Non-Equilibrium Thermodynamics-Based Convective Drying Model Applied to Oblate Spheroidal Porous Bodies: A Finite-Volume Analysis
Abstract
1. Introduction
2. Methodology
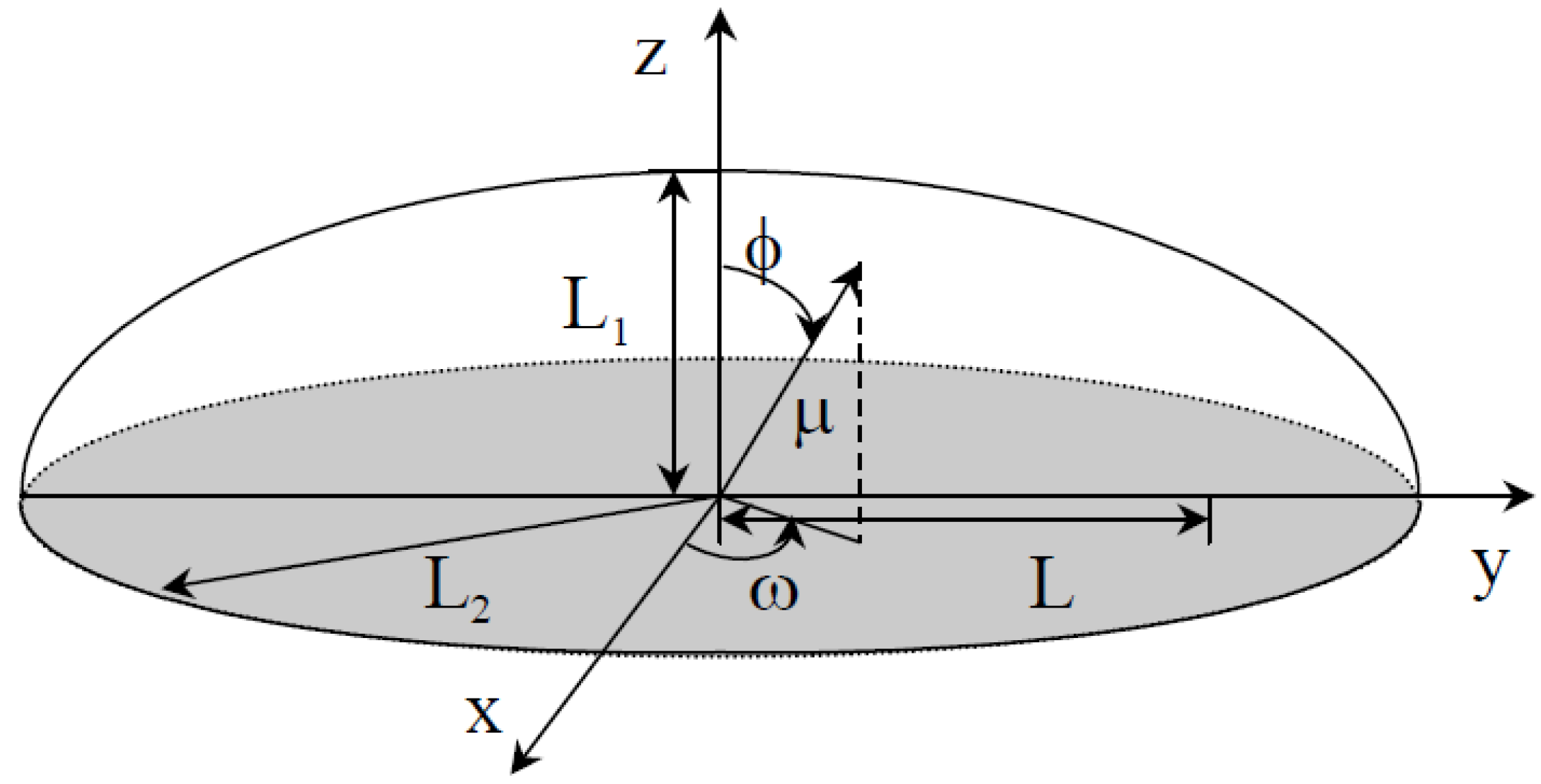
2.1. Mathematical Modeling
- (a)
- The solid is homogeneous and isotropic;
- (b)
- Mass transfer in the single particle occurs by diffusion of liquid and vapor, under decreasing drying rate;
- (c)
- At the beginning of the drying process, the distributions of the moisture and temperature content are considered uniform and symmetrical around the z-axis;
- (d)
- The thermophysical properties are variable during the drying process and dependent on the position and moisture content inside the material;
- (e)
- Volume shrinkage negligible;
- (f)
- No capillarity effect;
- (g)
- Moisture transfer inside the solid by liquid and vapor diffusion, and evaporation and convection on a solid surface;
- (h)
- Heat transfer inside the solid by conduction, evaporation, and convection on a solid surface.
- (a)
- Mass
- Initial:M(ξ, η, t = 0) = Mo.
- Symmetry planes: In mass transfer the angular and radial gradients of the moisture content are equal to zero in the symmetry planes.
- Free surface: The diffusive flux is equal to the convective flux of the moisture content on the surface of the oblate spheroid.
- (b)
- Heat
- InitialT(ξ,η, t = 0) = To = cte.
- Symmetry plane: Heat angular and radial gradients are equal to zero.
- Free surface: The diffusive flux is equal to the heat convective flux on the surface of the solid, more the energy to evaporate the water and the energy to heat the water vapor produced in the evaporation process. Then we can write:
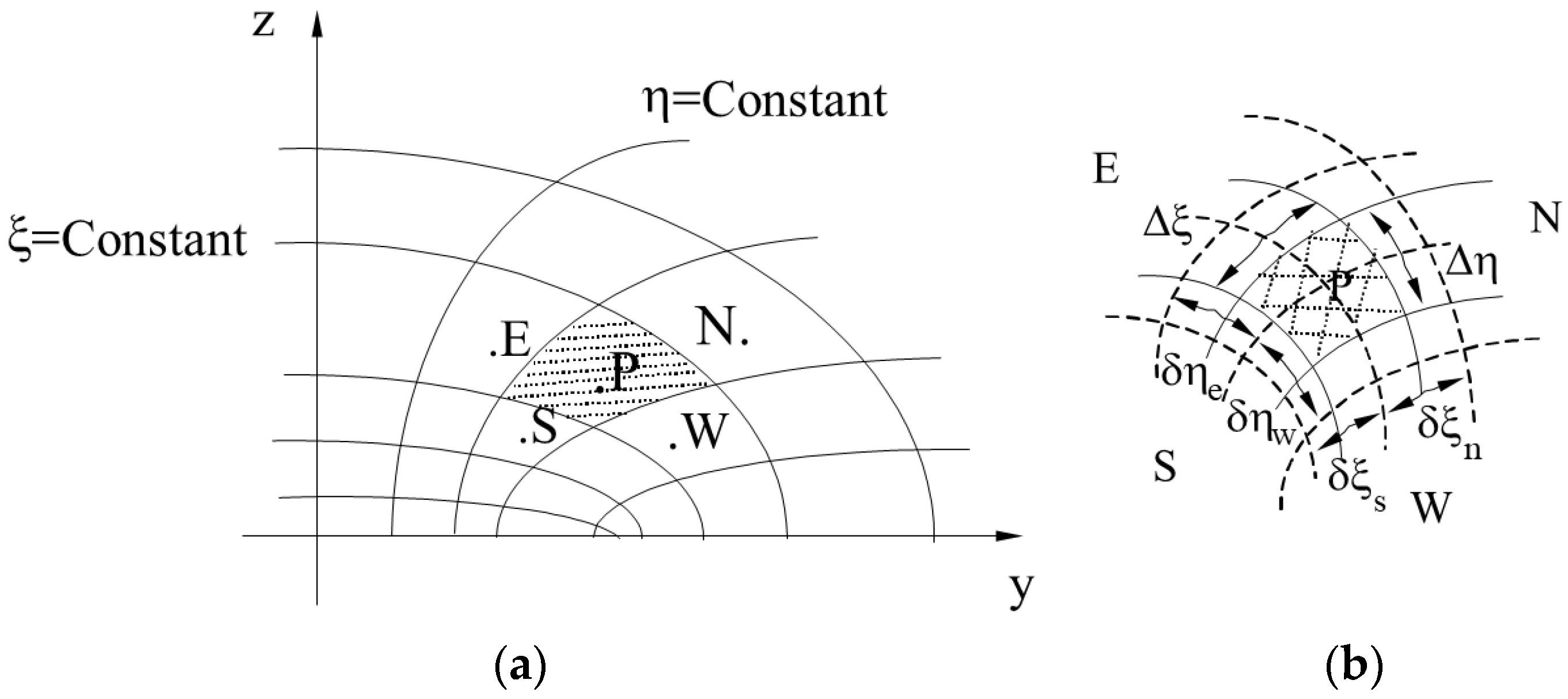
2.2. Numerical Solution of the Governing Equations
- Mass:
- Heat:where and are given in Appendix A. In this equation, Φ” is the flux of Φ per unit of the area obtained from the boundary condition imposed on the physical problem.
- Mass
- Heat
2.3. Application to Lentil Grain Drying
- Lentil grain dimension [5]: L1 = 1.4 × 10−3 m and L2 = 3.4 × 10−3 m
3. Results and Discussion
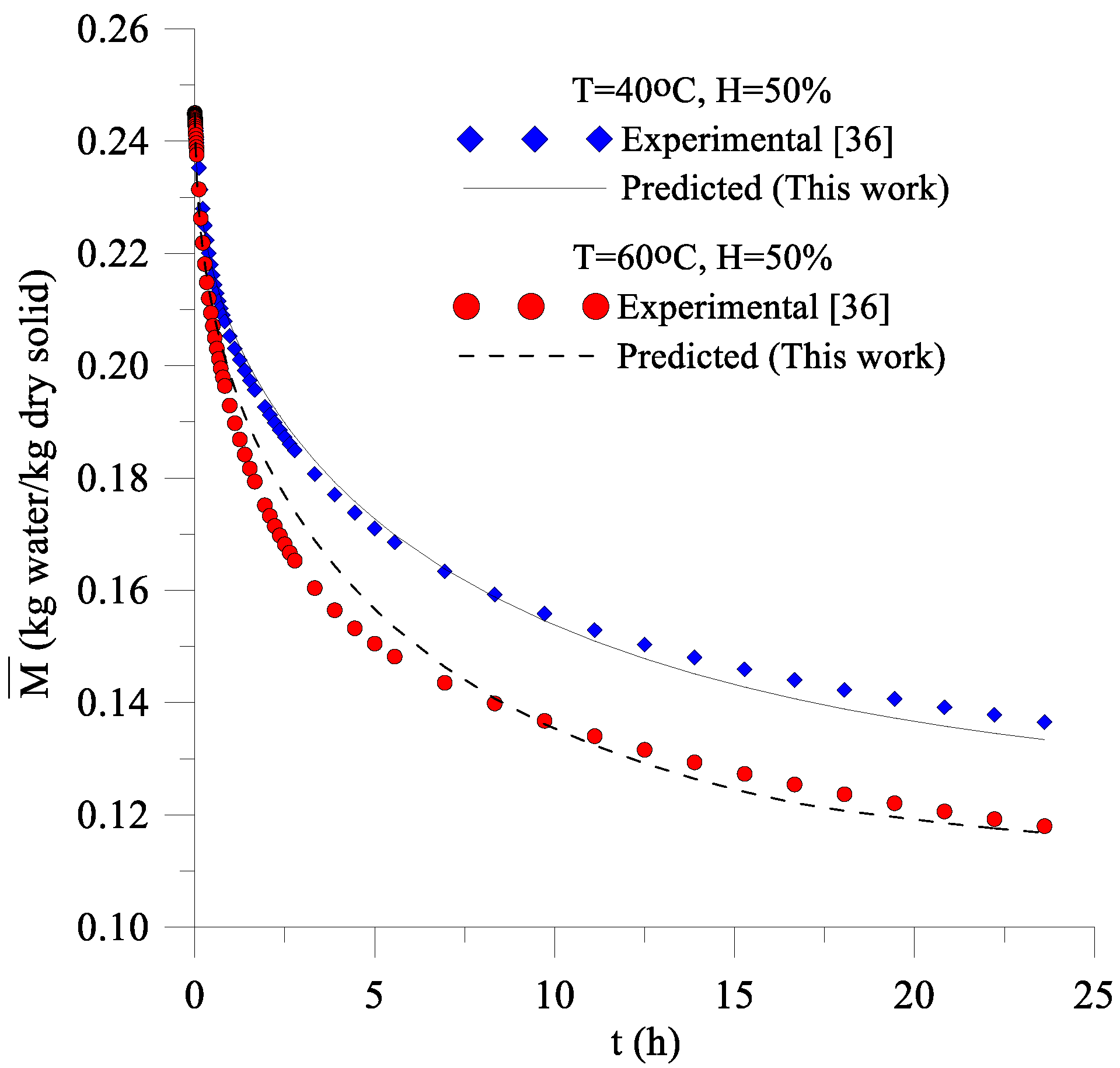
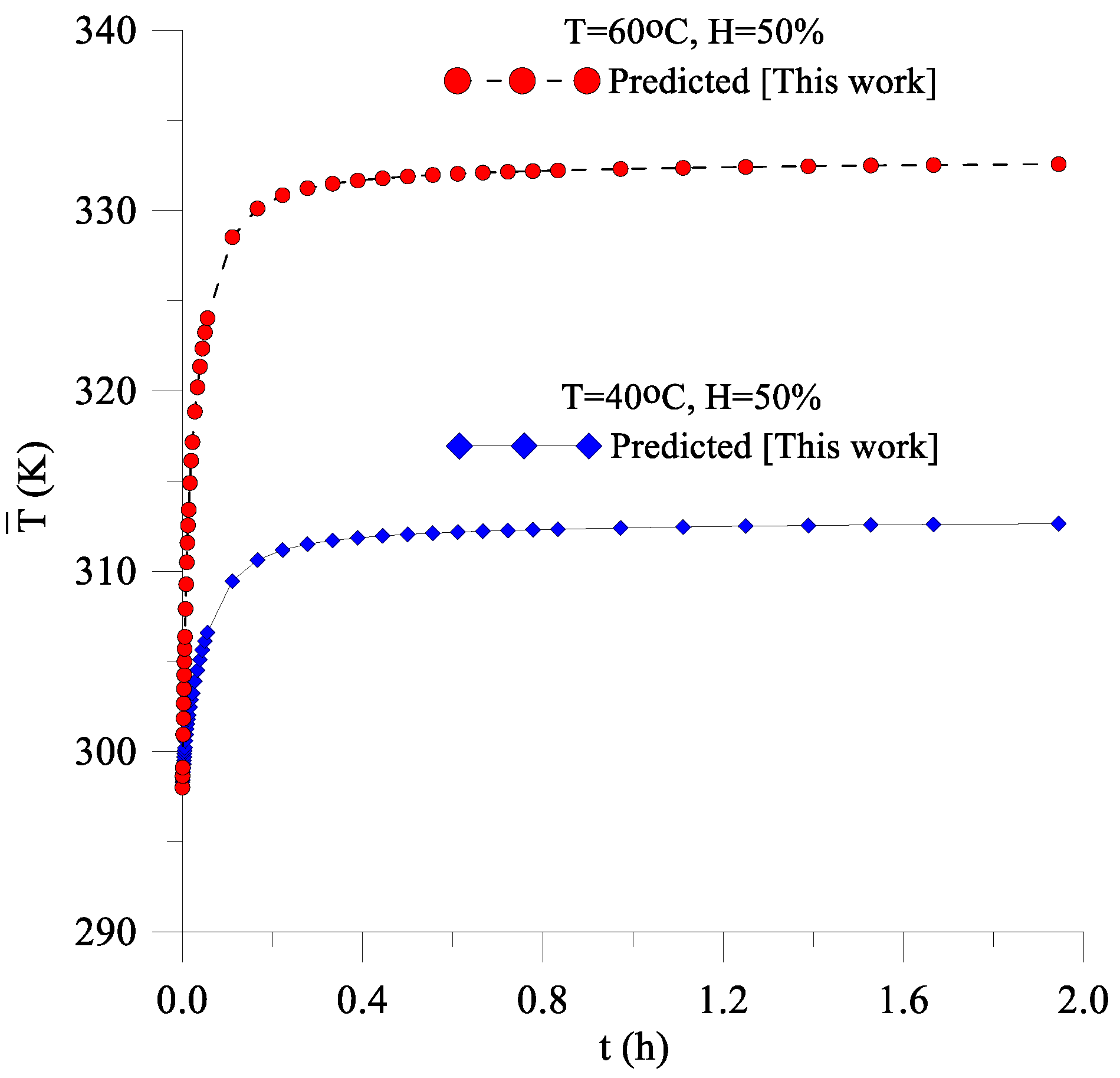
3.1. Drying and Heating Kinetics
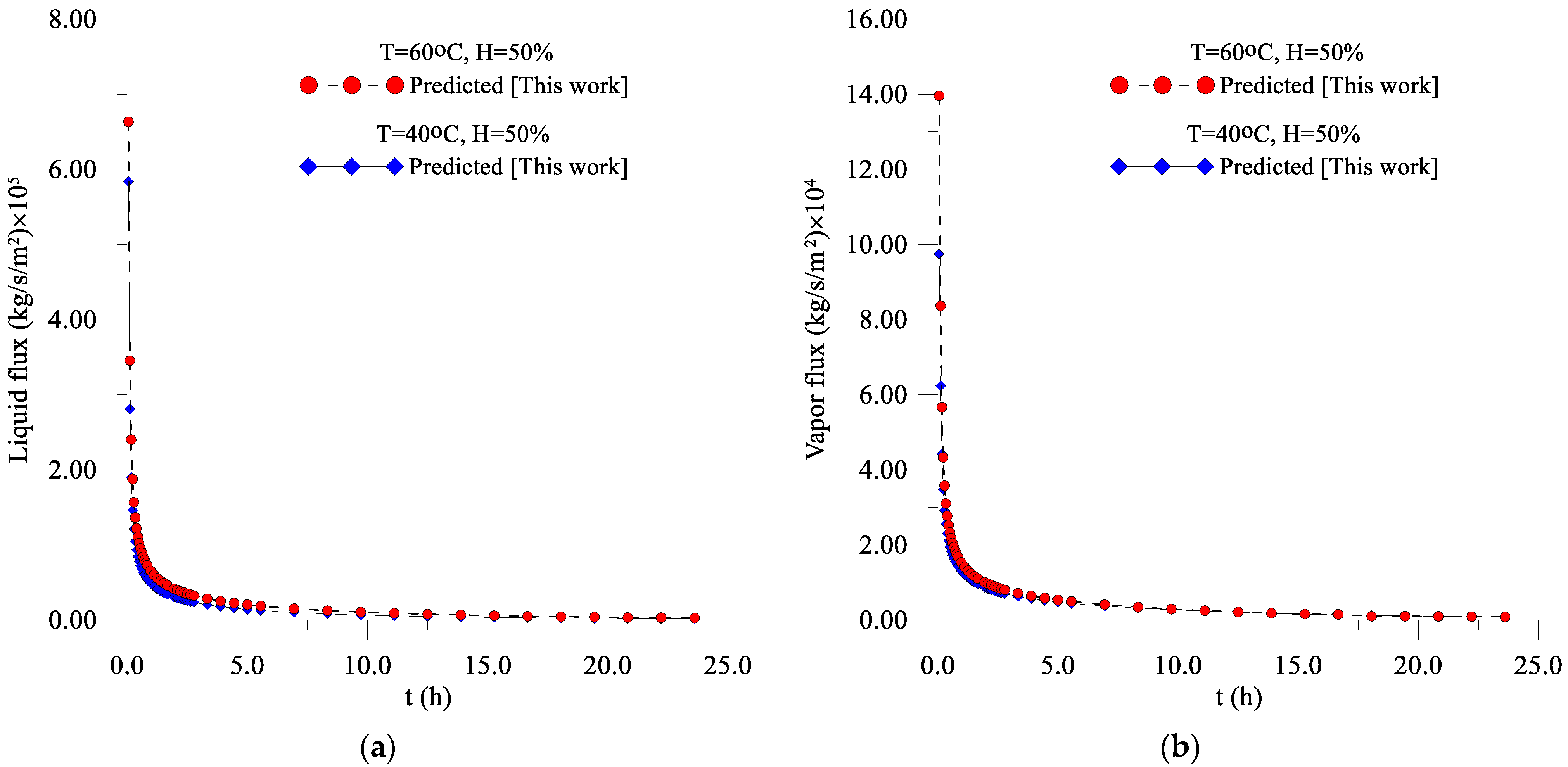
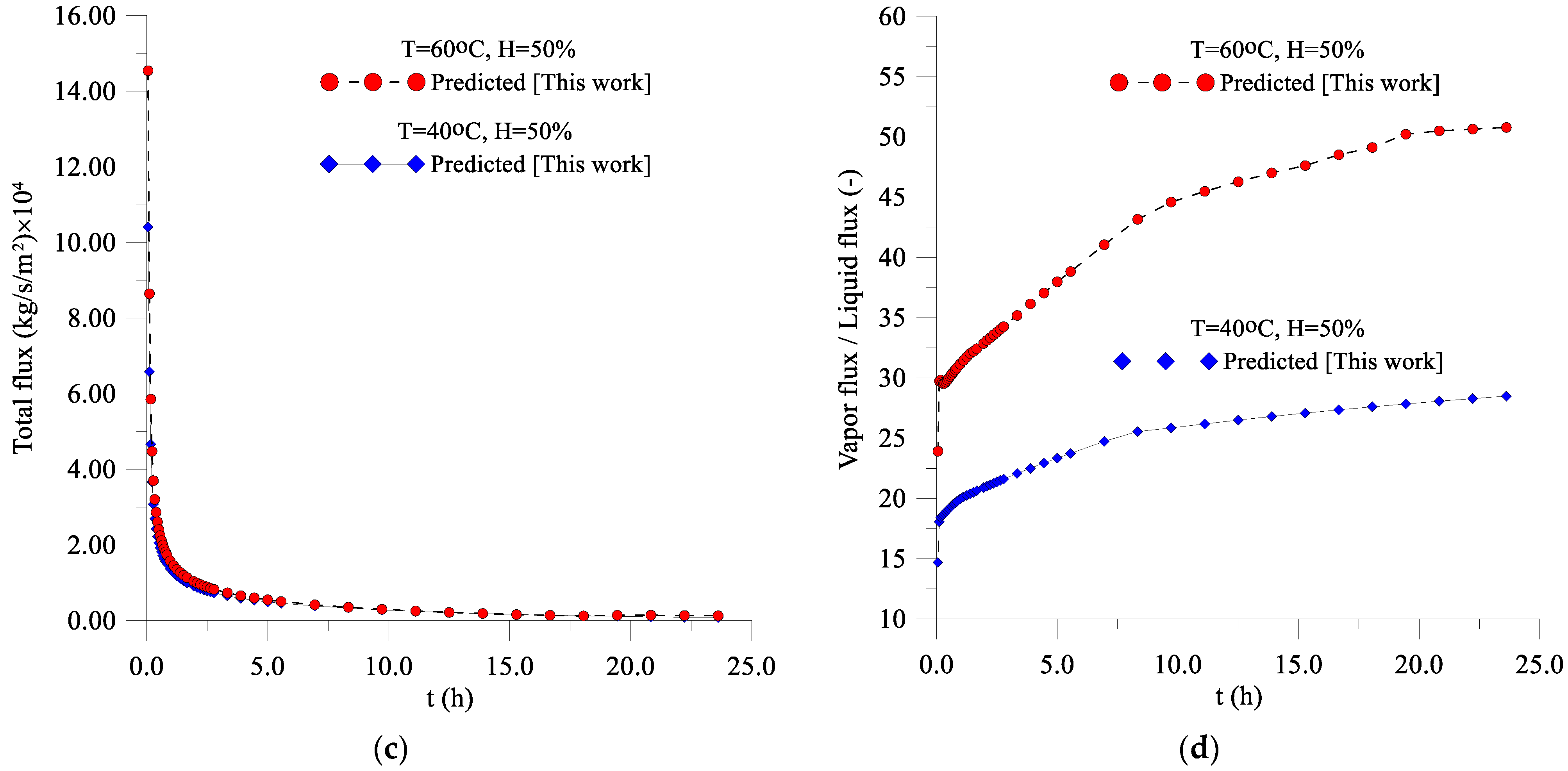
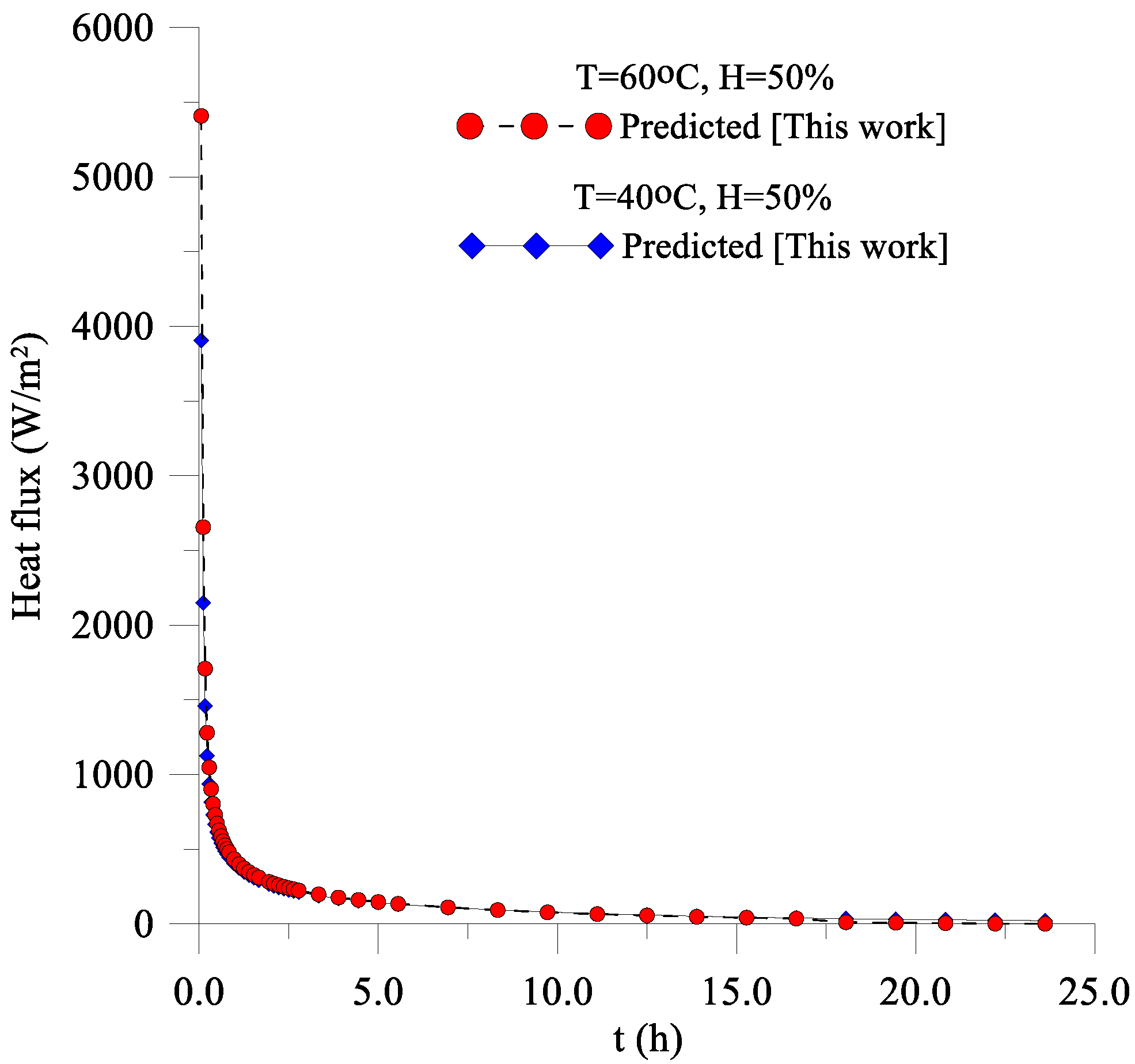
3.2. Fluxes of Liquid, Water Vapor, and Heat
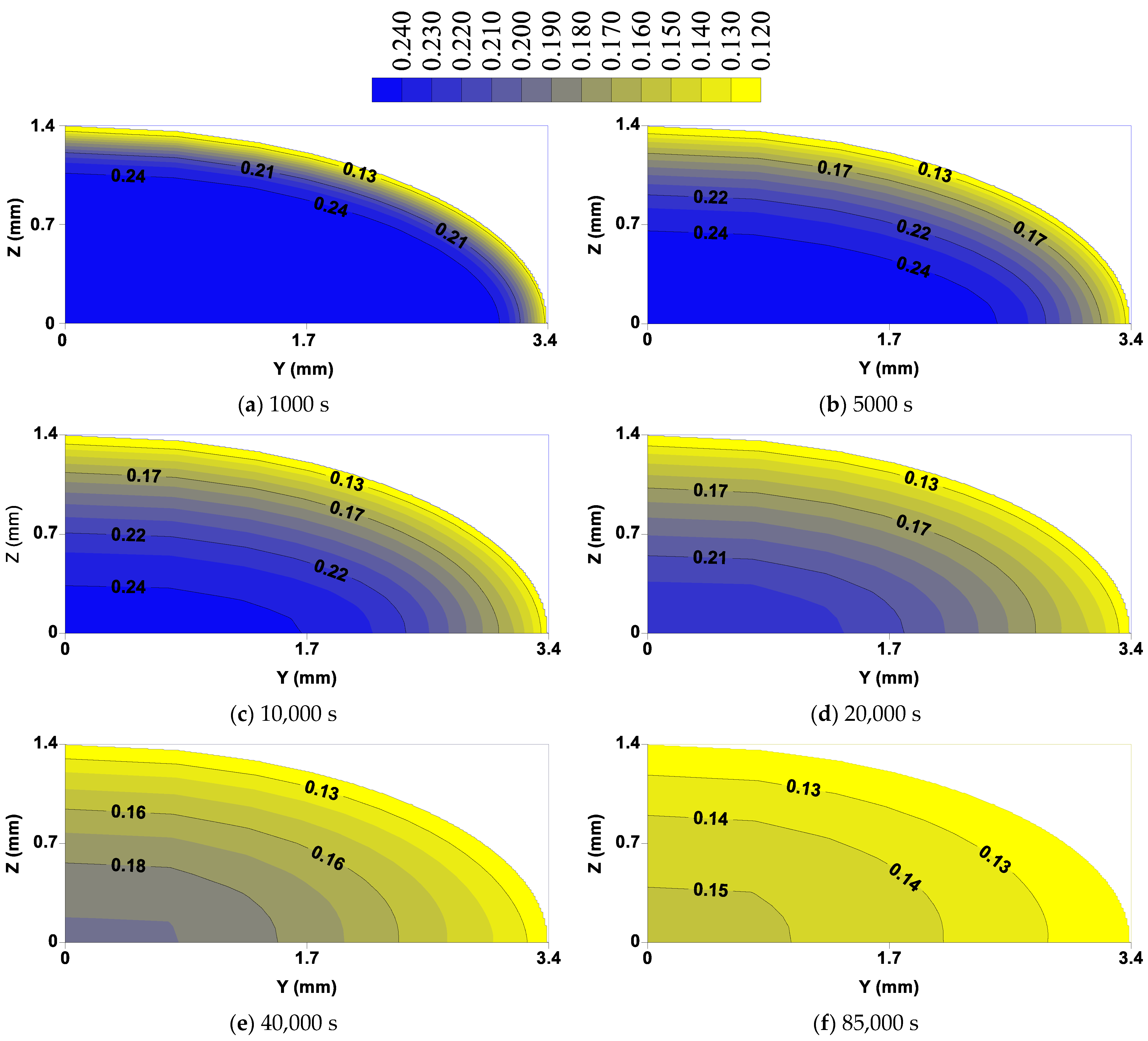
3.3. Distribution of Moisture Content and Temperature Inside the Lentil Grain
3.4. Transport Coefficients Evaluation (kl, kv, and hm)
4. Conclusions
- (a)
- The mathematical model proposed to predict transient diffusion in oblate spheroidal solids, and its numerical solution using the finite-volumes method with the convective condition on the surface was adequate since the predicted values of the average moisture content of the lentil grain along the process presented low deviation and variance when compared with the data of average moisture content obtained experimentally;
- (b)
- Inside the lentil grain, the dominant mass transfer mechanism is the vapor flux, on the surface of the solid, with a vapor flux/liquid flux ratio greater than 14.7 for T = 40 °C, and greater than 23.9 for T = 60 °C, growing with the drying time, especially at T = 60 °C;
- (c)
- The areas of lentil grain more susceptible to cracks are located on the surface and around the focal point due to the existence of higher thermal and hydric stresses originated by higher moisture and temperature gradients;
- (d)
- The liquid conductivity increases with the increase in the moisture content and decreases with the increase in temperature, while the vapor conductivity increases with the decrease in the moisture content and decreases with the increase in temperature due to the behavior of the saturation pressure in the pore inside the porous material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Silva, W.P.; Silva, C.D.P.S.; Lima, A.G.B. Uncertainty in the determination of equilibrium moisture content of agricultural products. Rev. Bras. Prod. Agroind. Gd. Espec. 2005, 7, 159–164. (In Portuguese) [Google Scholar]
- Park, K.J.; Antonio, G.C.; Oliveira, R.A.; Park, K.J.B. Process selection and drying equipments. In Brazilian Congress of Agricultural Engineering; 2006; Volume 35. SBEA/UFCG, Jaboticabal, Brazil (In Portuguese) [Google Scholar]
- Fellows, P.J. Food Processing Technology: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2009; ISBN 1845696344. [Google Scholar]
- Park, K.J.; Yado, M.K.M.; Brod, F.P.R. Drying studies of sliced pear bartlett (Pyrus sp.) sliced. Food Sci. Technol. 2001, 21, 288–292. (In Portuguese) [Google Scholar] [CrossRef][Green Version]
- Tang, J.; Sokhansanj, S. Moisture diffusivity in laird lentil seed components. Trans. ASAE 1993, 36, 1791–1798. [Google Scholar] [CrossRef]
- Tang, J.; Sokhansanj, S. Geometric changes in lentil seeds caused by drying. J. Agric. Eng. Res. 1993, 56, 313–326. [Google Scholar] [CrossRef]
- Wang, J.; Mujumdar, A.S.; Mu, W.; Feng, J.; Zhang, X.; Zhang, Q.; Fang, X.-M.; Gao, Z.-J.; Xiao, H.-W. Grape Drying: Current Status and Future Trends. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Hatamipour, M.S.; Mowla, D. Shrinkage of carrots during drying in an inert medium fluidized bed. J. Food Eng. 2002, 55, 247–252. [Google Scholar] [CrossRef]
- Karim, M.A.; Hawlader, M.N.A. Drying characteristics of banana: Theoretical modelling and experimental validation. J. Food Eng. 2005, 70, 35–45. [Google Scholar] [CrossRef]
- Li, C.; Li, B.; Huang, J.; Li, C. Energy and exergy analyses of a combined infrared radiation-counterflow circulation (IRCC) corn dryer. Appl. Sci. 2020, 10, 6289. [Google Scholar] [CrossRef]
- Carvalho, E.R.; Francischini, V.M.; Avelar, S.A.G.; Costa, J.C. Temperatures and periods of drying delay and quality of corn seeds harvested on the ears. J. Seed Sci. 2019, 41, 336–343. [Google Scholar] [CrossRef]
- Arora, S.; Bharti, S.; Sehgal, V.K. Convective drying kinetics of red chillies. Dry. Technol. 2006, 24, 189–193. [Google Scholar] [CrossRef]
- Gonelli, A.L.D.; Corrêa, P.C.; Resende, O.; dos Reis Neto, S.A. Study of moisture diffusion in wheat grain drying. Food Sci. Technol. 2007, 27, 135–140. (In Portuguese) [Google Scholar] [CrossRef][Green Version]
- Resende, O.; Corrêa, P.C.; Goneli, A.L.D.; Ribeiro, D.M. Physical properties of edible bean during drying: Determination and modelling. Ciência Agrotecnologia 2008, 32, 225–230. (In Portuguese) [Google Scholar] [CrossRef][Green Version]
- Doymaz, İ. Convective drying kinetics of strawberry. Chem. Eng. Process. Process. Intensif. 2008, 47, 914–919. [Google Scholar] [CrossRef]
- Kajiyama, T.; Park, K.J. Influence of feed initial moisture content on spray drying time. Rev. Bras. Prod. Agroind. 2008, 10, 1–8. (In Portuguese) [Google Scholar]
- Almeida, D.P.; Resende, O.; Costa, L.M.; Mendes, U.C.; de Fátima Sales, J. Drying kinetics of adzuki bean (Vigna angularis). Glob. Sci. Technol. 2009, 2, 72–83. (In Portuguese) [Google Scholar]
- Lima, W.M.P.B.; Lima, E.S.; Lima, A.R.C.; Oliveira Neto, G.L.; Oliveira, N.G.N.; Farias Neto, S.R.; Lima, A.G.B. Applying phenomenological lumped models in drying process of hollow ceramic materials. Defect Diffus. Forum 2020, 400, 135–145. [Google Scholar] [CrossRef]
- Fortes, M.; Okos, M.R. A non-equilibrium thermodynamics approach to transport phenomena in capillary porous media. Trans. ASAE 1981, 24, 756–760. [Google Scholar] [CrossRef]
- Luikov, A.V. Heat and Mass Transfer in Capillary-Porous Bodies; Pergamon Press: New York, NY, USA, 1966; ISBN 0080108326. [Google Scholar]
- Luikov, A.V. Systems of differential equations of heat and mass transfer in capillary-porous bodies. Int. J. Heat Mass Transf. 1975, 18, 1–14. [Google Scholar] [CrossRef]
- Fortes, M. A Non-Equilibrium Thermodynamics Approach to Transport Phenomena in Capillary Porous Media with Special Reference to Drying of Grains and Foods. Ph.D. Thesis, Perdue University, West Lafayette, IN, USA, 1978. [Google Scholar]
- Fortes, M.; Okos, M.R. Drying theories: Their bases and limitations as applied to foods and grains. In Advances in Drying; Hemisphere Publishing Corporation: Washington, DC, USA, 1980; Volume 1, pp. 119–154. [Google Scholar]
- de Oliveira, V.A.B.; Lima, A.G.B. de Drying of wheat based on the non-equilibrium thermodynamics: A numerical study. Dry. Technol. 2009, 27, 306–313. [Google Scholar] [CrossRef]
- Oliveira, V.A.B.; Lima, W.; Farias Neto, S.R.; Lima, A.G.B. Heat and mass diffusion and shrinkage in prolate spheroidal bodies based on non-equilibrium thermodynamics: A numerical investigation. J. Porous Media 2011, 14, 593–605. [Google Scholar] [CrossRef]
- Oliveira, V.A.B.; Lima, A.G.B.; Silva, C.J. Drying of wheat: A numerical study based on the non-equilibrium thermodynamics. Int. J. Food Eng. 2012, 8, 8. [Google Scholar] [CrossRef]
- Carmo, J.E.F.; Lima, A.G.B. Mass transfer inside oblate spheroidal solids: Modelling and simulation. Braz. J. Chem. Eng. 2008, 25, 19–26. [Google Scholar] [CrossRef]
- Melo, J.C.S.; Lima, A.G.B.; Pereira Silva, W.; Lima, W.M.P. Heat and Mass Transfer during Drying of Lentil Based on the Non-Equilibrium Thermodynamics: A Numerical Study. Defect Diffus. Forum 2015, 365, 285–290. [Google Scholar] [CrossRef]
- Melo, J.C.S.; Gomez, R.S.; Silva, J.B., Jr.; Queiroga, A.X.; Dantas, R.L.; Lima, A.G.B.; Silva, W.P. Drying of Oblate Spheroidal Solids via Model Based on the Non-Equilibrium Thermodynamics. Diffus. Found. 2020, 25, 83–98. [Google Scholar] [CrossRef]
- Magnus, W.; Oberhettinger, F.; Soni, R.P. Formulas and Theorems for the Special Functions of Mathematical Physics; Springer: Berlim, Germany, 1966; Volume 52, ISBN 3662117614. [Google Scholar]
- Maliska, C.R. Heat Transfer and Computational Fluid Mechanics; Grupo Gen-LTC: Rio de Janeiro, Brazil, 2017; ISBN 8521633351. (In Portuguese) [Google Scholar]
- Whitaker, S. Heat and mass transfer in granular porous media. Adv. Dry. 1980, 1, 23–61. [Google Scholar]
- Patankar, S. Numerical Heat Transfer and Fluid Flow; CRC Press: Boca Raton, FL, USA, 1980; ISBN 1482234211. [Google Scholar]
- Fortes, M.; Okos, M.R.; Barrett, J.R., Jr. Heat and mass transfer analysis of intra-kernel wheat drying and rewetting. J. Agric. Eng. Res. 1981, 26, 109–125. [Google Scholar] [CrossRef]
- Menkov, N.D. Moisture sorption isotherms of lentil seeds at several temperatures. J. Food Eng. 2000, 44, 205–211. [Google Scholar] [CrossRef]
- Tang, J.; Sokhansanj, S. A model for thin-layer drying of lentils. Dry. Technol. 1994, 12, 849–867. [Google Scholar] [CrossRef]
- Figliola, R.S.; Beasley, D.E. Theory and Design for Mechanical Measurements; John Wiley & Sons: New York, NY, USA, 2020; ISBN 1119723450. [Google Scholar]
- Silva, W.P.; Silva, C.M.D.P.S. LAB Fit Curve Fitting Software (Nonlinear Regression and Treatment of Data Program) V. 7.2.46 2009. Available online: www.labfit.net (accessed on 17 April 2021). (In Portuguese).
- Tang, J.; Sokhansanj, S.; Yannacopoulos, S.; Kasap, S.O. Specific heat capacity of lentil seeds by differential scanning calorimetry. Trans. ASAE 1991, 34, 517–522. [Google Scholar] [CrossRef]
- Cengel, Y. Heat and Mass Transfer: Fundamentals and Applications; McGraw-Hill: New York, NY, USA, 2014; ISBN 0077654765. [Google Scholar]


| Air | Lentil | ||||||
|---|---|---|---|---|---|---|---|
| Ta (°C) | Ha (%) | va (m/s) | Mo (% b.s) | Me (% b.s) | To (°C) | Te (°C) | t (s) |
| 40 | 50 | 0.3 | 24.5 | 12.1 | 25 | 40 | 86,400 |
| 60 | 50 | 0.3 | 24.5 | 10.1 | 25 | 60 | 86,400 |
| Case | a1 (----) | a2 (----) | hm (m/s) | hc (W/m2K) | ERMQ (kg/kg)2 | R2 | ||
|---|---|---|---|---|---|---|---|---|
| T (°C) | H (%) | |||||||
| 40 | 50 | 2.33 × 104 | 25.07 × 102 | 6.20 × 10−6 | 37.31 | 0.413 × 103 | 0.61 × 105 | 0.996 |
| 60 | 50 | 1.43 × 104 | 10.74 × 102 | 12.80 × 10−6 | 37.81 | 1.374 × 103 | 2.02 × 105 | 0.991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, J.C.S.; Delgado, J.M.P.Q.; Silva, W.P.; B. Lima, A.G.; Gomez, R.S.; Gomes, J.P.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Santos, I.B.; Machado, M.C.N.; et al. Non-Equilibrium Thermodynamics-Based Convective Drying Model Applied to Oblate Spheroidal Porous Bodies: A Finite-Volume Analysis. Energies 2021, 14, 3405. https://doi.org/10.3390/en14123405
Melo JCS, Delgado JMPQ, Silva WP, B. Lima AG, Gomez RS, Gomes JP, Figueirêdo RMF, Queiroz AJM, Santos IB, Machado MCN, et al. Non-Equilibrium Thermodynamics-Based Convective Drying Model Applied to Oblate Spheroidal Porous Bodies: A Finite-Volume Analysis. Energies. 2021; 14(12):3405. https://doi.org/10.3390/en14123405
Chicago/Turabian StyleMelo, João C. S., João M. P. Q. Delgado, Wilton P. Silva, Antonio Gilson B. Lima, Ricardo S. Gomez, Josivanda P. Gomes, Rossana M. F. Figueirêdo, Alexandre J. M. Queiroz, Ivonete B. Santos, Maria C. N. Machado, and et al. 2021. "Non-Equilibrium Thermodynamics-Based Convective Drying Model Applied to Oblate Spheroidal Porous Bodies: A Finite-Volume Analysis" Energies 14, no. 12: 3405. https://doi.org/10.3390/en14123405
APA StyleMelo, J. C. S., Delgado, J. M. P. Q., Silva, W. P., B. Lima, A. G., Gomez, R. S., Gomes, J. P., Figueirêdo, R. M. F., Queiroz, A. J. M., Santos, I. B., Machado, M. C. N., Lima, W. M. P. B., & Carmo, J. E. F. (2021). Non-Equilibrium Thermodynamics-Based Convective Drying Model Applied to Oblate Spheroidal Porous Bodies: A Finite-Volume Analysis. Energies, 14(12), 3405. https://doi.org/10.3390/en14123405









