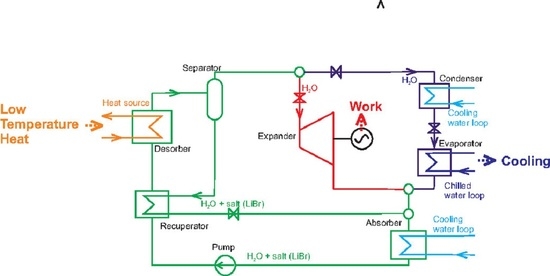
Absorption Power and Cooling Combined Cycle with an Aqueous Salt Solution as a Working Fluid and a Technically Feasible Configuration
Abstract
1. Introduction
- Separate thermodynamic cycles coupled by heat
- Separate thermodynamic cycles coupled by work
- Single-branch thermodynamic cycle for both power and thermally activated cooling
- Branched thermodynamic cycle for either power or thermally activated cooling
1.1. Separate Thermodynamic Cycles Coupled by Heat
1.2. Separate Thermodynamic Cycles Coupled by Work
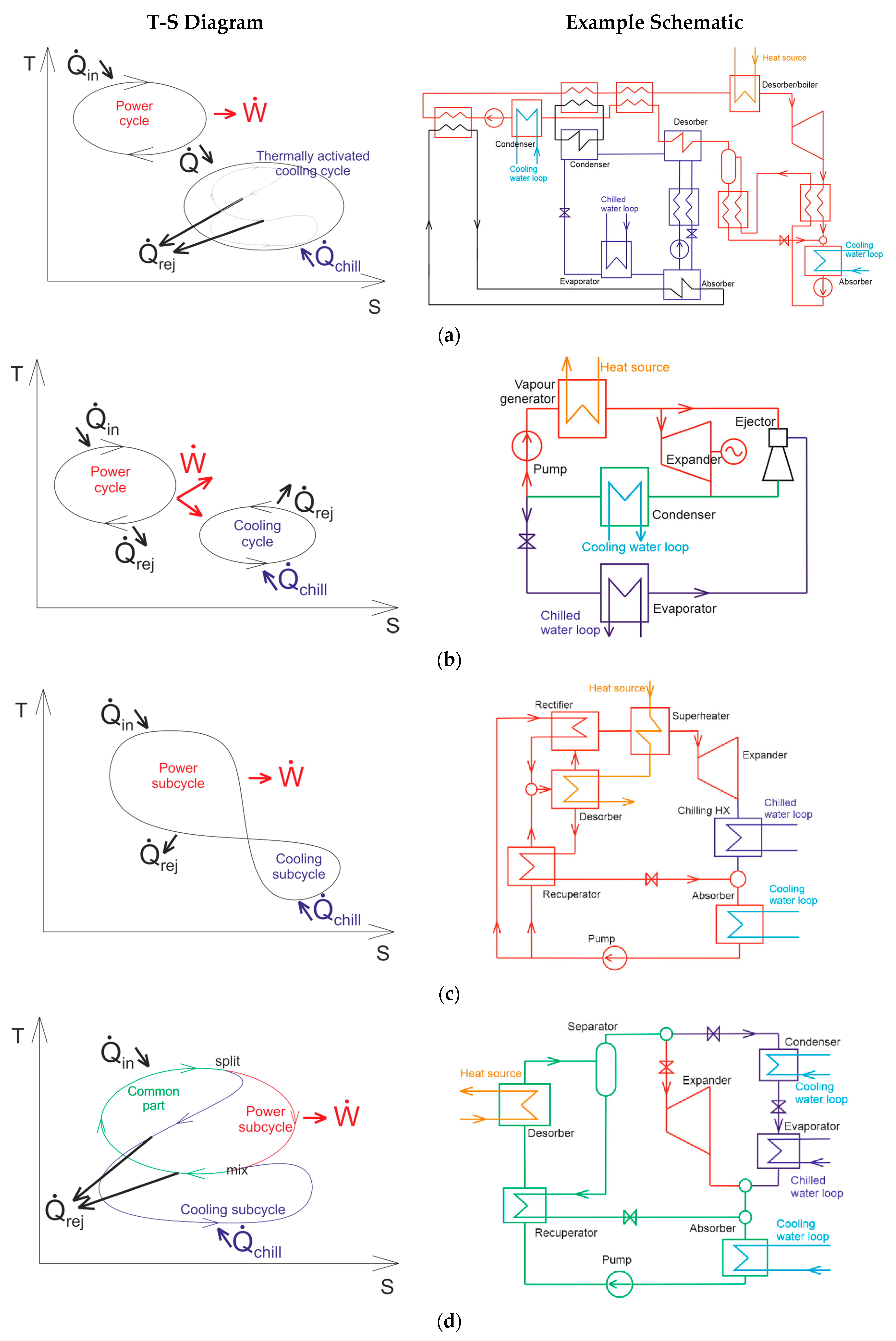
1.3. Single-Branch Thermodynamic Cycle for Both Power and Thermally Activated Cooling
1.4. Branched Thermodynamic Cycle for Either Power or Thermally Activated Cooling
1.5. Prospects for Aqueous Salt Solution Systems
2. Methods
2.1. Proposed Cycle Configuration
2.2. Calculation Method and Boundary Conditions
2.3. Performance Analysis Methods
3. Results and Discussion
3.1. Baseline APCC and Comparison with VCC–ORC
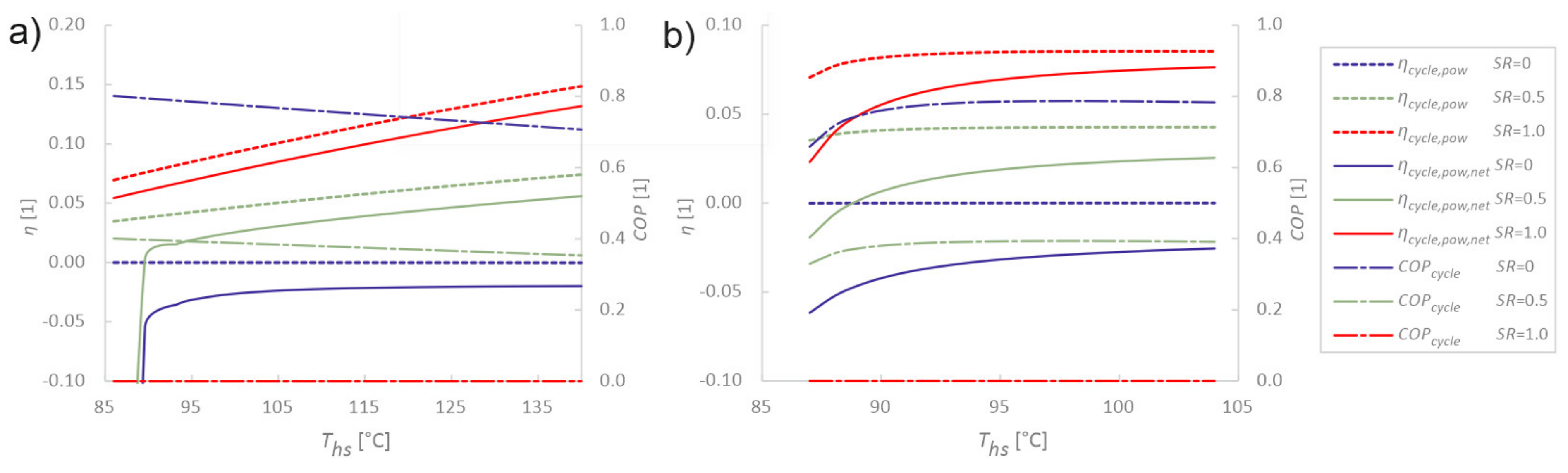
3.2. Sensitivity Analysis of Cycle Parameters
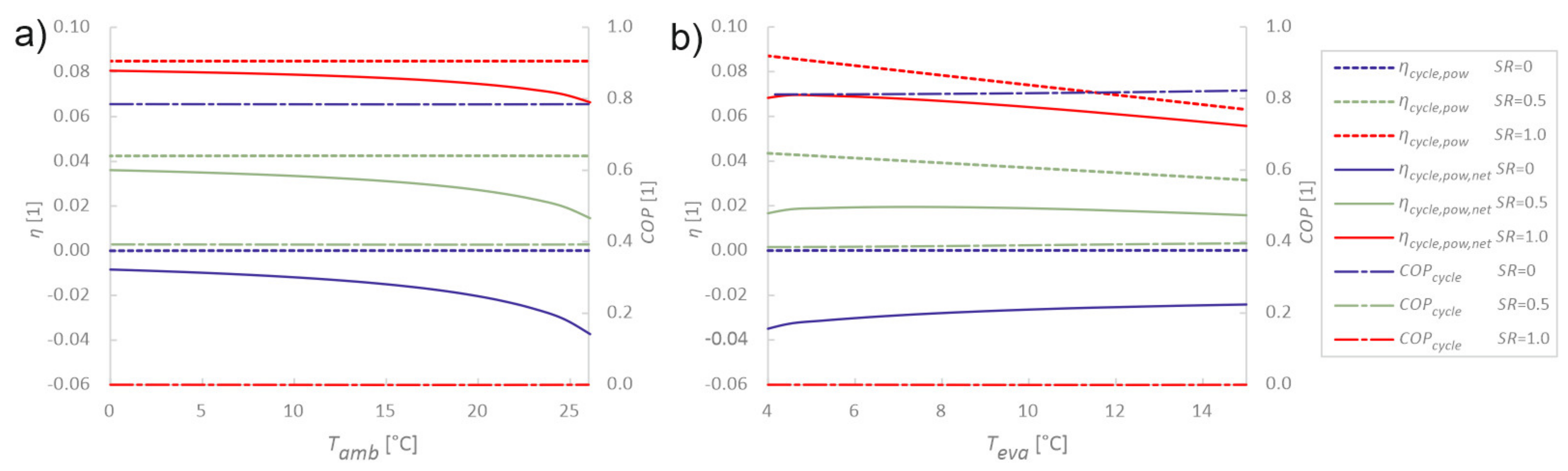
3.3. Sensitivity Analysis of Utilisation Parameters
3.4. Comparison of Working Fluids
3.5. Comparison of APCC with APC for Waste Heat Recovery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| COP | Coefficient of performance of the chiller | (1) |
| Exergy flux | (kW) | |
| h | Enthalpy | (kJ kg K−1) |
| Mass flow rate | (kg s−1) | |
| p | Pressure | (kPa) |
| Thermal power | (kW) | |
| RH | Relative humidity of air | (1) |
| s | Specific entropy | (kJ kg−1 K−1) |
| S | Entropy | (kJ K−1) |
| SR | Splitting ratio | (1) |
| T | Temperature | (°C/K) |
| v | Specific volume | (m3 kg−1) |
| Power | (kW) | |
| x | Vapour quality | (1) |
| Greek symbols | ||
| ξ | Mass concentration | (1) |
| η | Efficiency | (1) |
| Δ | Difference | (1) |
| Subscripts | ||
| 0 | Dead state, corresponding to ambient temperature | |
| abs | Absorber | |
| amb | Ambient | |
| cf | Chilled stream flow | |
| chill | Regarding chiller, cold production | |
| cond | Condenser | |
| comp | Compressor | |
| cw | Cooling water | |
| DC | Dry cooler | |
| exp | Expander | |
| hs | Heat source | |
| i | Numbering of streams | |
| in | Inlet | |
| is | Isentropic | |
| j | Fluid component | |
| out | Outlet | |
| pow | Regarding power production | |
| reg | Regenerator | |
| rej | Rejected | |
| sol | Solution | |
| util | Utilisation (of heat source) | |
| Abbreviations | ||
| APC | Absorption power cycle | |
| APCC | Absorption power and cooling combined cycle | |
| CHP | Combined heat and power | |
| CCP | Combined cooling and power | |
| CCHP | Combined cooling, heating, and power | |
| EES | Engineering equation solver | |
| ORC | Organic Rankine cycle | |
| VCC | Vapour compression chiller | |
| LiBr | Lithium bromide | |
| LiCl | Lithium chloride | |
| H2O | Water | |
| CaCl2 | Calcium carbonate | |
| NH3 | Ammonia | |
Appendix A
| Node | T (°C) | p (kPa) | h (kJ kg−1) | ṁ (kg s−1) | s (kJ kg−1 K−1) | ξ (1) | x (1) |
|---|---|---|---|---|---|---|---|
| 1 | 36.4 | 8.683 | 92 | 0.499 | 0.221 | 0.560 | subc |
| 2 | 79.1 | 8.683 | 179 | 0.499 | 0.484 | 0.560 | subc |
| 3 | 80.1 | 8.683 | 181 | 0.499 | 0.490 | 0.560 | 0 |
| 4 | 90.0 | 8.683 | 389 | 0.499 | 1.072 | 0.560 | 0.030 |
| 5 | 90.0 | 8.683 | 2669 | 0.035 | 8.463 | 0.000 | sup |
| 6 | 90.0 | 8.683 | 2669 | 0.018 | 8.463 | 0.000 | 1 |
| 7 | 5.0 | 0.8725 | 2417 | 0.018 | 8.689 | 0.000 | 0.963 |
| 8 | 90.0 | 8.683 | 2669 | 0.018 | 8.463 | 0.000 | sup |
| 9 | 43.1 | 8.683 | 180 | 0.018 | 0.613 | 0.000 | 0 |
| 10 | 5.0 | 0.8725 | 180 | 0.018 | 0.649 | 0.000 | 0.109 |
| 11 | 5.0 | 0.8725 | 2510 | 0.018 | 9.025 | 0.000 | 1 |
| 12 | 5.0 | 0.8725 | 2463 | 0.035 | 8.857 | 0.000 | 0.981 |
| 13 | 90.0 | 8.683 | 215 | 0.463 | 0.508 | 0.603 | 0 |
| 14 | 41.4 | 8.683 | 122 | 0.463 | 0.231 | 0.603 | subc |
| 15 | 41.4 | 0.8725 | 122 | 0.463 | 0.232 | 0.603 | 0.017 |
| 16 | 44.7 | 0.8725 | 288 | 0.499 | 0.847 | 0.560 | 0.028 |
| 17 | 36.4 | 0.8725 | 92 | 0.499 | 0.221 | 0.560 | 0 |
| 21 | 95.0 | 800 | 399 | 2.500 | 1.250 | ||
| 22 | 85.1 | 800 | 357 | 2.500 | 1.135 | ||
| 23 | 85.0 | 800 | 357 | 2.500 | 1.134 | ||
| 30 | 30.0 | 200 | 126 | 3.665 | 0.437 | ||
| 31 | 30.0 | 200 | 126 | 2.404 | 0.437 | ||
| 32 | 39.7 | 200 | 167 | 2.404 | 0.569 | ||
| 33 | 30.0 | 200 | 126 | 1.261 | 0.437 | ||
| 34 | 38.4 | 200 | 161 | 1.261 | 0.550 | ||
| 35 | 39.3 | 200 | 164 | 3.665 | 0.563 | ||
| 41 | 25.0 | 101.3 | 61 | 14.860 | 5.823 | ||
| 42 | 34.3 | 101.3 | 70 | 14.860 | 5.855 | ||
| 51 | 15.0 | 200 | 63 | 1.969 | 0.224 | ||
| 52 | 10.0 | 200 | 42 | 1.969 | 0.151 |
| Node | T (°C) | p (kPa) | h (kJ kg−1) | ṁ (kg s−1) | s (kJ kg−1 K−1) | x (1) |
|---|---|---|---|---|---|---|
| 1 | 43.3 | 841.7 | 257 | 0.524 | 1.192 | subc |
| 2 | 47.4 | 841.7 | 263 | 0.524 | 1.210 | subc |
| 3 | 82.5 | 841.7 | 313 | 0.524 | 1.358 | 0 |
| 4 | 82.5 | 841.7 | 463 | 0.524 | 1.781 | 1 |
| 5 | 53.9 | 275.3 | 447 | 0.524 | 1.794 | sup |
| 6 | 48.3 | 275.3 | 441 | 0.524 | 1.776 | sup |
| 7 | 43.0 | 275.3 | 436 | 0.524 | 1.759 | 1 |
| 8 | 43.0 | 275.3 | 257 | 0.524 | 1.192 | 0 |
| 11 | 35.0 | 831.5 | 249 | 0.158 | 1.165 | subc |
| 12 | 5.0 | 261.2 | 249 | 0.158 | 1.174 | 0.23 |
| 13 | 5.0 | 261.2 | 388 | 0.158 | 1.674 | 1 |
| 14 | 45.3 | 831.5 | 415 | 0.158 | 1.691 | sup |
| 15 | 43.0 | 831.5 | 412 | 0.158 | 1.682 | 1 |
| 16 | 43.0 | 831.5 | 260 | 0.158 | 1.202 | 0 |
| 21 | 95.0 | 800 | 399 | 2.500 | 1.250 | |
| 22 | 87.5 | 800 | 367 | 2.500 | 1.163 | |
| 23 | 85.0 | 800 | 357 | 2.500 | 1.134 | |
| 30 | 30.0 | 200 | 126 | 3.584 | 0.437 | |
| 31 | 30.0 | 200 | 126 | 2.811 | 0.437 | |
| 32 | 38.0 | 200 | 159 | 2.811 | 0.546 | |
| 33 | 38.2 | 200 | 160 | 2.811 | 0.549 | |
| 34 | 30.0 | 200 | 126 | 0.773 | 0.437 | |
| 35 | 38.0 | 200 | 159 | 0.773 | 0.546 | |
| 36 | 38.2 | 200 | 160 | 0.773 | 0.548 | |
| 37 | 38.2 | 200 | 160 | 3.584 | 0.549 | |
| 41 | 25.0 | 101.3 | 61 | 14.530 | 5.823 | |
| 42 | 33.2 | 101.3 | 69 | 14.530 | 5.852 | |
| 51 | 15.0 | 200 | 63 | 1.052 | 0.224 | |
| 52 | 10.0 | 200 | 42 | 1.052 | 0.151 |
| Node | T (°C) | p (kPa) | h (kJ kg−1) | ṁ (kg s−1) | s (kJ kg−1 K−1) | ξ (1) | x (1) |
|---|---|---|---|---|---|---|---|
| 1 | 35.5 | 8.089 | 88 | 0.502 | 0.218 | 0.555 | subc |
| 2 | 77.5 | 8.089 | 174 | 0.502 | 0.479 | 0.555 | subc |
| 3 | 77.6 | 8.089 | 174 | 0.502 | 0.479 | 0.555 | 0 |
| 4 | 90.0 | 8.089 | 435 | 0.502 | 1.211 | 0.555 | 0.030 |
| 5 | 90.0 | 8.089 | 2669 | 0.045 | 8.496 | 0.000 | sup |
| 6 | 90.0 | 8.089 | 2669 | 0.022 | 8.496 | 0.000 | 1 |
| 7 | 5.0 | 0.8725 | 2424 | 0.022 | 8.715 | 0.000 | 0.963 |
| 8 | 90.0 | 8.089 | 2669 | 0.022 | 8.496 | 0.000 | sup |
| 9 | 41.7 | 8.089 | 175 | 0.022 | 0.595 | 0.000 | 0 |
| 10 | 5.0 | 0.8725 | 175 | 0.022 | 0.629 | 0.000 | 0.109 |
| 11 | 5.0 | 0.8725 | 2510 | 0.022 | 9.025 | 0.000 | 1 |
| 12 | 5.0 | 0.8725 | 2467 | 0.045 | 8.870 | 0.000 | 0.981 |
| 13 | 90.0 | 8.089 | 218 | 0.458 | 0.502 | 0.609 | 0 |
| 14 | 40.5 | 8.089 | 124 | 0.458 | 0.224 | 0.609 | subc |
| 15 | 40.5 | 0.8725 | 124 | 0.458 | 0.224 | 0.609 | 0.017 |
| 16 | 45.9 | 0.8725 | 331 | 0.502 | 0.993 | 0.555 | 0.028 |
| 17 | 35.5 | 0.8725 | 88 | 0.502 | 0.218 | 0.555 | 0 |
| 21 | 95.0 | 800 | 399 | 2.500 | 1.250 | ||
| 22 | 82.5 | 800 | 346 | 2.500 | 1.105 | ||
| 23 | 82.5 | 800 | 346 | 2.500 | 1.105 | ||
| 30 | 30.0 | 200 | 126 | 4.624 | 0.437 | ||
| 31 | 30.0 | 200 | 126 | 2.717 | 0.437 | ||
| 32 | 40.8 | 200 | 171 | 2.717 | 0.582 | ||
| 33 | 30.0 | 200 | 126 | 1.907 | 0.437 | ||
| 34 | 37.0 | 200 | 155 | 1.907 | 0.532 | ||
| 35 | 39.2 | 200 | 164 | 4.624 | 0.562 | ||
| 41 | 25.0 | 101.3 | 61 | 18.750 | 5.823 | ||
| 42 | 34.2 | 101.3 | 70 | 18.750 | 5.855 | ||
| 51 | 15.0 | 200 | 63 | 2.480 | 0.224 | ||
| 52 | 10.0 | 200 | 42 | 2.480 | 0.151 |
| Node | T (°C) | p (kPa) | h (kJ kg−1) | ṁ (kg s−1) | s (kJ kg−1 K−1) | x (1) |
|---|---|---|---|---|---|---|
| 1 | 43.3 | 725.7 | 257 | 0.905 | 1.192 | subc |
| 2 | 46.2 | 725.7 | 261 | 0.905 | 1.205 | subc |
| 3 | 76.6 | 725.7 | 304 | 0.905 | 1.334 | 0 |
| 4 | 76.6 | 725.7 | 460 | 0.905 | 1.777 | 1 |
| 5 | 52.3 | 275.3 | 445 | 0.905 | 1.788 | sup |
| 6 | 48.3 | 275.3 | 441 | 0.905 | 1.776 | sup |
| 7 | 43.0 | 275.3 | 436 | 0.905 | 1.759 | 1 |
| 8 | 43.0 | 275.3 | 257 | 0.905 | 1.192 | 0 |
| 11 | 35.0 | 831.5 | 249 | 0.237 | 1.165 | subc |
| 12 | 5.0 | 261.2 | 249 | 0.237 | 1.174 | 0.23 |
| 13 | 5.0 | 261.2 | 388 | 0.237 | 1.674 | 1 |
| 14 | 45.3 | 831.5 | 415 | 0.237 | 1.691 | sup |
| 15 | 43.0 | 831.5 | 412 | 0.237 | 1.682 | 1 |
| 16 | 43.0 | 831.5 | 260 | 0.237 | 1.202 | 0 |
| 21 | 95.0 | 800 | 399 | 2.500 | 1.250 | |
| 22 | 81.6 | 800 | 342 | 2.500 | 1.094 | |
| 23 | 77.9 | 800 | 327 | 2.500 | 1.050 | |
| 30 | 30.0 | 200 | 126 | 3.584 | 0.437 | |
| 31 | 30.0 | 200 | 126 | 4.854 | 0.437 | |
| 32 | 38.0 | 200 | 159 | 4.854 | 0.546 | |
| 33 | 38.2 | 200 | 160 | 4.854 | 0.549 | |
| 34 | 30.0 | 200 | 126 | 1.155 | 0.437 | |
| 35 | 38.0 | 200 | 159 | 1.155 | 0.546 | |
| 36 | 38.2 | 200 | 160 | 1.155 | 0.548 | |
| 37 | 38.2 | 200 | 160 | 6.009 | 0.549 | |
| 41 | 25.0 | 101.3 | 61 | 24.360 | 5.823 | |
| 42 | 33.2 | 101.3 | 69 | 24.360 | 5.852 | |
| 51 | 15.0 | 200 | 63 | 1.571 | 0.224 | |
| 52 | 10.0 | 200 | 42 | 1.571 | 0.151 |
References
- Johnson, I.; Choate, W.T.; Davidson, A. Waste Heat Recovery. Technology and Opportunities in U.S. Industry; BCS, Inc.: Laurel, MD, USA, 2008. [Google Scholar] [CrossRef]
- US Department of Energy. Chapter 6: Innovating Clean Energy Technologies in Advanced Manufacturing. Technology Assessments. Waste Heat Recovery Systems. Quadrennial Technology Review; US Department of Energy: Washington, DC, USA, 2015.
- Brückner, S.; Liu, S.; Miró, L.; Radspieler, M.; Cabeza, L.F.; Lävemann, E. Industrial waste heat recovery technologies: An economic analysis of heat transformation technologies. Appl. Energy 2015, 151, 157–167. [Google Scholar] [CrossRef]
- Saha, B.K.; Chakraborty, B.; Dutta, R. Estimation of waste heat and its recovery potential from energy-intensive industries. Clean Technol. Environ. Policy 2020, 22, 1–20. [Google Scholar] [CrossRef]
- D’Adamo, I.; Rosa, P. Current state of renewable energies performances in the European Union: A new reference framework. Energy Convers. Manag. 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Ibarra-Bahena, J.; Romero, R. Performance of Different Experimental Absorber Designs in Absorption Heat Pump Cycle Technologies: A Review. Energies 2014, 7, 751–766. [Google Scholar] [CrossRef]
- Nasr Isfahani, R.; Sampath, K.; Moghaddam, S.; Isfahani, R.N.; Sampath, K.; Moghaddam, S. Nanofibrous membrane-based absorption refrigeration system. Int. J. Refrig. 2013, 36, 2297–2307. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.W.; Lee, H.; Chung, J.T.; Kang, Y.T. Optimal design of generators for H2O/LiBr absorption chiller with multi-heat sources. Energy 2019, 167, 47–59. [Google Scholar] [CrossRef]
- Ribatski, G.; Jacobi, A. Falling-film evaporation on horizontal tubes—A critical review. Int. J. Refrig. 2005, 28, 635–653. [Google Scholar] [CrossRef]
- García-Hernando, N.; Almendros-Ibáñez, J.; Ruiz, G.; de Vega, M. On the pressure drop in Plate Heat Exchangers used as desorbers in absorption chillers. Energy Convers. Manag. 2011, 52, 1520–1525. [Google Scholar] [CrossRef][Green Version]
- Marcos, J.; Izquierdo, M.; Lizarte, R.; Palacios, E.; Ferreira, C.A.I. Experimental boiling heat transfer coefficients in the high temperature generator of a double effect absorption machine for the lithium bromide/water mixture. Int. J. Refrig. 2009, 32, 627–637. [Google Scholar] [CrossRef]
- Lizarte, R.; Izquierdo, M.; Marcos, J.; Palacios, E. An innovative solar-driven directly air-cooled LiBr–H 2 O absorption chiller prototype for residential use. Energy Build. 2012, 47, 1–11. [Google Scholar] [CrossRef]
- Deng, J.; Wang, R.; Han, G. A review of thermally activated cooling technologies for combined cooling, heating and power systems. Prog. Energy Combust. Sci. 2011, 37, 172–203. [Google Scholar] [CrossRef]
- Demirkaya, G. Theoretical and Experimental Analysis of Power and Cooling Cogeneration Utilizing Low Temperature Heat Sources. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2011. [Google Scholar]
- Wu, D.; Wang, R. Combined cooling, heating and power: A review. Prog. Energy Combust. Sci. 2006, 32, 459–495. [Google Scholar] [CrossRef]
- Wang, J.; Han, Z.; Guan, Z. Hybrid solar-assisted combined cooling, heating, and power systems: A review. Renew. Sustain. Energy Rev. 2020, 133, 110256. [Google Scholar] [CrossRef]
- Wegener, M.; Malmquist, A.; Isalgué, A.; Martin, A. Biomass-fired combined cooling, heating and power for small scale applications—A review. Renew. Sustain. Energy Rev. 2018, 96, 392–410. [Google Scholar] [CrossRef]
- Ayou, D.S.; Bruno, J.C.; Saravanan, R.; Coronas, A. An overview of combined absorption power and cooling cycles. Renew. Sustain. Energy Rev. 2013, 21, 728–748. [Google Scholar] [CrossRef]
- Ayou, D.S.; Bruno, J.C.; Coronas, A. Combined absorption power and refrigeration cycles using low- and mid-grade heat sources. Sci. Technol. Built Environ. 2015, 21, 934–943. [Google Scholar] [CrossRef]
- Demirkaya, G.; Padilla, R.V.; Goswami, D.Y. A review of combined power and cooling cycles. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 534–547. [Google Scholar] [CrossRef]
- Demierre, J.; Favrat, D.; Schiffmann, J.; Wegele, J. Experimental investigation of a Thermally Driven Heat Pump based on a double Organic Rankine Cycle and an oil-free Compressor-Turbine Unit. Int. J. Refrig. 2014, 44, 91–100. [Google Scholar] [CrossRef]
- Cho, H.; Smith, A.D.; Mago, P. Combined cooling, heating and power: A review of performance improvement and optimisation. Appl. Energy 2014, 136, 168–185. [Google Scholar] [CrossRef]
- Ahmadi, P.; Dincer, I.; Rosen, M.A. Exergo-environmental analysis of an integrated organic Rankine cycle for trigeneration. Energy Convers. Manag. 2012, 64, 447–453. [Google Scholar] [CrossRef]
- Chaiyat, N.; Kiatsiriroat, T. Analysis of combined cooling heating and power generation from organic Rankine cycle and absorption system. Energy 2015, 91, 363–370. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, O. Thermoeconomic analysis of SOFC-GT-VARS-ORC combined power and cooling system. Int. J. Hydrog. Energy 2019, 44, 27575–27586. [Google Scholar] [CrossRef]
- Rashidi, J.; Ifaei, P.; Esfahani, I.J.; Ataei, A.; Yoo, C.K. Thermodynamic and economic studies of two new high efficient power-cooling cogeneration systems based on Kalina and absorption refrigeration cycles. Energy Convers. Manag. 2016, 127, 170–186. [Google Scholar] [CrossRef]
- Rashidi, J.; Yoo, C.K. Exergetic and exergoeconomic studies of two highly efficient power-cooling cogeneration systems based on the Kalina and absorption refrigeration cycles. Appl. Therm. Eng. 2017, 124, 1023–1037. [Google Scholar] [CrossRef]
- Prigmore, D.; Barber, R. Cooling with the sun’s heat Design considerations and test data for a Rankine Cycle prototype. Sol. Energy 1975, 17, 185–192. [Google Scholar] [CrossRef]
- Wang, H.; Peterson, R.; Harada, K.; Miller, E.; Ingram-Goble, R.; Fisher, L.; Yih, J.; Ward, C. Performance of a combined organic Rankine cycle and vapor compression cycle for heat activated cooling. Energy 2011, 36, 447–458. [Google Scholar] [CrossRef]
- Patel, B.; Desai, N.B.; Kachhwaha, S.; Jain, V.; Hadia, N. Thermo-economic analysis of a novel organic Rankine cycle integrated cascaded vapor compression–absorption system. J. Clean. Prod. 2017, 154, 26–40. [Google Scholar] [CrossRef]
- Oliveira, A.; Afonso, C.; Matos, J.; Riffat, S.; Nguyen, M.; Doherty, P. A combined heat and power system for buildings driven by solar energy and gas. Appl. Therm. Eng. 2002, 22, 587–593. [Google Scholar] [CrossRef]
- Novotny, V.; Vodicka, V.; Mascuch, J.; Kolovratnik, M. Possibilities of water-lithium bromide absorption power cycles for low temperature, low power and combined power and cooling systems. Energy Procedia 2017, 129, 818–825. [Google Scholar] [CrossRef]
- Kutlu, C.; Erdinc, M.T.; Li, J.; Wang, Y.; Su, Y. A study on heat storage sizing and flow control for a domestic scale solar-powered organic Rankine cycle-vapour compression refrigeration system. Renew. Energy 2019, 143, 301–312. [Google Scholar] [CrossRef]
- Aphornratana, S.; Sriveerakul, T. Analysis of a combined Rankine-vapour-compression refrigeration cycle. Energy Convers. Manag. 2010, 51, 2557–2564. [Google Scholar] [CrossRef]
- Wang, J.; Dai, Y.; Sun, Z. A theoretical study on a novel combined power and ejector refrigeration cycle. Int. J. Refrig. 2009, 32, 1186–1194. [Google Scholar] [CrossRef]
- Habibzadeh, A.; Rashidi, M.; Galanis, N. Analysis of a combined power and ejector-refrigeration cycle using low temperature heat. Energy Convers. Manag. 2013, 65, 381–391. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Li, H.; Yu, Z. Theoretical analysis of a combined power and ejector refrigeration cycle using zeotropic mixture. Appl. Energy 2015, 160, 912–919. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, N.; Zhao, L.; Deng, S.; Li, H.; Yu, Z. Analysis of a novel combined power and ejector-refrigeration cycle. Energy Convers. Manag. 2016, 108, 266–274. [Google Scholar] [CrossRef]
- Sadeghi, M.; Yari, M.; Mahmoudi, S.M.S.; Jafari, M. Thermodynamic analysis and optimisation of a novel combined power and ejector refrigeration cycle—Desalination system. Appl. Energy 2017, 208, 239–251. [Google Scholar] [CrossRef]
- Ghaebi, H.; Rostamzadeh, H.; Matin, P.S. Performance evaluation of ejector expansion combined cooling and power cycles. Heat Mass Transf. 2017, 53, 2915–2931. [Google Scholar] [CrossRef]
- Xu, F.; Goswami, D.Y.; Bhagwat, S.S. A combined power/cooling cycle. Energy 2000, 25, 233–246. [Google Scholar] [CrossRef]
- Demirkaya, G.; Padilla, R.V.; Fontalvo, A.; Lake, M.; Lim, Y.Y. Thermal and Exergetic Analysis of the Goswami Cycle Integrated with Mid-Grade Heat Sources. Entropy 2017, 19, 416. [Google Scholar] [CrossRef]
- Hasan, A.; Goswami, D.; Vijayaraghavan, S. First and second law analysis of a new power and refrigeration thermodynamic cycle using a solar heat source. Sol. Energy 2002, 73, 385–393. [Google Scholar] [CrossRef]
- Abed, H.; Atashkari, K.; Niazmehr, A.; Jamali, A. Thermodynamic optimisation of combined power and refrigeration cycle using binary organic working fluid. Int. J. Refrig. 2013, 36, 2160–2168. [Google Scholar] [CrossRef]
- Martin, C.; Goswami, D. Effectiveness of cooling production with a combined power and cooling thermodynamic cycle. Appl. Therm. Eng. 2006, 26, 576–582. [Google Scholar] [CrossRef]
- Fontalvo, A.; Pinzon, H.; Duarte, J.; Bula, A.; Quiroga, A.G.; Padilla, R.V. Exergy analysis of a combined power and cooling cycle. Appl. Therm. Eng. 2013, 60, 164–171. [Google Scholar] [CrossRef]
- Padilla, R.V.; Demirkaya, G.; Goswami, D.Y.; Stefanakos, E.; Rahman, M.M. Analysis of power and cooling cogeneration using am-monia-water mixture. Energy 2010, 35, 4649–4657. [Google Scholar] [CrossRef]
- Demirkaya, G.; Padilla, R.V.; Goswami, D.Y.; Stefanakos, E.; Rahman, M.M. Analysis of a combined power and cooling cycle for low-grade heat sources. Int. J. Energy Res. 2010, 35, 1145–1157. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Goswami, D.Y. A combined power and cooling cycle modified to improve resource utilization efficiency using a distillation stage. Energy 2006, 31, 1177–1196. [Google Scholar] [CrossRef]
- Sadrameli, S.; Goswami, D. Optimum operating conditions for a combined power and cooling thermodynamic cycle. Appl. Energy 2007, 84, 254–265. [Google Scholar] [CrossRef]
- Chen, H.; Goswami, D.Y. Simulation of a Thermodynamic Cycle with Organic Absorbents and CO2 as a Working Fluid. In Proceedings of the 2008 AIChE Annual Meeting, Philadelphia, PA, USA, 16–21 November 2008. [Google Scholar]
- Vijayaraghavan, S.; Goswami, D.Y. Organic working fluids for a combined power and cooling cycle. J. Energy Resour. Technol. 2005, 127, 125–130. [Google Scholar] [CrossRef]
- Demirkaya, G.; Padilla, R.V.; Fontalvo, A.; Bula, A.; Goswami, D.Y. Experimental and Theoretical Analysis of the Goswami Cycle Operating at Low Temperature Heat Sources. J. Energy Resour. Technol. 2018, 140, 072005. [Google Scholar] [CrossRef]
- Tamm, G.; Goswami, D.Y. Novel combined power and cooling thermodynamic cycle for low temperature heat sources, part II: Experimental investigation. J. Sol. Energy Eng. Trans. 2003, 125, 223–229. [Google Scholar] [CrossRef]
- Muye, J.; Ayou, D.S.; Saravanan, R.; Coronas, A. Performance study of a solar absorption power-cooling system. Appl. Therm. Eng. 2016, 97, 59–67. [Google Scholar] [CrossRef]
- Shankar, R.; Srinivas, T. Performance investigation of Kalina cooling cogeneration cycles. Int. J. Refrig. 2018, 86, 163–185. [Google Scholar] [CrossRef]
- Shankar, R.; Srinivas, T. Development and analysis of a new integrated power and cooling plant using LiBr–H2O mixture. Sadhana 2014, 39, 1547–1562. [Google Scholar] [CrossRef]
- Rivera, W.; Sánchez-Sánchez, K.; Hernández-Magallanes, J.A.; Jiménez-García, J.C.; Pacheco, A. Modeling of Novel Thermodynamic Cycles to Produce Power and Cooling Simultaneously. Processes 2020, 8, 320. [Google Scholar] [CrossRef]
- Erickson, D.C.; Anand, G.; Kyung, I. Heat-Activated Dual-Function Absorption Cycle. ASHRAE Trans. Symp. 2004, 110, 515–524. [Google Scholar]
- Jawahar, C.; Saravanan, R.; Bruno, J.C.; Coronas, A. Simulation studies on gax based Kalina cycle for both power and cooling applications. Appl. Therm. Eng. 2013, 50, 1522–1529. [Google Scholar] [CrossRef]
- Praveen Kumar, G.; Saravanan, R.; Coronas, A. Experimental studies on combined cooling and power system driven by low-grade heat sources. Energy 2017, 128, 801–812. [Google Scholar] [CrossRef]
- López-Villada, J.; Ayou, D.S.; Bruno, J.C.; Coronas, A. Modelling, simulation and analysis of solar absorption power-cooling systems. Int. J. Refrig. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Okwose, C.F.; Abid, M.; Ratlamwala, T.A.H. Performance analysis of compressor-assisted two-stage triple effect absorption refrigeration cycle for power and cooling. Energy Convers. Manag. 2021, 227, 113547. [Google Scholar] [CrossRef]
- Shokati, N.; Ranjbar, F.; Yari, M. A comprehensive exergoeconomic analysis of absorption power and cooling cogeneration cycles based on Kalina, part 1: Simulation. Energy Convers. Manag. 2018, 158, 437–459. [Google Scholar] [CrossRef]
- Ventas, R.; Lecuona, A.; Vereda, C.; Rodriguez-Hidalgo, M. Performance analysis of an absorption double-effect cycle for power and cold generation using ammonia/lithium nitrate. Appl. Therm. Eng. 2017, 115, 256–266. [Google Scholar] [CrossRef]
- Wang, J.; Dai, Y.; Gao, L. Parametric analysis and optimisation for a combined power and refrigeration cycle. Appl. Energy. 2008, 85, 1071–1085. [Google Scholar] [CrossRef]
- Zhang, N.; Cai, R.; Lior, N. A Novel Ammonia-Water Cycle for Power and Refrigeration Cogeneration. Aerospace 2004, 183–196. [Google Scholar] [CrossRef]
- Zhang, N.; Lior, N. Methodology for thermal design of novel combined refrigeration/power binary fluid systems. Int. J. Refrig. 2007, 30, 1072–1085. [Google Scholar] [CrossRef]
- Parikhani, T.; Ghaebi, H.; Rostamzadeh, H. A novel geothermal combined cooling and power cycle based on the absorption power cycle: Energy, exergy and exergoeconomic analysis. Energy 2018, 153, 265–277. [Google Scholar] [CrossRef]
- Herold, K.E.; Radermacher, R.; Klein, S.A. Absorption Chillers and Heat Pumps; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Younès, R.; Zeidan, H.; Harb, H.; Ghaddar, A. Optimal design and economical study for solar air-conditioning by absorption chillers. In Proceedings of the International IIR Conference on Latest Development in Refrigerated Storage, Transportation and Display of Food Products, Amman, Jordan, 28–30 March 2005. [Google Scholar]
- Weiss, W.; Spörk-Dür, M. Solar Heat Worldwide. Global Market Development and Trends in 2018. Detailed Market Figures 2017; AEE Institute for Sustainable Technologies: Gleisdorf, Austria, 2019. [Google Scholar]
- Ibrahim, O.M.; Klein, S. Absorption power cycles. Energy 1996, 21, 21–27. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Zhang, Y. A review of research on the Kalina cycle. Renew. Sustain. Energy Rev. 2012, 16, 5309–5318. [Google Scholar] [CrossRef]
- Shankar, R.; Srinivas, T. Options in Kalina Cycle Systems. Energy Procedia 2016, 90, 260–266. [Google Scholar] [CrossRef]
- Jonsson, M. Advanced Power Cycles with Mixture as the Working Fluid. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2003. [Google Scholar]
- Micak, H.A. An introduction to the Kalina cycle. In Proceedings of the 1996 International Joint Power Generation Conference, Houston, TX, USA, 13–17 October 1996. [Google Scholar]
- Novotny, V.; Kolovratnik, M. Absorption power cycles for low-temperature heat sources using aqueous salt solutions as working fluids. Int. J. Energy Res. 2016, 41, 952–975. [Google Scholar] [CrossRef]
- Eller, T.; Heberle, F.; Brüggemann, D. Second law analysis of novel working fluid pairs for waste heat recovery by the Kalina cycle. Energy 2017, 119, 188–198. [Google Scholar] [CrossRef]
- Novotny, V.; Vitvarova, M.; Kolovratnik, M. Absorption Power Cycles with Various Working Fluids for Exergy-Efficient Low-Temperature Waste Heat Recovery. Smart Sustain. Plan. Cities Reg. 2018, 99–111. [Google Scholar] [CrossRef]
- Hernando, N.G.; de Vega, M.; Soria-Verdugo, A.; Sanchez, S. Energy and exergy analysis of an absorption power cycle. Appl. Therm. Eng. 2013, 55, 69–77. [Google Scholar] [CrossRef]
- Shokati, N.; Ranjbar, F.; Yari, M. A comparative analysis of rankine and absorption power cycles from exergoeconomic viewpoint. Energy Convers. Manag. 2014, 88, 657–668. [Google Scholar] [CrossRef]
- Ozcan, H.; Dincer, I. Thermodynamic Analysis of an Integrated SOFC, Solar ORC and Absorption Chiller for Tri-generation Applications. Fuel Cells 2013, 13, 781–793. [Google Scholar] [CrossRef]
- Styliaras, V. A mixed cycle for converting heat to mechanical work. Heat Recover. Syst. CHP 1995, 15, 749–753. [Google Scholar] [CrossRef]
- Novotný, V.; Szucs, D.J.; Spale, J.; Vodicka, V.; Mascuch, J.; Kolovratník, M. Absorption power cycle with libr solution working fluid-design of the proof-of-concept unit. In Proceedings of the 5th International Seminar on ORC Power Systems, Athens, Greece, 9–11 September 2019. [Google Scholar]
- Novotný, V.; Vitvarová, M.; Jakobsen, J.P.; Kolovratník, M. Analysis and Design of Novel Absorption Power Cycle Plants. In Proceedings of the ASME 2016 10th International Conference on Energy Sustainability, Charlotte, NC, USA, 26–30 June 2016; p. V001T13A005. [Google Scholar]
- Klein, S.A. EES–Engineering Equation Solver, Version V10.354-3D, 2018-1-24. F-Chart Software. Available online: http://fchartsoftware.com (accessed on 15 June 2021).
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Klein, S.A. Thermodynamic properties of ammonia-water mixtures. Trans. Soc Heat Refrig. Air Cond. Eng. 1993, 99, 1495. [Google Scholar]
- Pátek, J.; Klomfar, J. Thermodynamic properties of the LiCl-H2O system at vapor-liquid equilibrium from 273 K to 400 K. Int. J. Refrig. 2008, 31, 287–303. [Google Scholar] [CrossRef]
- Kretzschmar., H.-J.; Stoecker, I.; Kunick, M.; Hasch, S. Property Library for Mixtures of Water/Lithium Bromide FluidEES with LibWaLi for Engineering Equation Solver; KCE: Taoyuan City, Taiwan, 2011. [Google Scholar]
- Kim, D.S.; Ferreira, C.A.I. A Gibbs Energy Equation for LiBr/H2O Solutions. In Proceedings of the 6th IIR-Gustav Lorentzen Conference, Glasgow, UK, 29 August–1 September 2004. [Google Scholar]
- Conde, M.R. Properties of aqueous solutions of lithium and calcium chlorides: Formulations for use in air conditioning equipment design. Int. J. Therm. Sci. 2004, 43, 367–382. [Google Scholar] [CrossRef]
- Weiß, A.; Popp, T.; Zinn, G.; Preißinger, M.; Brüggemann, D. A micro-turbine-generator-construction-kit (MTG-c-kit) for small-scale waste heat recovery ORC-Plants. Energy 2019, 181, 51–55. [Google Scholar] [CrossRef]
- Weiß, A.P.; Popp, T.; Müller, J.; Hauer, J.; Brüggemann, D.; Preißinger, M. Experimental characterization and comparison of an axial and a cantilever micro-turbine for small-scale Organic Rankine Cycle. Appl. Therm. Eng. 2018, 140, 235–244. [Google Scholar] [CrossRef]
- DiPippo, R. Geothermal Power Plants: Principles, Applications, Case Studies, and Environmental Impact; Butterworth Heinemann: Oxford, UK, 2012. [Google Scholar]
- An, Q.; Lemort, V.; Zhai, H.; An, Q.; Shi, L.; Lemort, V.; Quoilin, S. Categorisation and analysis of heat sources for organic Rankine cycle systems. Renew. Sustain. Energy Rev. 2016, 64, 790–805. [Google Scholar]
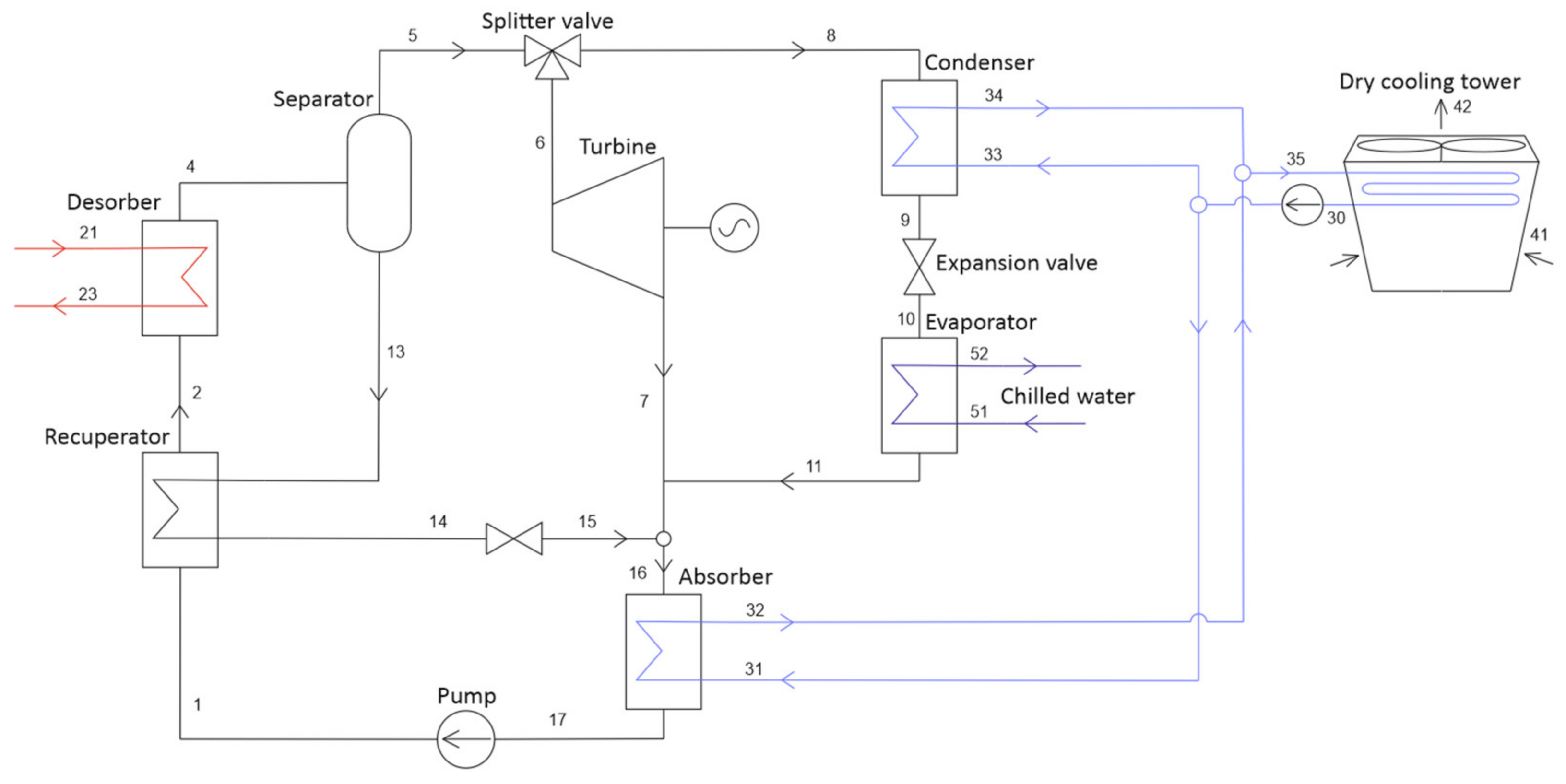


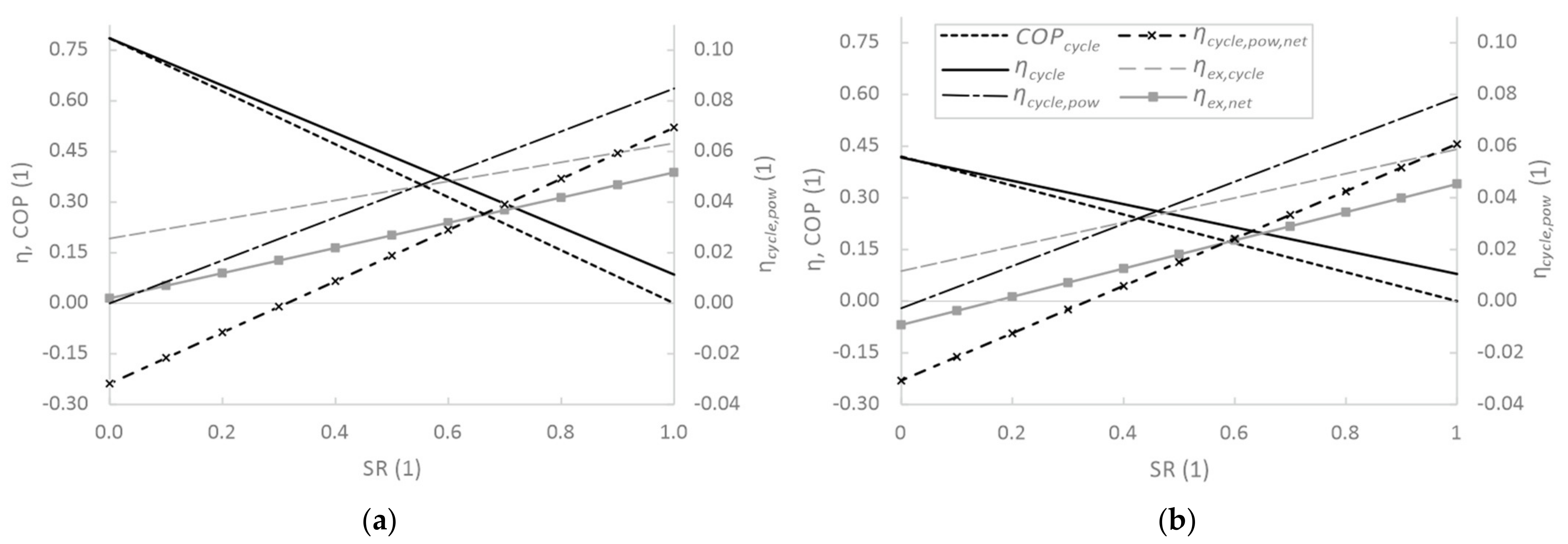

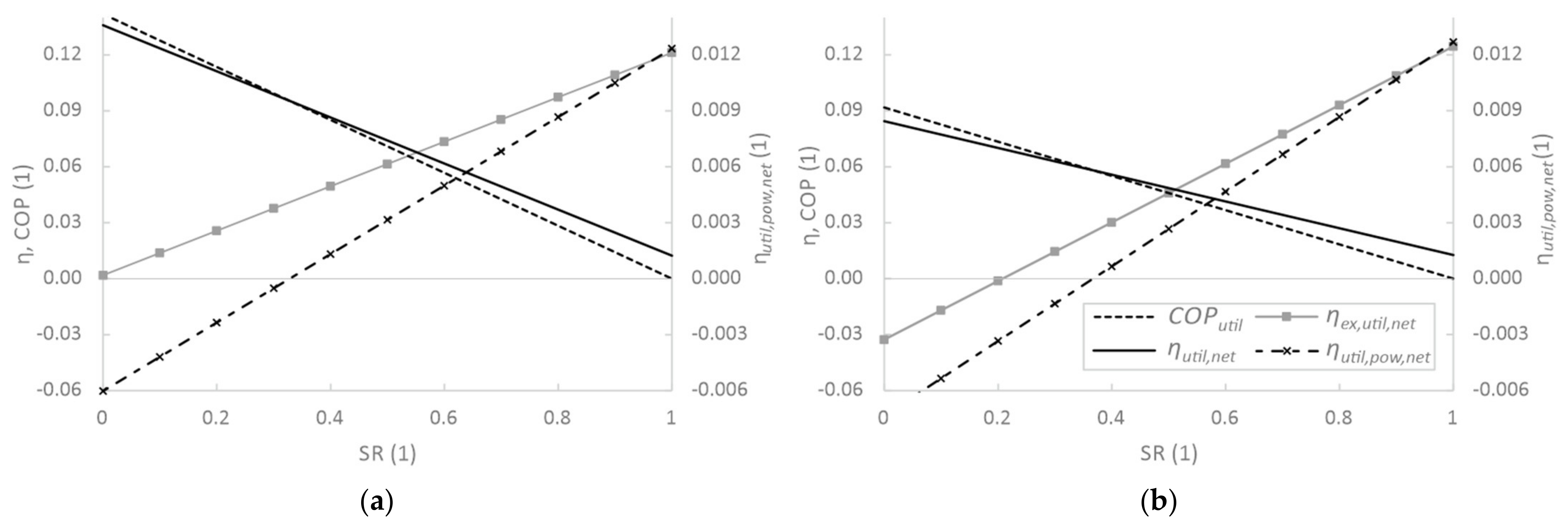


| Tamb | pamb | RHamb | Ths | Teva | T51 | ΔTmin, HX |
|---|---|---|---|---|---|---|
| (°C) | (kPa) | (%) | (°C) | (°C) | (°C) | (°C) |
| 25 | 101.325 | 70 | 95 | 5 | 15 | 5 |
| ṁhs | phs | pcw | ΔpDC | ηis | ξsol,1 | Ths, out |
| (kg s−1) | (kPa) | (kPa) | (kPa) | (%) | (%) | (°C) |
| 2.5 | 8 000 | 200 | 0.15 | 80 | 56 | 85 |
| Parameter | Unit | APCC | ORC–VCC |
|---|---|---|---|
| % | 43.5 | 24.8 | |
| % | 4.24 | 3.81 | |
| 1 | 0.39 | 0.21 | |
| % | 1.88 | 1.51 | |
| % | 33.3 | 26.4 | |
| % | 20.1 | 13.6 | |
| kW | 41.3 | 22.0 | |
| kW | 105.1 | 105.1 | |
| kW | 141.9 | 123.1 | |
| kW | 43.3 | 3.0 | |
| kW | 4.46 | 8.58 | |
| kW | −0.003 | −0.29 | |
| kW | 0 | −4.29 | |
| kW | −2.48 | −2.42 | |
| kW | 1.98 | 1.58 |
| ξ1 (1) | ξ2 (1) | p1 (kPa) | p2 (kPa) | SR (1) | (1) | (%) | (%) | (%) | (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| H2O-LiBr | 0.56 | 0.60 | 8.7 | 0.87 | 0 | 0.79 | 0.0 | −3.2 | 19.2 | 1.5 |
| 0.5 | 0.39 | 4.2 | 1.9 | 33.3 | 20.1 | |||||
| 1 | 0.00 | 8.5 | 6.9 | 47.4 | 38.8 | |||||
| H2O-LiCl | 0.44 | 0.52 | 9.1 | 0.87 | 0 | 0.79 | 0.0 | −3.1 | 19.3 | 2.2 |
| 0.5 | 0.40 | 4.4 | 2.0 | 34.0 | 21.0 | |||||
| 1 | 0.00 | 8.7 | 7.1 | 48.7 | 39.7 | |||||
| H2O-CaCl2 | 0.56 | 0.68 | 7.7 | 0.87 | 0 | 0.74 | 0.0 | −3.3 | 18.2 | −0.3 |
| 0.5 | 0.37 | 3.8 | 1.6 | 30.4 | 17.9 | |||||
| 1 | 0.00 | 7.6 | 6.5 | 42.6 | 36.3 | |||||
| NH3-H2O | 0.50 | 0.45 | 1512.2 | 458.6 | 0 | 0.56 | −0.9 | −3.6 | 8.3 | −6.3 |
| 0.5 | 0.28 | 3.1 | 1.0 | 23.9 | 12.2 | |||||
| 1 | 0.00 | 7.1 | 5.5 | 39.6 | 30.7 |
| ξ1 (1) | ξ2 (1) | p1 (kPa) | p2 (kPa) | SR (1) | (1) | (%) | (%) | (%) | (%) | (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O-LiBr | 0.56 | 0.61 | 8.1 | 0.87 | 0 | 0.14 | 0.0 | −0.6 | 14.2 | 13.6 | 0.2 |
| 0.5 | 0.07 | 0.7 | 0.3 | 7.8 | 7.4 | 6.2 | |||||
| 1 | 0.00 | 1.5 | 1.2 | 1.5 | 1.2 | 12.1 | |||||
| H2O-LiCl | 0.43 | 0.52 | 9.2 | 0.87 | 0 | 0.13 | 0.0 | −0.5 | 12.5 | 13.0 | 0.7 |
| 0.5 | 0.07 | 0.7 | 0.3 | 6.9 | 7.2 | 6.2 | |||||
| 1 | 0.00 | 1.4 | 1.2 | 1.2 | 1.4 | 11.7 | |||||
| H2O-CaCl2 | 0.55 | 0.67 | 8.0 | 0.87 | 0 | 0.10 | 0.0 | −0.5 | 10.6 | 11.0 | 0.0 |
| 0.5 | 0.05 | 0.6 | 0.2 | 5.8 | 6.1 | 4.7 | |||||
| 1 | 0.00 | 1.1 | 1.0 | 1.0 | 1.1 | 9.4 | |||||
| NH3-H2O | 0.51 | 0.43 | 1417.7 | 455.1 | 0 | 0.11 | −0.1 | −0.7 | 10.5 | 10.0 | −2.0 |
| 0.5 | 0.05 | 0.6 | 0.2 | 6.0 | 5.5 | 4.4 | |||||
| 1 | 0.00 | 1.4 | 1.1 | 1.4 | 1.1 | 10.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotny, V.; Szucs, D.J.; Špale, J.; Tsai, H.-Y.; Kolovratnik, M. Absorption Power and Cooling Combined Cycle with an Aqueous Salt Solution as a Working Fluid and a Technically Feasible Configuration. Energies 2021, 14, 3715. https://doi.org/10.3390/en14123715
Novotny V, Szucs DJ, Špale J, Tsai H-Y, Kolovratnik M. Absorption Power and Cooling Combined Cycle with an Aqueous Salt Solution as a Working Fluid and a Technically Feasible Configuration. Energies. 2021; 14(12):3715. https://doi.org/10.3390/en14123715
Chicago/Turabian StyleNovotny, Vaclav, David J. Szucs, Jan Špale, Hung-Yin Tsai, and Michal Kolovratnik. 2021. "Absorption Power and Cooling Combined Cycle with an Aqueous Salt Solution as a Working Fluid and a Technically Feasible Configuration" Energies 14, no. 12: 3715. https://doi.org/10.3390/en14123715
APA StyleNovotny, V., Szucs, D. J., Špale, J., Tsai, H.-Y., & Kolovratnik, M. (2021). Absorption Power and Cooling Combined Cycle with an Aqueous Salt Solution as a Working Fluid and a Technically Feasible Configuration. Energies, 14(12), 3715. https://doi.org/10.3390/en14123715