In Situ Recrystallization of Co-Evaporated Cu(In,Ga)Se2 Thin Films by Copper Chloride Vapor Treatment towards Solar Cell Applications
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussions
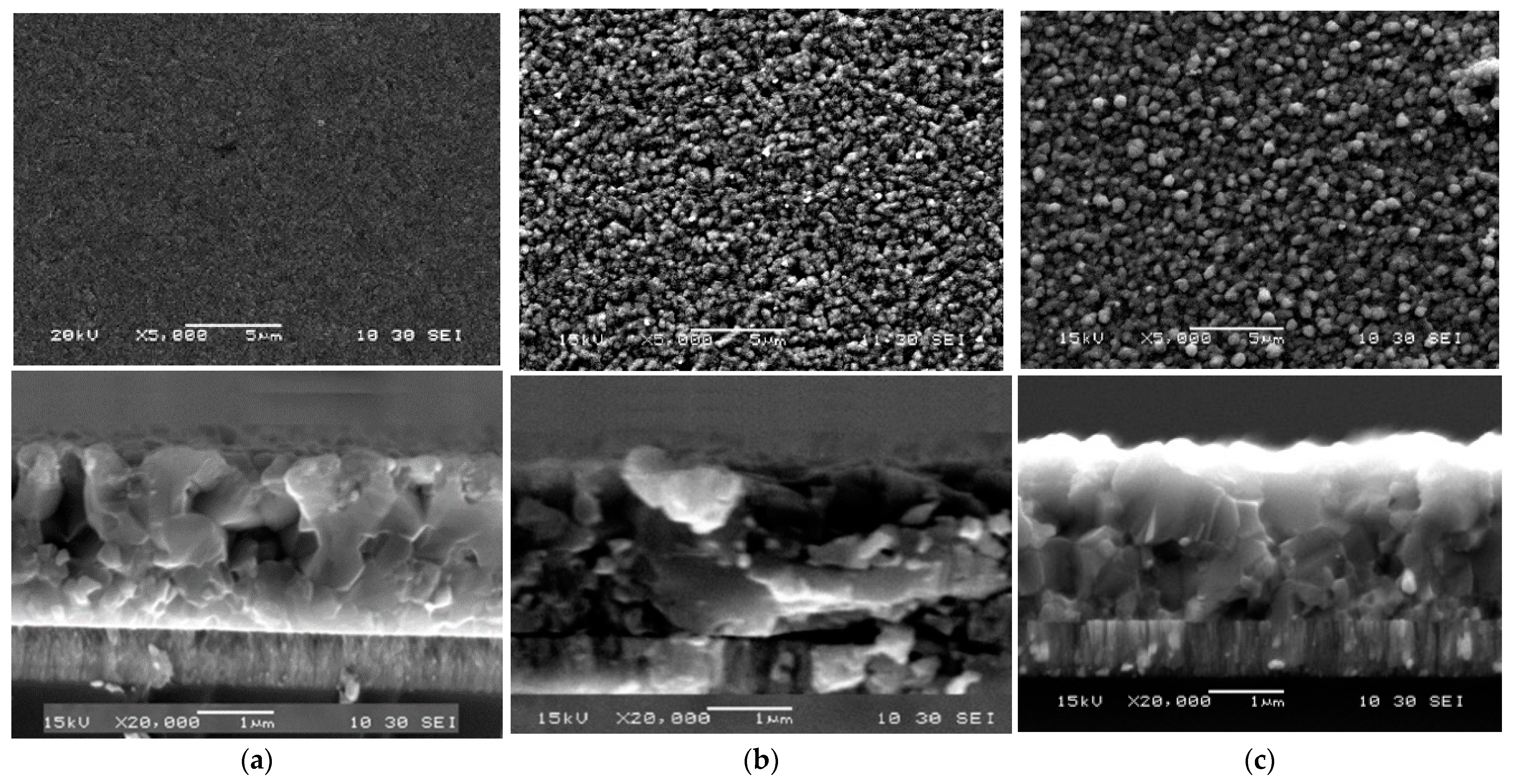
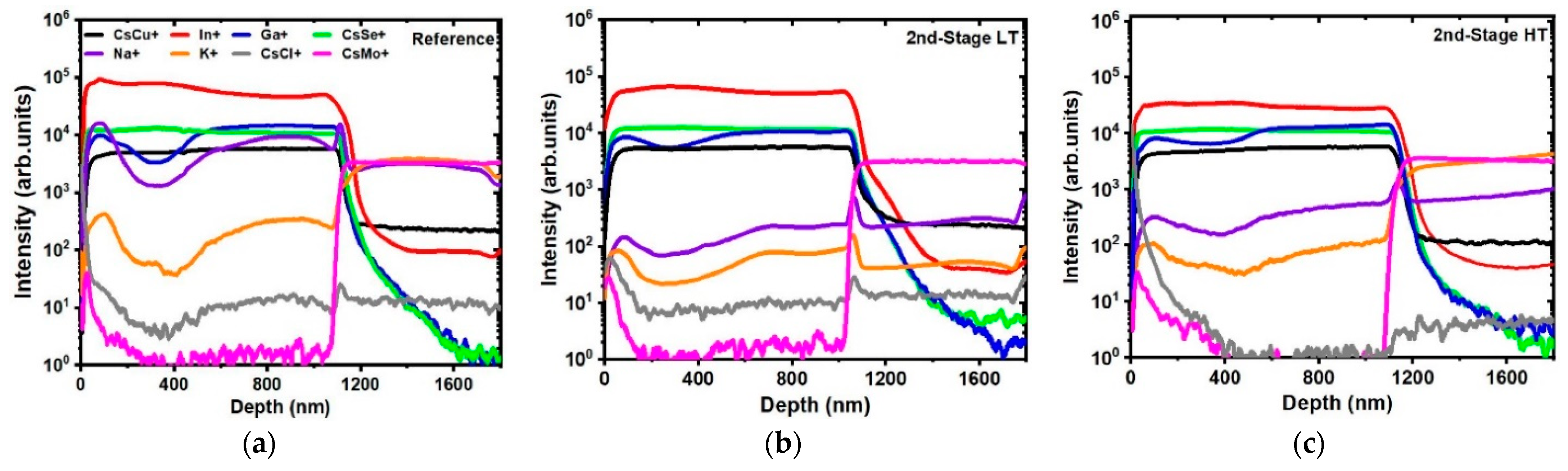
3.1. Second Stage Temperature Dependence
3.2. Third Stage Temperature Dependence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaelin, M.; Rudmann, D.; Tiwari, A.N. Low cost processing of CIGS thin film solar cells. Sol. Energy 2004, 77, 749–756. [Google Scholar] [CrossRef]
- Niki, S.; Contreras, M.; Repins, I.; Powalla, M.; Kushiya, K.; Ishizuka, S.; Matsubara, K. CIGS absorbers and processes. Prog. Photovolt. Res. Appl. 2010, 18, 453–466. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 56). Prog. Photovolt. Res. Appl. 2020, 28, 629–638. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Kou, X.L.; Cai, Y.A.; Liu, W.; Yu, T.; Pang, J.B.; Li, C.J.; Sun, Y. Effect of substrate temperature on the structural and electrical properties of CIGS films based on the one-stage co-evaporation process. Semicond. Sci. Technol. 2010, 25, 055007. [Google Scholar] [CrossRef]
- McCandless, B.E.; Moulton, L.V.; Birkmire, R.W. Recrystallization and sulfur diffusion in CdCl2-treated CdTe/CdS thin films. Prog. Photovolt. Res. Appl. 1997, 5, 249–260. [Google Scholar] [CrossRef]
- Kato, T. Cu(In,Ga)(Se,S)2 solar cell research in Solar Frontier: Progress and current status. Jpn. J. Appl. Phys. 2017, 56, 04CA02. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, D.; Zhao, M.; Gong, Q.; Guo, L.; Ouyang, L.; Sun, R.; Wei, Y.; Zhan, S. The effects of annealing temperature on CIGS solar cells by sputtering from quaternary target with Se-free post annealing. Appl. Surf. Sci. 2017, 413, 175–180. [Google Scholar] [CrossRef]
- Rodriguez-Alvarez, H.; Barreau, N.; Kaufmann, C.A.; Weber, A.; Klaus, M.; Painchaud, T.; Schock, H.W.; Mainz, R. Recrystallization of Cu(In,Ga)Se2 thin films studied by X-ray diffraction. Acta Mater. 2013, 61, 4347–4353. [Google Scholar] [CrossRef]
- Poudel, D.; Belfore, B.; Karki, S.; Rajan, G.; Soltanmohammad, S.; Rockett, A.; Marsillac, S. Assessment of Cu(In,Ga)Se2 Solar Cells Degradation due to Water Ingress Effect on the CdS Buffer Layer. J. Energy Power Technol. 2021, 3. [Google Scholar] [CrossRef]
- Noufi, R.; Gabor, A.M.; Tuttle, J.R.; Tennant, A.L.; Contreras, M.A.; Albin, D.S.; Carapella, J.J. Method of Fabricating High-Efficiency Cu(In,Ga)(SeS)2 Thin Films for Solar Cells. U.S. Patent 5,441,897, 15 August 1995. [Google Scholar]
- Chirilă, A.; Reinhard, P.; Pianezzi, F.; Bloesch, P.; Uhl, A.R.; Fella, C.; Kranz, L.; Keller, D.; Gretener, C.; Hagendorfer, H.; et al. Potassium-induced surface modification of Cu(In,Ga)Se2 thin films for high-efficiency solar cells. Nat. Mater. 2013, 12, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Rudmann, D.; Da Cunha, A.F.; Kaelin, M.; Kurdesau, F.; Zogg, H.; Tiwari, A.N.; Bilger, G. Efficiency enhancement of Cu(In,Ga)Se2 solar cells due to post-deposition Na incorporation. Appl. Phys. Lett. 2004, 84, 1129–1131. [Google Scholar] [CrossRef]
- Karki, S.; Deitz, J.I.; Rajan, G.; Soltanmohammad, S.; Poudel, D.; Belfore, B.; Bhandari, G.; Grassman, T.J.; Rockett, A.; Marsillac, S. Impact of water ingress on molybdenum thin films and its effect on Cu(In,Ga)Se2 Solar Cells. IEEE J. Photovolt. 2019, 10, 696–702. [Google Scholar] [CrossRef]
- Poudel, D.; Karki, S.; Belfore, B.; Rajan, G.; Atluri, S.S.; Soltanmohammad, S.; Rockett, A.; Marsillac, S. Degradation Mechanism Due to Water Ingress Effect on the Top Contact of Cu(In,Ga)Se2 Solar Cells. Energies 2020, 13, 4545. [Google Scholar] [CrossRef]
- Ayala, O.; Belfore, B.; Ashrafee, T.; Akwari, J.; Rajan, G.; Karki, S.; Poudel, D.; Marsillac, S. Theoretical Analysis of Experimental Data of Sodium Diffusion in Oxidized Molybdenum Thin Films. Energies 2021, 14, 2479. [Google Scholar] [CrossRef]
- Rajan, G.; Belfore, B.; Karki, S.; Poudel, D.; Kahoui, H.; Lanham, N.; Palmiotti, E.; Soltanmohammad, S.; Rockett, A.; Marsillac, S. Impact of Post-Deposition Recrystallization by Alkali Fluorides on Cu(In,Ga)Se2 Thin-Film Materials and Solar Cells. Thin Solid Films 2019, 690, 137526. [Google Scholar] [CrossRef]
- Belfore, B.; Poudel, D.; Palmiotti, E.; Rajan, G.; Karki, S.; Rockett, A.; Marsillac, S. Ex-Situ Recrystallization of CIGS via Metal Halides. In Proceedings of the 47th IEEE Photovoltaic Specialists Conference (PVSC), Calgary, AB, Canada, 15 June–21 August 2020; pp. 1102–1104. [Google Scholar] [CrossRef]
- Feurer, T.; Reinhard, P.; Avancini, E.; Bissig, B.; Löckinger, J.; Fuchs, P.; Carron, R.; Weiss, T.P.; Perrenoud, J.; Stutterheim, S.; et al. Progress in thin film CIGS photovoltaics–Research and development, manufacturing, and applications. Prog. Photovolt. Res. Appl. 2017, 25, 645–667. [Google Scholar] [CrossRef]
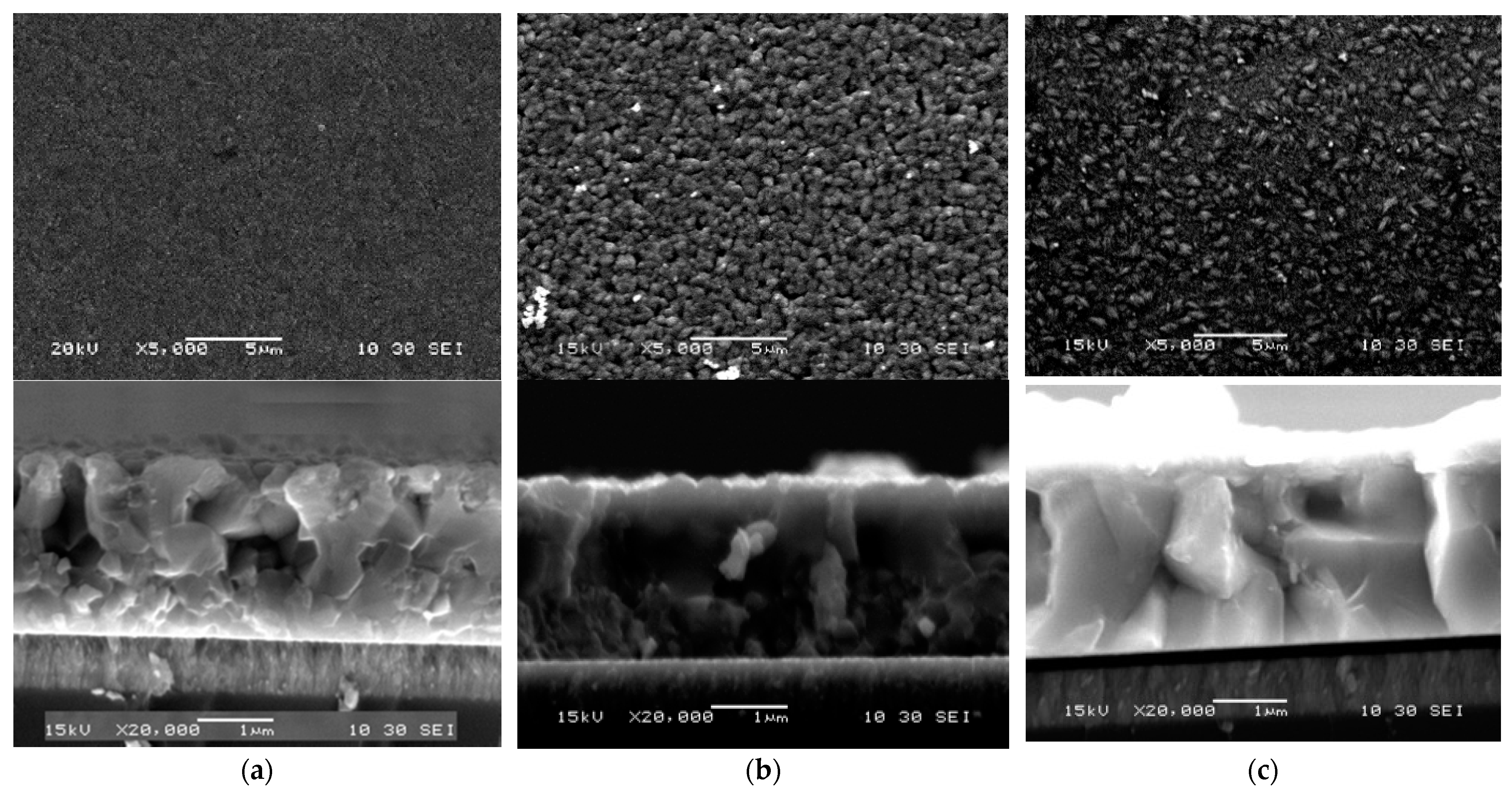
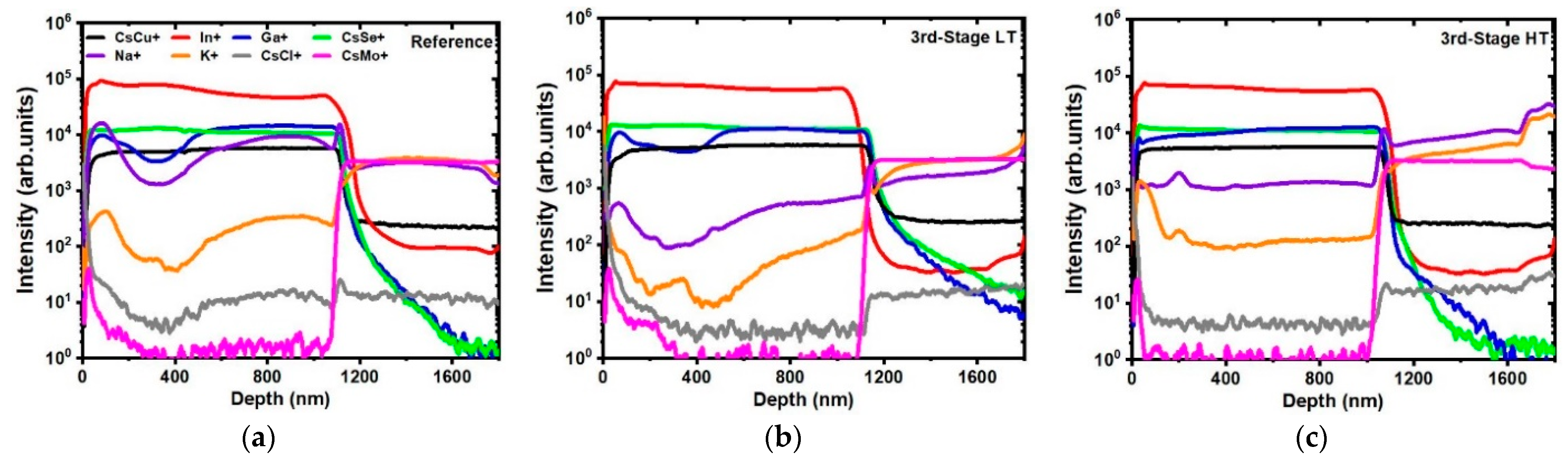
| Samples | First Stage TSS (°C) | Second Stage TSS (°C) | Third Stage TSS (°C) |
|---|---|---|---|
| Reference Sample | 350 | 400 | 450 |
| Second Stage Low Temperature (LT) | 350 | 350 | 400 |
| Second Stage High Temperature (HT) | 350 | 400 | 400 |
| Third Stage Low Temperature (LT) | 350 | 400 | 425 |
| Third Stage High Temperature (HT) | 350 | 400 | 450 |
| Samples | Reference | Second Stage LT | Second Stage HT | ||||||
|---|---|---|---|---|---|---|---|---|---|
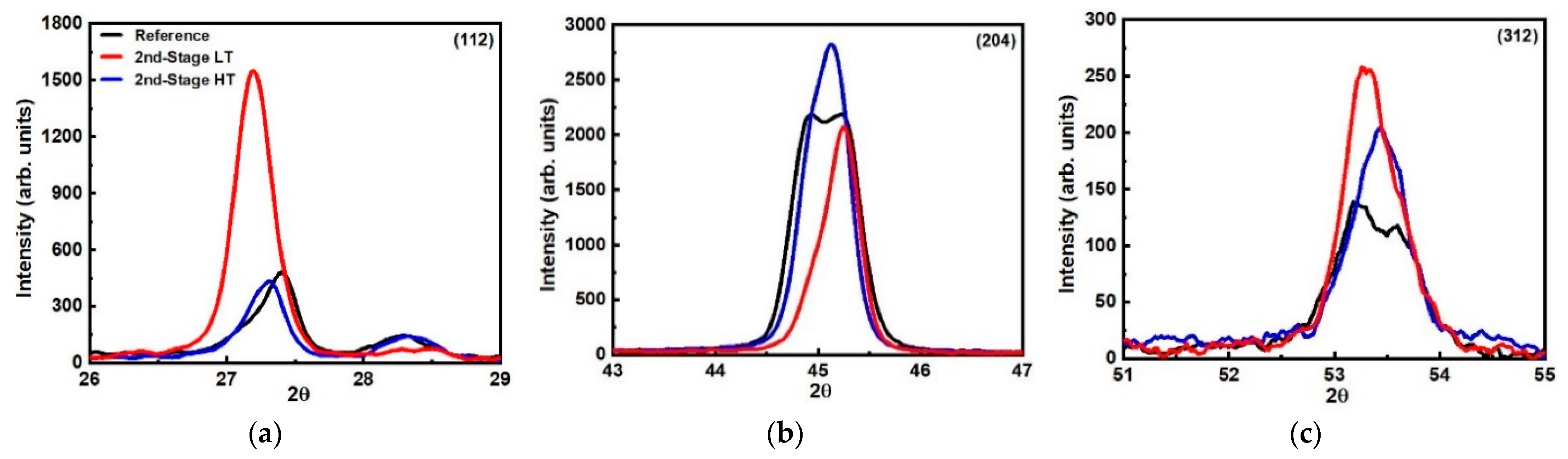
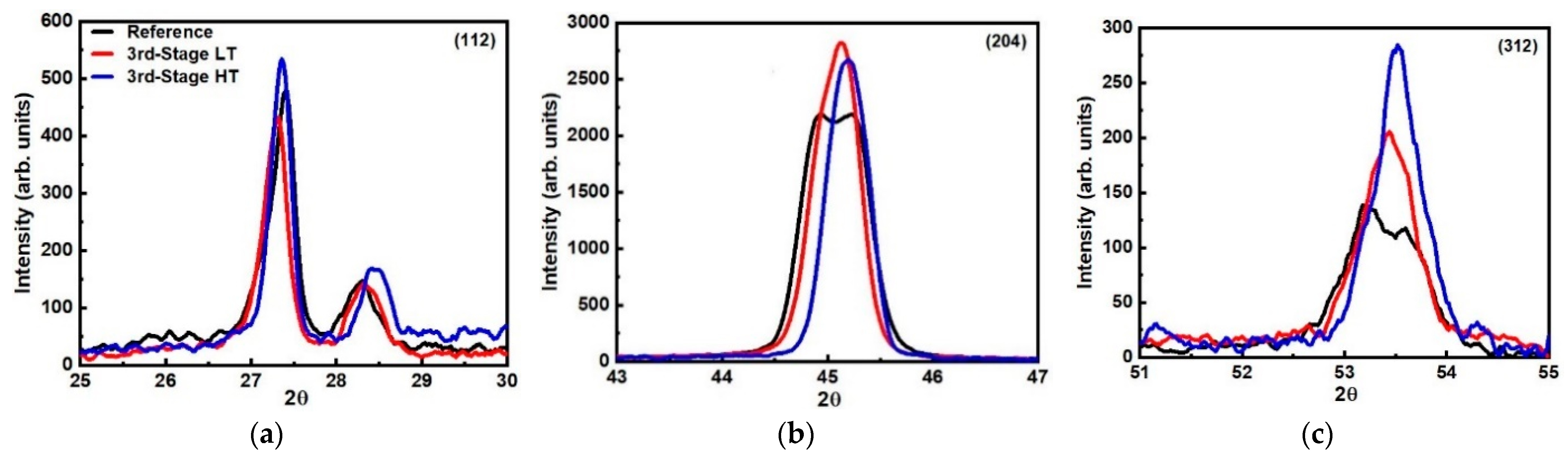
| Peaks | (112) | (204) | (312) | (112) | (204) | (312) | (112) | (204) | (312) |
| Angles (deg) | 27.4 | 44.8/45.3 | 53.3 | 27.1 | 45.2 | 53.5 | 27.3 | 44.9/45.1 | 53.4 |
| FWHM (deg) | 0.32 | 0.30/0.56 | 0.82 | 0.30 | 0.41 | 0.58 | 0.30 | 0.25/0.44 | 0.54 |
| Integrated Intensity | 225 | 329/1521 | 120 | 693 | 1150 | 207 | 213 | 350/1602 | 141 |
| Crystallite Size (nm) | 26.7 | 29.9/16.0 | 11.3 | 28.4 | 21.9 | 16.0 | 28.4 | 35.9/20.4 | 17.2 |
| Cu/III (XRF) | 0.89 | 0.88 | 0.88 | ||||||
| Ga/III (XRF) | 0.34 | 0.33 | 0.32 | ||||||
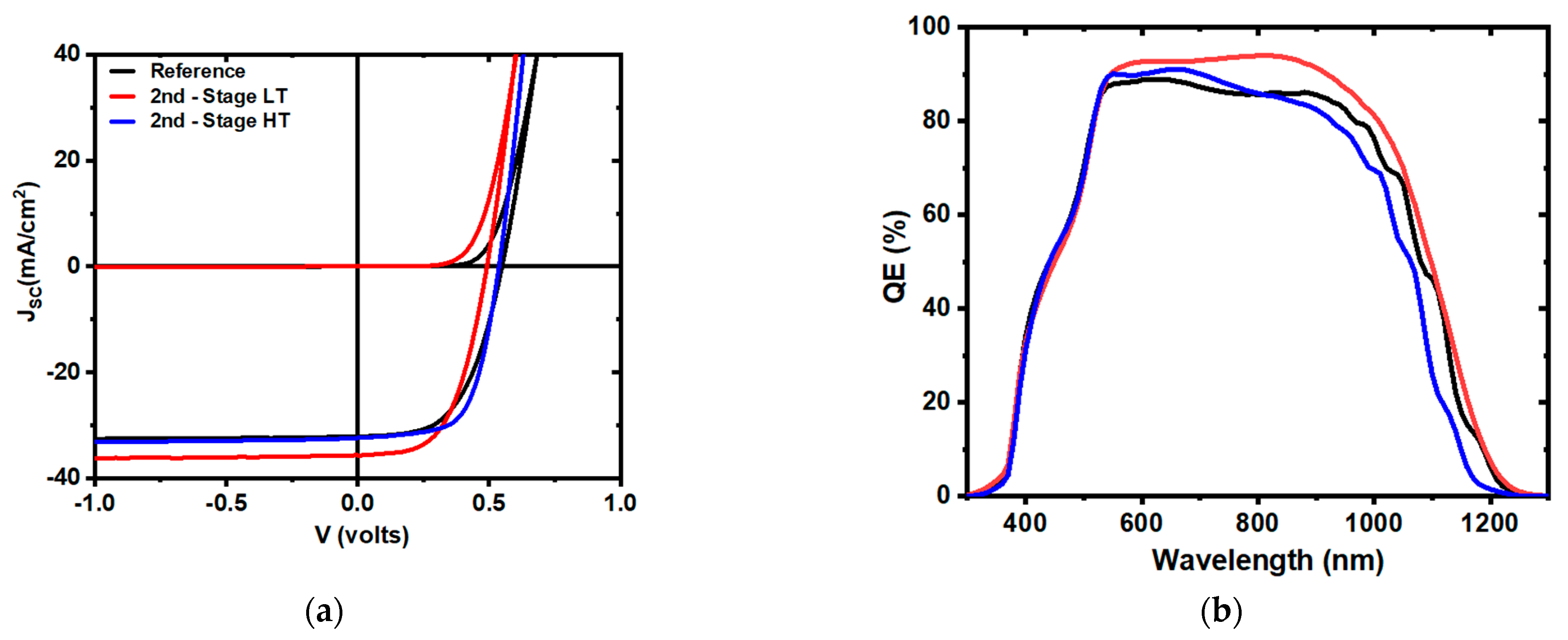
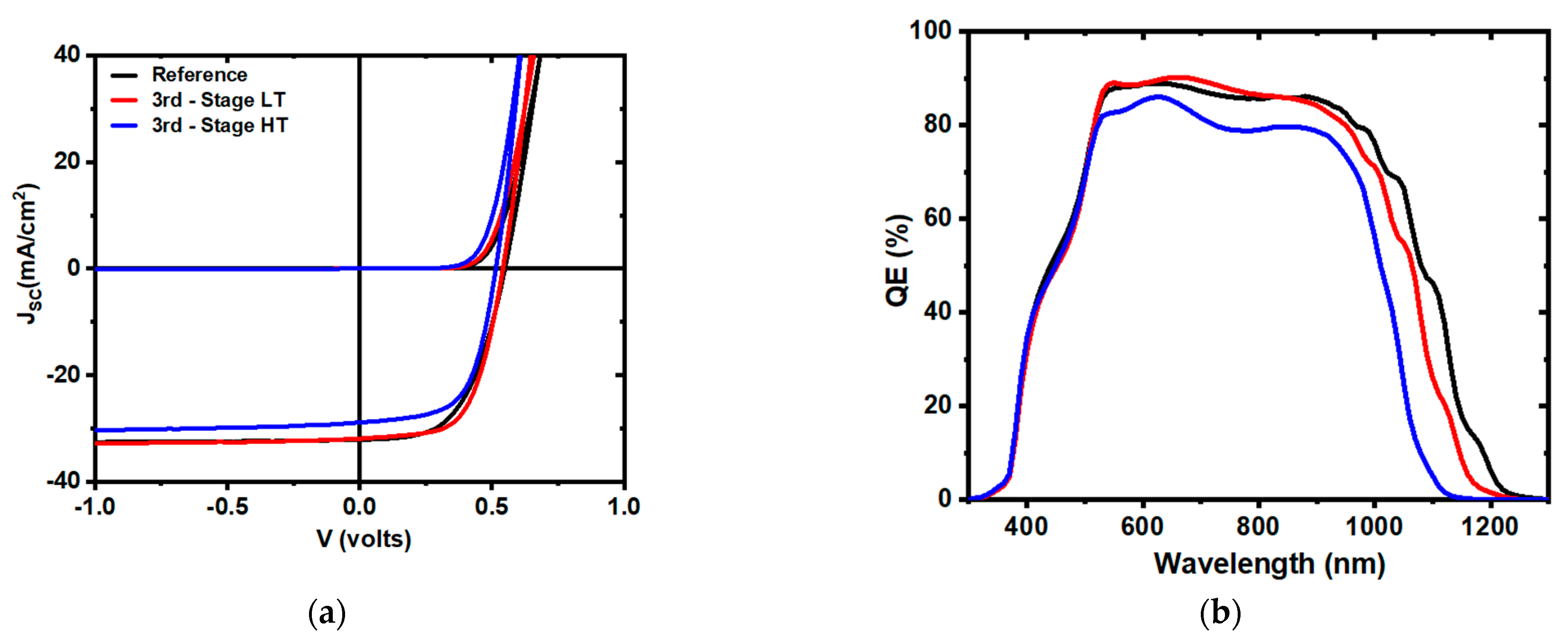
| Sample | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) | Jo (mA/cm2) | A | RSH (Ω cm2) | RS (Ω cm2) | Eg (QE) |
|---|---|---|---|---|---|---|---|---|---|
| Reference | 0.55 | 32.0 | 54.8 | 9.6 | 5.4 × 10−8 | 1.8 | 1.5 × 104 | 2.0 | 1.09 |
| Second Stage LT | 0.49 | 35.6 | 55.5 | 9.7 | 5.1 × 10−8 | 1.7 | 1.8 × 104 | 1.9 | 1.08 |
| Second Stage HT | 0.54 | 32.3 | 63.3 | 11.0 | 2.0 × 10−8 | 1.6 | 5.2 × 104 | 1.3 | 1.13 |
| Samples | Reference | Third Stage LT | Third Stage HT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peaks | (112) | (204) | (312) | (112) | (204) | (312) | (112) | (204) | (312) |
| Angles (deg) | 27.4 | 44.8/45.3 | 53.3 | 27.3 | 44.8/45.2 | 53.4 | 27.4 | 45.1 | 53.5 |
| FWHM (deg) | 0.32 | 0.30/0.56 | 0.82 | 0.29 | 0.24/0.45 | 0.55 | 0.25 | 0.40 | 0.53 |
| Integrated Intensity | 225 | 329/1521 | 120 | 200 | 342/1615 | 143 | 242 | 1600 | 189 |
| Crystallite Size (nm) | 26.7 | 29.9/16.0 | 11.3 | 29.4 | 37.4/20.0 | 16.9 | 34.1 | 22.4 | 17.5 |
| Cu/III | 0.89 | 0.88 | 0.89 | ||||||
| Ga/III | 0.34 | 0.33 | 0.34 | ||||||
| Sample | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) | Jo (mA/cm2) | A | RSh (Ω cm2) | RS (Ω cm2) | Eg (QE) |
|---|---|---|---|---|---|---|---|---|---|
| Reference | 0.55 | 32.0 | 54.8 | 9.6 | 5.4 × 10−8 | 1.8 | 1.5 × 104 | 2.0 | 1.09 |
| Third Stage LT | 0.54 | 31.9 | 61.0 | 10.5 | 2.2 × 10−8 | 1.6 | 4.8 × 104 | 1.6 | 1.13 |
| Third Stage HT | 0.51 | 28.8 | 61.0 | 9.0 | 5.1 × 10−8 | 1.7 | 1.6 × 104 | 1.7 | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, D.; Belfore, B.; Ashrafee, T.; Palmiotti, E.; Karki, S.; Rajan, G.; Lepetit, T.; Rockett, A.; Marsillac, S. In Situ Recrystallization of Co-Evaporated Cu(In,Ga)Se2 Thin Films by Copper Chloride Vapor Treatment towards Solar Cell Applications. Energies 2021, 14, 3938. https://doi.org/10.3390/en14133938
Poudel D, Belfore B, Ashrafee T, Palmiotti E, Karki S, Rajan G, Lepetit T, Rockett A, Marsillac S. In Situ Recrystallization of Co-Evaporated Cu(In,Ga)Se2 Thin Films by Copper Chloride Vapor Treatment towards Solar Cell Applications. Energies. 2021; 14(13):3938. https://doi.org/10.3390/en14133938
Chicago/Turabian StylePoudel, Deewakar, Benjamin Belfore, Tasnuva Ashrafee, Elizabeth Palmiotti, Shankar Karki, Grace Rajan, Thomas Lepetit, Angus Rockett, and Sylvain Marsillac. 2021. "In Situ Recrystallization of Co-Evaporated Cu(In,Ga)Se2 Thin Films by Copper Chloride Vapor Treatment towards Solar Cell Applications" Energies 14, no. 13: 3938. https://doi.org/10.3390/en14133938
APA StylePoudel, D., Belfore, B., Ashrafee, T., Palmiotti, E., Karki, S., Rajan, G., Lepetit, T., Rockett, A., & Marsillac, S. (2021). In Situ Recrystallization of Co-Evaporated Cu(In,Ga)Se2 Thin Films by Copper Chloride Vapor Treatment towards Solar Cell Applications. Energies, 14(13), 3938. https://doi.org/10.3390/en14133938








