1. Introduction
The widespread development of electronics allows for the production of portable devices characterized by high functionality. In the case of devices intended for the armed forces or other services operating in difficult environmental conditions, it becomes particularly important to attain the smallest possible dimensions and weight of devices, along with the longest possible period of their operation between either the replacement of the power source or the need to recharge it. With the current level of electronic systems integration, the most common element determining the weight and dimensions of the device is the power source itself. What follows is, on the one hand, the need to search for energy-saving solutions, characterized by a minimized electricity demand, and on the other hand, to seek light and more efficient power sources. Currently, lithium-ion (Li-ion) batteries are commonly used as a power source in these types of applications. Li-ion batteries have several advantages, on account of which they enjoy great popularity. The key benefits are high energy density, high durability, and low self-discharge, while the shortcomings of Li-ion batteries include their sensitivity to overcharging and deep discharge [
1,
2,
3,
4].
The constant development of lithium-ion technology gradually eliminates the disadvantages of this type of battery, making them safe to use. A number of Li-ion battery types have been developed which differ mainly in as far as the electrode material is concerned. In the research described in this article, LiFePO
4 batteries were used in which the risk of uncontrolled temperature increase in the case of overcharging was reduced, as compared to cobalt-based cells [
5].
To obtain the required power source parameters, batteries are connected into packs. The parallel connection allows for higher capacity to be obtained [
6], while the series connection provides increased output voltage. Only batteries of the same type, from one production lot and characterized by the same state of charge should be combined into packs [
7]. Differing states of batteries (state-of-charge (SoC), their capacity, internal impedance) result in an uneven distribution of energy accumulated in the cells of the pack, which may lead to their overcharging or deep (excessive) discharge and, consequently, to battery damage. For these reasons, the state-of-health (SoH) of the cells used in the pack is an important parameter to be taken into account. In practice, however, it is very difficult to determine the current SoH level. Due to irreversible electrochemical reactions, as batteries are being used, their parameters gradually degrade [
8,
9]. Many external factors influence the rapidity and magnitude of these changes. Therefore, in the pack, the aging processes taking place may vary from battery to battery. The rate of capacity-fade and changes in internal resistance depends, inter alia, on the battery SoC and temperature [
10]. If there are differences in the capacities of the cells, the discrepancy deepens in subsequent charge/discharge cycles, leading either to overcharging or over-discharge of the lowest capacity cell. Maintaining an overcharged or over-discharged state leads to cell damage [
11]. To guard against such situations, battery management systems (BMS) are used, which protect the cells against overcharging, excessive discharge, and overload [
12,
13,
14]. It is made possible due to the parameters of individual cells being constantly monitored by the BMS systems. SoC is a crucial parameter, the precise determination of which has a substantial impact on the effectiveness of the BMS system. For the precise determination of SoC, many methods have been developed based on complex modeling of Li-ion cells taking into account inter alia: SoH, the internal resistance of the cell, and other parameters [
15,
16,
17]. For a BMS system, precise SoC determination is of particular importance in complex, high-capacity energy storage systems. In simple, small-capacity systems, the most economical and commonly used solutions are based on charge counting (CC) [
15].
This article presents the results of research on the charging and discharging processes of a LiFePO4 battery pack, which is practically applied in portable devices with low energy consumption, e.g., mine-laying and mine-clearing equipment. It was examined whether the differences in the capacities of individual batteries from one production batch could lead to their operation in conditions not recommended by the manufacturer, leading to the risk of overcharging or excessive discharge of the cells comprising the pack. The impact of successive charge-discharge cycles on the energy spread stored in individual cells was analyzed. Then, the influence of the BMS on the charging-discharging process, including the energy distribution between the cells, efficiency, and cycle duration, was verified.
The tests were performed in conditions similar to those occurring during the operation of the battery pack with the configuration available in a real dual-use device. The research was aimed at determining the potential causes of failure of a battery pack with no protection offered by the BMS systems and checking the available methods of tackling this issue.
Among the novelty elements of the proposed solutions in this article in respect to literature, the following should be distinguished:
Testing the process of charging and discharging a pack, in which one of the batteries is characterized by different initial energy;
Testing the process of charging the pack immediately following the discharge cycle, without the relaxation period;
Indication of the possibility of protecting the battery pack in the discharge process solely based on information on the voltage of the entire pack.
2. Materials and Methods
2.1. Comparison of the State of Batteries in a Pack
The basic parameter allowing the comparison of the state of batteries is the battery state of charge (SoC). SoC determines the amount of energy stored in the battery relative to its nominal capacity. This parameter cannot be measured directly, however, there are several methods allowing the SoC to be inferred indirectly. In the case of the tested batteries, the methods of assessing physicochemical properties of the battery (electrolyte, electrodes) are not applicable [
18]. The methods which use voltage, current, and temperature measurement can be applied in practice [
19].
Battery voltage (U
B) depends on SoC, and thus theoretically, this dependence can be used to determine SoC. However, in LiFePO
4 batteries in a wide range of SoC, the voltage characteristic changes with SoC decreasing very slightly [
20]. This makes it difficult to accurately determine the current state of the battery at each point. Clear U
B changes occur at a low SoC level [
20,
21,
22]. Moreover, this characteristic largely depends on current and temperature, which makes its application difficult. The elimination of the influence of current is possible by measuring the battery’s open-circuit voltage (OCV, V
OC). However, immediately after the load is disconnected, the VOC changes for the time necessary for the battery to reach electrochemical equilibrium [
23], which is not clearly defined, and that, in many applications, makes it difficult to use this parameter in practice.
Another method used to estimate SoC is to calculate the charge present in the battery from the measurement of the current and time during the charging or discharging. To determine the absolute value of the SoC, it is necessary to know the initial state of SoC prior to charging/discharging [
8,
15,
16,
19,
21]. If the SoC is not known, it is possible to calculate the SoC change during the charging/discharging cycle according to the following relationship:
where C
n denotes nominal battery capacity, I is charge/discharge current, t is time, and T denotes charge/discharge time.
In the case of a battery packs connected in series, this method will not allow the determination of the degree of the SoC variation between individual cells, as the current flowing through all the cells is the same.
The energy spread between individual cells of the pack can be determined by measuring the current and voltage at each cell during charging/discharging and calculating the energy supplied by or drawn by the batteries, according to the following Formula (2):
where E
B represents energy supplied by/drawn by the battery, U
B is the voltage measured at the battery terminals, I denotes charging/discharging current of the battery pack, t denotes time, and T—charging/discharging time.
For ideal batteries, the energy drawn during charging would be equal to the energy supplied to the receiver during discharge. Obviously, battery efficiency is less than 100%, and one of the main factors responsible is its internal resistance. According to a commonly used Li-ion battery equivalent model, the series resistance value depends, to a large extent, on the SoC, and it increases with a decrease in the SoC value [
5,
17,
18,
24]. This fact leads to an increase in the differences in the energies stored in batteries of varying SoC levels.
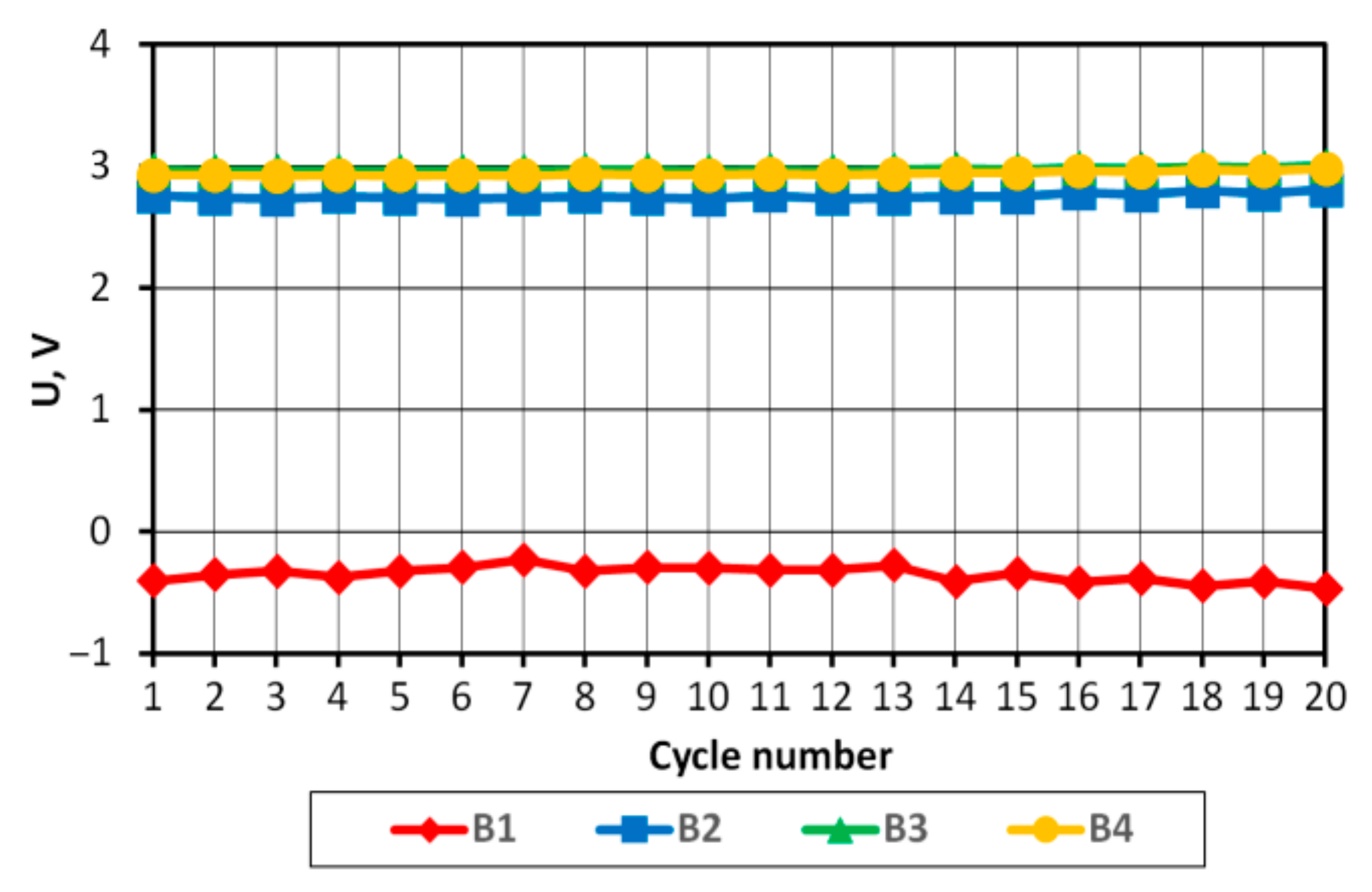
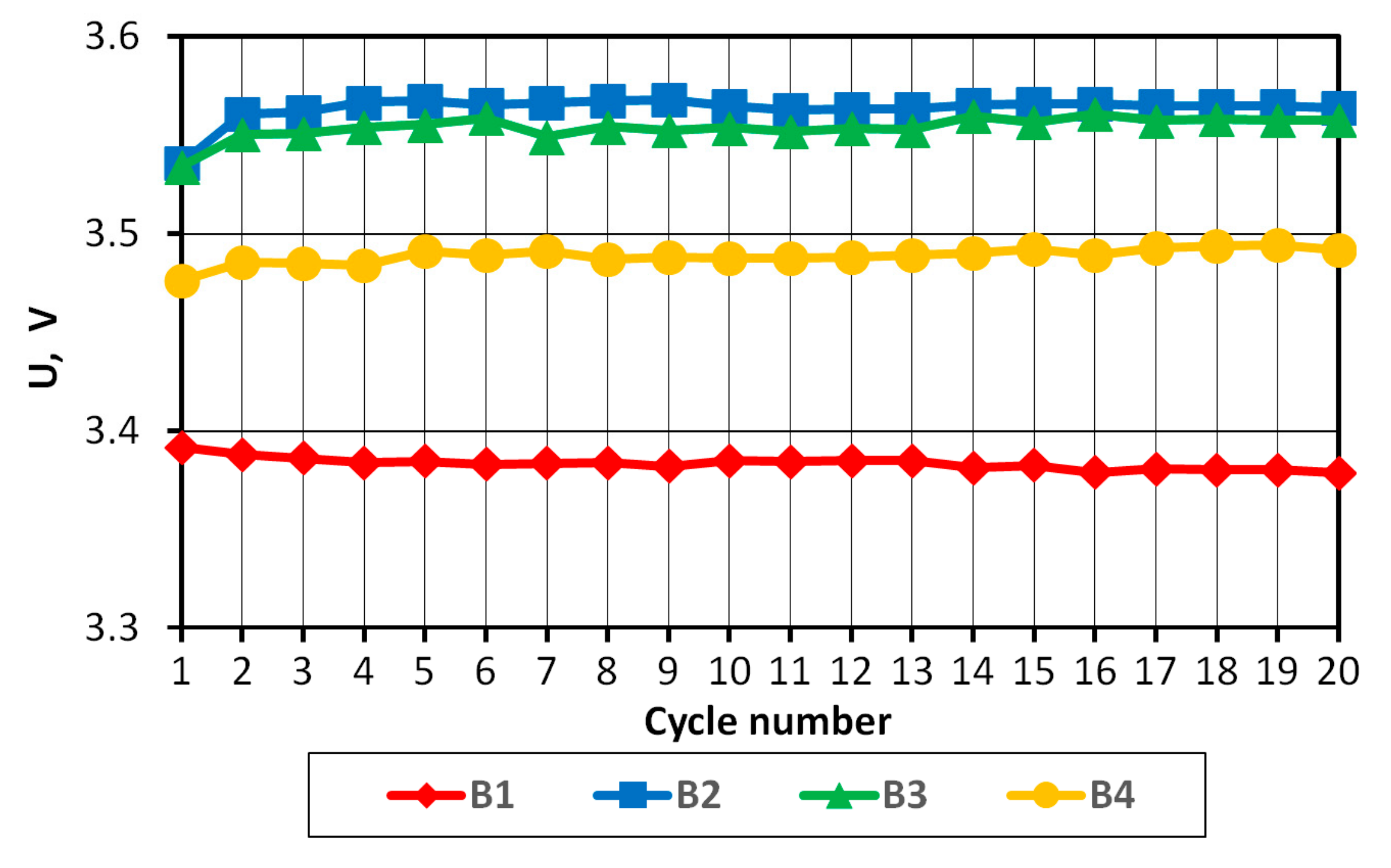
Based on the analysis presented above, the measurement of voltage UB and energy EB was used to determine the degree of SoC variation of the batteries comprising the tested pack. Throughout the research, efforts were not aimed at determining the exact SoC value, but rather at investigating the degree to which individual batteries differ after successive charging/discharging cycles. UB of the batteries at the end of charging and discharging cycles was compared.
The E
B energy for each cell was calculated by numerical integration using the rectangle method according to the following formula:
where E
Bn is the energy supplied by or drawn by the battery B
n, U
Bn denotes voltage measured at the battery terminals B
n, I is battery pack charging/discharging current, t is time, T denotes charging/discharging time, and Δt is the time between subsequent measurements, m = T/Δt.
2.2. Cell Balancing System
One of the tasks of battery management systems (BMS) is ensuring that the battery condition is balanced [
25]. There are numerous techniques for achieving equilibrium, but they are broadly divided into active and passive methods. Active methods are used in high power systems, where the dissipation of large amounts of energy for battery cell balancing would be unacceptable [
7,
12]. In the case of batteries used in portable devices, the energy stored by them is relatively low, and in this area, practically only passive balancing systems are used. The operation of these systems consists mainly of controlling the voltages of individual batteries during charging/discharging and adding additional loads in parallel to individual cells by controlling the FET keys according to a specific algorithm [
12]. This is the principle behind the BMS, constructed on the basis of the ABLIC (Tokyo, Japan) S-8254A system used during the study.
2.3. Measurement System
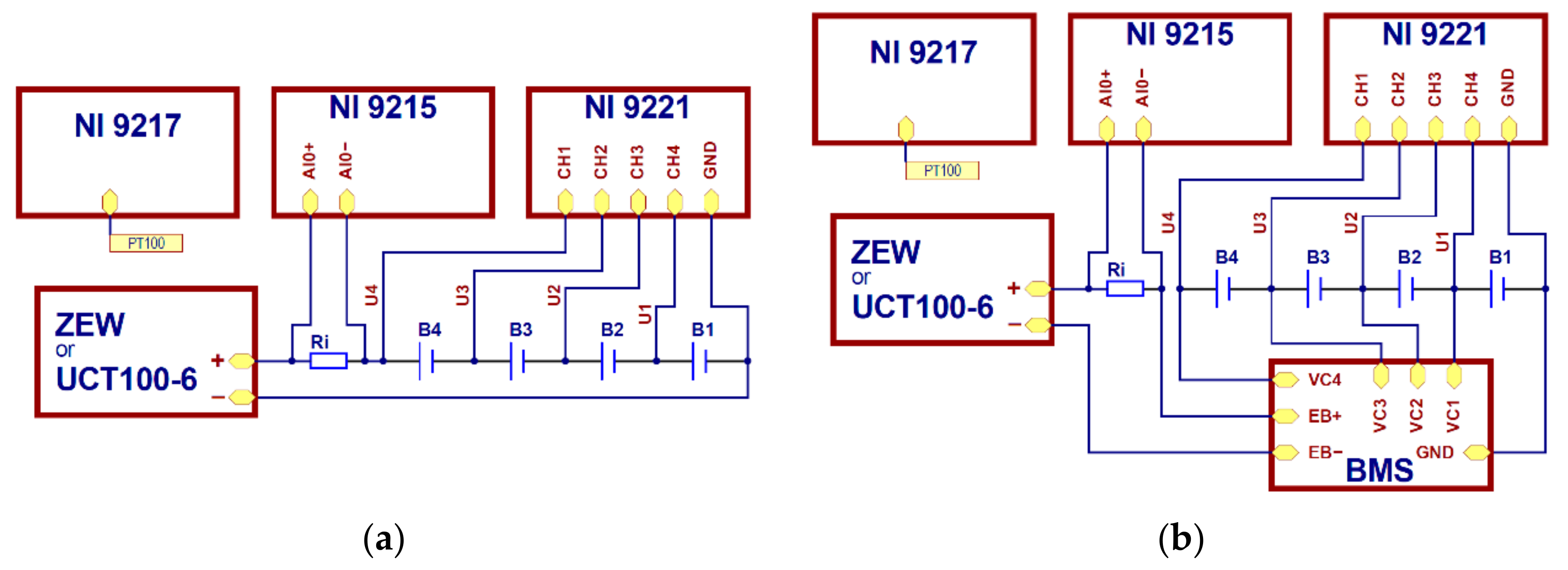
The tests were carried out with the use of commonly available APR 18650M 1A batteries manufactured by A123 Systems company (Novi, MI, USA). Fresh batteries, never used before, were used for the tests. Based on previous measurements of the properties of APR 18650M 1A batteries, carried out by the authors on lots from various deliveries, it appears that the state of charge of the new batteries was approximately 50%. Within one lot, it was found that there were variations between the individual units of up to 50 mAh. For these reasons, the battery marked as B1 was discharged by 50 mAh, which was about 4.5% of the nominal capacity (1100 mAh), for the tests. Then the batteries were connected in series to form a pack (
Figure 1).
According to
Section 2.1, to determine the condition of individual batteries in the pack, voltage measurements for individual batteries had to be conducted and measurements of the current flowing through the cells during the charging and discharging processes. Measurements were carried out in the arrangement shown in
Figure 2 using the following measurement modules: NI 9221, NI 9215, and NI 9217 by the National Instruments company (NI). The NI 9221 module enabled voltage measurements with a resolution of 12 bits, whereas the NI 9215 allowed for differential measurements with a 16-bit resolution.
U1 ÷ U4 voltage measurements were conducted using the NI 9221 module, while the NI 9215 module was used to measure Ui on the Ri resistor, whose resistance equals 10 mΩ. All the measurements were recorded every 5 s.
Voltage values for individual batteries were calculated based on the following dependences:
where UB1 ÷ UB4 voltage for B1 ÷ B4 batteries, U1 ÷ U4 voltage measures in points indicated in
Figure 2. The current intensity was calculated using the following formula:
The charging and discharging of the battery pack was performed with the use of two types of chargers:
1. ZEW-type battery charger (constructed by the authors, based on the charger used in electric detonation systems);
2. UCT100-6 battery-charging station by the VOLTCRAFT company (Wollerau, Switzerland).
The ZEW-type charger test results allowed the authors to evaluate the operation of the batteries in their actual operating conditions. The use of the UCT100-6 universal charger provided the means of checking whether the change of charging parameters had a significant impact on the behavior of the batteries.
In both devices, the charging process followed the CC-CV method [
9]. In the first charging stage, the charging current (CC) was constant, and after the voltage threshold value was reached, a shift was made to the constant voltage (CV) charging method at the charger output until the charging current dropped to the minimum threshold value. Charging parameters cannot be changed in the ZEW charger. The charging current of the CC phase was 0.5 A, while the voltage in the CV phase equaled 14 V.
The discharge process in the ZEW-type charger was carried out by attaching a constant resistance load to the battery pack. Due to the high constant voltage characteristic of LiFePO4 batteries in a wide range of SoC changes; also the current during discharge remained constant and amounted to 0.8 A. The UCT100-6 charger enables the regulation and stabilization of the discharge current.
4. Conclusions
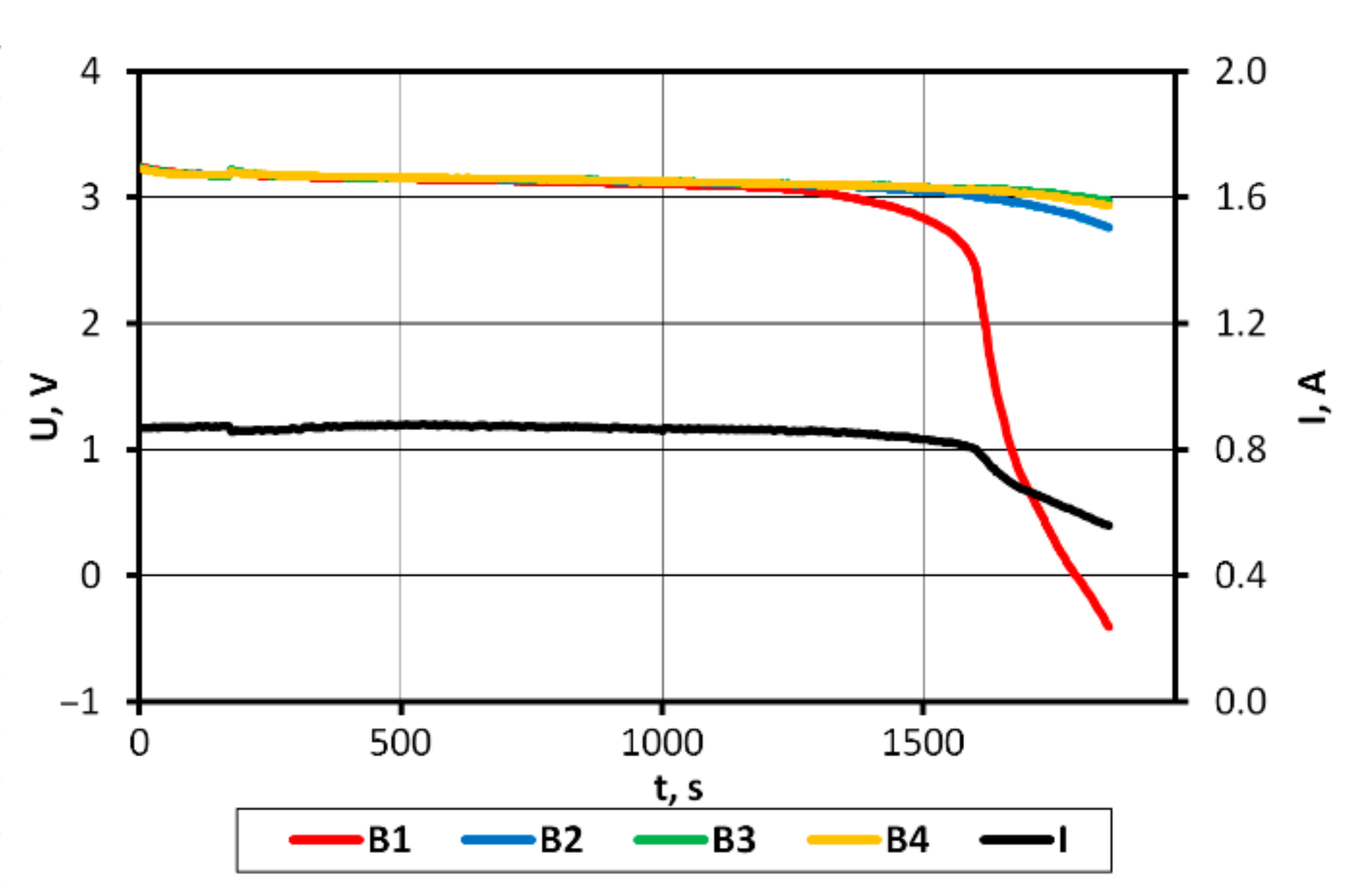
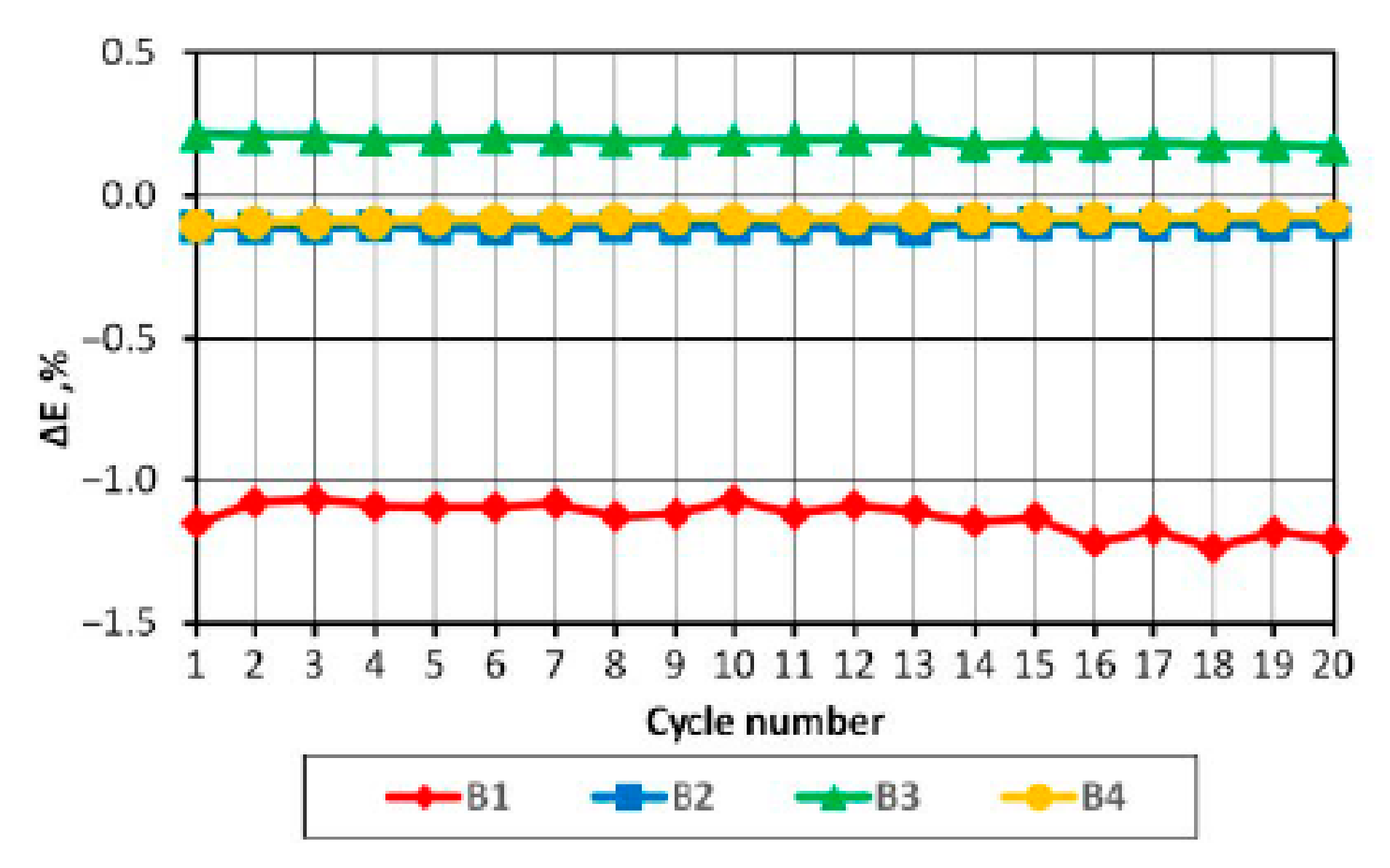
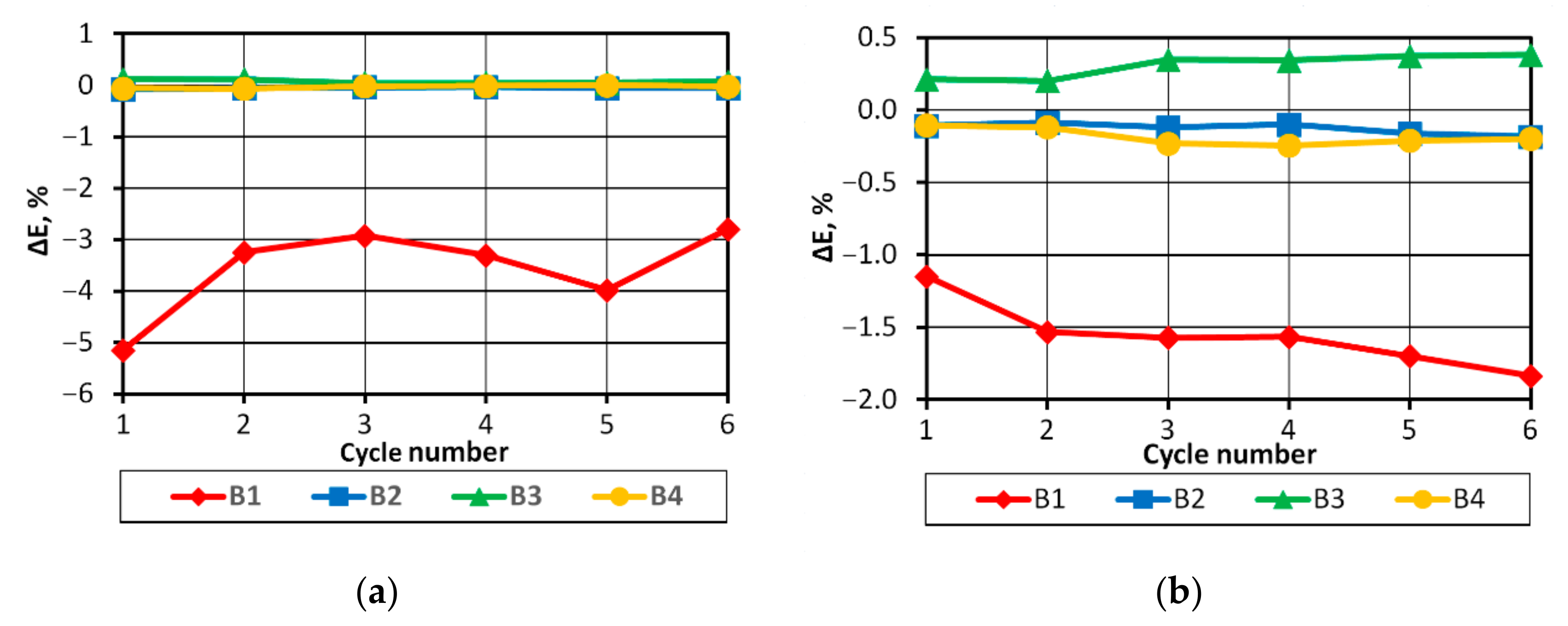
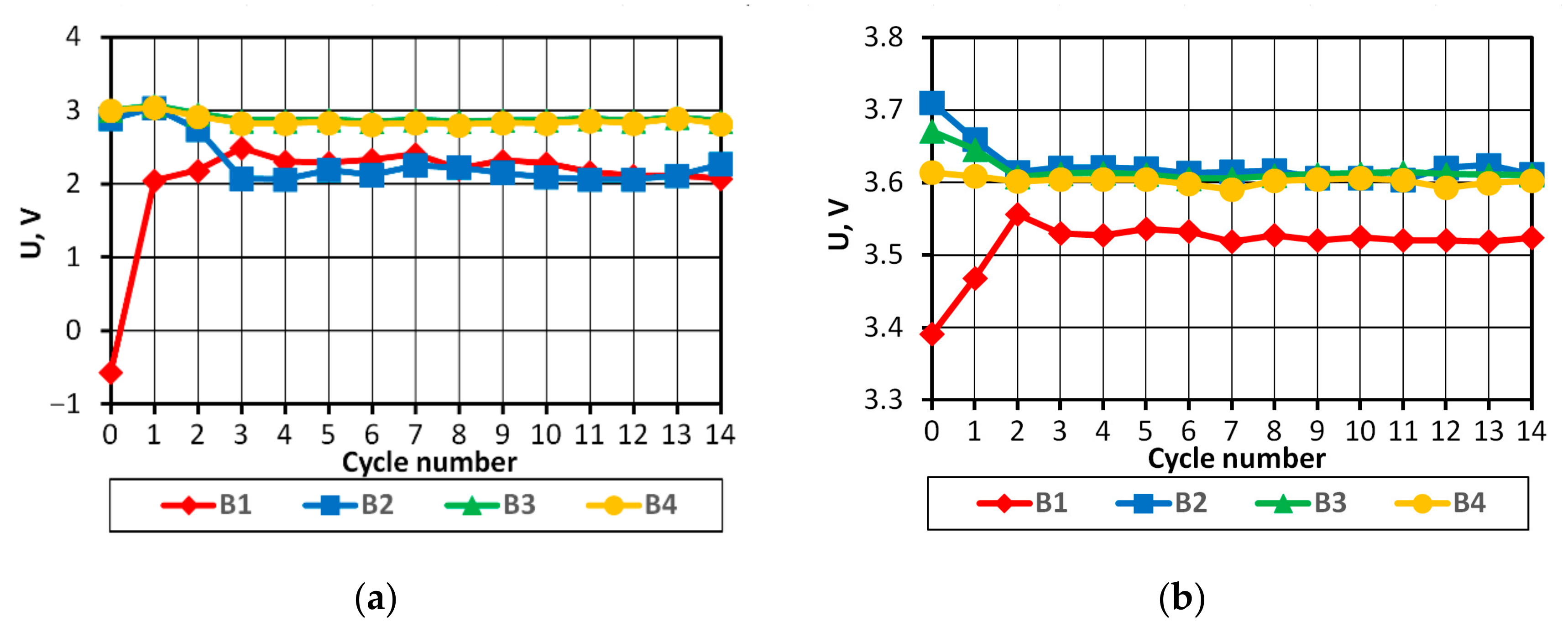
On the basis of the results obtained, it can be concluded that in the case of a pack consisting of four series-connected APR 18650M 1A batteries with a SoC difference of 3%, the batteries in the final discharge/charging phases operate in non-recommended ranges, which may consequently lead to partial or complete cell degradation.
BMS solved the problem of providing batteries with optimal operating conditions, however, when energy drawn from the batteries is very low, they can significantly affect the energy efficiency of the power supply system.
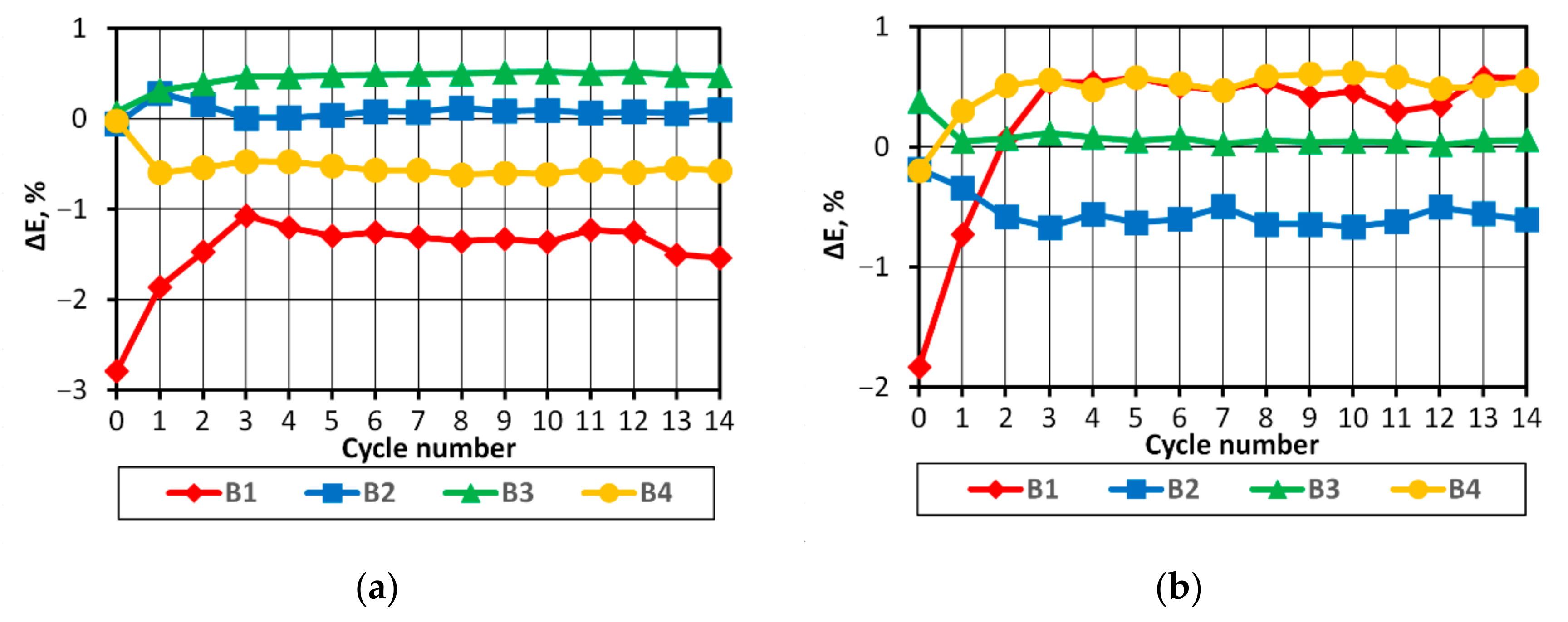
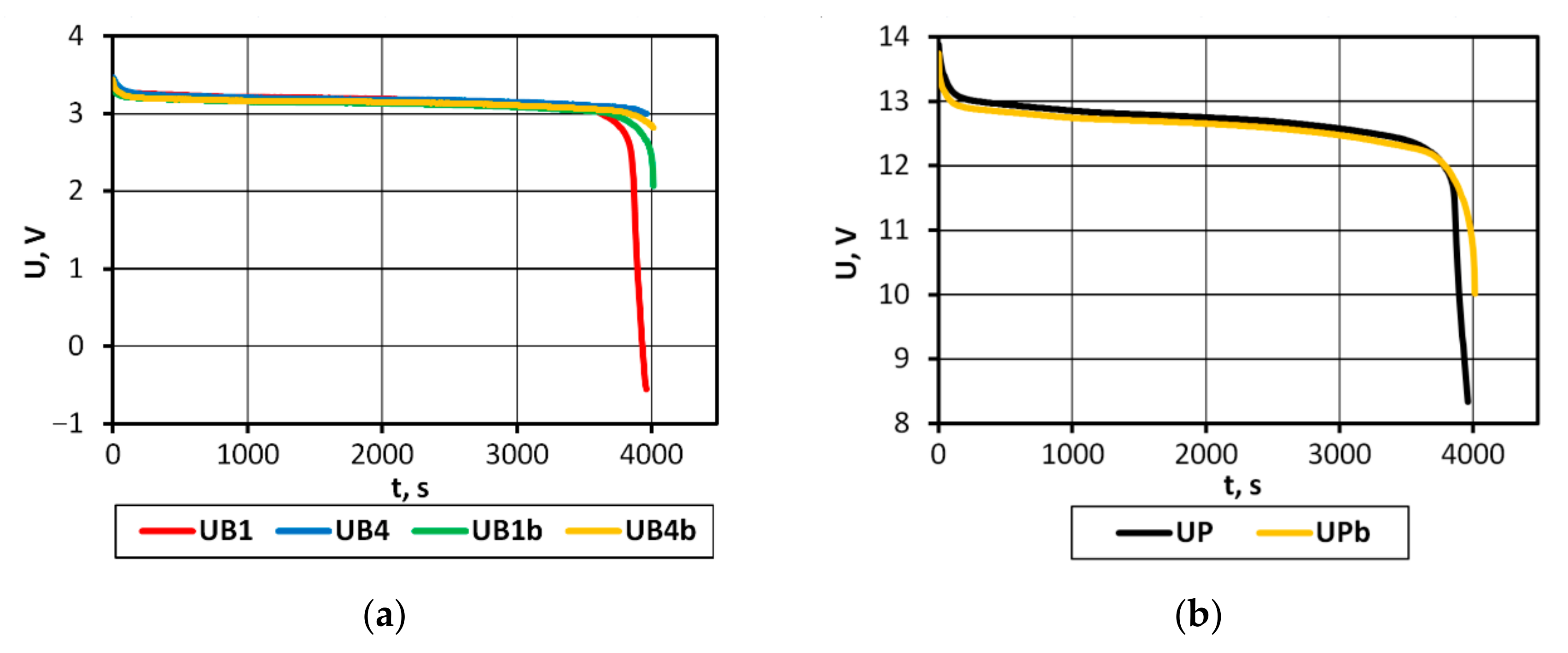
The analysis of the discharge voltage characteristics of the packs characterized by varying levels of cell balance (
Figure 12) shows that the comparison of the rate of voltage changes in the final discharge phase will allow SoC imbalance to be detected in the studied battery pack. It seems that these dependencies might be used to develop a protection system against damage to the entire pack. This requires continued research in this area.
From the practical point of view, the findings from this study suggest that with the correct selection of batteries combined in the pack, the initial differences in the energy stored in the batteries are of vital importance. It is possible to develop a system protecting the battery pack in the discharge process, one which could operate solely based on information on the voltage of the entire pack.