Torrefaction and Thermochemical Properties of Agriculture Residues
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation and Procedure
2.2. Torrefaction Procedure
2.3. Composition Analysis
2.4. Thermogravimetric Analysis
3. Results and Discussion
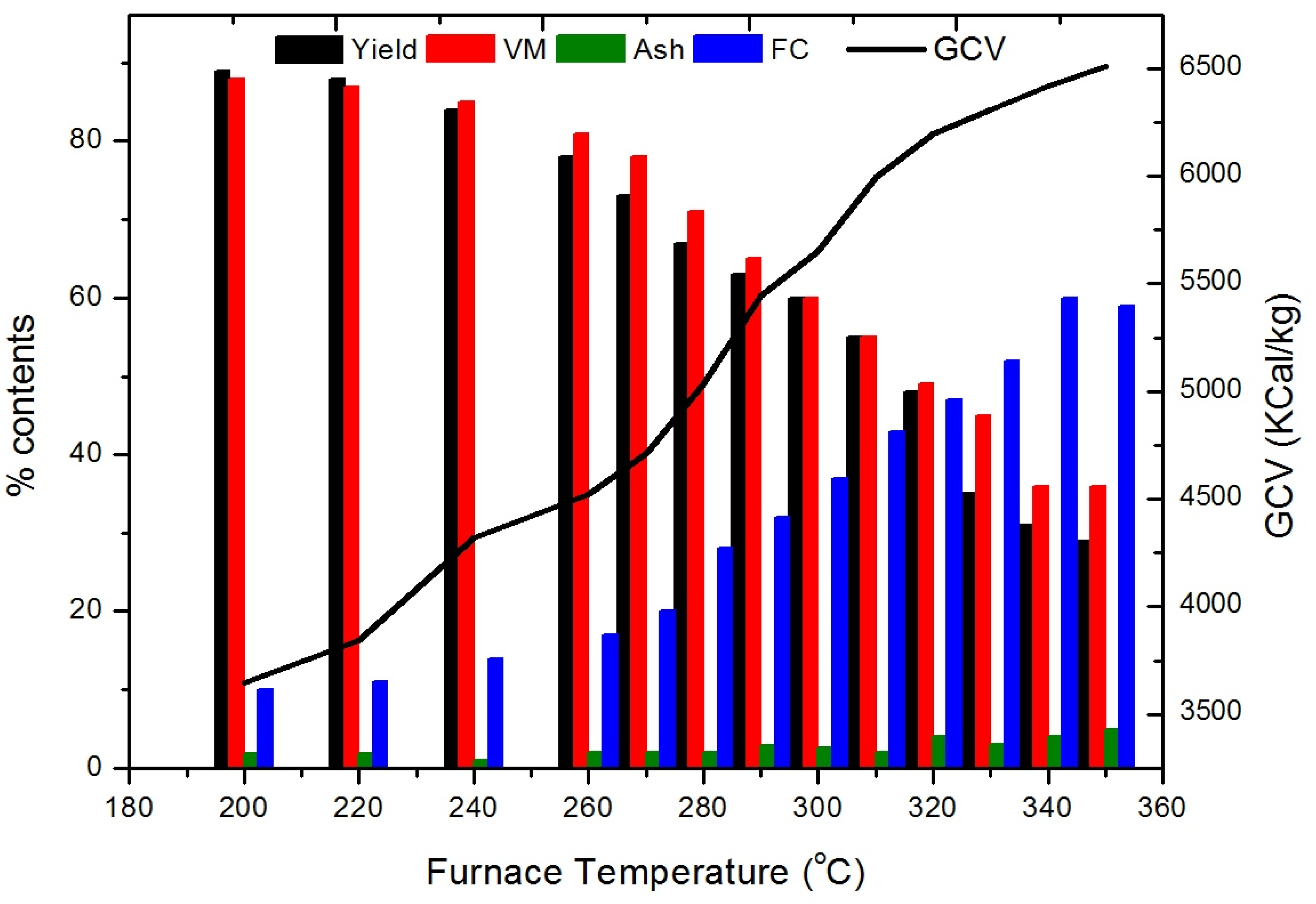
3.1. Effect of Temperature
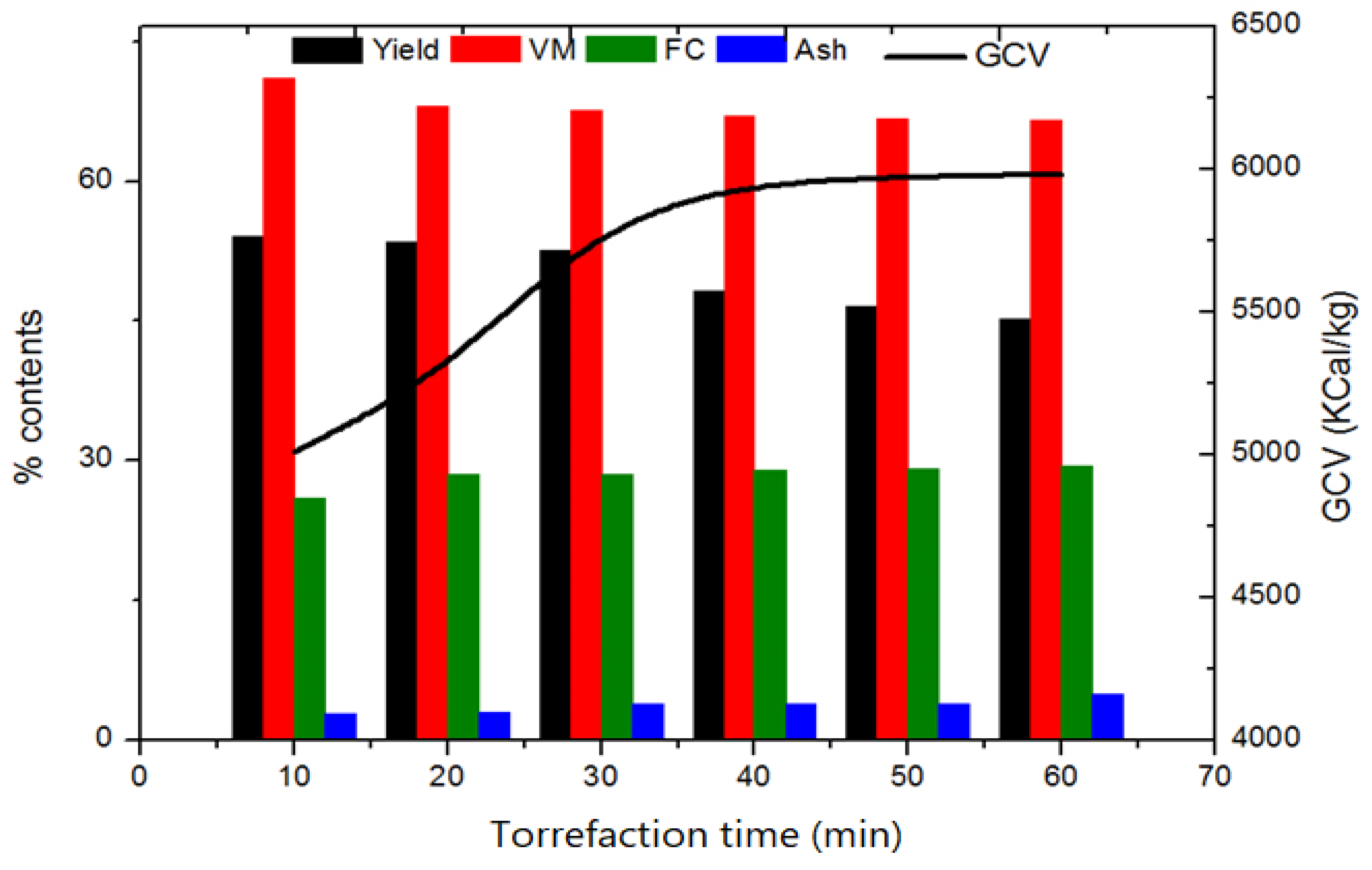
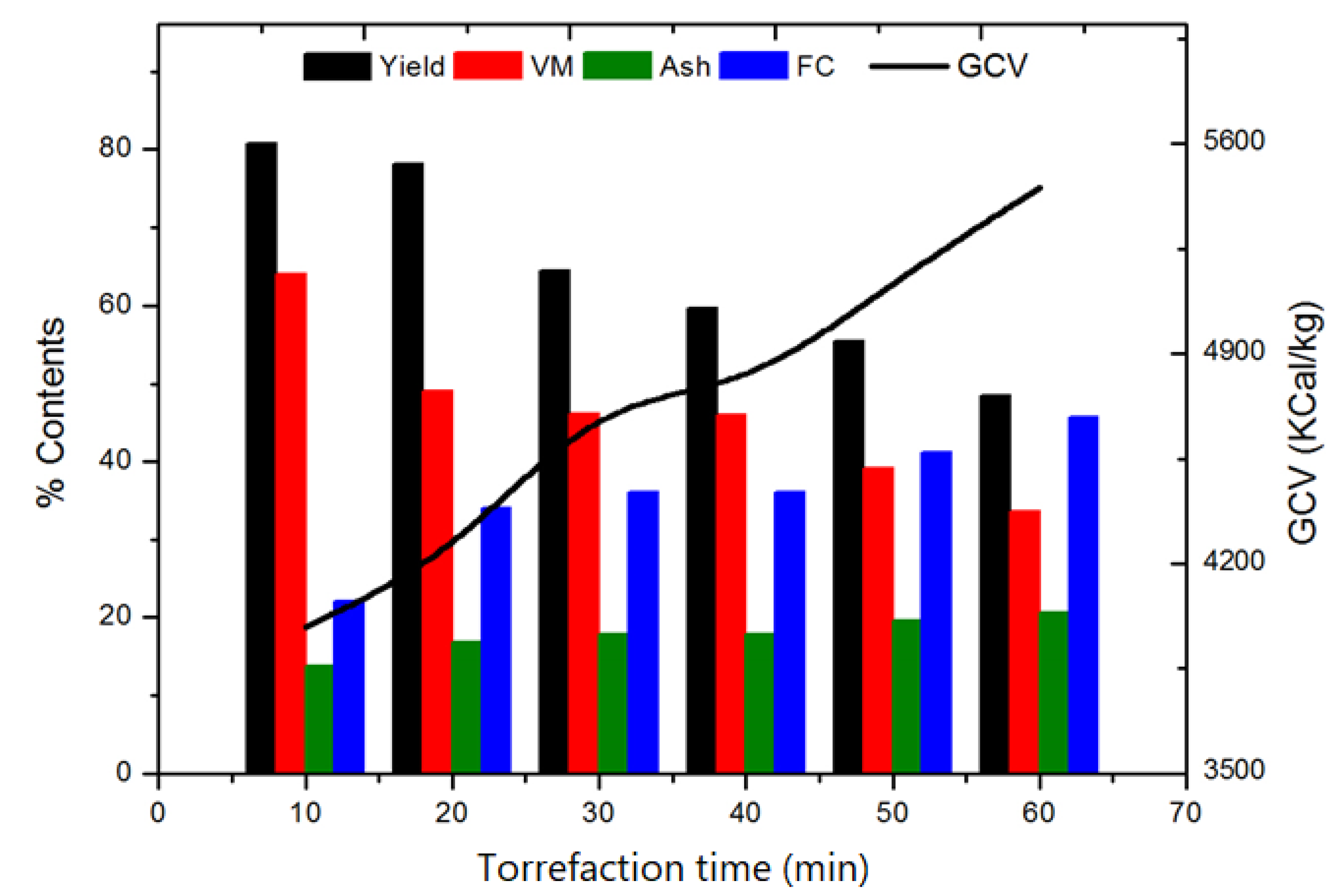
3.2. Effect of Residence Time
3.3. Compositional Analysis
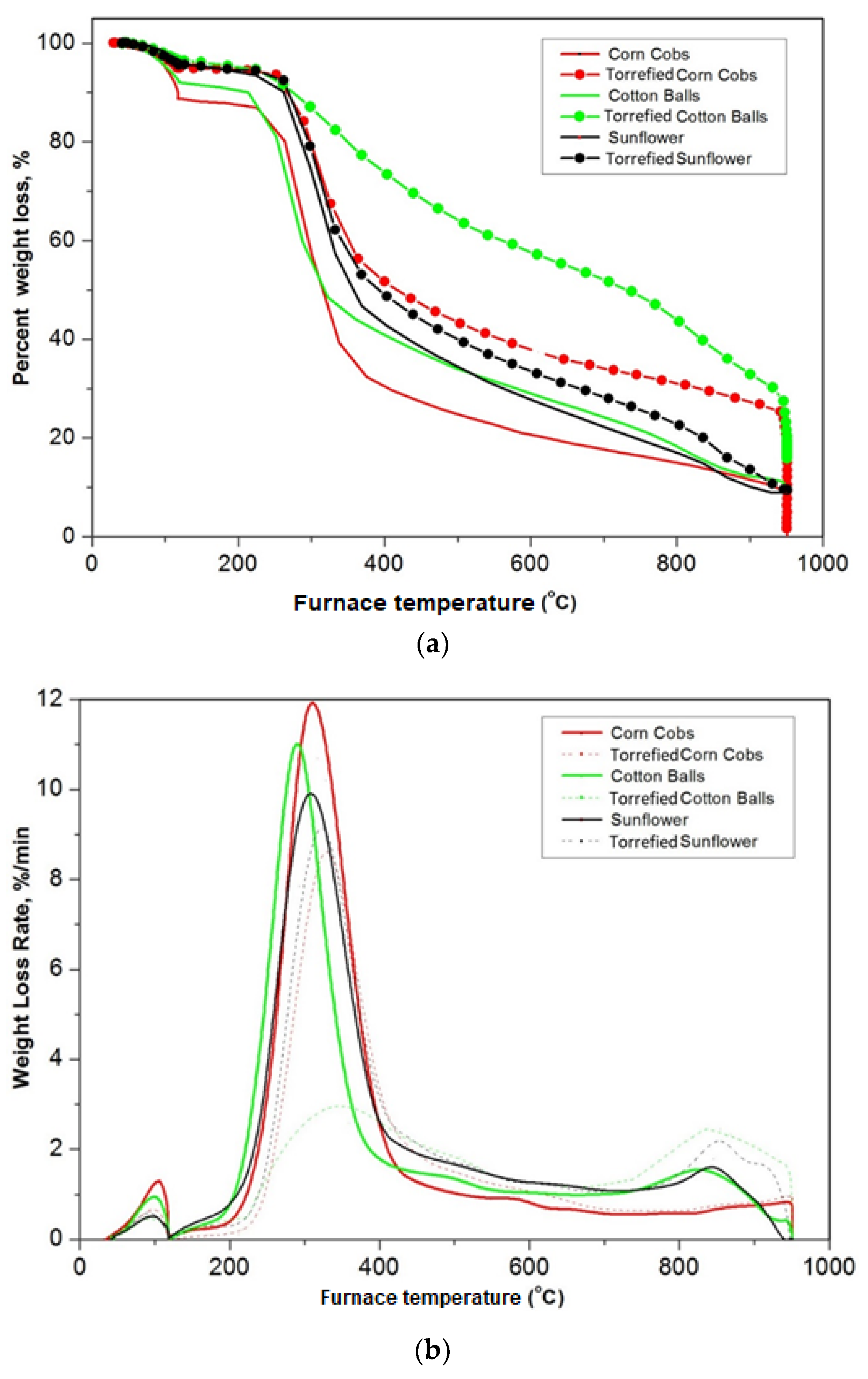
3.4. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. An economic analysis of biogas-biomethane chain from animal residues in Italy. J. Clean. Prod. 2019, 230, 888–897. [Google Scholar] [CrossRef]
- Cheng, F.; Luo, H.; Colosi, L.M. Slow pyrolysis as a platform for negative emissions technology: An integration of machine learning models, life cycle assessment, and economic analysis. Energy Convers. Manag. 2020, 223, 113258. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.–J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.; Show, P.; Ong, H.; Chang, J.-S.; Ho, S.-H.; Culaba, A.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Samadi, S.; Ghobadian, B.; Nosrati, M. Prediction and estimation of biomass energy from agricultural residues using air gasification technology in Iran. Renew. Energy 2020, 149, 1077–1091. [Google Scholar] [CrossRef]
- Abdelhady, S.; Shalaby, M.; Shaban, A. Techno-Economic Analysis for the Optimal Design of a National Network of Agro-Energy Biomass Power Plants in Egypt. Energies 2021, 14, 3063. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Colin, B.; Pétrissans, A.; Pétrissans, M. A study of hygroscopic property of biomass pretreated by torrefaction. Energy Procedia 2019, 158, 32–36. [Google Scholar] [CrossRef]
- Akhtar, J.; Yaseen, A.; Munir, S. Effect of rice husk co-combustion with coal on gaseous emissions and combustion efficiency. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 1010–1018. [Google Scholar] [CrossRef]
- Meng, J.; Park, J.; Tilotta, D.; Park, S. The effect of torrefaction on the chemistry of fast-pyrolysis bio-oil. Bioresour. Technol. 2012, 111, 439–446. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ding, L.; Gong, Y.; Li, W.; Wei, J.; Yu, G. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis. Bioresour. Technol. 2019, 280, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kai, X.; Meng, Y.; Yang, T.; Li, B.; Xing, W. Effect of torrefaction on rice straw physicochemical characteristics and particulate matter emission behavior during combustion. Bioresour. Technol. 2019, 278, 1–8. [Google Scholar] [CrossRef]
- Sumaira, K.; Nawaz, C.; Shahid, M.; Hafiza, S. Effect of torrefaction conditions on the physicochemical characterization of agricultural wastes (sugar cane baggase). Waste Manag. 2019, 88, 280–290. [Google Scholar]
- Di, B. Product distribution from pyrolysis of wood and agricultural residues. Ind. Eng. Chem. Res. 1999, 38, 2216–2224. [Google Scholar]
- Irfan, M.; Gulsher, M.; Abbas, S.; Syed, Q.; Nadeem, M.; Baig, S. Effect of various pretreatment conditions on enzymatic saccharification. Songklanakarin J. Sci. Technol. 2011, 33, 397–404. [Google Scholar]
- Ranzi, E.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Pierucci, S.; Sommariva, S. Chemical Kinetics of Biomass Pyrolysis. Energy Fuels 2008, 22, 4292–4300. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.; Gibbs, B. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef]
- Ghetti, P.; Ricca, L.; Angelini, L. Thermal analysis of biomass and corresponding pyrolysis products. Fuel 1996, 75, 565–573. [Google Scholar] [CrossRef]
- Ayoub, G.; Akhtar, J.; Rana, M.; Qasim, M.; Sheikh, N.; Munir, S. Investigation of forestry wastes: Torrefaction and their thermal properties for use in energy recovery schemes. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Welker, C.M.; Balasubramanian, V.K.; Petti, C.; Rai, K.M.; DeBolt, S.; Mendu, V. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef] [Green Version]
- Poudel, J.; Karki, S.; Oh, S.C. Valorization of Waste Wood as a Solid Fuel by Torrefaction. Energies 2018, 11, 1641. [Google Scholar] [CrossRef] [Green Version]
- Anukam, A.I.; Goso, B.P.; Okoh, O.O.; Mamphweli, S.N. Studies on Characterization of Corn Cob for Application in a Gasification Process for Energy Production. J. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.-Y.; Feng, X.-B.; Cao, J.-P.; Tang, W.; Wang, Z.-H.; Yang, Z.; Zhao, J.-P.; Zhang, L.-Y.; Wang, Y.-J.; Zhao, X.-Y. Catalytic Conversion of Coal and Biomass Volatiles: A Review. Energy Fuels 2020, 34, 10307–10363. [Google Scholar] [CrossRef]
- Cahyanti, M.; Doddapaneni, T.; Madissoo, M.; Pärn, L.; Virro, I.; Kikas, T. Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies 2021, 14, 2774. [Google Scholar] [CrossRef]
- Karlsson, H.; Ahlgren, S.; Sandgren, M.; Passoth, V.; Wallberg, O.; Hansson, P.-A. A systems analysis of biodiesel production from wheat straw using oleaginous yeast: Process design, mass and energy balances. Biotechnol. Biofuels 2016, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Olugbade, T.O.; Ojo, O.T. Biomass Torrefaction for the Production of High-Grade Solid Biofuels: A Review. BioEnergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.d.O.; Nunes, L.J.R. Future Perspectives of Biomass Torrefaction: Review of the Current State-Of-The-Art and Research Development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef] [Green Version]
- Sumaira, K.; Shahid, M.; Nawaz, C.; Hafiza, S. Physicochemical characterization of Thar coal and torrefied corn cob. Energy Exploration and Exploitation 2019, 37, 1286–1305. [Google Scholar] [CrossRef]
- Bajcar, M.; Zaguła, G.; Saletnik, B.; Tarapatskyy, M.; Puchalski, C. Relationship between Torrefaction Parameters and Physicochemical Properties of Torrefied Products Obtained from Selected Plant Biomass. Energies 2018, 11, 2919. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yan, R.; Chin, T.; Liang, D.T.; Chen, A.H.; Zheng, C. Thermogravimetric Analysis−Fourier Transform Infrared Analysis of Palm Oil Waste Pyrolysis. Energy Fuels 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Dacres, O.D.; Tong, S.; Li, X.; Zhu, X.; Edreis, E.M.; Liu, H.; Luo, G.; Worasuwannarak, N.; Kerdsuwan, S.; Fungtammasan, B.; et al. Pyrolysis kinetics of biomasses pretreated by gas-pressurized torrefaction. Energy Convers. Manag. 2019, 182, 117–125. [Google Scholar] [CrossRef]
- Munir, S.; Sattar, H.; Nadeem, A.; Azam, M. Thermal and kinetic performance analysis of corncobs, Falsa sticks, and Chamalang coal under oxidizing and inert atmospheres. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Talero, G.; Rincón, S.; Gómez, A. Torrefaction of oil palm residual biomass: Thermogravimetric characterization. Fuel 2019, 242, 496–506. [Google Scholar] [CrossRef]



| Sample | Moisture % | Volatile Matter (VM) % | Fixed Carbon (FC) % | Ash % | Sulfur % | GCV (Kcal/kg) |
|---|---|---|---|---|---|---|
| Maize Stalks (Corn Cobs) | 6.9 | 88 | 4.12 | 0.4 | 0.51 | 3600 |
| Cotton Stalks (Cotton Balls) | 6.23 | 75.24 | 5.49 | 13.03 | 0.44 | 3696 |
| Sunflower Stalks | 5.8 | 73 | 6.1 | 9.34 | 0.37 | 3435 |
| State of Sample | Type of Biomass | Extractives (%) | Hemicellulose (%) | Cellulose (%) | Lignin (%) |
|---|---|---|---|---|---|
| Raw | Corn Cobs | 7 | 27 | 38 | 22 |
| Cotton Balls | 7.4 | 29.83 | 32.59 | 24 | |
| Sunflower | 12.33 | 31.97 | 33.31 | 17.64 | |
| Torrefied | Corn Cobs | 5 | 18 | 35 | 36 |
| Cotton Balls | 5.6 | 24.22 | 29.44 | 34.2 | |
| Sunflower | 9.55 | 29.35 | 31 | 24.73 |
| Sample Type | Sample Name | Temperature Range (°C) | Peak Temperature (°C) | Maximum Weight Loss Rate (%/min) | Average Weight Loss Rate (%/min) | CCF |
|---|---|---|---|---|---|---|
| Original | Corn Cob | 225–411 | 300 | 13.67 | 5.11 | 4.59942 × 10−6 |
| Cotton | 213–427 | 288 | 13.32 | 4.65 | 4.7403 × 10−6 | |
| Sunflower | 205–425 | 317 | 10.69 | 9.14 | 5.68835 × 10−11 | |
| Torrefied | Corn Cob | 252–436 | 326 | 9.85 | 3.82 | 1.81753 × 10−6 |
| Cotton | 213–565 | 359 | 3.12 | 1.9 | 3.63961 × 10−7 | |
| Sunflower | 216–434 | 328 | 10.2 | 8.9 | 9.7401 × 10−12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, J.; Imran, M.; Ali, A.M.; Nawaz, Z.; Muhammad, A.; Butt, R.K.; Jillani, M.S.; Naeem, H.A. Torrefaction and Thermochemical Properties of Agriculture Residues. Energies 2021, 14, 4218. https://doi.org/10.3390/en14144218
Akhtar J, Imran M, Ali AM, Nawaz Z, Muhammad A, Butt RK, Jillani MS, Naeem HA. Torrefaction and Thermochemical Properties of Agriculture Residues. Energies. 2021; 14(14):4218. https://doi.org/10.3390/en14144218
Chicago/Turabian StyleAkhtar, Javaid, Muhammad Imran, Arshid Mahmood Ali, Zeeshan Nawaz, Ayyaz Muhammad, Rehan Khalid Butt, Maria Shahid Jillani, and Hafiz Amir Naeem. 2021. "Torrefaction and Thermochemical Properties of Agriculture Residues" Energies 14, no. 14: 4218. https://doi.org/10.3390/en14144218
APA StyleAkhtar, J., Imran, M., Ali, A. M., Nawaz, Z., Muhammad, A., Butt, R. K., Jillani, M. S., & Naeem, H. A. (2021). Torrefaction and Thermochemical Properties of Agriculture Residues. Energies, 14(14), 4218. https://doi.org/10.3390/en14144218






