A Novel Condition Monitoring Procedure for Early Detection of Copper Corrosion Problems in Oil-Filled Electrical Transformers
Abstract
:1. Introduction
2. Materials and Methods
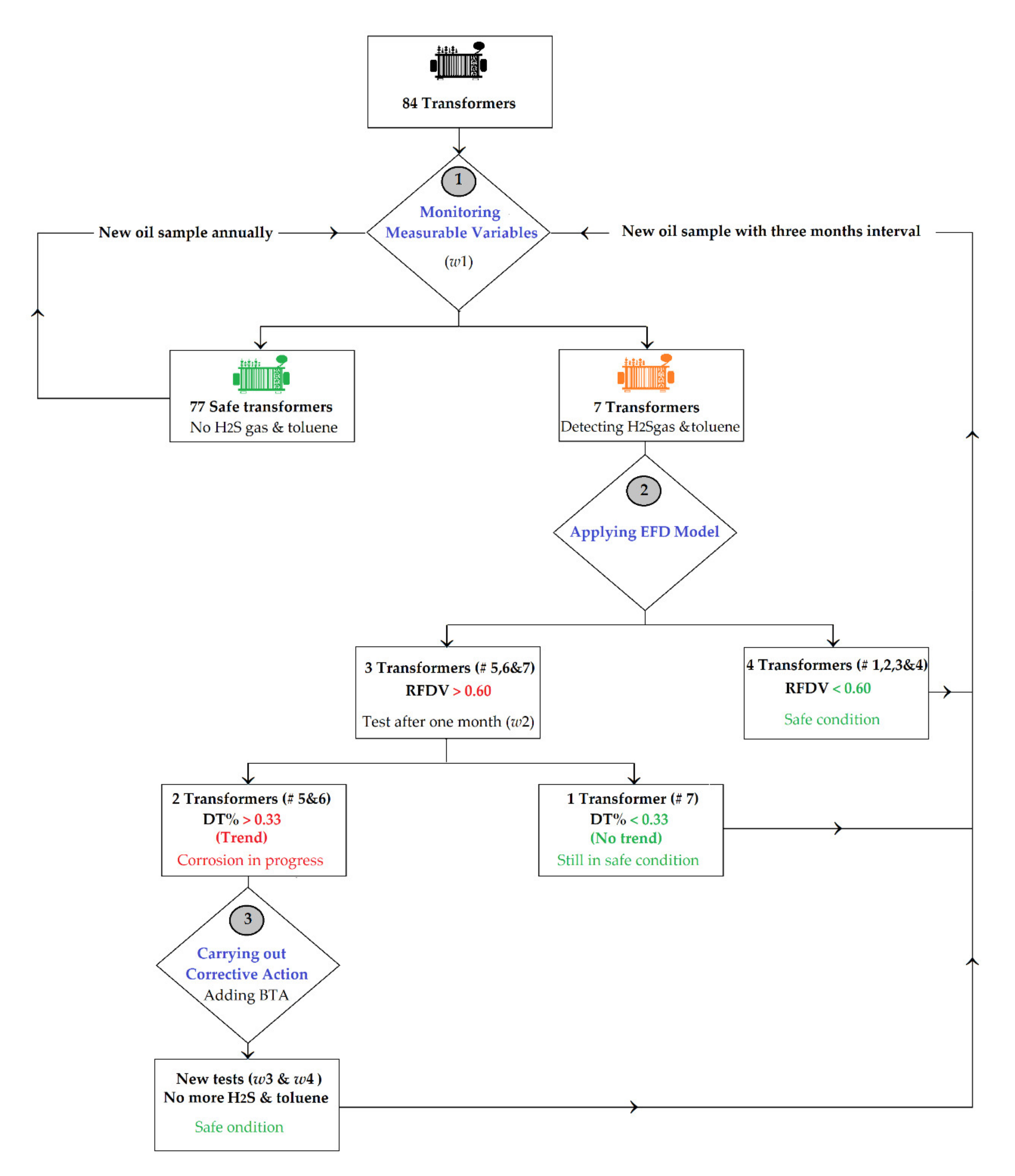
2.1. The CM Procedure
- 1.
- Monitoring the measurable variables: The mechanism of the corrosion reaction was established in [3] as two reactions. Reaction (1): dibenzyl disulfide (DBDS) depletes to benzyl mercaptan (BM) at the overheating condition and presence of proton H+. Reaction (2): The BM decomposes in the presence of Cu ions as a catalyst and proton H+ at overheating conditions to form sulfur deposits as copper sulfide (Cu2S) on the copper windings associated with the by-products, H2S gas and toluene; see Figure 2.
- 2.
- Applying the Early Fault Diagnosis (EFD) Model: After identifying the transformers with suspected copper corrosion propagation where H2S gas and toluene are generated coinciding with depleting DBDS and BM, a fault trend chart can be created based on measured values of H2S gas and toluene. This chart can track the corrosion fault progression during the useful life of the transformers. The regular periodic schedule of oil analysis in a normal condition is annually [37]. As soon the H2S gas and toluene are generated in the oil, the recommended periodic schedule could be within a three month interval [3,6] or according to the maintenance plan. The fault trend chart is based on a novel numerical method in order to track the copper corrosion problems and select the correct time of corrective actions [6]. The numerical method includes the following calculations:
- Caution Limit (CL) of the H2S gas and toluene, which were defined as 1 and 2 ppm, respectively [3].
- Warning Limit (WL), which was estimated as 50% of the CL value as an indication of starting a fault [6].
- Alarm Limit (AL), which was estimated as 80% of the CL based on an experimental investigation, showed that the acceptable relative error in the oil analysis and uncertainty could be up to 20% [38].
- Relative Fault Detection Value (RFDV), which is the difference between the first measured value (w1) of H2S gas or toluene and WL relative to the WL [6]; see Equation (2):
- Daily Trend (DT%) is the trend of the increase of the measured value per day and is calculated based on first measured value (w1) and second one (w2); see Equation (3):
- 3.
- Carrying out corrective action: The main corrective action is adding benzene triazole-type metal passivators, an anticorrosion additive, to the insulating oil in-service; Benzo Triazole (called BTA) or Toluiltriazole-dialkylamine (called Irgamet-39). These passivators are usually recommended to suppress the corrosion reaction throughout by neutralizing the activity of the catalyst Cu ions [40,41,42], see Figure 3. The optimal concentration limit value of BTA and Irgamet-39 are 50 and 150 ppm, respectively [40]. However, exceeding the mentioned optimal concentration limit value can lead to the formation of a high amount of undesirable flammable hydrogen gas (H2) in the oil, especially with Irgamet-39 compared with lower amounts when using BTA [43], see Figure 3, and acceleration of oxidation process in transformers [40]. Hence, an optimal amount of a passivator should be added at the correct time. On the other hand, adding a passivator after the values of the H2S gas and toluene have exceeded their caution limits will suppress further anticipated corrosion reaction but will not reduce the sulfur deposits which have already occurred on the copper windings [40].
2.2. Empirical Study Design
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | Alarm Limit |
| BTA | Benzo Triazole |
| CBM | Condition-Based Maintenance |
| CCD | Covered Conductor Deposition |
| CL | Caution Limit |
| CM | Condition Monitoring |
| DBDS | Dibenzyl disulfide |
| DT | Daily Trend % |
| EFD | Early Fault Diagnosis |
| HI | Health Index % |
| H2S | Hydrogen sulfide gas |
| Irgamet 39 | Toluiltriazole-dialkylamine |
| MPM | Markov Prediction Model |
| PCB | Polychlorinated biphenyl |
| PPM | Part per million |
| PSMD | Primary Substation Maintenance Department |
| RAT | Relative Alarm Threshold |
| RFDV | Relative Fault Detection Value |
| RSD | Relative Standard Deviation % |
| SDM | Statistical Distribution Model |
| Uexp. | Expanded uncertainty |
| WL | Warning Limit |
References
- Gao, S.; Yang, L. Effects of sulfur corrosion on the properties of oil–paper insulation induced by dibenzyl disulfide. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1089–1097. [Google Scholar] [CrossRef]
- Flora, S.D.; Kumari, M.K.; Rajan, S.J. A New Approach to Study the Effects of Copper Sulphide on Electric Stress Distribution in Paper Oil Insulation of Transformers. In Proceedings of the IEEE Conference Electrical Insulation, Philadelphia, PA, USA, 8–11 June 2014. [Google Scholar]
- Jadim, R.; Kans, M.; Rehman, S.; Alhems, L. A relevant condition monitoring of corrosive sulfur deposition on the windings of oil-filled power transformers. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1736–1742. [Google Scholar] [CrossRef]
- Jadim, R.; Ingwald, A.; Al-Najjar, B. Review Study of Condition Monitoring and Maintenance Approaches for Diagnosis Corrosive Sulphur Deposition in Oil-Filled Electrical Transformers. In Proceedings of the New Paradigm of Industry 4.0, Studies in Big Data 64, Bhubaneswar, India, 27 September 2018. [Google Scholar]
- El-Harbawi, M.; Al-Mubaddel, F. Risk of fire and explosion in electrical substations due to the formation of flammable mixtures. Sci. Rep. Nat. Res. 2020, 10, 6295. [Google Scholar] [CrossRef]
- Jadim, R.; Kans, M.; Schulte, J.; Alhattab, M.N.; Alhendi, M.H.; Bushehry, A.H. On approaching relevant cost-effective sustainable maintenance of mineral oil-filled electrical transformers. Energies 2021, 14, 3670. [Google Scholar] [CrossRef]
- Fitzgerald, E.F.; Standfast, S.J.; Youngblood, L.G.; Melius, J.M.; Janerich, D.T. Assessing the health effects of potential exposure to PCBs, Dioxins, and Furans from electrical transformer fires. Arch. Environ. Health Int. J. 1986, 41, 368–376. [Google Scholar] [CrossRef]
- Jasiulewicz-Kaczmarek, M. The Role and Contribution of Maintenance in Sustainable Manufacturing. In Proceedings of the International Conference 7th IFAC on Manufacturing Modelling, Management, and Control, International Federation of Automatic Control, Saint Petersburg, Russia, 19–21 June 2013. [Google Scholar]
- Vahidi, F.; Tenbohlen, S. Statistical Failure Analysis of European Substation Transformers. In Proceedings of the Conference ETG-Fachbericht—Diagnostik Elektrischer Betriebsmittel, Berlin, Germany, 25–26 November 2014. [Google Scholar]
- Martin, D.; Beckett, C.; Brown, J.; Nielsen, S. Analysis and mitigation of Australian and New Zealand power transformer failures resulting in fires and explosion. IEEE Electr. Insul. Mag. 2019, 35, 7–14. [Google Scholar] [CrossRef]
- Guide for Transformer Fire Safety Practices; CIGRE Brochure 537, Working Group A2.33; CIGRE publication: Paris, France, 2013.
- Ng, A.K.-L. Risk Assessment of Transformer Fire Protection in a Typical New Zealand High-Rise Building. Master’s Thesis, Department of Civil Engineering, University of Canterbury, Christchurch, New Zealand, 2007. [Google Scholar]
- ChemSpider, Chemical Structure Database. Available online: http://www.chemspider.com (accessed on 5 June 2021).
- Schectera, A.; Birnbaumb, L.; Ryanc, J.J.; Constabled, J.D. Dioxins: An overview. Environ. Res. 2006, 10, 419–428. [Google Scholar] [CrossRef]
- Wierckx, N.; Koopman, F.; Harald; Ruijssenaars, H.J.; de Winde, J.H. Microbial degradation of furanic compounds: Biochemistry, genetics, and impact. Appl. Microbiol. Biotechnol. 2011, 92, 1095–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phadungthin, R.; Haema, J. Development of Risk Optimization Model for High Voltage Substation Transformer Maintenance. In Proceedings of the IEEE Asia-Pacific Power and Energy Engineering Conference, Brisbane, QLD, Australia, 15–18 November 2015. [Google Scholar]
- Prajapati, A.; Bechtel, J.; Ganesan, S. Condition based maintenance: A survey. J. Qual. Maint. Eng. 2012, 18, 384–400. [Google Scholar] [CrossRef]
- Niu, G.; SukYang, B.; Pecht, M. Development of an optimized condition-based maintenance system by data fusion and reliability-centred maintenance. Reliab. Eng. Syst. Saf. 2010, 95, 786–796. [Google Scholar] [CrossRef]
- Shina, J.; Junb, H. On condition-based maintenance policy. J. Comput. Des. Eng. 2015, 2, 119–127. [Google Scholar] [CrossRef] [Green Version]
- EN 13306. Standard for Maintenance-Maintenance Terminology. Available online: https://infostore.saiglobal.com/en-gb/Standards/BS-EN-13306-2017-232245_SAIG_BSI_BSI_543804/ (accessed on 1 January 2017).
- Al-Najjar, B. On establishing cost-effective condition-based maintenance: Exemplified for vibration-based maintenance in case companies. J. Qual. Maint. Eng. 2012, 18, 401–416. [Google Scholar] [CrossRef]
- Akshatha, A.; Anjana, K.; Ravindra, D.; Vishwanath, G.; Rajan, J.S. Study of Copper Corrosion in Transformers due to Sulphur in Oil Using Chemical Methods. In Proceedings of the International Conference IEEE on Electrical Insulation and Dielectric Phenomena (CEIDP), Montreal, QC, Canada, 14–17 October 2012. [Google Scholar]
- Scatiggio, F.; Tumiatti, V.; Maina, R.; Tumiatti, M.; Pompili, M.; Bartnikas, R. Corrosive sulphur induced failures in oil-filled electrical power transformers and shunt reactors. IEEE J. Mag. 2009, 24, 1240–1248. [Google Scholar]
- Smith, J.R.; Sen, P.K. Corrosive Sulfur in Transformer Oil. In Proceedings of the Industry Applications Society Annual Meeting (IAS), Houston, TX, USA, 3–7 October 2010. [Google Scholar]
- Amaro, P.S.; Holt, A.F.; Facciotti, M.; Pilgrim, J.A.; Lewin, P.L.; Brown, R.C.D.; Wilson, G.; Jarman, P.N. Impact of Corrosive Sulfur in Transformer Insulation Paper. In Proceedings of the IEEE Electrical Insulation Conference, Ottawa, ON, Canada, 2–5 June 2013. [Google Scholar]
- Ren, S.; Xu, Y.; Cao, X.; Zhong, L.; Yu, Q.; Jeanjean, R. A research Summary of Corrosive Sulfur in Mineral Oils. In Proceedings of the IEEE 9th International Conference on Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009. [Google Scholar]
- Scatiggio, F.; Tumiatti, V.; Maina, R.; Tumiatti, M.; Pompili, M.; Bartnikas, R. Corrosive sulfur in insulating oils: Its detection and correlated power apparatus failures. IEEE Trans. Power Deliv. 2008, 23, 508–509. [Google Scholar] [CrossRef]
- Akshatha, A.; Rajan, J.S.; Viswanath, G.R.; Ravindara, D.; Naidu, C.J.; Singh, K.P. Estimation of Sulphur Compounds in Transformer Oil. In Proceedings of the IEEE Conference, Power and Energy Systems (ICPS), Chennai, India, 22–24 December 2011. [Google Scholar]
- Test Methods for Quantitative Determination of Corrosive Sulfur Compounds in Unused and Used Insulating Liquids-Part 1: Test Method for Quantitative Determination of Dibenzyldisulfide (DBDS). In IEC 62697-1, 1st ed.; International Electrotechnical Commission: Geneva, Switzerland, 2012.
- Insulating Liquids-Test Method for Detection of Potentially Corrosive Sulphur in Used and Unused Insulating Oil. In IEC 62535, 1st ed.; International Electrotechnical Commission: Geneva, Switzerland, 2008.
- Amaro, P.S.; Facciotti, M.; Holt, A.F.; Pilgrim, J.A.; Lewin, P.L.; Brown, R.C.D.; Wilson, G.; Jarman, P. Tracking Copper Sulfide Formation in Corrosive Transformer Oil. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Shenzhen, China, 20–23 October 2013. [Google Scholar]
- Amaro, P.S.; Facciotti, M.; Holt, A.F.; Pilgrim, J.A.; Lewin, P.L.; Brown, R.C.D.; Wilson, G.; Jarman, P. X-ray fluorescence as a condition monitoring tool for copper and corrosive sulphur species in insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 701–708. [Google Scholar] [CrossRef]
- Copper Sulphide Long Term Mitigation and Risk Assessment; CIGRE Brochure 625, Group A2.40; CIGRE Publication: Paris, France, 2015.
- ASTM D5623. Standard Test Method for Sulfur Compounds in Light Petroleum Liquids by Gas Chromatography and Sulfur Selective Detection; American Society for Testing and Materials: West Conshohocken, PA, USA, 7 January 2019. [Google Scholar]
- ASTM UOP163. Hydrogen Sulfide and Mercaptan Sulfur in Liquid Hydrocarbons by Potentiometric Titration; American Society for Testing and Materials: West Conshohocken, PA, USA, 1 January 2010. [Google Scholar]
- ASTM D5580. Standard Test Method for Determination of Benzene, Toluene, Ethylbenzene, p/m-Xylene, o-Xylene, C9 and Heavier Aromatics, and Total Aromatics in Finished Gasoline by Gas Chromatography; American Society for Testing and Materials: West Conshohocken, PA, USA, 1 April 2021. [Google Scholar]
- Mineral Insulating Oils in Electrical Equipment—Supervision and Maintenance Guidance. In IEC 60422, 4th ed.; International Electrotechnical Commission: Geneva, Switzerland, 2013.
- Arrhenius, K.; Fischer, A.; Buker, O.; Adrien, H.; El Masri, A.; Lestremaub, F.; Robinsonc, T. Analytical methods for the determination of oil carryover from CNG/biomethane refuelling stations recovered in a solvent. R. Soc. Chem. 2020, 10, 11907–11917. [Google Scholar]
- IEC 60599, 2nd ed. Mineral Oil-Filled Electrical Equipment in Service—Guidance on the Interpretation of Dissolved and Free Gases Analysis. In Proceedings of the International Electrotechnical Commission, Geneva, Switzerland, 31 March 1999.
- Rehman, S.; Alhems, L.; Jadim, R.; Faraj, B.; Balasubramanian, K.; Al Mutairi, S.; Al-Yemni, K.; Shinde, V.; Al-Hsaien, A. Maximum acceptable concentrations of DBDS, sulfur mercaptan and optimal concentration of passivators for safe and prolonged operation of power transformers. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2438–2442. [Google Scholar] [CrossRef]
- Kato, F.; Amimoto, T.; Nishiura, R.; Mizuno, K.; Toyama, S. Suppressive effect and its duration of Triazole-based passivators on copper sulfide deposition on kraft paper in transformer. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 1915–1921. [Google Scholar] [CrossRef]
- Wiklund, P.; Levin, M.; Pahlavanpour, B. Copper dissolution and metal passivators in insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2007, 23, 6–14. [Google Scholar] [CrossRef]
- Kato, F.; Amimoto, T.; Nishiura, R.; Mizuno, K.; Toyama, S. Hydrogen generation by adding passivator for suppressing copper-sulfide deposition in transformers. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1931–1937. [Google Scholar] [CrossRef]
- Yin, R.K. Case Study Research and Applications: Design and Methods, 6th ed.; SAGE Publications: Los Angeles, CA, USA, 2018. [Google Scholar]
- Kulkarni, S.V.; Khaparde, S.A. Transformer Engineering Design and Practice, 1st ed.Marcel Dekker Press: New York, NY, USA, 2004. [Google Scholar]
- Cong, H.; Pan, H.; Qian, D.; Zhao, H.; Li, Q. Reviews on sulphur corrosion phenomenon of the oil–paper insulating system in mineral oil transformer. High Volt. 2021, 6, 193–209. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L.; Ke, T. Failure mechanism of transformer oil-immersed cellulosic insulation induced by sulfur corrosion. Cellulose 2020, 27, 7157–7174. [Google Scholar] [CrossRef]
- Guide for the Repair of Power and Distribution Transformers; EASA AR 200; Electrical Apparatus Service Association: St. Louis, MO, USA, 2011.
- Tronstad, I.; Roel, C.M.; Glomm, W.R.; Blekkan, E.A.; Ese, M.G. Ageing and corrosion of paper insulated copper windings: The effect of Irgamet 39 in aged insulated oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 345–358. [Google Scholar] [CrossRef]
- Matejkova, M.; Kastanek, F.; Maleterova, Y.; Kuzilek, V.; Kosanova, L.; Solcova, O. Removal of corrosive sulfur from insulating oils by natural sorbent and liquid-liquid extraction. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2383–2389. [Google Scholar] [CrossRef]
- Atanasova-Hoehlein, I.; Heinzig, P.; Mezhvynskiy, V.; Nuernberg, D.E.; Thiess, U. Method for Removing Corrosive Sulfur Compounds from a Transformer Oil. U.S. Patent Application 13/125,128, 20 April 2011. [Google Scholar]
- Dahlund, M.O. Method for Removal of Reactive Sulfur from Insulating Oil. U.S. Patent Application 60/566,606, 16 October 2008. [Google Scholar]
- Mohd Selva, A.; Azis, N.; Yahaya, M.S.; Ab Kadir, M.Z.A.; Jasni, J.; Yang Ghazali, Y.Z.; Talib, M.A. Application of Markov model to estimate individual condition parameters for transformers. Energies 2018, 11, 2114. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Parlikad, A. A Markovian model for power transformer maintenance. Electr. Power Energy Syst. 2018, 99, 175–182. [Google Scholar] [CrossRef]
- Yahaya, M.S.; Azis, N.; Selva, A.M.; Ab Kadir, M.Z.A.; Jasni, J.; Hairi, M.H.; Ghazali, Y.Z.Y.; Talib, M.A. Effect of pre-determined maintenance repair rates on the health index state distribution and performance condition curve based on the Markov prediction model for sustainable transformers asset management strategies. Sustainability 2018, 10, 3399. [Google Scholar] [CrossRef] [Green Version]
- Mohd Selva, A.; Azis, N.; Shariffudin, N.S.; Ab Kadir, M.Z.A.; Jasni, J.; Yahaya, M.S.; Talib, M.A. Application of statistical distribution models to predict health index for condition-based management of transformers. Appl. Sci. 2021, 11, 2728. [Google Scholar] [CrossRef]



| Measurable Variable | CL | WL | AL | RAT |
|---|---|---|---|---|
| H2S gas | 1.0 ppm | 0.50 | 0.80 | 0.60 |
| Toluene | 2.0 ppm | 1.00 | 1.60 | 0.60 |
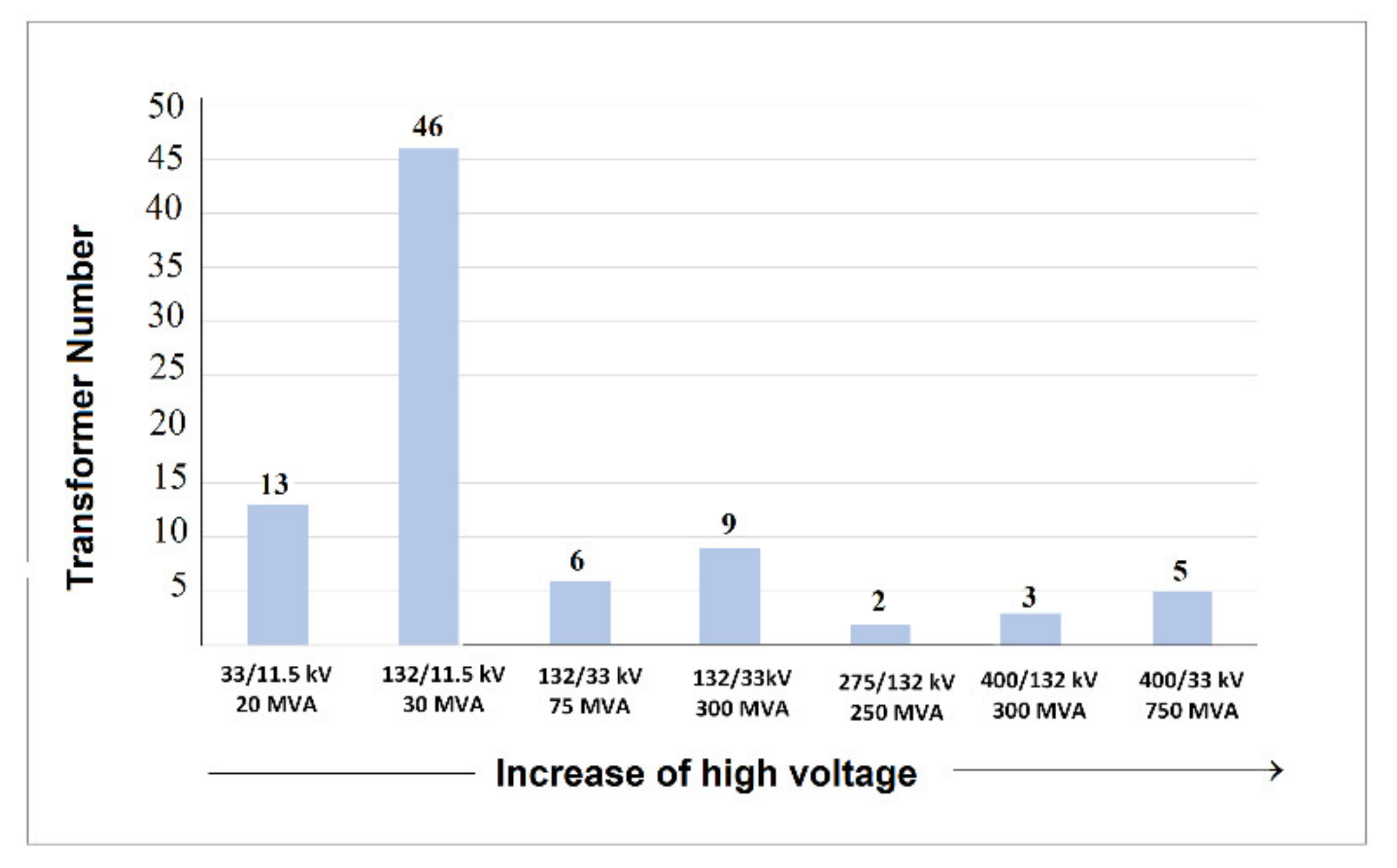
| No. | Transformer Serial Number/ID | Voltage HV/LV, kV | Power Rating, MVA | Substation Name | DBDS (ppm) | BM (ppm) | H2S Gas w1 (ppm) | Toluene w1 (ppm) | RFDV |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8235120102/Tr2 | 132/33 | 300 | Fifth ring road | 1.0 | <0.1 | 0.20 | 0.30 | <0.60 |
| 2 | S251625/Tr1 | 132/11.5 | 30 | Mahbola-A | 3.0 | <0.1 | 0.42 | 0.25 | <0.60 |
| 3 | 111353/Tr1 | 132/33 | 300 | Omirya-W | <0.1 | 0.23 | 0.30 | 0.93 | <0.60 |
| 4 | 07MD970101/Tr1 | 132/11.5 | 30 | S. Alabdullah | <0.1 | 0.33 | 0.10 | 0.45 | <0.60 |
| 5 | M0036/Tr1 | 132/11.5 | 30 | Mishref-A | 9.0 | 0.90 | 0.83 | 1.62 | >0.60 |
| 6 | M0037/Tr2 | 132/11.5 | 30 | Mishref-A | 6.0 | 0.80 | 0.81 | 1.70 | >0.60 |
| 7 | M0038/Tr3 | 132/11.5 | 30 | Mishref-A | 4.3 | <0.1 | 0.88 | 1.68 | >0.60 |
| No. | Transformer Serial Number/ID | Toluene w2 (ppm) | H2S Gas w2 (ppm) | DT (%) |
|---|---|---|---|---|
| 5 | M0036/Tr1 | 1.93 | 0.92 | >0.33 |
| 6 | M0037/Tr2 | 1.90 | 0.90 | >0.33 |
| 7 | M0038/Tr3 | 1.69 | 0.92 | <0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadim, R.; Kans, M.; Alhattab, M.; Alhendi, M. A Novel Condition Monitoring Procedure for Early Detection of Copper Corrosion Problems in Oil-Filled Electrical Transformers. Energies 2021, 14, 4266. https://doi.org/10.3390/en14144266
Jadim R, Kans M, Alhattab M, Alhendi M. A Novel Condition Monitoring Procedure for Early Detection of Copper Corrosion Problems in Oil-Filled Electrical Transformers. Energies. 2021; 14(14):4266. https://doi.org/10.3390/en14144266
Chicago/Turabian StyleJadim, Ramsey, Mirka Kans, Mohammed Alhattab, and May Alhendi. 2021. "A Novel Condition Monitoring Procedure for Early Detection of Copper Corrosion Problems in Oil-Filled Electrical Transformers" Energies 14, no. 14: 4266. https://doi.org/10.3390/en14144266






