1. Introduction
Nowadays, the concept of circular economy has become the fundamental economy model of developed regions, and it is spread and intensively implemented all over the world [
1,
2]. One of its most important aspects is to increase the rate of reuse, recycling, and recovery of resources, which allows for the optimum utilization of available raw materials, residues, and wastes and results in energy saving and reduction of environmental emissions, especially greenhouse gases (GHGs) to the atmosphere [
3]. Due to this fact, actions related with the circular economy approach are also implemented to metallurgical processes, which are regarded as some of the most energy-demanding and materials-consuming industrial branches. Early studies on the circular economy in iron and steel enterprises discuss the evaluation of cleaner production, further supplemented with the concept of “efficiency” introduced to evaluate the level of the circular economy. These include both overall resource efficiency and iron resource efficiency [
4].
There are two main routes of steel production of industrial importance currently used worldwide. A total of 70% of the world steel is produced by the route of blast furnace (BF), in which iron ore is reduced to pig iron, followed by pig iron conversion into steel in a basic oxygen furnace (BOF). Inputs of this route are mainly iron ore, coal, limestone, and, to a much lower extent, steel scrap. The second route, within which 30% of the world’s steel is produced, is based on the electric arc furnace (EAF), which involves scrap steel as feedstock as well as electricity as energy source [
5]. Additionally, in this route, carbon sources are of great importance, as, in modern electric arc furnaces, the share of energy input from fossil fuels such as natural gas and coal is over 40% of the total energy input. Solid carbon sources, such as coal, petrol, coke, etc., are used in the EAF as slag foaming agents [
6].
Significant efforts and commitments are undertaken within both European and worldwide activities in steel industry by introducing innovative actions on high-performance products and increasing process efficiency by also reducing its environmental impacts [
5]. For example, the use of residual coke structural fractions in the blast furnace (BF) process is commonly implemented in the sector. In the case of the iron ore sintering process, there are preliminary actions related with the possibility of recovery of iron from zinced blast furnace or basic oxygen furnace slurries or their reuse in other industrial branches. Other actions are focused on the use of novel fuels suitable to substitute conventional fossil fuels currently used in iron ore sintering processes [
7,
8,
9,
10,
11,
12,
13]. The latter is in agreement with the creation of industrial symbiosis (IS), targeted on the development of synergy routes between different sectors. Through the IS concept implementation, byproducts, residues, or wastes from one sector (e.g., agriculture, biomass processing) may become valuable feedstocks to other sectors (e.g., iron and steel production) [
5].
The sintering process is an important raw material preparation step for the production of hot metal in blast furnaces [
14]. It is a thermal agglomeration process (1300–1480 °C) of a mixture of iron ore mineral fines (0.5–8.0 mm) and other materials, such as byproducts of the iron and steelmaking industry, fluxes, slag-forming elements, and fossil fuels (coke breeze, i.e., the fraction of coke of the smallest grain size below 12 mm). The objective of the process is to obtain a load (12–35 mm) with the suitable physical–chemical and mechanical properties, which can be further processed in a blast furnace to produce pig iron [
15]. Sintering is the most economic and widely used agglomeration process to prepare iron ore fines for blast furnace use. Compared with pellets, production of sinter is cheaper, and compared with lump ore, fluxed sinter is often more reducible with better softening characteristics [
14].
Sintering is an energy-intensive (1.4–2.6 MWh/t [
16]) and complex process, in which a number of parameters have to be taken into account [
17]. The basic fuel for the iron ore sintering process is coke breeze (CB), the use of which can be supported by substitutional fuels, e.g., anthracite [
18]. However, the number of actions dedicated to substitution of conventional fuels by other ones, preferably of renewable or bio (or generally green) features, are taken. Among them, chars derived from residual biomass, formed during pyrolysis of selected feedstocks (biodegradable residues or wastes), are of high interest. Pyrolysis is a thermal process during which organic substances, at the absence of oxygen, are thermochemically processed to gaseous, liquid, and solid products. Hence, in the case of residues, their transformation to useable materials, e.g., a fuel for metallurgical process, takes place, and thus such the processing of residues fully agrees with the circular economy concept.
Residual biomass, especially from the agricultural sector, is regarded as a valuable, renewable energy source. European agriculture annually generates 442,000 Gg of residues (dry matter), which correspond to 46% of overall agriculture biomass production. Most of the residues are generated during production of cereals (74% of total agricultural residue production) and oil-bearing crops (17%). The main producers of residual biomass in Europe are France (83,600 Gg/year, dry matter), Germany (59,200 Gg/year, dry matter), and Poland (45,500 Gg/year, dry matter). In the case of the use of biomass for energetic purposes, agricultural biomass in Europe represents 27% of the overall biomass supply [
19]. It is estimated that Polish resources of biomass, which could be used as a renewable energy source, could deliver 835 PJ/year (overall biomass potential), while nowadays they deliver 263 PJ/year [
20]. The unfavorable features of biomass, such as poor grinding capability, low energy density, high moisture content, irregular structure (shape and size), and non-uniform composition, usually limit its direct use as a fuel, especially in metallurgical processes.
It has been shown that the direct utilization of biomass for replacement of coal in iron and steel processes is affected by improper storage and unavailability of technological developments required for biomass fuel. Additionally, high moisture content lowering the biomass heating value is also disadvantageous, while it can be improved by proper storage and pretreatment section. Conversion methods should also be considered in order to enable the use of any type of biomass [
17]. There are also some other disadvantages related with the composition and properties of biomass, which include high oxygen content in biomass, presence of water-soluble fractions, alkaline, and halogen elements, and some hazardous trace elements, also connected with highly variable composition and properties of biomass. Another issue is the indefinite availability of sustainable biomass resources [
21]. In literature, there is research focused on technological, economic, and environmental analysis of substitution of coke breeze (CB) with raw biomass in the iron ore sintering process. The impact of the biomass share in sintering mixture on process parameters and final sinter quality has been investigated. It was found that the addition of biomass up to 10 wt.% in fuel (as the substitute of CB) resulted in the decrease of permeability, and thus in the decrease of the process capacity. Moreover, the increase of overall fuel consumption (coke breeze and biomass) was observed, which adversely affected the economy of the process. The increase of CO
2, CO, and NO
x in exhaust gases was also observed. Hence, it was concluded that the use of raw biomass in the sintering process was inefficient [
22,
23,
24].
One of the methods enabling the improvement of biomass properties and, thus, increasing the rate of the residual biomass utilization as a fuel is its pyrolysis. The main benefits gained by the process are the increase of calorific value, the decrease of transport and storage costs, and the reduction of energy consumption required for grinding and milling. Biomass pyrolysis allows to decrease the moisture content below 3 wt.%, while the calorific value and carbon content are increased by 15–25 wt.%, which makes the produced (bio)char far more attractive than the feedstock used to its production [
25].
The biomass-derived chars have great potential in lowering the net CO
2 emissions of integrated (BF–BOF route) steel plants. The properties of chars produced by pyrolysis of biomass can be tailored in regard to the further application and supplementing stream (e.g., coke breeze for sintering, coal blend for cokemaking, coal injectant for the blast furnace, recarburizing of steel, etc.), thus resulting in optimal performance and greater value-in-use of the char [
26]. Moreover, it would be a circular “return to basic”, as, at the beginning of iron, and the next steel manufacture, this was biomass, which was used as a fuel for the process. However, the industrialization era made hard coal to be a so-far unbeatable material commonly applied in the metallurgical industry [
27].
The substitution of CB with residual biomass char (or other bio residual/waste stream) in iron ore sintering processes can be the next step toward circular economy implementation [
3]. The char formed during pyrolysis of bioresidues is characterized by significant carbon content, even up to 80 wt.%, which is comparable with the C content in CB. This feature makes the material suitable for iron sintering processes. However, in order to substitute coke breeze with the (bio)char, the latter should also be characterized by low content of both volatile matter and ash. The preliminary research on the substitution of conventional fuels with biomass and biomass-derived materials are already available in the literature. There are several aspects which need to be taken into account while evaluating chars derived from biomass toward their use in iron sintering. These include composition, especially content of chlorine, alkali, and volatile matter, as well as emission.
The research on the impact of fuel mixture comprised of wood charcoal (WdCh), sawdust (SD), and coke breeze (CB) on final sinter quality and usability in metallurgical processes was carried out in [
28]. It was shown that the share of SD in fuel streams comprising SD and CB should not exceed 10 wt.%, while in the case of WdCh–CB and WdCh–SD–CB fuel mixtures, the shares could be 30 wt.% –70 wt.% and 20 wt.% –10 wt.% –70 wt.%, respectively. The research also showed that the complete substitution of CB with biomass-derived fuel was not possible because of the low energy density values. However, at the established substitutional fuel content, the properties of final sinter fulfilled the required quality standards [
29]. In another study, the phase composition of sinters obtained by replacing a part of coke breeze (CB) with charcoal (Ch) or walnut shell (WnSh) substitute was examined. The stream of fuel applied in the research comprised of CB and 44 and 86 wt.% of Ch or 35 and 42 wt.% of WnSh. It was shown that the replacement of part of the CB in the sintering mixture with the biomass-based fuels changed the sintering conditions, which led to the change in ratio of some phases. However, regarding the composition of the final sinter, it was concluded that the use of studied fuels had no negative impact on the phase composition of produced sinters [
30].
Research involving raw biomass and different biochars as substitutional fuels for the sintering process were also carried out together with investigation on the use of bituminous coal, pitch, and graphite for this purpose. According to the results, the obtained sinter was characterized by the desired quality, similar to one obtained with the use of coke breeze. However, it was found that, due to environmental emissions, the raw biomass could not be directly applied as a fuel for the sintering process, as its use would require the additional exhaust gas cleaning installation. The application of char, on the other hand, was more beneficial, as it improved the efficiency of the process while it limited the emission of NO
x, SO
x, and dusts. Nevertheless, biomass-derived char required additional monitoring of granulometric composition and moisture content, as the rate of its combustion could be too high [
22].
As the raw biomass is shown to be unsuitable substitutional fuel for iron ore sintering, the pyrolyzed residual biomass can be considered for this purpose. However, one must take into account the type of the raw biomass, its affinity to and composition after pyrolysis, and its impact on sintering process emissions. In the article, the results of the research on the use of chars produced by pyrolysis of residual biomass (biochars) as a substitute of coke breeze in the iron sintering process are presented. Three types of biomass were examined, i.e., pelletized sawmill sawdust (SDP), woodchips (WdC), and sunflower husks (SH). The effect of the alternative fuel impact on both sinter quality and usability in the metallurgical process, as well as environmental emissions, was checked. With regard to the latter, the system for exhaust gas cleaning was tested in relation to emission limitation. Moreover, processes involved in the research, i.e., biomass pyrolysis and iron ore sintering with the use of biochars, were carried out in pilot and semi-industrial-scale installations, respectively, to effectively simulate actual conditions and assess the obtained results. The improvement of environmental effects in the form of GHGs emission limitation in the proposed solution results from the fact that conventional fossil fuel-derived carbon is substituted with carbon of biomass origin. Hence, the limitation is proportional to the share of substitutional fuel in the sintering fuel stream. The same approach is stated for the pyrolysis process. The emission, which results from the combustion of volatile components released from biomass during pyrolysis (the energy source of the pyrolysis process), is also regarded as the emission from renewable sources.
3. Results and Discussion
3.1. Physicochemical Properties of Residual Biomass-Derived Chars
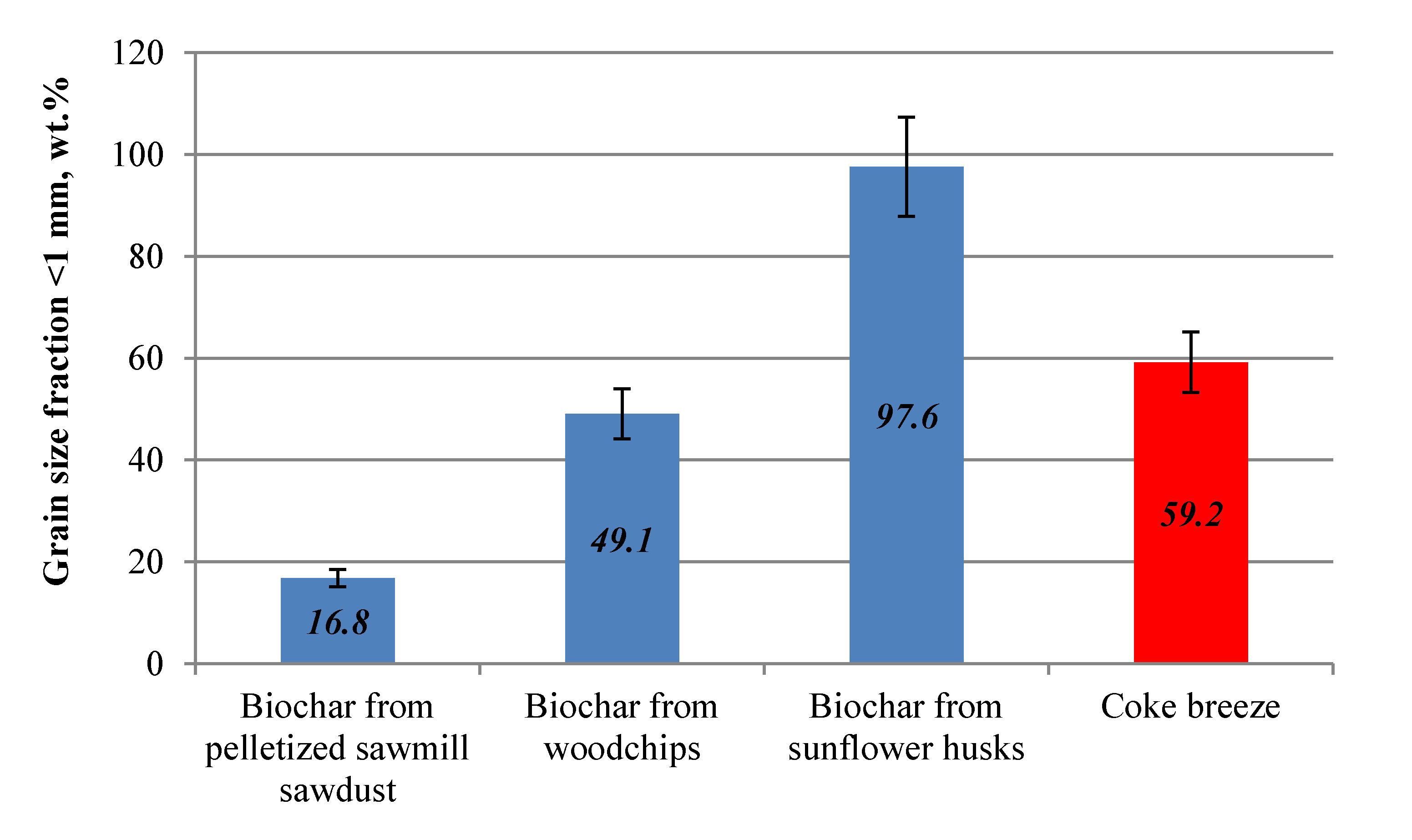
The chars produced from different residual biomass types were characterized according to grain size, bulk density, and chemical composition. The grain size is one of the factors that significantly affects the course of CB combustion. Many studies and industrial practices show that coarse-grained CB causes delayed combustion, which leads to higher heat energy consumption during the process. On the other hand, fine-grained fuel reduces permeability of the sinter mix and, therefore, the efficiency of the process itself. Due to this fact, the use of CB fraction with grains sized below 1 mm is avoided. In
Figure 4, photographs of produced chars are presented together with average grain size (AGS).
In
Table 4, the grain size analysis results are presented together with the characteristics of coke breeze conventionally used as a fuel for ores sintering processes, while in
Figure 5, the overall share of smallest (undesired) grain fraction of biochars and coke breeze is presented.
The smallest size of grains was measured for biochar from SH, for which the average grain size was equal to 0.28 mm, while the share of fractions <1 mm reached ca. 98 wt.%. The biochar from WdC was characterized by grain size similar to CB, with average value equal to 1.26 mm (for CB it was 1.45 mm). However, the share of smallest grain fraction <1 mm was slightly lower than in the case for coke breeze, and reached ca. 50 wt.% (60 wt.% for CB), which was advantageous. The best granularity with regard to sintering process preferences was obtained for biochar from residual SDP. Its average grain size reached 3.37 mm, with a very low content of smallest grain fraction <1 mm of 17 wt.%. Such granularity of the fuel, according to literature data [
18], was expected to positively influence the permeability of the sintering mixture.
In
Table 5, the chemical composition of biochars and coke breeze is presented.
The content of elemental carbon in biochars from SDP (82 wt.%) was comparable to the parameter value in CB (83.8 wt.%), while in the case of biochars from WdC and SH, the value of the parameter was lower and reached 75 wt.% and 73 wt.%, respectively. Moreover, all biochars were characterized by elevated content of volatile matter (from 9.7 to 25 wt.%) with regard to the CB characteristic (1.58 wt.%). The high content of volatile matter was not advantageous due to both technological issues of sintering process reported in literature and the increase of hydrocarbons content in the exhaust gas.
On the other hand, the lower content of ballast, i.e., SiO2 (from 0.48 to 3.24 wt.%), in comparison with CB (6.24 wt.%), was the beneficial feature of all produced biochars. Moreover, relatively high content of CaO in biochars (from 0.66 to 1.66 wt.%) would allow for the reduction of the required amount of additional flux (calcium oxide), which was also favorable. With regard to CaO and SiO2 balance, related to the fuel basicity, the best composition was observed for biochar from SH (1.66 wt.% CaO and 0.48 wt.% SiO2), and the next was for biochar from WdC (0.90 wt.% CaO and 0.64 wt.% of SiO2), while in the case of biochar from SDP, it was not so beneficial, as the content of CaO was 0.66 wt.% at SiO2 content 3.24 wt.%.
Another advantageous feature of biochars was the very low content of substances undesired in the sintering process, i.e., sodium, zinc, and copper. Despite this fact, it was noticed that the content of potassium in all biochars, especially in one derived from sunflower husks, was quite high in the range of 0.15 wt.% to 1.43 wt.%. Both sodium and potassium are found to cause problems in the dedusting stage of gas cleaning, as when they react with chlorine the resistance of dust to separation increases, and thus the efficiency of electrofilters decreases. The final advantage of biochars was related to the low content of sulfur, which was in the range of 0.15 to 0.33 wt.%, while the value determined for CB was 0.9 wt.%.
Considering the physicochemical composition of produced biochars (especially grain size and volatile matter content), it was concluded that materials derived from pelletized residual sawdust and residual wood chips would be the most preferable fuel for iron ore sintering processes. Nevertheless, tests with the use of all types of biochars were carried out.
3.2. The Impact of Biochars on Sintering Process Parameters and Sinter Properties
In
Table 6, the parameters of the sintering process and the properties of produced sinters obtained at different (low ~12.3 wt.% and high ~27.8 wt.%) share of biochars in the fuel are presented. The sintering processes were repeated three times (or more, if significant differences in obtained results were observed) and the presented results are the averaged values. The detailed experimental data can be found in
Table A1, presented in
Appendix A.
The data shown in
Table 6 indicates that the substitution of CB fuel with biochars in the investigated range leads to production of sinter of properties mostly comparable to ones observed for conventionally produced material. The key parameters crucial for evaluation of the sintering process (productivity, fuel consumption) and most advantageous effects resulting from biochars use are discussed below.
It was observed that the introduction of 27.8 wt.% SDP biochar to the fuel stream increased the production efficiency by 5.6%, i.e., from 37.73 Mg/m2/24 h to 39.86 Mg/m2/24 h. In the case of 12.3 wt.% share of pyrolyzed WdC, the production efficiency was higher by 2.5% (it increased from 37.73 Mg/m2/24 h for CB to 38.66 Mg/m2/24 h for mixed fuel). The high share of biochar from sunflower husks in the fuel caused the production efficiency decrease of 9.3% (from 37.73 Mg/m2/24 h for CB to 34.20 Mg/m2/24 h for mixed fuel). Hence, it was concluded that, with regard to production efficiency, the share of substitutional fuel in the fuel stream should not exceed ~30 wt.% in the case of biochars derived from residual SDP or residual WdC, while in the case of biochar from SH, its share in fuel had to be kept below 10 wt.%.
In the case of fuel consumption, it was observed that at the lower share of biochars in the fuel stream, the parameter value increased by max. 1.3% with the use of biochar from residual SDP, while it decreased by 2.2% with the use of pyrolyzed WdC. The noticeable increase of fuel consumption was noted for all fuel streams containing higher shares of biochars, and it was in the range of 1.5% for biochar from SDP to 3.7% for biochar from SH (a 2.3% increase was noted for biochar from residual WdC). Thus, with regard to unit fuel consumption, it was concluded, similarly as in the case of production efficiency, that the share of substitutional fuel in the fuel stream should not exceed ~30 wt.% in the case of biochars derived from residual SDP or residual WdC, while in the case of char from SH, its share in fuel had to be kept below 10 wt.%.
A very interesting phenomenon noted for all sinters produced with the use of substitutional fuel was the decrease of FeO content in comparison to sinter produced with the use of CB only, for which the value of parameter was 9.3 wt.%. In the case of low shares of biochars in the fuel stream, the content of FeO in final sinters varied from 6.18 to 7.13 wt.%, while at high shares of biochars in the fuel stream, it was even lower and ranged from 5.46 to 6.40 wt.%. With regard to further BF processing of sinters, it is a very desired feature, as sinters of lower FeO content reveal better reductive properties and, thus, the smaller amount of coke is required for metallic iron recovery. The better reducibility of sinters produced with the use of biochars was confirmed by determination of reducibility index (RI), which showed that the dR/dt ratio of sinters with biochars containing fuel was in the range from 0.98 to 1.17%/min, while for sinter produced with CB fuel, it was 0.96%/min. The measurements of reduction degradation index (RDI) also showed the more advantageous properties of sinters produced with the use of biochars, which revealed better resistance towards reductive conditions. The lower value of RDI index is preferable, as it means that the sinter is less vulnerable to crushing and formation of fraction <3.15 mm at BF operational conditions. In the discussed research, the sinter produced with the use of only CB contained 17.7 wt.% of fraction <15 mm, while for sinters produced with the use of biochars it was 13.8–16.1 wt.% (for low shares in the fuel stream) and from 12.2–17.4 wt.% (for high shares in the fuel stream).
Other beneficial features of the use of biochars in the sintering process were much lower levels of alkalis, chlorine, and zinc in the produced sinter. The sinters produced with the biochars also revealed better strength, measured as ISO T (the share of grain fraction >6.3 mm [
33]). The highest value of the parameter was obtained for low share of char from residual WdC and reached 70.76 wt.%, and the next highest was for the low share of char from SDP, i.e., 70.57 wt.%, while for the sinter produced with the use of CB only, it was 68.17 wt.%. Simultaneously, abrasion ISO A (the share of fraction <0.5 mm [
33]) was practically the same as the one measured for sinter produced with the conventional fuel. This was even more interesting due to the fact that the ISO T and ISO A were usually worsening at lowering FeO content in the sinter, as, according to the practice, the better reducibility is, the worse the strength of the sinter observed. Hence, the use of biochars in sinter production could actually improve both strength and reduction properties of the final product.
These observations are in agreement with other research discussed in the literature. For example, in [
34], biochar powder with grain size of 5–10 mm was used to replace 6 wt.% and 12 wt.% of CB in the fuel and it was found that the sinter products could meet the requirements of blast furnace, although the tumble strength was reduced. In research involving the use of straw-derived biochar, due to different combustibility of CB and biomaterial, there was a considerable degradation in sinter quality when more than 20 wt.% of CB was substituted [
35]. In the review on biochar use in metallurgy [
36], the authors state that the use of biochar in the sintering stage enabled higher sintering productivity, but led to weaker strength of sinter, which was opposite to the discussed research. However, the cases discussed in the review cover up to 40 wt.% share of biochar in the fuel stream. Thus, it can be concluded that the usability of biochar in sintering processes possesses individual character, as it strongly depends on biochar quality and its amount in the fuel stream.
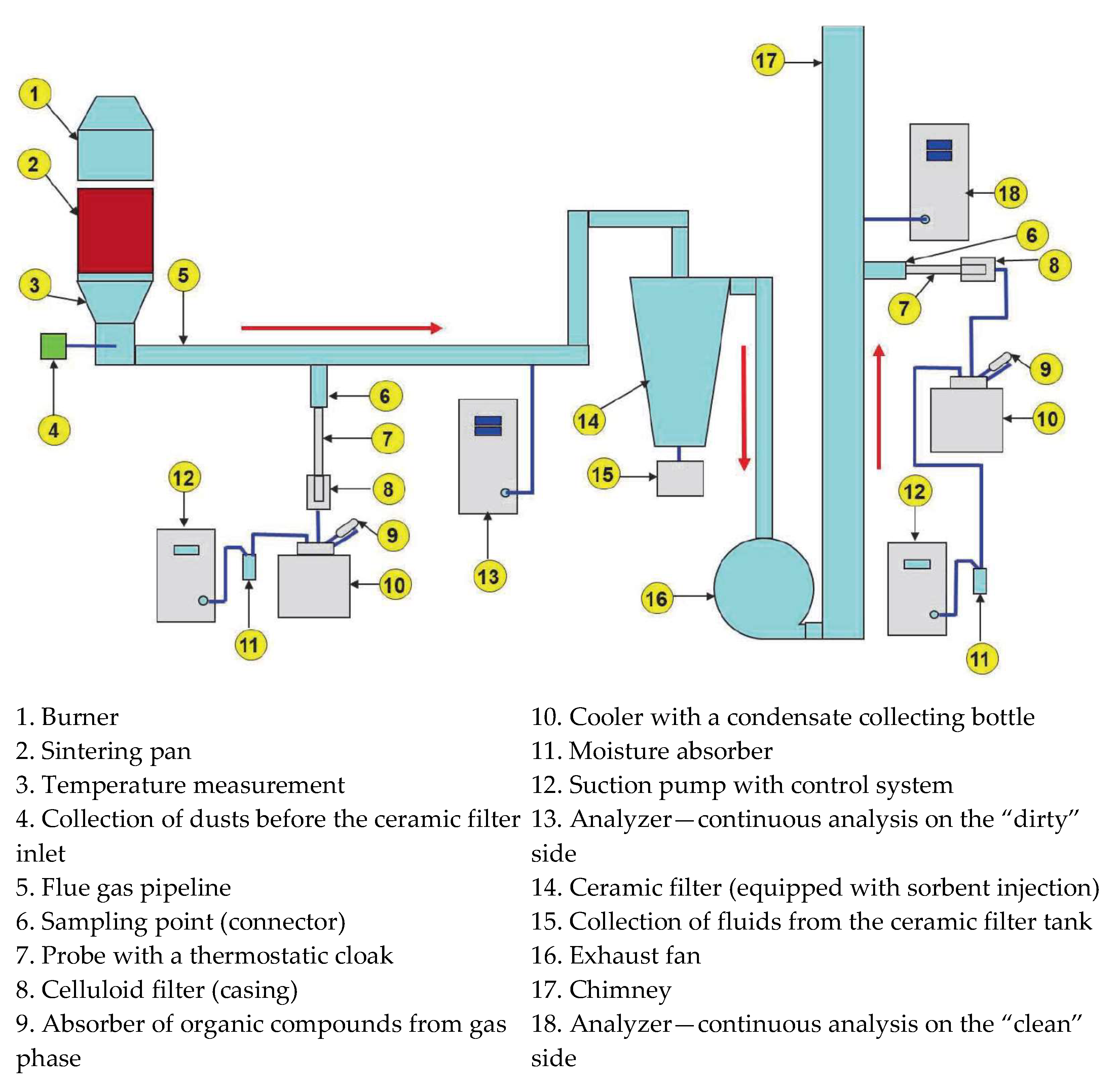
3.3. The Impact of Chars Derived from Residual Biomass on Flue Gases Properties
The impact of a given fuel on a sintering process has to be recognized not only in regard to the final sinter properties, but also in regard to environmental aspects, including emissions. Hence, during the performance of sintering tests, the continuous analyses of exhaust gas before and after catalytic ceramic filter were carried out, covering the parameters such as O
2, CO, CO
2, NO
x, SO
2, and CH
4, while the content of dust was measured periodically. The obtained results of flue gases composition are presented in
Table 7, while in
Figure 6 and
Figure 7, exemplary emission plots are shown.
The measurements of exhaust gas composition allowed us to observe that the use of biochars in the fuel stream resulted in the slight increase of CO
2 and NO
x content (CO
2 8.48–9.31 vol.%; NO
x 218–273 ppm) in comparison with the exhaust gas generated during sintering with fuel composed only of CB (CO
2 8.24 vol.%; NO
x 216 ppm). The exception was the content of NO
x in exhaust gas formed during the process with the use of SDP biochar, for which the measured concentrations were 190 ppm (high share) and 206 ppm (low share). The amount of CO determined during tests with biochars (1.10–1.32 vol.%) was also slightly higher than one for tests with CB only (0.96 vol.%), which could result from the incomplete combustion of biochars during the process. The elevated content of methane (17–45 ppm) noted for fuel mixtures with regard to conventional single component fuel (19 ppm) was probably related to the higher content of volatile matter observed for biochars. On the other hand, lower emissions of SO
2 (46–103 ppm) in comparison with CB (134 ppm) were obtained, which was the result of the lower content of sulfur in pyrolyzed residual biomasses. The periodical measurements of dust content in exhaust gas generated during the sintering process with biomass indicated an increased value of the parameter (265–342 mg/Nm
3) in comparison with standard CB fuel (222 mg/Nm
3). In reference to literature data, it was reported that the partial substitution of coke breeze with charcoal resulted in higher concentration of CO
x and lower concentration of SO
x and NO
x in the exhaust gas. The higher concentration of CO and CO
2 was attributed to the higher charcoal addition compared to coke breeze to achieve the return fine balance and sinter quality. The lower concentration of SO
x and NO
x was attributed to the lower content of S and N in charcoal compared to that of CB [
37]. Hence, as in the case of sinter quality, the effect of biochar on emission depends strongly on the biochar quality and process conditions. Additionally, according to [
38,
39], the use of biochar in the sintering could not be directly followed by a reduction in the greenhouse gas emissions, but the net CO
2 emission balance could be reduced by 5–15%, mainly because of the biomass carbon neutrality.
The application of catalytic ceramic filter in the semi-industrial-scale iron ores sintering process enabled the significant reduction of pollutants emitted to the atmosphere. It was found that the additional use of hydrated lime sorbent (HLS) allowed for complete sorption of sulfur compounds present in the exhaust gas, as at any stage of emission measurements their concentration was below the detection limit (<1.0 ppm (
Figure 6)). On the other hand, the presence of SO
2 was noted in exhaust gas before the catalytic ceramic filter, which is shown in
Figure 7 (green line). The applied HLS was probably also responsible for the decrease of CO
2, as the amount of the compound in the gas decreased from 8.24–9.31 vol.% to 4.03–4.58 vol.%. It was a very desired feature, considering emission limitations of the sintering process. Another advantageous effect was the reduction of NO
x content by 40–53%, which was obtained due to the presence of the catalyst placed on the ceramic surface of the filter. The content of CO and CH
4 was also significantly decreased (to 0.57–0.68 vol.% and to 9–32 ppm, respectively). Thus, it was concluded that the applied catalytic ceramic filter enabled integrated elimination of pollutants by their transformation to CO
2, N
2, and H
2O. Simultaneously, it allowed for very efficient dedusting of the cleaned gas, as the final concentration of dust was always below 1.0 mg/Nm
3 (limit of detection), regardless of the fuel composition and process configuration, which corresponded with up to 99.7% of dedusting efficiency.
As iron ores sintering is the main source of emission of dibenzodioxins/dibenzofurans in integrated steel plants [
27,
40], the samples of dusts from the catalytic ceramic filter were collected and analyzed with regard to dioxins and furans (PCDD/PCDF) content, as well as to polyaromatic hydrocarbons (PAHs). The dust form during process with CB fuel was also analyzed. Each time, 85 g of the sample taken for testing was analyzed. The results of the analysis are presented in
Table 8. For comparative purposes, samples of HLS were also analyzed with regard to the content of these specific pollutants [
41].
Hydrated lime sorbent (HLS) injected to the exhaust gas stream contained trace amounts of PAHs (0.019 mg/sample) and did not contain PCDD/F. For the use of fuel composed of only CB, the content of PAHs was at the level of 1.6 mg/sample, while the concentration of dioxins and furans was 155.2 ng/sample. The amount of PAHs noted for biochars containing fuel was 1.5–3.9 times higher, which was caused by the significantly higher content of volatile matter.
The highest content of PCDD/F (by up to 70% with regard to other tested biochars and CB), equal to 264.3 ng/sample and accompanied with the highest toxic equivalent TEQ = 29, was noted in the biochar derived from SH. The elevated content of the pollutants (ca. 8% higher with regard to CB) was also noted for residual WdC and reached 166.8 ng/sample. The lowest concentration of PCDD/F, 116.7 nm/sample (25% lower than CB), was measured for biochar from SDP. Hence, considering amounts of PAHs and PCDD/F formed during the process, and appearing in the process gas, it was concluded that biochars from residual WdC or SDP could be efficiently used as substitutional fuel for iron ores sintering processes. Moreover, one must notice that the gas cleaning system involved in the research, comprising of catalytic ceramic filter and hydrated lime sorbent injection, enabled a 90% reduction of PAHs concentration and min. 95% reduction of PCDD/F [
18], and the final quality of exhaust gas corresponded to emission requirements established in dedicated legal acts.