Biomethane Potential of Selected Organic Waste and Sewage Sludge at Different Temperature Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- GTS, which was obtained from the grease traps that are installed at the facility of a meat processing plant in the Silesian region of Poland. The content of VS. for this type of substrate was determined as 57.80%. The C/N ratio was 12.8.
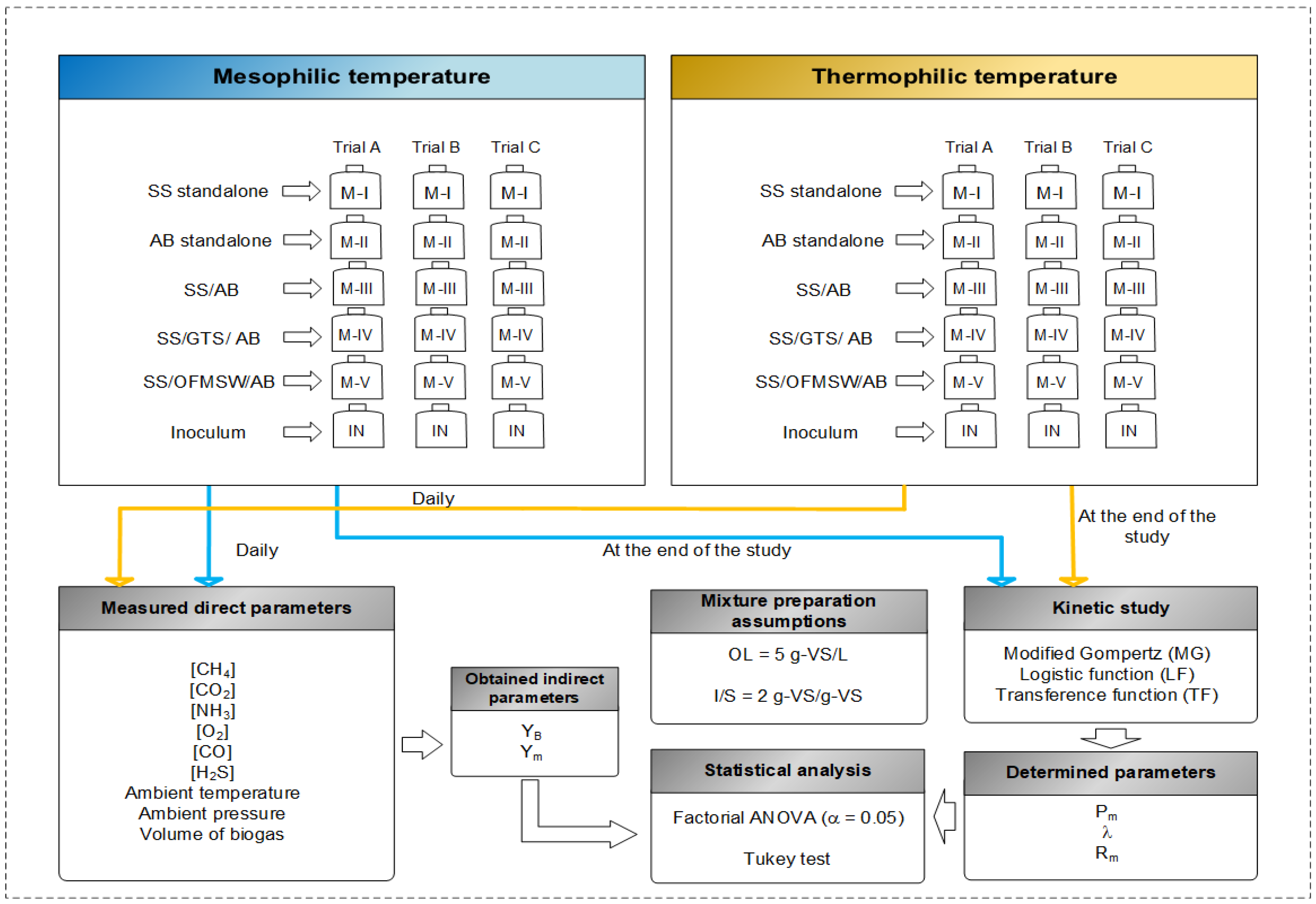
2.2. Experimental Procedure
2.2.1. BMP Assay
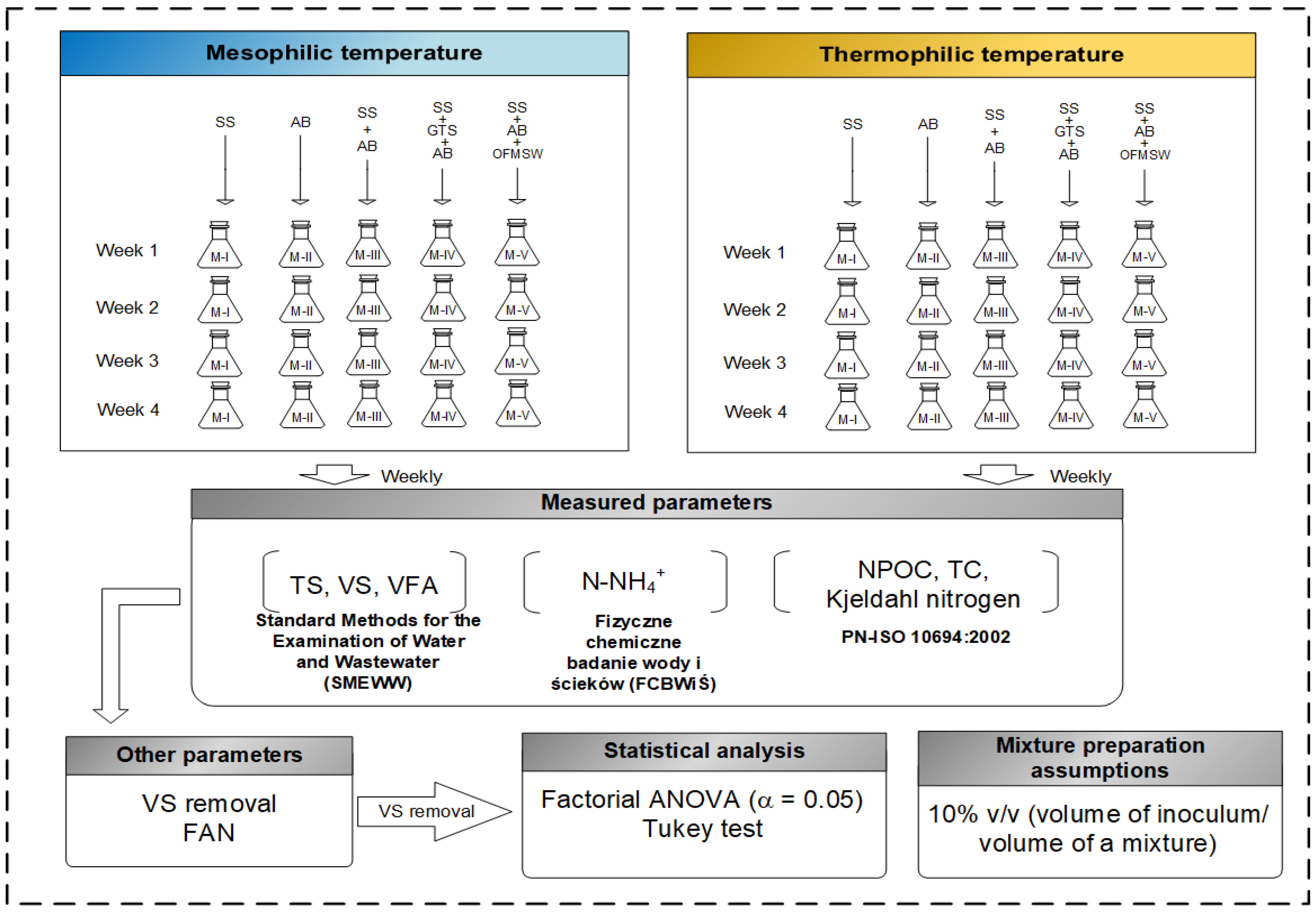
2.2.2. Physicochemical Analysis of Mixtures
2.3. Kinetic Study and Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
3.2. BMP Assay
3.3. Kinetic Study
3.4. Statistical Analysis
4. Conclusions
- Application of GTS and AB substrates for SS co-digestion with feed composition 60/30/10% (SS/GTS/AB) improved both YB and Ym parameters for 35.13% and 88.37%, respectively, at mesophilic temperature and 35.42% and 71.09% at thermophilic one in comparison to standalone SS; however, its λ was the second highest one compared to other mixtures. This mixture also had the highest potential methane yield, particularly at thermophilic temperature.
- Application of OFMSW and AB for SS co-digestion shows rapid adaptation as proved by the kinetic study (its λ was the lowest one at both temperatures among other studied mixtures).
- Thermophilic temperature significantly increased N-NH4+ and FAN concentration.
- All the applied models proved that they can be used for efficient evaluation of three component AcD; however, the best fitting was obtained for the MG model (9 of 10 cases) and the worst fitting for the LF (9 of 10 cases).
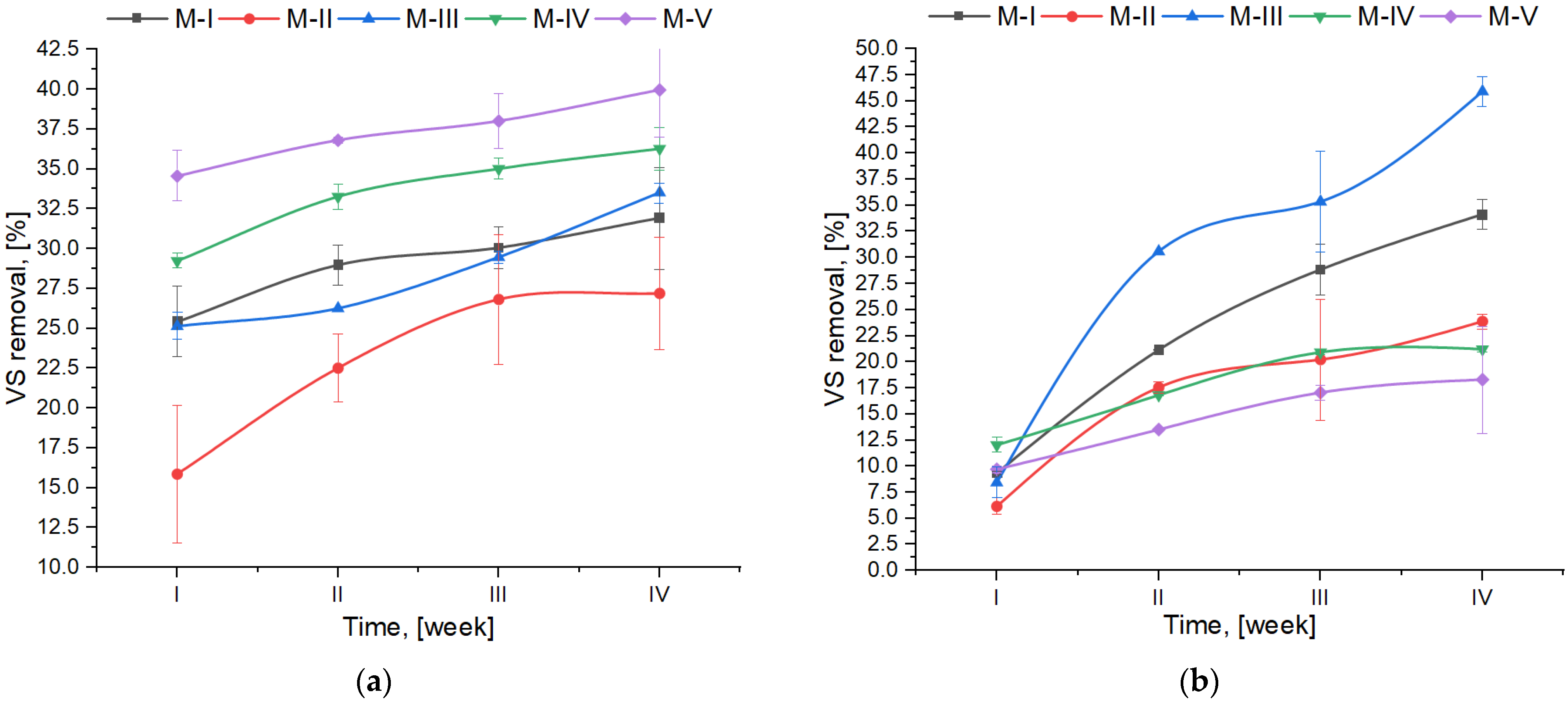
- VS removal was much slower at thermophilic temperature, and the values were lower for 64% (as an average for all mixtures) at the first thermophilic digestion week in comparison to the mesophilic one. As confirmed by the statistical analysis, temperature influenced this parameter the most.
- Statistical analysis confirmed that the YB results depended highly on the choice of substrates (factor “mixture”) for mixture preparation and operational temperature of the digestion as was also proved with the Tukey HSD test.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Seadi, T.; Rutz, D.; Prassl, H.; Kottner, M.; Finsterwalder, T.; Silke Volk, R.J. Biogas-Handbook; Al Seadi, T., Ed.; University of Southern Denmark: Esbjerg, Denmark, 2008; ISBN 9788799296200. [Google Scholar]
- Calise, F.; Cappiello, F.L.; D’Accadia, M.D.; Infante, A.; Vicidomini, M. Modeling of the anaerobic digestion of organic wastes: Integration of heat transfer and biochemical aspects. Energies 2020, 13, 2702. [Google Scholar] [CrossRef]
- Iacovidou, E.; Ohandja, D.G.; Voulvoulis, N. Food waste co-digestion with sewage sludge—Realising its potential in the UK. J. Environ. Manag. 2012, 112, 267–274. [Google Scholar] [CrossRef]
- Kouas, M.; Torrijos, M.; Sousbie, P.; Harmand, J.; Sayadi, S. Modeling the anaerobic co-digestion of solid waste: From batch to semi-continuous simulation. Bioresour. Technol. 2019, 274, 33–42. [Google Scholar] [CrossRef]
- Giordano, A.; Di Capua, F.; Esposito, G.; Pirozzi, F. Long-term biogas desulfurization under different microaerobic conditions in full-scale thermophilic digesters co-digesting high-solid sewage sludge. Int. Biodeterior. Biodegradation 2019, 142, 131–136. [Google Scholar] [CrossRef]
- Neczaj, E.; Grosser, A.; Worwąg, M. Boosting production of methane from sewage sludge by addition of grease trap sludge. Environ. Prot. Eng. 2013, 39, 125–133. [Google Scholar] [CrossRef]
- Bień, J.; Matysiak, B.; Wystalska, K. Stabilization and Dewatering of Sewage Sludge; Wydawnictwo Politechniki Częstochowskiej: Częstochowa, Poland, 1999; ISBN 8371930615. (In Polish) [Google Scholar]
- Neczaj, E.; Bien, J.; Grosser, A.; Worwag, M.; Kacprzak, M. Anaerobic treatment of sewage sludge and grease trap sludge in continuous co-digestion. Glob. Nest J. 2012, 14, 141–148. [Google Scholar]
- Grosser, A. Determination of methane potential of mixtures composed of sewage sludge, organic fraction of municipal waste and grease trap sludge using biochemical methane potential assays. a comparison of BMP tests and semi-continuous trial results. Energy 2018, 143, 488–499. [Google Scholar] [CrossRef]
- Chernysh, Y.; Roy, I.; Chubur, V.; Shulipa, Y.; Roubík, H. Co-digestion of poultry litter with cellulose-containing substrates collected in the urban ecosystem. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Poeschl, M.; Ward, S.; Owende, P. Prospects for expanded utilization of biogas in Germany. Renew. Sustain. Energy Rev. 2010, 14, 1782–1797. [Google Scholar] [CrossRef]
- Bux, F.; Chisti, Y. Algae Biotechnology: Products and Processes; Springer International Publishing: Cham, Switzerland, 2016; ISBN 3319123343. [Google Scholar]
- Tsapekos, P.; Alvarado-Morales, M.; Kougias, P.G.; Konstantopoulos, K.; Angelidaki, I. Co-digestion of municipal waste biopulp with marine macroalgae focusing on sodium inhibition. Energy Convers. Manag. 2019, 180, 931–937. [Google Scholar] [CrossRef]
- Davidsson, Å.; Lövstedt, C.; la Cour Jansen, J.; Gruvberger, C.; Aspegren, H. Co-digestion of grease trap sludge and sewage sludge. Waste Manag. 2008, 28, 986–992. [Google Scholar] [CrossRef]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic co-digestion of sludge and organic food waste-performance, inhibition, and impact on the microbial community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef] [Green Version]
- Grosser, A.; Neczaj, E.; Singh, B.R.; Almås, Å.R.; Brattebø, H.; Kacprzak, M. Anaerobic digestion of sewage sludge with grease trap sludge and municipal solid waste as co-substrates. Environ. Res. 2017, 155, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Wagdy, R.; Said, N. Evaluation of biogas production from anaerobic co-digestion of sewage sludge with microalgae and agriculture wastes. BioResources 2019, 14, 8405–8412. [Google Scholar]
- Solé-Bundó, M.; Garfí, M.; Ferrer, I. Pretreatment and co-digestion of microalgae, sludge and fat oil and grease (FOG) from microalgae-based wastewater treatment plants. Bioresour. Technol. 2020, 298, 122563. [Google Scholar] [CrossRef] [PubMed]
- Al Seadi, T.; Lukehurst, C. Quality management of digestate from biogas plants used as fertiliser. IEA Bioenergy 2012, 37, 40. [Google Scholar]
- Minister of the Environment. Regulation of the Minister of the Environment of 6 February 2015 on the Municipal 572 Sewage Sludge (J. L. 2015, No. 0, Item. 257). 2015. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20150000257/O/D20150257.pdf (accessed on 24 June 2021). (In Polish)
- Ministry of the Environment Regulation of the Minister of the Environment of 20 January 2015 Regarding the 576 R10 Recovery Process. 2015. (Journal of Laws 2015 Item 132). 2015. Available online: http://www.fao.org/faolex/577results/details/en/c/LEX-FAOC151955/ (accessed on 24 June 2021). (In Polish).
- Epstein, G.; Smale, D.A. Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecol. Evol. 2017, 7, 8624–8642. [Google Scholar] [CrossRef]
- Global Invasive Species Database. 2021. Available online: http://www.iucngisd.org/gisd/?st=100ss&fr=1&str=&lang=EN (accessed on 22 June 2021).
- Sosnowski, P.; Klepacz-Smolka, A.; Kaczorek, K.; Ledakowicz, S. Kinetic investigations of methane co-fermentation of sewage sludge and organic fraction of municipal solid wastes. Bioresour. Technol. 2008, 99, 5731–5737. [Google Scholar] [CrossRef]
- Grosser, A. The influence of decreased hydraulic retention time on the performance and stability of co-digestion of sewage sludge with grease trap sludge and organic fraction of municipal waste. J. Environ. Manag. 2017, 203, 1143–1157. [Google Scholar] [CrossRef]
- Petracchini, F.; Liotta, F.; Paolini, V.; Perilli, M.; Cerioni, D.; Gallucci, F.; Carnevale, M.; Bencini, A. A novel pilot scale multistage semidry anaerobic digestion reactor to treat food waste and cow manure. Int. J. Environ. Sci. Technol. 2018, 15, 1999–2008. [Google Scholar] [CrossRef]
- Zhang, E.; Li, J.; Zhang, K.; Wang, F.; Yang, H.; Zhi, S.; Liu, G. Anaerobic digestion performance of sweet potato vine and animal manure under wet, semi-dry, and dry conditions. AMB Express 2018, 8, 45. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Alleman, J.E. Free Ammonia-Nitrogen Calculator & Information. Available online: http://home.eng.iastate.edu/~jea/w3-research/free-ammonia/nh3.html (accessed on 20 June 2021).
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Massé, D.I.; Rajagopal, R.; Singh, G. Technical and operational feasibility of psychrophilic anaerobic digestion biotechnology for processing ammonia-rich waste. Appl. Energy 2014, 120, 49–55. [Google Scholar] [CrossRef]
- Yun, Y.M.; Kim, D.H.; Cho, S.K.; Shin, H.S.; Jung, K.W.; Kim, H.W. Mitigation of ammonia inhibition by internal dilution in high-rate anaerobic digestion of food waste leachate and evidences of microbial community response. Biotechnol. Bioeng. 2016, 113, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Spasiano, D.; Giordano, A.; Adani, F.; Fratino, U.; Pirozzi, F.; Esposito, G. High-solid anaerobic digestion of sewage sludge: Challenges and opportunities. Appl. Energy 2020, 278, 115608. [Google Scholar] [CrossRef]
- Hermanowicz, W.; Dożańska, W.; Dojlido, J.; Koziorowski, B.; Zerbe, J. Physico-Chemical Analysis of Water and Sewage; Arkady: Warszawa, Poland, 1999; ISBN 978-83-213-4067-8. (In Polish) [Google Scholar]
- Jenkins, S.H. Standard Methods for the Examination of Water and Wastewater. Water Res. 1982, 16, 1495–1496. [Google Scholar] [CrossRef]
- Jha, A.K.; Li, J.; Zhang, L.; Ban, Q.; Jin, Y. Comparison between wet and dry anaerobic digestions of cow dung under mesophilic and thermophilic conditions. Adv. Water Resour. Prot. 2013, 1, 28–38. [Google Scholar]
- Zhao, H.W.; Viraraghavan, T. Analysis of the performance of an anaerobic digestion system at the Regina wastewater treatment plant. Bioresour. Technol. 2004, 95, 301–307. [Google Scholar] [CrossRef]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; de Castro, J.S.; de do Couto, E.A.C.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci. Total Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Olsson, J.; Feng, X.M.; Ascue, J.; Gentili, F.G.; Shabiimam, M.A.; Nehrenheim, E.; Thorin, E. Co-digestion of cultivated microalgae and sewage sludge from municipal waste water treatment. Bioresour. Technol. 2014, 171, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Elango, D.; Pulikesi, M.; Baskaralingam, P.; Ramamurthi, V.; Sivanesan, S. Production of biogas from municipal solid waste with domestic sewage. J. Hazard. Mater. 2007, 141, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Luostarinen, S.; Luste, S.; Sillanpää, M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Girault, R.; Bridoux, G.; Nauleau, F.; Poullain, C.; Buffet, J.; Peu, P.; Sadowski, A.G.; Béline, F. Anaerobic co-digestion of waste activated sludge and greasy sludge from flotation process: Batch versus CSTR experiments to investigate optimal design. Bioresour. Technol. 2012, 105, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Guendouz, J.; Buffière, P.; Cacho, J.; Carrère, M.; Delgenes, J.-P. Dry anaerobic digestion in batch mode: Design and operation of a laboratory-scale, completely mixed reactor. Waste Manag. 2010, 30, 1768–1771. [Google Scholar] [CrossRef]
- Kelly, R.J.; Shearer, B.D.; Kim, J.; Goldsmith, C.D.; Hater, G.R.; Novak, J.T. Relationships between analytical methods utilized as tools in the evaluation of landfill waste stability. Waste Manag. 2006, 26, 1349–1356. [Google Scholar] [CrossRef]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Owens, J.M.; Chynoweth, D.P. Biochemical methane potential of municipal solid waste (MSW) components. Water Sci. Technol. 1993, 27, 1–14. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Migliore, G.; Alisi, C.; Sprocati, A.R.; Massi, E.; Ciccoli, R.; Lenzi, M.; Wang, A.; Cremisini, C. Anaerobic digestion of macroalgal biomass and sediments sourced from the Orbetello lagoon, Italy. Biomass Bioenergy 2012, 42, 69–77. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Trobajo, R.; Caiola, N.; Ibáñez, C.; Fabregat, A.; Bengoa, C. Biogas production from sewage sludge and microalgae co-digestion under mesophilic and thermophilic conditions. Renew. Energy 2015, 75, 374–380. [Google Scholar] [CrossRef]
- Hallaji, S.M.; Kuroshkarim, M.; Moussavi, S.P. Enhancing methane production using anaerobic co-digestion of waste activated sludge with combined fruit waste and cheese whey. BMC Biotechnol. 2019, 19, 19. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Obata, O.; Ditchfield, A.; Hatton, A.; Akunna, J. Investigating the impact of inoculum source on anaerobic digestion of various species of marine macroalgae. Algal Res. 2020, 46, 101803. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Strömberg, S.; Nistor, M.; Liu, J. Towards eliminating systematic errors caused by the experimental conditions in Biochemical Methane Potential (BMP) tests. Waste Manag. 2014, 34, 1939–1948. [Google Scholar] [CrossRef]
- Raposo, F.; De Rubia, M.A.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2011, 16, 861–877. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, F.; Fernández-Cegrí, V.; de la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Ponsá, S.; Gea, T.; Sánchez, A. Anaerobic co-digestion of the organic fraction of municipal solid waste with several pure organic co-substrates. Biosyst. Eng. 2011, 108, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef] [PubMed]
- El-Mashad, H.M. Kinetics of methane production from the codigestion of switchgrass and Spirulina platensis algae. Bioresour. Technol. 2013, 132, 305–312. [Google Scholar] [CrossRef] [PubMed]


| Substrate | TS (%) | VS (%) | VS/TS (–) | C/N (–) |
|---|---|---|---|---|
| AB | 88.70 | 60.08 | 0.67 | 9.36 |
| OFMSW | 20.20 | 19.70 | 0.96 | 29.3 ± 0.7 |
| GTS | 58.40 | 57.80 | 0.99 | 12.8 |
| Digested sludge (inoculum) | 2.61 (1)–2.84 (2) | 1.38 (1)–1.59 (2) | 0.53 (1)–0.56 (2) | 8.65 (2)–8.85 (1) |
| Mixture | Percentage Composition of Each Mixture Based on VS. of Each Substrate | Mesophilic Digestion | Thermophilic Digestion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AB | SS | GTS | OFMSW | |||||||||
| TS (%) | VS (%) | VS/TS (−) | C/N (−) | TS (%) | VS (%) | VS/TS (−) | C/N (−) | |||||
| M-I | – | 100 | – | – | 3.84 | 3.08 | 0.80 | 10 | 3.22 | 2.42 | 0.75 | 10.3 |
| M-II | 100 | – | – | – | 4.76 | 3.20 | 0.67 | 8.3 | 4.72 | 3.03 | 0.64 | 8.2 |
| M-III | 10 | 90 | – | – | 4.02 | 3.15 | 0.78 | 11.6 | 3.46 | 2.56 | 0.74 | 10 |
| M-IV | 10 | 60 | 30 | – | 5.54 | 4.51 | 0.81 | 9.9 | 4.99 | 3.99 | 0.79 | 11.7 |
| M-V | 10 | 60 | – | 30 | 5.26 | 4.25 | 0.80 | 10.5 | 4.83 | 3.82 | 0.79 | 10.8 |
| Mixture | YB (N mL/g-VSadd) | Ym (N mL-CH4/g-VSadd) | ||
|---|---|---|---|---|
| M | T | M | T | |
| M-I | 474.61 ± 11.94 bc | 497.23 ± 33.31 bcd | 138.47 ± 4.70 bcd | 161.13 ± 13.11 de |
| M-II | 456.61 ± 13.60 bc | 397.64 ± 36.06 b | 97.50 ± 2.85 b | 108.79 ± 5.73 bc |
| M-III | 451.30 ± 9.98 bc | 452.11 ± 8.97 bc | 138.04 ± 2.94 bcd | 138.57 ± 5.38 bcd |
| M-IV | 641.34 ± 33.84 e | 673.36 ± 8.97 e | 260.83 ± 15.02 f | 275.66 ± 4.11 f |
| M-V | 512.55 ± 30.05 cd | 592.74 ± 98.36 de | 147.18 ± 7.88 cd | 203.07 ± 47.49 e |
| IN | 78.74 ± 0.00 a | 36.84 ± 1.08 a | 4.22 ± 0.00 a | 0.49 ± 0.01 a |
| Mixture | Model | Pm (N mL-CH4/g-VSadd) | Rm (N mL-CH4 × g-VSadd−1 × d−1) | λ (d) | R2 (−) | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | T | M | T | M | T | M | T | ||
| M-I | MG | 135.80 | 157.11 | 32.33 | 47.42 | 1.074 | 1.099 | 0.9985 | 0.9986 |
| LF | 141.52 | 168.14 | 36.21 | 50.46 | 0.409 | 0.388 | 0.9825 | 0.9775 | |
| TF | 134.53 | 155.30 | 32.64 | 47.78 | 1.229 | 1.212 | 0.9958 | 0.9957 | |
| M-II | MG | 96.70 | 70.33 | 29.77 | −6.18 | 1.433 | 93.036 | 0.9995 | 0 |
| LF | 100.70 | 122.99 | 28.40 | 24.77 | 0.464 | 0.866 | 0.9685 | 0.9374 | |
| TF | 95.93 | 107.52 | 29.65 | 39.49 | 1.544 | 2.975 | 0.9983 | 0.9997 | |
| M-III | MG | 136.17 | 138.08 | 36.23 | 51.85 | 0.921 | 0.856 | 0.9985 | 0.9995 |
| LF | 141.29 | 144.29 | 41.93 | 56.41 | 0.356 | 0.258 | 0.9822 | 0.9777 | |
| TF | 134.77 | 137.05 | 36.08 | 51.45 | 1.033 | 0.934 | 0.9943 | 0.997 | |
| M-IV | MG | 260.56 | 275.93 | 37.54 | 74.34 | 1.234 | 1.507 | 0.9995 | 0.9994 |
| LF | 277.23 | 298.17 | 43.91 | 75.72 | 0.606 | 0.532 | 0.9882 | 0.969 | |
| TF | 256.36 | 272.79 | 40.84 | 74.01 | 1.624 | 1.633 | 0.9968 | 0.999 | |
| M-V | MG | 146.71 | 191.69 | 42.78 | 49.69 | 0.808 | 0.853 | 0.9995 | 0.9952 |
| LF | 151.12 | 206.25 | 51.28 | 58.03 | 0.289 | 0.349 | 0.9811 | 0.984 | |
| TF | 145.45 | 188.83 | 42.19 | 50.18 | 0.907 | 0.979 | 0.997 | 0.9904 | |
| Parameter | Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| Time | Mixt. | Temp. | Time/Mixt. | Time/Temp. | Mixt./Temp. | Time/Mixt./Temp. | ||
| VS removal | F | 218.39 | 57.66 | 602.79 | 9.34 | 41.55 | 113.57 | 8.37 |
| p | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| YB | F | 0.693 | 13.953 | 0.630 | 0.072 | 0.250 | 3.345 | 0.073 |
| p | 0.558967 | 0.000000 | 0.429667 | 0.999992 | 0.860842 | 0.013855 | 0.999990 | |
| Ym | F | 57.537 | 1.118 | 10.650 | 0.293 | 3.391 | 0.475 | 0.311 |
| p | 0.000000 | 0.346796 | 0.001620 | 0.988921 | 0.012927 | 0.700691 | 0.985666 | |
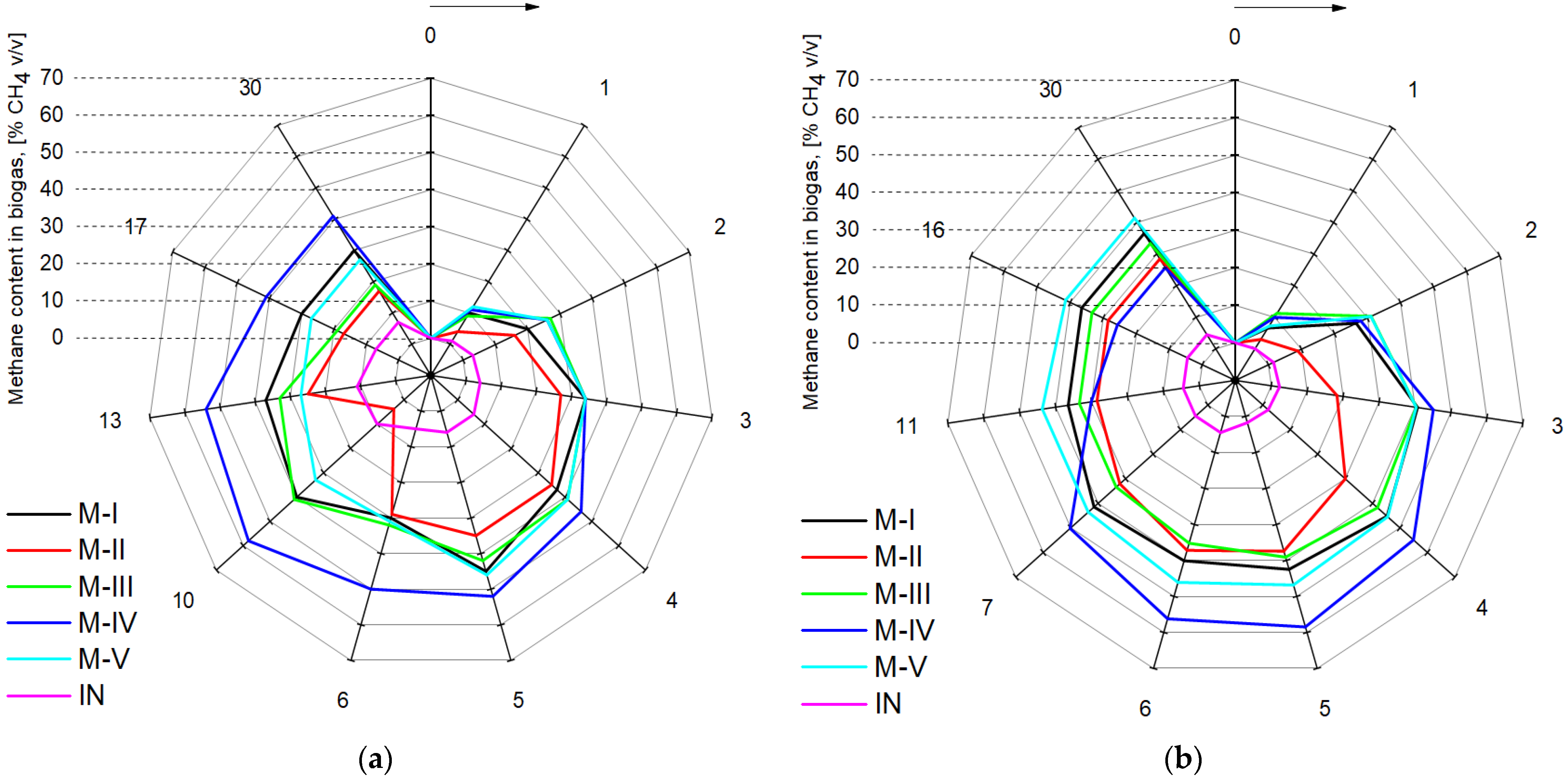
| Variation of methane in biogas | F | 25.262 | 19.073 | 59.791 | 3.209 | 14.373 | 7.360 | 3.261 |
| p | 0.000000 | 0.000000 | 0.000000 | 0.000866 | 0.000000 | 0.000042 | 0.000738 | |
| Parameter | Factor | Mixture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VS removal | Mixt. | M-V | M-IV | M-II | M-II | M-I | M-III | M-I | M-IV | M-V | M-III |
| Temp. | T | T | T | M | M | M | T | M | M | T | |
| Mean | 18.32 | 21.24 | 23.90 | 27.21 | 31.93 | 33.53 | 34.16 | 36.28 | 39.96 | 45.93 | |
| a | **** | **** | **** | ||||||||
| b | **** | **** | **** | ||||||||
| c | **** | **** | **** | **** | |||||||
| d | **** | **** | **** | **** | |||||||
| e | **** | **** | **** | **** | |||||||
| f | **** | **** | |||||||||
| YB | Mixt. | MV | MIV | MII | MII | MI | MIII | MI | MIV | MV | MIII |
| Temp. | T | T | T | M | M | M | T | M | M | T | |
| Mean | 316.40 | 388.01 | 396.35 | 443.99 | 444.63 | 449.70 | 457.21 | 502.33 | 614.21 | 660.51 | |
| a | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | |
| Ym | Mixt. | MV | MIV | MII | MII | MI | MIII | MI | MIV | MV | MIII |
| Temp. | T | T | T | M | M | M | T | M | M | T | |
| Mean | 92.31 | 97.50 | 103.72 | 122.06 | 135.04 | 143.24 | 147.51 | 156.68 | 247.83 | 275.66 | |
| a | **** | **** | **** | **** | **** | **** | **** | **** | |||
| b | **** | **** | **** | **** | **** | ||||||
| c | **** | **** | |||||||||
| Variation of methane in biogas | Mixt. | MV | MIV | MII | MII | MI | MIII | MI | MIV | MV | MIII |
| Temp. | T | T | T | M | M | M | T | M | M | T | |
| Mean | 5.00 | 17.00 | 25.70 | 27.00 | 28.42 | 30.00 | 33.40 | 36.58 | 41.00 | 41.40 | |
| a | **** | ||||||||||
| b | **** | ||||||||||
| c | **** | **** | **** | ||||||||
| d | **** | **** | **** | ||||||||
| e | **** | **** | |||||||||
| f | **** | **** | |||||||||
| g | **** | **** | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smetana, G.; Neczaj, E.; Grosser, A. Biomethane Potential of Selected Organic Waste and Sewage Sludge at Different Temperature Regimes. Energies 2021, 14, 4217. https://doi.org/10.3390/en14144217
Smetana G, Neczaj E, Grosser A. Biomethane Potential of Selected Organic Waste and Sewage Sludge at Different Temperature Regimes. Energies. 2021; 14(14):4217. https://doi.org/10.3390/en14144217
Chicago/Turabian StyleSmetana, German, Ewa Neczaj, and Anna Grosser. 2021. "Biomethane Potential of Selected Organic Waste and Sewage Sludge at Different Temperature Regimes" Energies 14, no. 14: 4217. https://doi.org/10.3390/en14144217
APA StyleSmetana, G., Neczaj, E., & Grosser, A. (2021). Biomethane Potential of Selected Organic Waste and Sewage Sludge at Different Temperature Regimes. Energies, 14(14), 4217. https://doi.org/10.3390/en14144217







