2)center dot 12H(2)O and Its Catalytic Activity in CO Oxidation Reaction. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 71–75. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Sohail, M.; Zaman, N.; Usman, M. Nanocomposites of cobalt benzene tricarboxylic acid MOF with rGO: An efficient and robust electocatalyst for oxygen evaluation reaction (OER). Renew. Energy 2020, 156, 1040–1054. [Google Scholar] [CrossRef]
- Zheng, W.; Bi, W.; Gao, X.; Zhang, Z.; Yuan, W.; Li, L. A nickel and cobalt bimetal organic framework with high capacity as an anode material for lithium-ion batteries. Sustain. Energy Fuels 2020, 4, 5757–5764. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Qiu, L.-G.; Wang, W.; Li, Z.-Q.; Xu, T.; Wu, Z.-Y.; Jiang, X. Kinetics of oxidation of hydroquinone to p-benzoquinone catalyzed by microporous metal-organic frameworks M3(BTC)2 [M = copper(II), cobalt(II), or nickel(II); BTC = benzene-1,3,5-tricarboxylate] using molecular oxygen. Transit. Met. Chem. 2009, 34, 263–268. [Google Scholar] [CrossRef]
- Yan, W.; Han, L.-J.; Jia, H.-L.; Shen, K.; Wang, T.; Zheng, H.-G. Three Highly Stable Cobalt MOFs Based on “Y”-Shaped Carboxylic Acid: Synthesis and Absorption of Anionic Dyes. Inorg. Chem. 2016, 55, 8816–8821. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Ramachandran, R.; Zhao, C.; Wang, F. Influence of synthesis temperature on cobalt metal-organic framework (Co-MOF) formation and its electrochemical performance towards supercapacitor electrodes. J. Solid State Electrochem. 2018, 22, 3873–3881. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Montero Lanzuela, E.; Navalon, S.; Garcia, H. Cobalt-Based Metal Organic Frameworks as Solids Catalysts for Oxidation Reactions. Catalysts 2021, 11, 95. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Bordiga, S. Increasing the stability of Mg2(dobpdc) metal-organic framework in air through solvent removal. Mater. Chem. Front. 2017, 1, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.C.; Vitillo, J.G.; Babucci, M.; Hoffman, A.S.; Boubnov, A.; Beauvais, M.L.; Chen, Z.; Cramer, C.J.; Chapman, K.W.; Bare, S.R.; et al. Structure, Dynamics, and Reactivity for Light Alkane Oxidation of Fe(II) Sites Situated in the Nodes of a Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 18142–18151. [Google Scholar] [CrossRef]
- Bonino, F.; Lamberti, C.; Chavan, S.; Vitillo, J.G.; Bordiga, S. Characterization of MOFs. 1. Combined Vibrational and Electronic Spectroscopies. In Metal Organic Frameworks as Heterogeneous Catalysts; Llabrés i Xamena, F.X., Gascon, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2013; pp. 76–142. [Google Scholar]
- Ethiraj, J.; Bonino, F.; Lamberti, C.; Bordiga, S. H2S interaction with HKUST-1 and ZIF-8 MOFs: A multitechnique study. Microporous Mesoporous Mater. 2015, 207, 90–94. [Google Scholar] [CrossRef]
- Alves, I.C.B.; Santos, J.R.N.; Viegas, D.S.S.; Marques, E.P.; Lacerda, C.A.; Zhang, L.; Zhang, J.J.; Marques, A.L.B. Nanoparticles of Fe2O3 and Co3O4 as Efficient Electrocatalysts for Oxygen Reduction Reaction in Acid Medium. J. Braz. Chem. Soc. 2019, 30, 2681–2690. [Google Scholar] [CrossRef]
- Rajeevgandhi, C.; Sathiyamurthy, K.; Guganathan, L.; Savithiri, S.; Bharanidharan, S.; Mohan, K. Experimental and theoretical investigations on the spinel structure of Co(2)O(3)nanoparticles synthesized via simple co-precipitation method. J. Mater. Sci. Mater. Electron. 2020, 31, 16769–16779. [Google Scholar] [CrossRef]


| Item | Co(NO3)2·6H2O (g) | BTC (g) | DMF (mL) | H2O (mL) | T (°C) | Reaction Time (min) |
|---|---|---|---|---|---|---|
| Batch 1 | 1.4551 | 0.5250 | 100 | 50 | 140 | 120 |
| Batch 2 | 2.9102 | 1.0508 | 100 | 50 | 140 | 120 |
| Batch 3 | 1.4551 | 0.5250 | 100 | 50 | 140 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ethiraj, J.; Surya, V.; Selvam, P.; Vitillo, J.G. Water-Driven Structural Transformation in Cobalt Trimesate Metal-Organic Frameworks. Energies 2021, 14, 4751. https://doi.org/10.3390/en14164751
Ethiraj J, Surya V, Selvam P, Vitillo JG. Water-Driven Structural Transformation in Cobalt Trimesate Metal-Organic Frameworks. Energies. 2021; 14(16):4751. https://doi.org/10.3390/en14164751
Chicago/Turabian StyleEthiraj, Jayashree, Vinayagam Surya, Parasuraman Selvam, and Jenny G. Vitillo. 2021. "Water-Driven Structural Transformation in Cobalt Trimesate Metal-Organic Frameworks" Energies 14, no. 16: 4751. https://doi.org/10.3390/en14164751
APA StyleEthiraj, J., Surya, V., Selvam, P., & Vitillo, J. G. (2021). Water-Driven Structural Transformation in Cobalt Trimesate Metal-Organic Frameworks. Energies, 14(16), 4751. https://doi.org/10.3390/en14164751