Effects of Kaolin Addition on Mechanical Properties for Cemented Coal Gangue-Fly Ash Backfill under Uniaxial Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
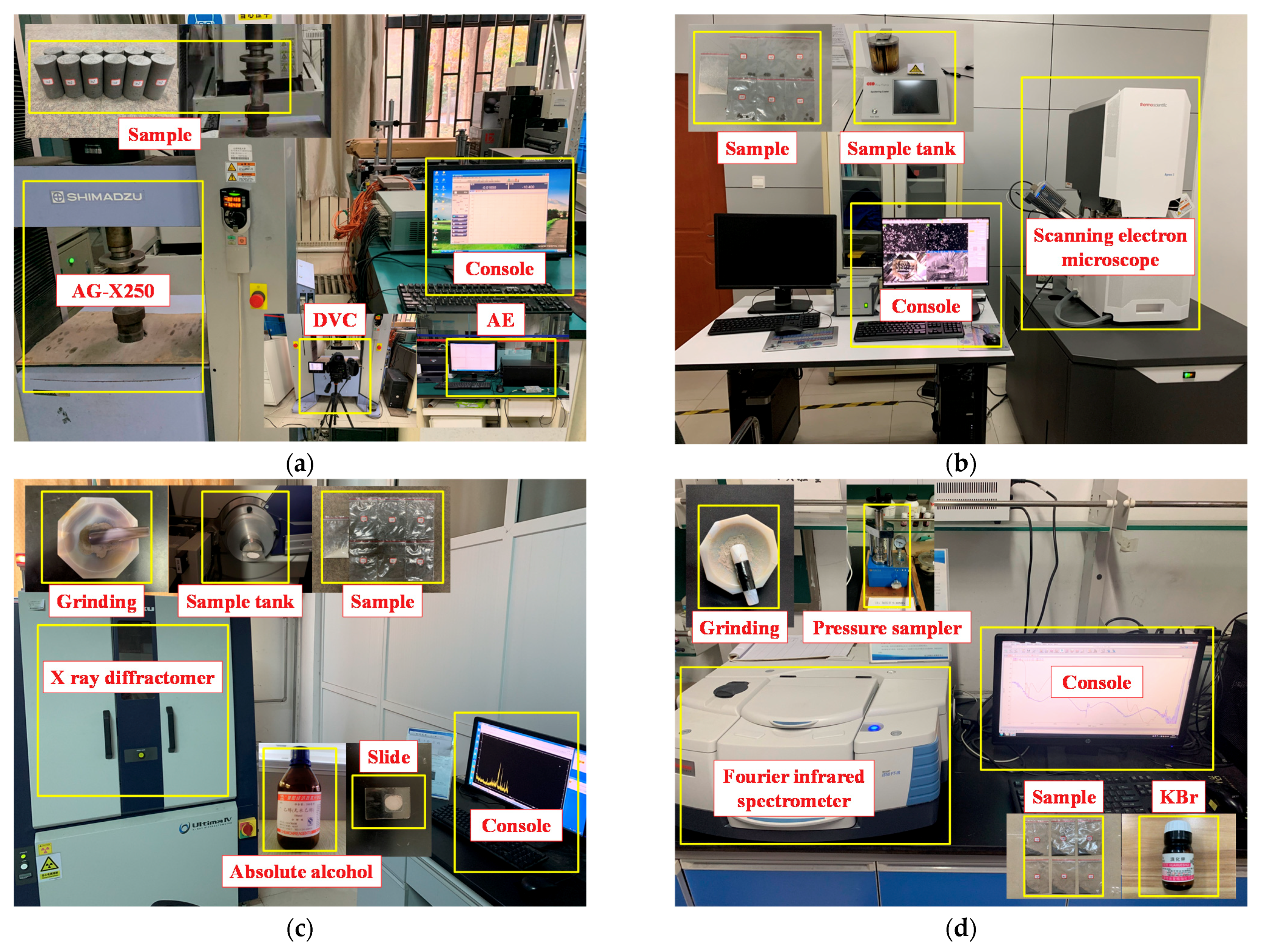
2.3. Test Method
2.3.1. Uniaxial Compression Tests
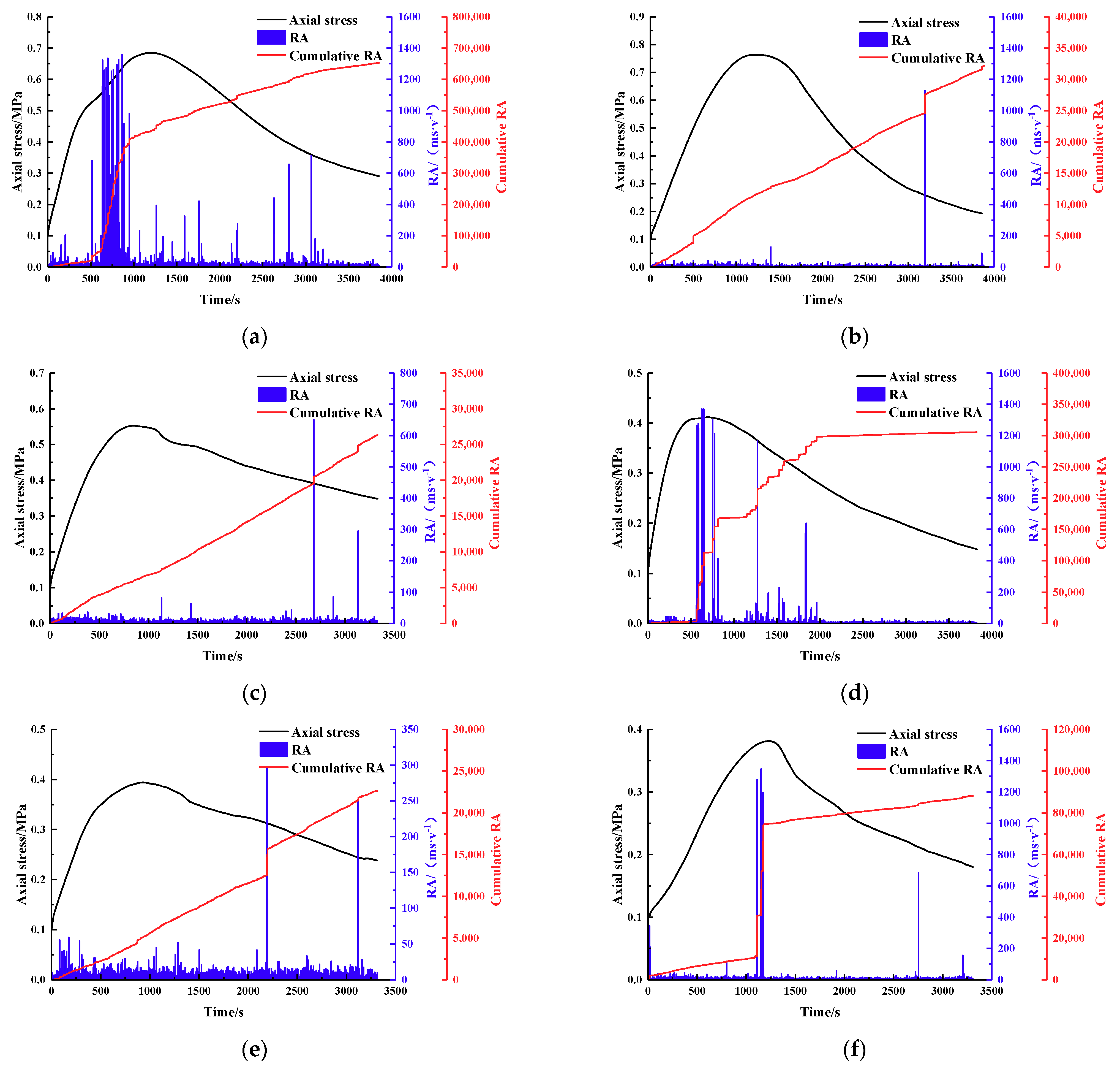
2.3.2. Acoustic Emission Experiment
2.3.3. Scanning Electron Microscopy Experiment
2.3.4. X-ray Diffraction Experiment
2.3.5. Fourier-Transform Infrared Spectroscopy Experiment
3. Results
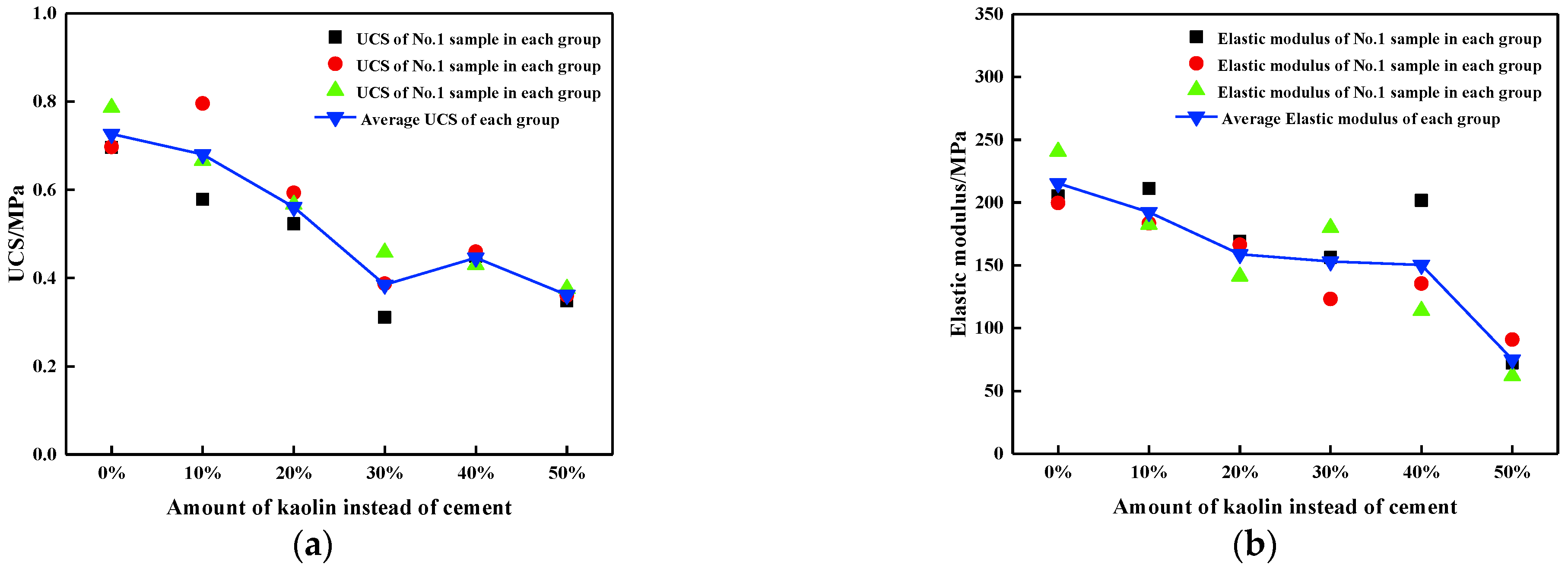
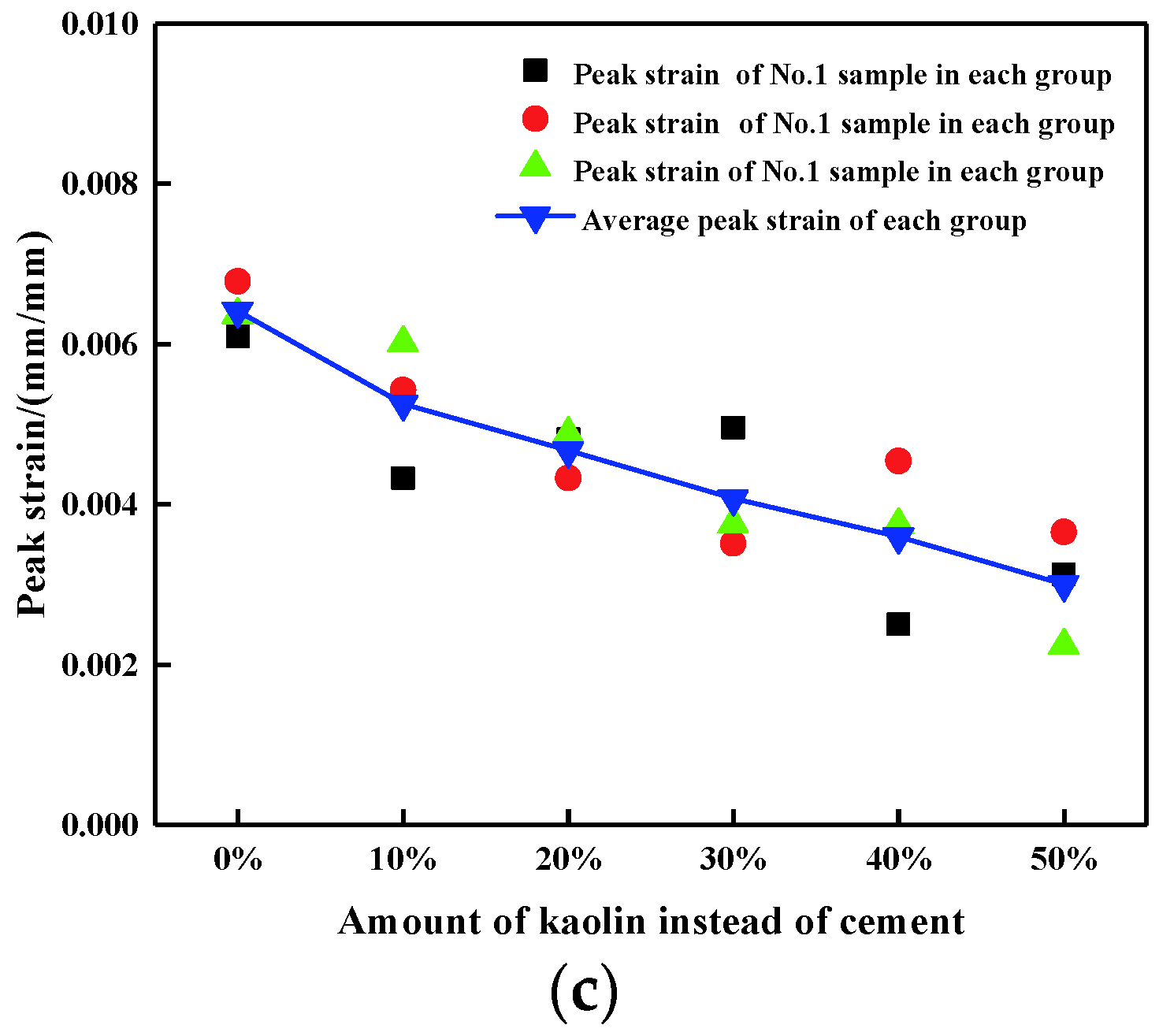
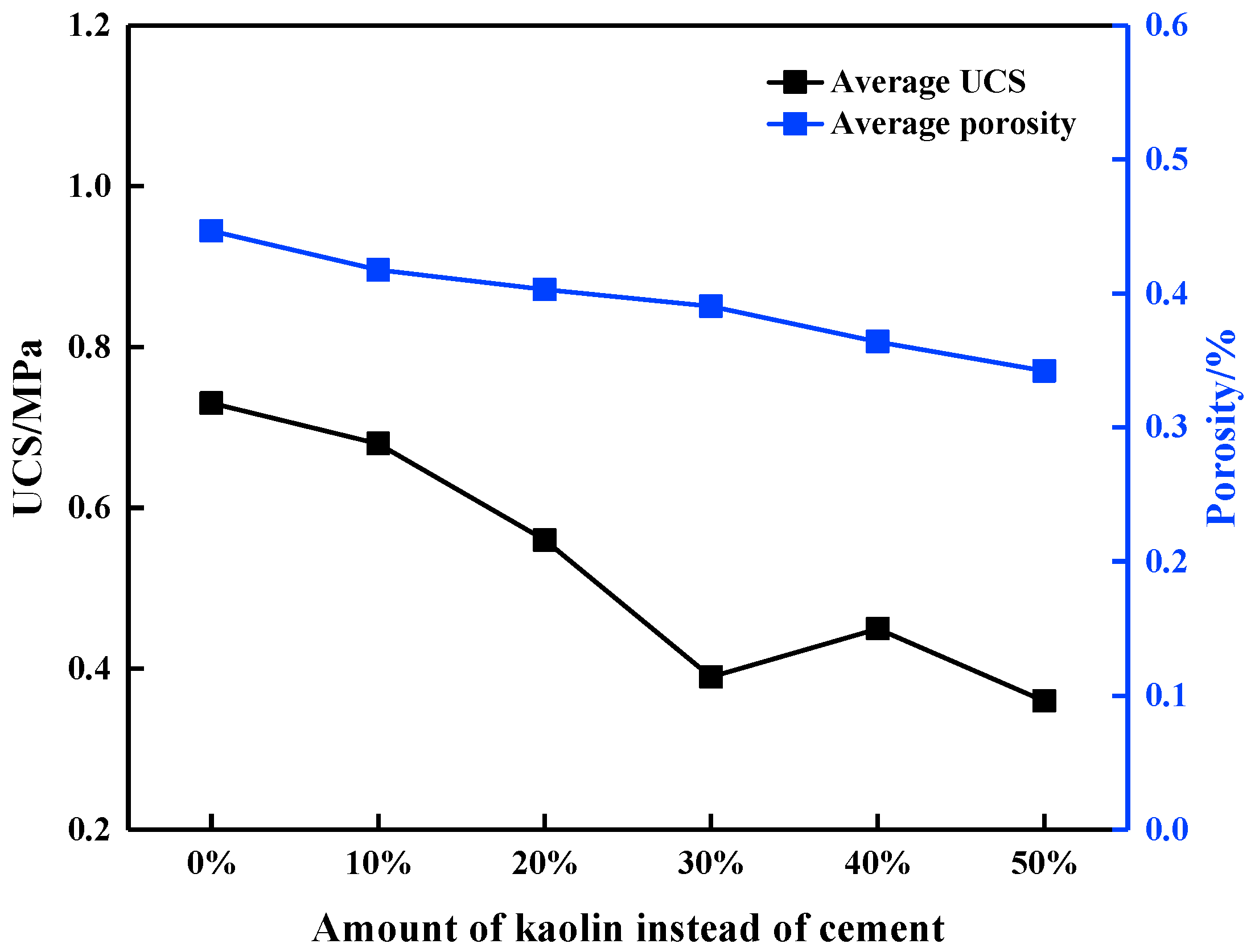
3.1. Uniaxial Compression Test Results
3.2. Macro Failure Characteristics
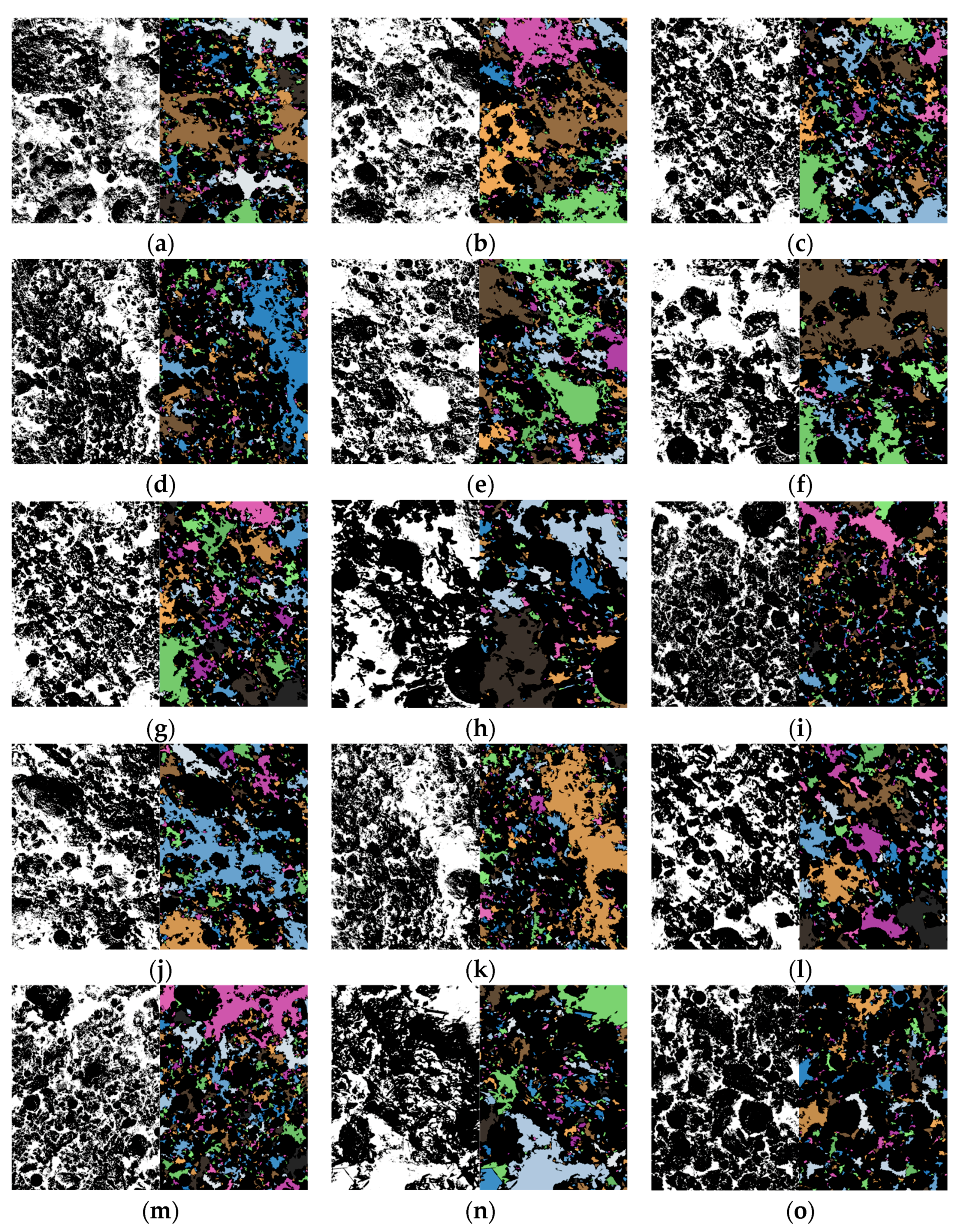
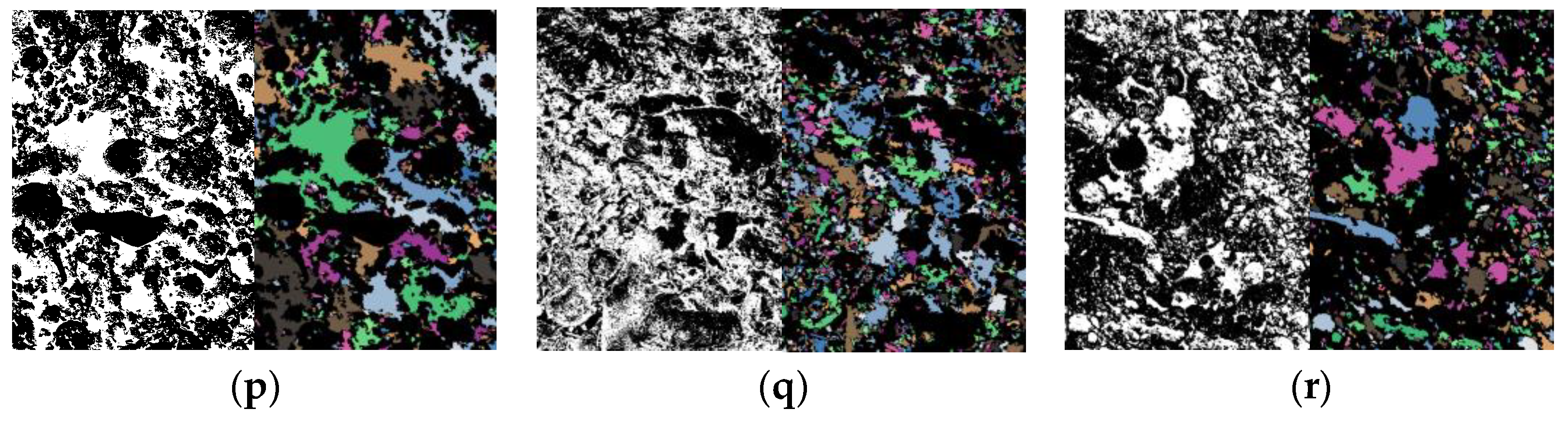
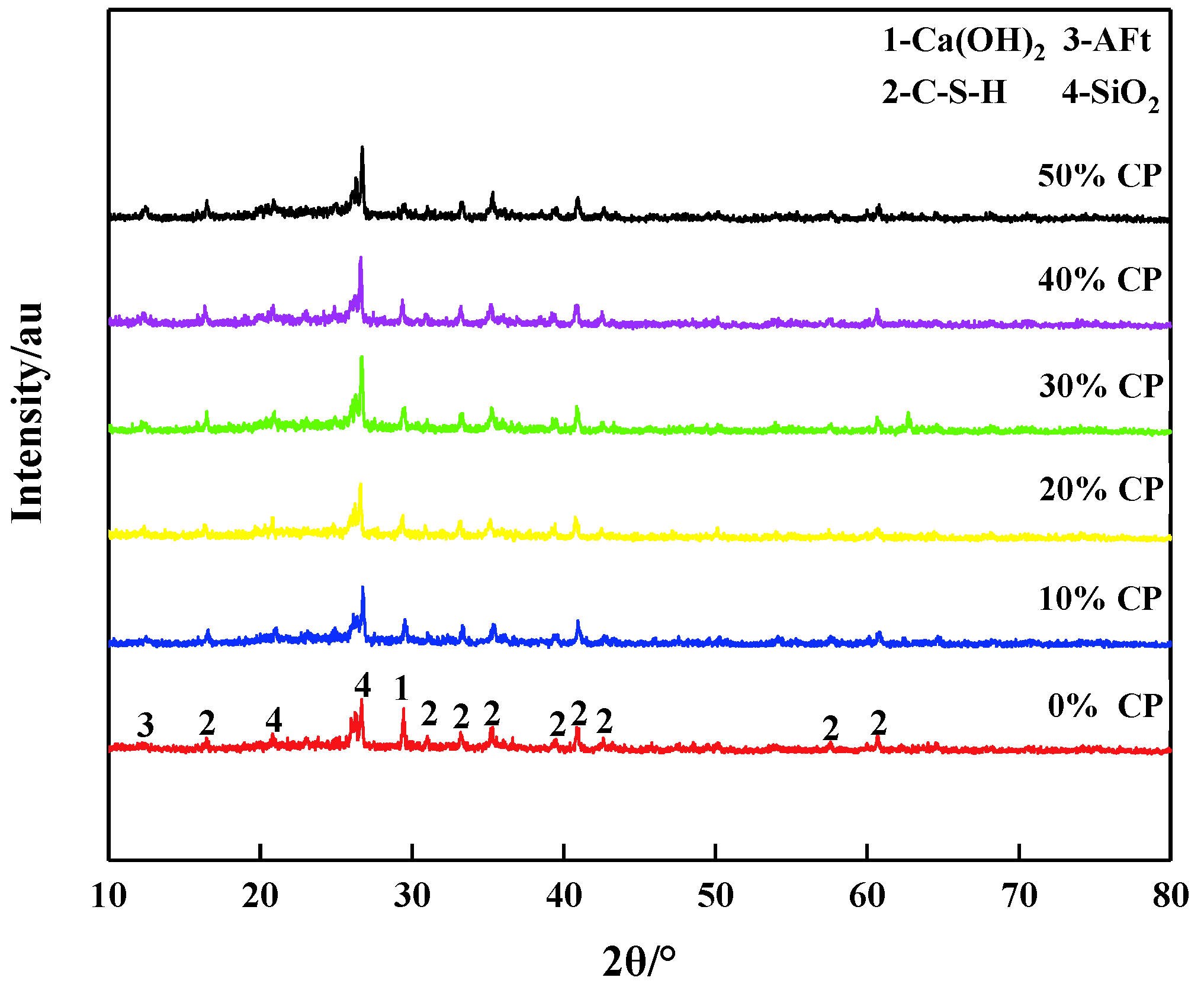
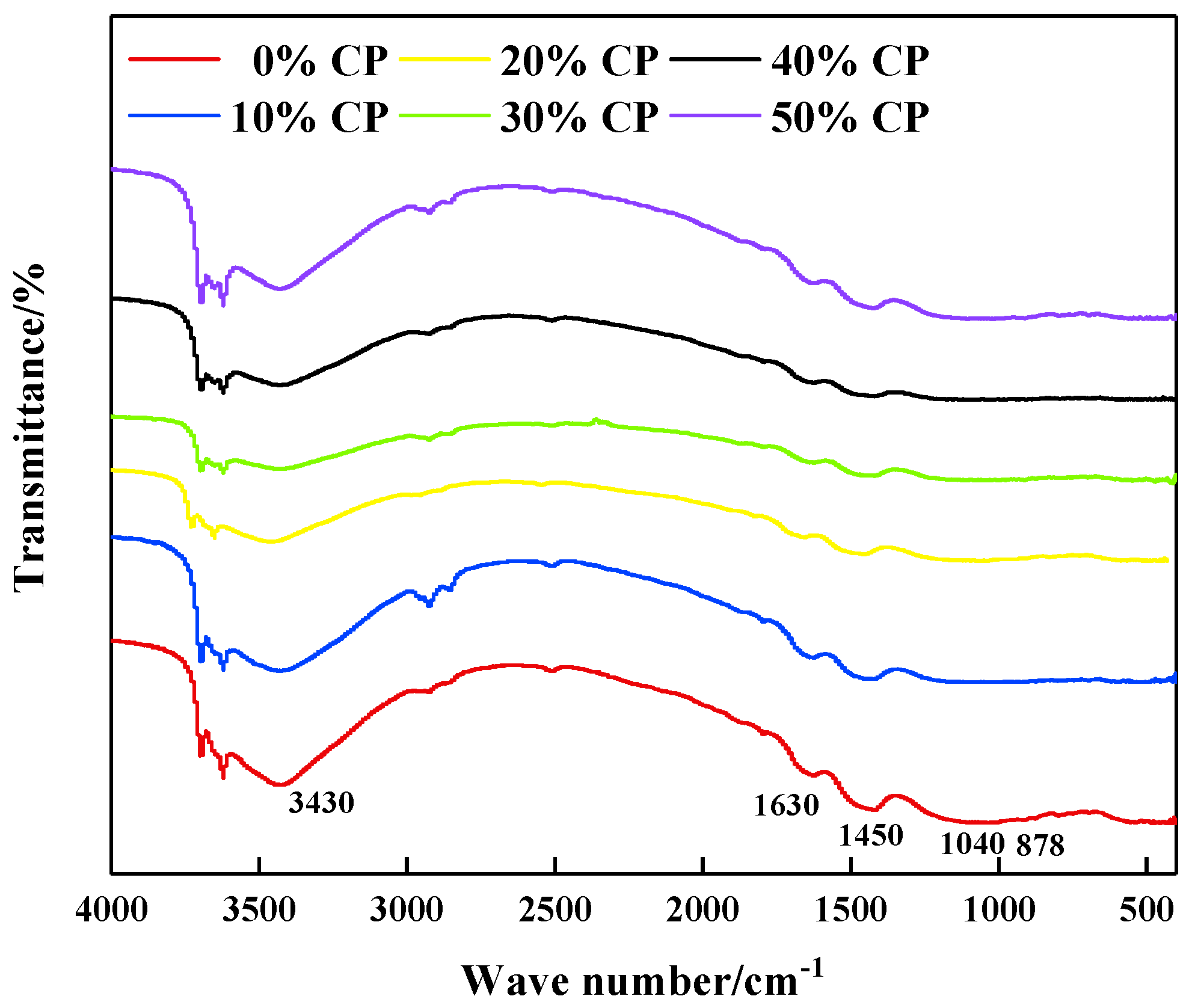
3.3. Microstructure Characteristics
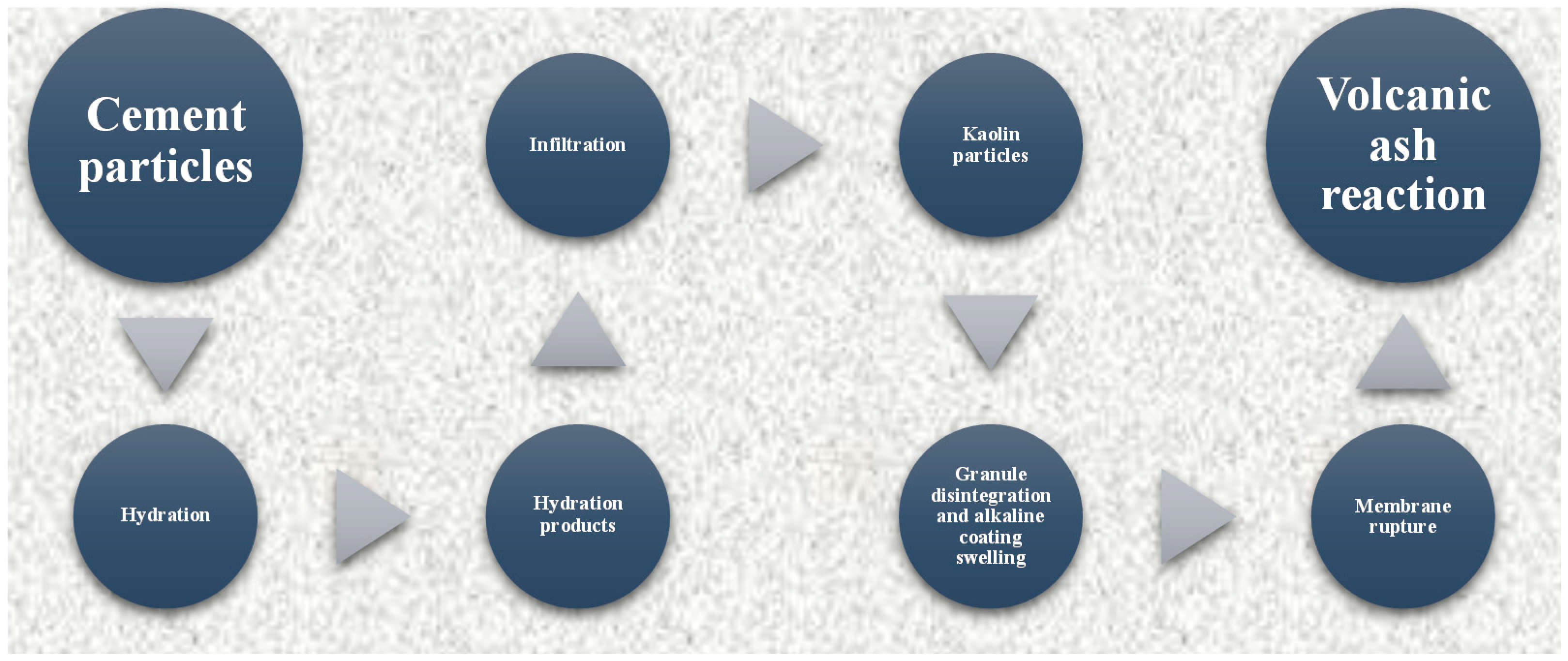
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miao, X.X.; Ju, F.; Huang, Y.L.; Guo, G.L. New progress and prospect of backfill mining theory and technology. J. China Univ. Min. Technol. 2015, 44, 391–399, 429. [Google Scholar]
- Liu, Y.; Guo, Y.L.; Li, H.; Wang, H.Y. Experimental study on the influence of construction waste recycled aggregate on the transportation performance of mine CGFB. J. Shandong Univ. Sci. Technol. Nat. Sci. Ed. 2020, 39, 59–65. [Google Scholar]
- Sun, X.K. Development status and prospect of green filling mining in mines. Coal Sci. Technol. 2020, 48, 48–55. [Google Scholar]
- Liu, J.G.; Li, X.W.; He, T. Application status and development of filling mining in China. J. China Coal Soc. 2020, 45, 141–150. [Google Scholar]
- Mo, Z.Y.; Liu, Y.L.; Wang, D.G.; Li, Z.J.; Bai, L.G. Research progress on mechanical properties of metakaolin cement based materials. Chin. Ceram. Soc. 2018, 37, 911–917. [Google Scholar]
- Cao, Y.D.; Li, Y.X.; Zhang, J.S.; Cao, Z.; Sun, C.B. Effects of fineness and calcination temperature on pozzolanic activity and microstructure of coal gangue. Chin. Ceram. Soc. 2017, 45, 117–122. [Google Scholar]
- Cheng, H.L.; Yang, F.H.; Ma, B.G.; Zhang, J.; Hao, L.W.; Liu, G.Q. Composite activation of high alumina gangue and analysis of its pozzolanic effect. J. Build. Mater. 2016, 19, 248–254. [Google Scholar]
- Lin, C.; Li, J.X.; Chen, P.X.; Chen, G. Study on the influence of metakaolin on the compressive strength of concrete. Guangdong Arch. Civ. Eng. 2019, 26, 70–72. [Google Scholar]
- Hou, L.Y.; Yang, A.R. Study on the influence of mineral admixtures on cement hydration properties. Water Res. Hydropower Eng. 2020, 51, 198–204. [Google Scholar]
- Pang, J.Y.; Chen, X.P. Mechanical properties test of concrete with high active mineral admixture. Chin. Ceram. Soc. 2020, 39, 3143–3151. [Google Scholar]
- Xi, Y.S. Effect of metakaolin admixture on workability and early mechanical properties of cement mortar. China Concr. 2019, 8, 72–76. [Google Scholar]
- Jiang, G.; Rong, Z.D.; Sun, W. Effect of metakaolin on properties of high performance cement mortar. J. Southeast Univ. Nat. Sci. Ed. 2015, 45, 121–125. [Google Scholar]
- Li, F.H.; Zhang, G.B.; Zhou, H.Y.; Zhou, S.; Li, G.H. Inhibitory effect of high activity metakaolin and fly ash on alkali aggregate reaction. J. Build. Mater. 2017, 20, 876–880. [Google Scholar]
- Dong, M.; Ma, J.D.; Li, Y.F. Effect of metakaolin admixture on strength of cement mortar. J. Mater. Sci. Eng. 2020, 38, 295–300. [Google Scholar]
- Liu, Y.Y. Application and Theoretical Study of Coal Series Kaolin Activation and Cement Admixture. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2018. [Google Scholar]
- Wang, H. Process Mineralogy and Beneficiation Test of Sandy Kaolin. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2013. [Google Scholar]
- Yu, W. Mechanism of Metakaolin on Hydration Products of Cement Paste. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2013. [Google Scholar]
- Ebenezer, A.; Emmanuel, N.; Benjamin, A.T.; Stephen, K.A.; George, N.B.; Joanna, A.M.H.; Michael, O.P. Synthesis and Characterization of Modified Kaolin-Bentonite Composites for Enhanced Fluoride Removal from Drinking Water. Adv. Mater. Sci. Eng. 2021, 2021, 6679422. [Google Scholar]
- Chen, M.N.; Chen, X.L.; Zhang, C.Y.; Cui, B.Z.; Li, Z.W.; Zhao, D.Y.; Wang, Z. Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers 2021, 13, 1204. [Google Scholar] [CrossRef]
- Partha, D.; Tadikonda, V.B. Kaolin based protective barrier in municipal landfills against adverse chemo-mechanical loadings. Sci. Rep. 2021, 11, 1–12. [Google Scholar]
- Chen, M.C.; Fang, W.; Xu, K.C. Effect of ceramic admixtures on performance of cement mortar. Chin. Ceram. Soc. 2015, 34, 793–798. [Google Scholar]
- Wang, Z.Y.; Yao, S.Y. Structure and properties of animal bone ash doped Na2O-CaO-SiO2 Wollastonite Glass ceramics. J. Shandong Univ. Sci. Technol. Nat. Sci. Ed. 2020, 39, 48–55. [Google Scholar]
- Zhang, H.W.; Elsworth, D.; Wan, Z.J. Failure response of composite rock-coal samples. Geomech. Geophys. Geo-Energy Geo-Resour. 2018, 4, 175–192. [Google Scholar] [CrossRef]
- Yin, D.W.; Chen, S.J.; Liu, X.Q.; Ma, H.F. Effect of joint angle in coal on failure mechanical behavior of roof rock-coal combined body. Q. J. Eng. Geol. Hydrogeol. 2018, 51, 202–209. [Google Scholar] [CrossRef]
- Yin, D.W.; Chen, S.J.; Chen, B.; Liu, X.Q.; Ma, H. Strength and failure characteristics of the rock-coal combined body with single joint in coal. Geomech. Eng. 2018, 15, 1113–1124. [Google Scholar]
- Lu, Y.W.; Sun, C.P.; Shen, B.T. Experimental study on damage evolution and crack propagation characteristics of sandstone under combined stress. J. Shandong Univ. Sci. Technol. Nat. Sci. Ed. 2020, 39, 37–45. [Google Scholar]
- Wang, Q.; Jiang, Z.H.; Jiang, B.; Gao, H.K.; Huang, Y.B.; Zhang, P. Research on an automatic roadway formation method in deep mining areas by roof cutting with high-strength bolt-grouting. Int. J. Rock Mech. Min. 2020, 128, 104264. [Google Scholar] [CrossRef]
- Yin, S.J.; Chen, S.J.; Ge, Y.; Liu, R. Mechanical properties of rock–coal bi-material samples with different lithologies under uniaxial loading. J. Mater. Res. Technol. 2021, 10, 322–338. [Google Scholar] [CrossRef]
- ASTM E569/E569M-13. Standard Practice for Acoustic Emission Monitoring of Structures during Controlled Stimulation; ASTM International: West Conshohocken, PA, USA, 2013; Available online: www.astm.org (accessed on 10 March 2021).
- Sukpyo, K.; Hyeju, K.; Byoungky, L. Hydration Properties of Cement with Liquefied Red Mud Neutralized by Nitric Acid. Materials 2021, 14, 2641. [Google Scholar] [CrossRef]
- Geuntae, H.; Sangwoo, O.; Seongcheol, C.; Won-Jong, C.; Young-Jin, K.; Chiwon, S. Correlation between the Compressive Strength and Ultrasonic Pulse Velocity of Cement Mortars Blended with Silica Fume: An Analysis of Microstructure and Hydration Kinetics. Materials 2021, 14, 2476. [Google Scholar] [CrossRef]
- Cao, K.; Wang, L.; Xu, Y.; Shen, W.F.; Wang, H. The Hydration and Compressive Strength of Cement Mortar Prepared by Calcium Acetate Solution. Adv. Civ. Eng. 2021, 2021, 8817725. [Google Scholar] [CrossRef]
- Tragazikis, I.K.; Kordatou, T.Z.; Exarchos, D.A.; Dalla, P.T.; Matikas, T.E. Monitoring the Hydration Process in Carbon Nanotube Reinforced Cement-Based Composites Using Nonlinear Elastic Waves. Appl. Sci. 2021, 11, 1720. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, G.J.; Liu, Y.Z. Early Hydration Heat of Calcium Sulfoaluminate Cement with Influences of Supplementary Cementitious Materials and Water to Binder Ratio. Materials 2021, 14, 642. [Google Scholar] [CrossRef]
- Shiotani, T.; Ohtsu, M.; Ikeda, K. Detection and evaluation of AE waves due to rock deformation. Constr. Build. Mater. 2001, 15, 235–246. [Google Scholar] [CrossRef]
- Liu, C.; Shi, B.; Zhou, J.; Tang, C. Qunication and characteriation of microporosity by image processing, geometric measurement and statistical methods: Application on SEM images of clay materials. Appl. Clay Sci. 2011, 54, 97–106. [Google Scholar] [CrossRef]
- Liu, C.; Tang, C.; Shi, B.; Suo, W. Automatic quantification of crack patterns by image processing. Comput. Geosci. 2013, 57, 77–80. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Q.; Shi, B.; Gu, Y.F. Digital image recognition method of rock particle and pore system and its application. Geotech. Eng. 2018, 40, 925–931. [Google Scholar]
- Urfels, S.; Bauer, J.; Vogel, H. General method of specimen preparation for the quantitative microscopic characterization of nano- and microparticles. Mater. Charact. 2019, 153, 60–68. [Google Scholar] [CrossRef]
- Farnama, Y.; Geikerb, M.R.; Bentzc, D.; Weiss, J. Acoustic emission waveform characterization of crack origin and mode in fractured and ASR damaged concrete. Cem. Concr. Compos. 2015, 60, 135–145. [Google Scholar] [CrossRef]
- Zingg, L.; Briffaut, M.; Baroth, J.; Malecot, Y. Influence of cement matrix porosity on the triaxial behaviour of concrete. Cem. Concr. Res. 2016, 80, 52–59. [Google Scholar] [CrossRef]
- Chen, G.; Xu, P.; Mi, G.; Zhu, J. Compressive strength and cracking of composite concrete in hot-humid environments based on microscopic quantitative analysis. Constr. Build. Mater. 2019, 225, 441–451. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhang, N.; Li, H.; Zhao, X.B.; Liu, X.M. Study on hydration characteristics of red mud based paste like filling materials. Chin. J. Chem. Eng. 2017, 39, 1640–1646. [Google Scholar]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Cioffi, R.; Colangelo, F. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of iron rich cements using red mud. Cem. Concr. Res. 1997, 27, 1037–1046. [Google Scholar] [CrossRef]






| Raw Material | Chemical Composition and Content/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Na2O | SO3 | K2O | MgO | TiO2 | MnO | Fe2O3 | ZnO | BaO | |
| Cement | 15.722 | 12.340 | 51.643 | 2.177 | 3.025 | 1.203 | 8.706 | 1.031 | 0.190 | 3.891 | 0.072 | — |
| Fly ash | 37.855 | 39.724 | 6.214 | 1.456 | 1.221 | 2.118 | 3.914 | 2.479 | 0.055 | 4.856 | 0.107 | — |
| Gangue | 39.987 | 30.177 | 11.727 | 1.758 | 2.886 | 2.643 | 3.088 | 2.531 | — | 4.928 | — | 0.276 |
| Kaolin | 42.153 | 47.704 | — | — | — | 5.063 | 1.276 | 2.079 | — | 1.725 | — | — |
| Addition | Number | UCS (MPa) | Elastic Modulus (MPa) | Peak Strain (mm/mm) |
|---|---|---|---|---|
| 0% | A-1 | 0.70 | 205.47 | 0.0061 |
| A-2 | 0.70 | 199.66 | 0.0068 | |
| A-3 | 0.79 | 240.75 | 0.0064 | |
| Average | 0.73 | 215.29 | 0.0064 | |
| 10% | B-1 | 0.58 | 211.20 | 0.0043 |
| B-2 | 0.80 | 183.47 | 0.0054 | |
| B-3 | 0.67 | 182.44 | 0.0060 | |
| Average | 0.68 | 192.37 | 0.0053 | |
| 20% | C-1 | 0.52 | 169.18 | 0.0048 |
| C-2 | 0.59 | 166.39 | 0.0043 | |
| C-3 | 0.57 | 141.19 | 0.0049 | |
| Average | 0.56 | 158.92 | 0.0047 | |
| 30% | D-1 | 0.31 | 156.33 | 0.0050 |
| D-2 | 0.39 | 123.17 | 0.0035 | |
| D-3 | 0.46 | 179.88 | 0.0038 | |
| Average | 0.39 | 153.13 | 0.0041 | |
| 40% | E-1 | 0.45 | 201.66 | 0.0025 |
| E-2 | 0.46 | 135.53 | 0.0045 | |
| E-3 | 0.43 | 113.93 | 0.0037 | |
| Average | 0.45 | 150.37 | 0.0036 | |
| 50% | F-1 | 0.35 | 72.36 | 0.0031 |
| F-2 | 0.36 | 90.85 | 0.0037 | |
| F-3 | 0.38 | 61.91 | 0.0022 | |
| Average | 0.36 | 75.04 | 0.0030 |
| Addition | Number | Region Percentage (Porosity) |
|---|---|---|
| 0% | A-1 | 43.26% |
| A-2 | 49.45% | |
| A-3 | 41.26% | |
| Average | 44.66% | |
| 10% | B-1 | 33.52% |
| B-2 | 45.21% | |
| B-3 | 46.56% | |
| Average | 41.76% | |
| 20% | C-1 | 41.27% |
| C-2 | 39.64% | |
| C-3 | 39.94% | |
| Average | 40.28% | |
| 30% | D-1 | 39.08% |
| D-2 | 39.54% | |
| D-3 | 38.47% | |
| Average | 39.03% | |
| 40% | E-1 | 37.08% |
| E-2 | 36.04% | |
| E-3 | 36.05% | |
| Average | 36.39% | |
| 50% | F-1 | 35.07% |
| F-2 | 34.84% | |
| F-3 | 32.77% | |
| Average | 34.23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yin, D.; Zhu, C.; Wang, F.; Jiang, N.; Zhang, Z. Effects of Kaolin Addition on Mechanical Properties for Cemented Coal Gangue-Fly Ash Backfill under Uniaxial Loading. Energies 2021, 14, 3693. https://doi.org/10.3390/en14123693
Li F, Yin D, Zhu C, Wang F, Jiang N, Zhang Z. Effects of Kaolin Addition on Mechanical Properties for Cemented Coal Gangue-Fly Ash Backfill under Uniaxial Loading. Energies. 2021; 14(12):3693. https://doi.org/10.3390/en14123693
Chicago/Turabian StyleLi, Faxin, Dawei Yin, Chun Zhu, Feng Wang, Ning Jiang, and Zhen Zhang. 2021. "Effects of Kaolin Addition on Mechanical Properties for Cemented Coal Gangue-Fly Ash Backfill under Uniaxial Loading" Energies 14, no. 12: 3693. https://doi.org/10.3390/en14123693
APA StyleLi, F., Yin, D., Zhu, C., Wang, F., Jiang, N., & Zhang, Z. (2021). Effects of Kaolin Addition on Mechanical Properties for Cemented Coal Gangue-Fly Ash Backfill under Uniaxial Loading. Energies, 14(12), 3693. https://doi.org/10.3390/en14123693








