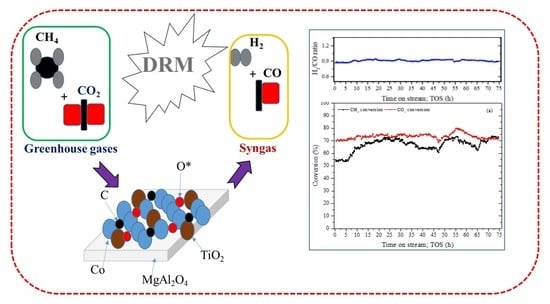
Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production
Abstract
1. Introduction
2. Materials and Methods
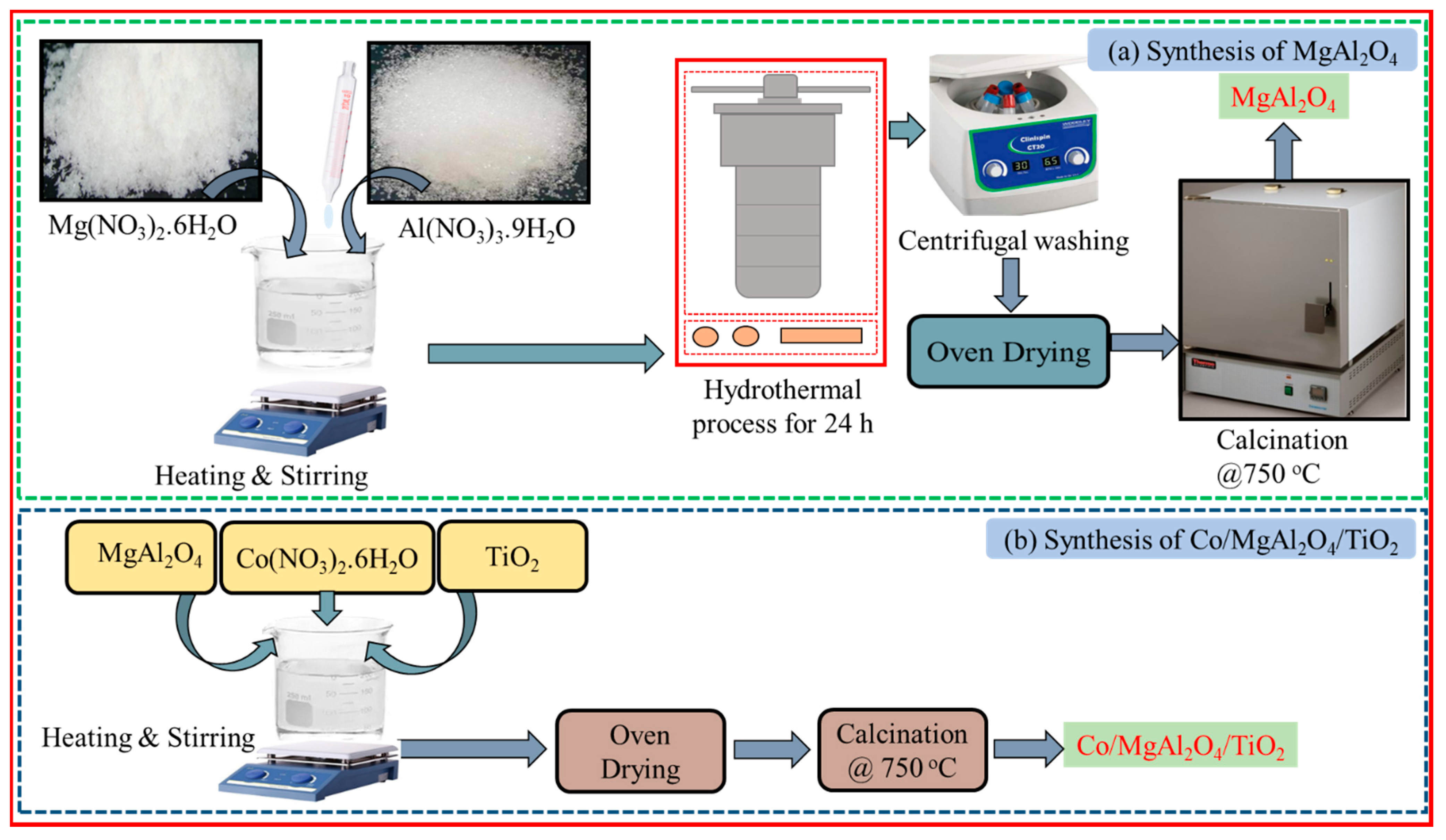
2.1. Synthesis of MgAl2O4 and TiO2 Nanoparticles
2.2. Preparation of Co/TiO2–MgAl2O4 Nanocomposite
2.3. Materials Characterisation
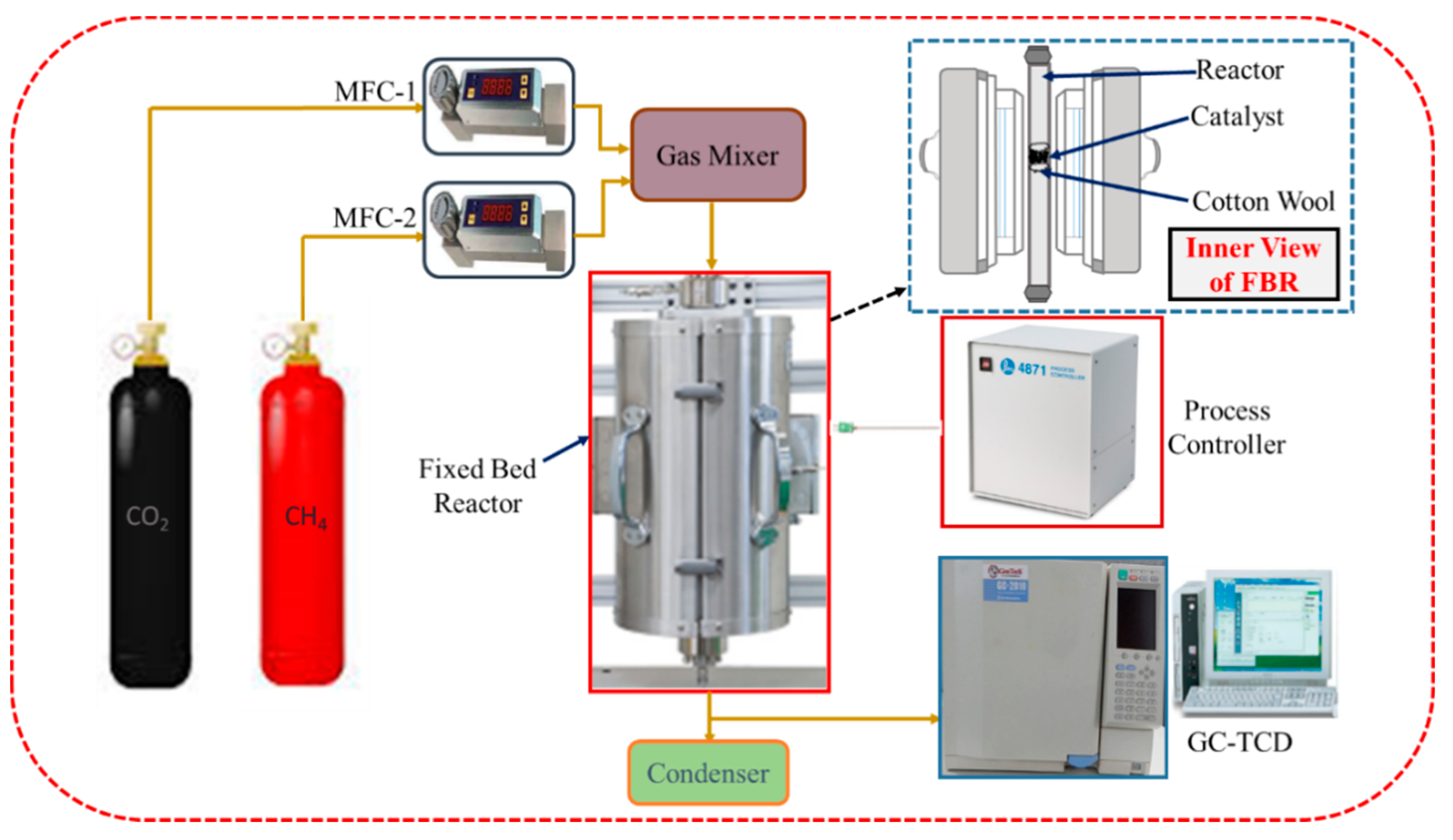
2.4. Experimental Setup
2.5. Catalytic Activity Calculations
3. Results and Discussion
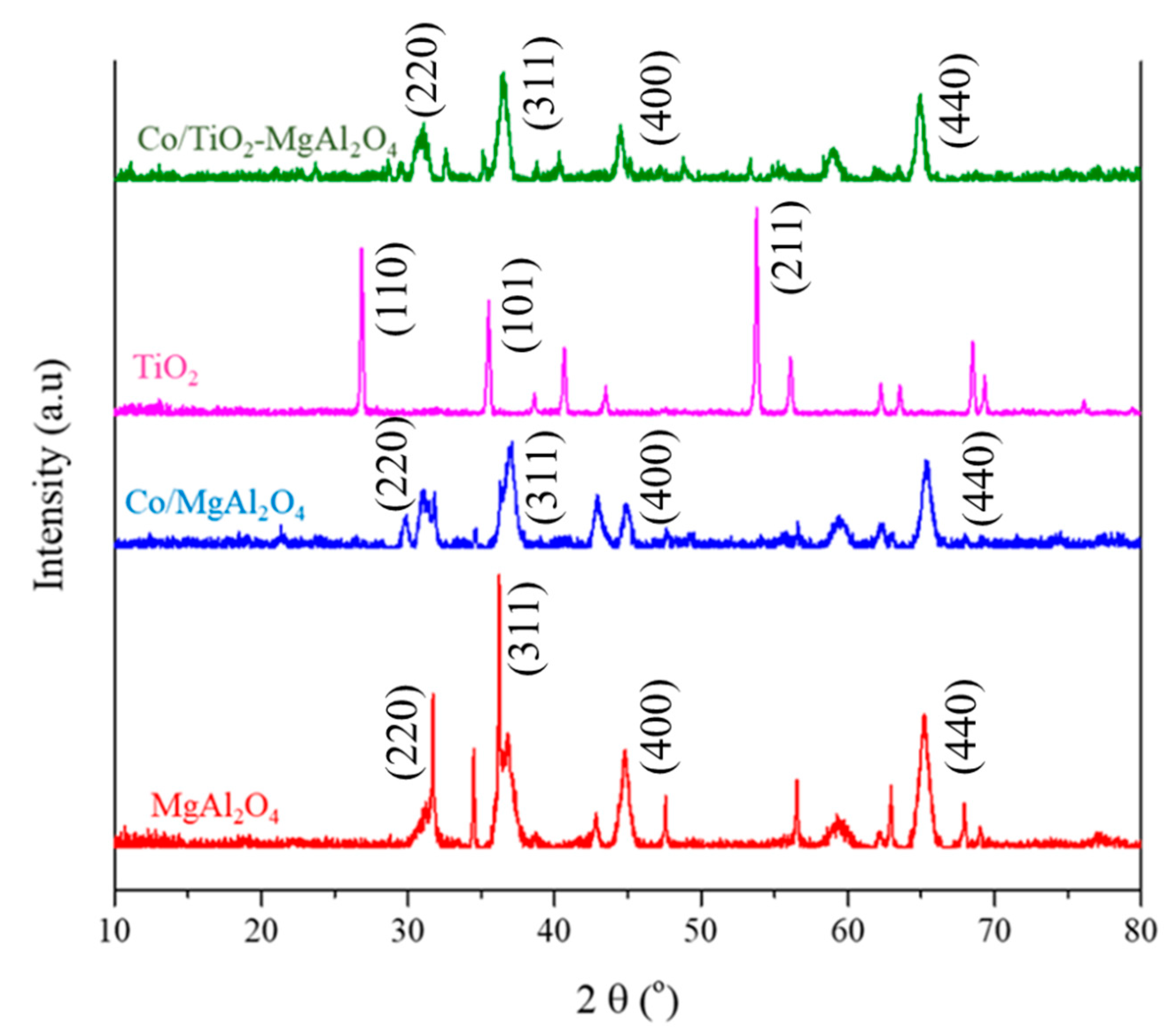
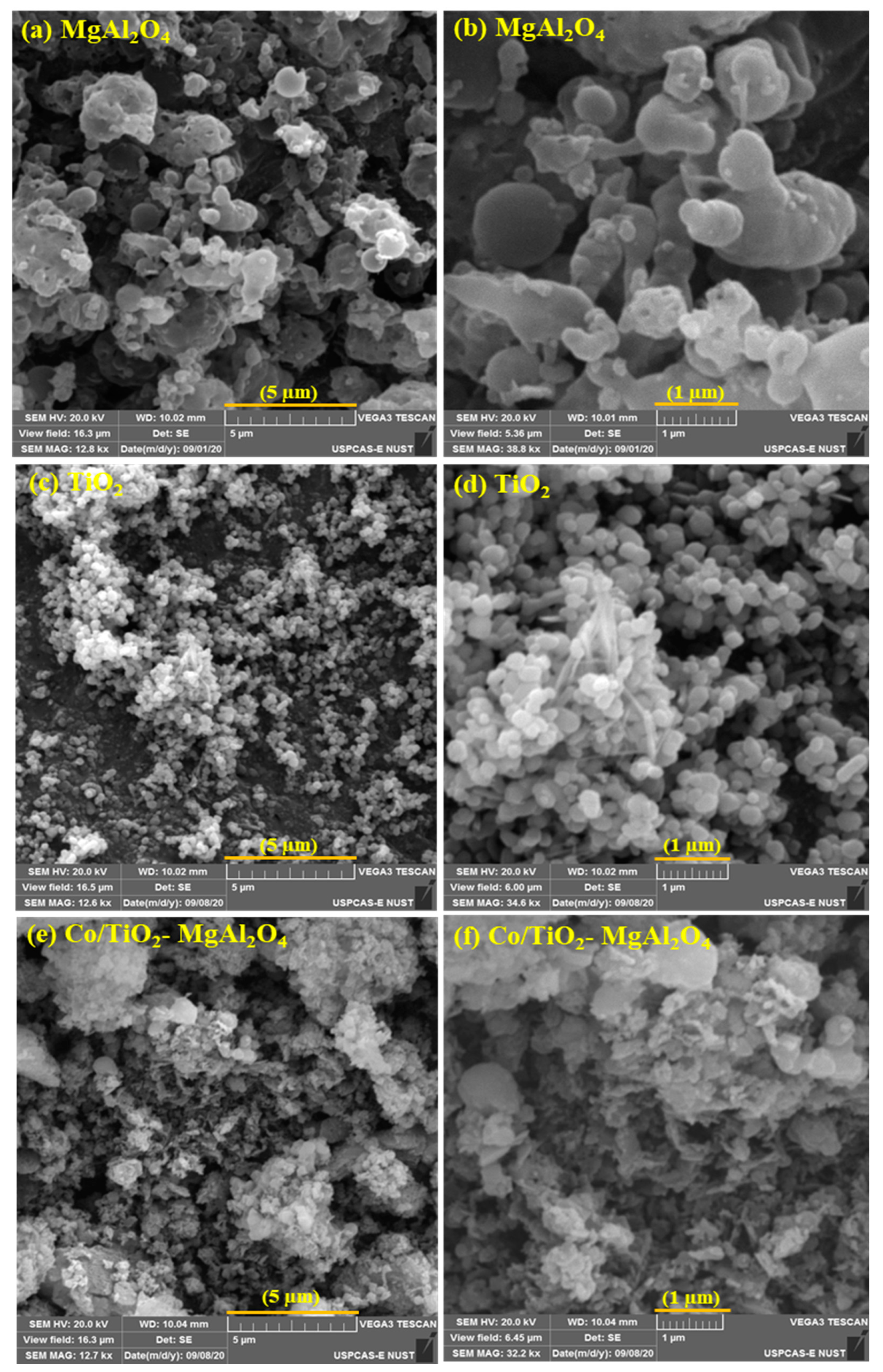
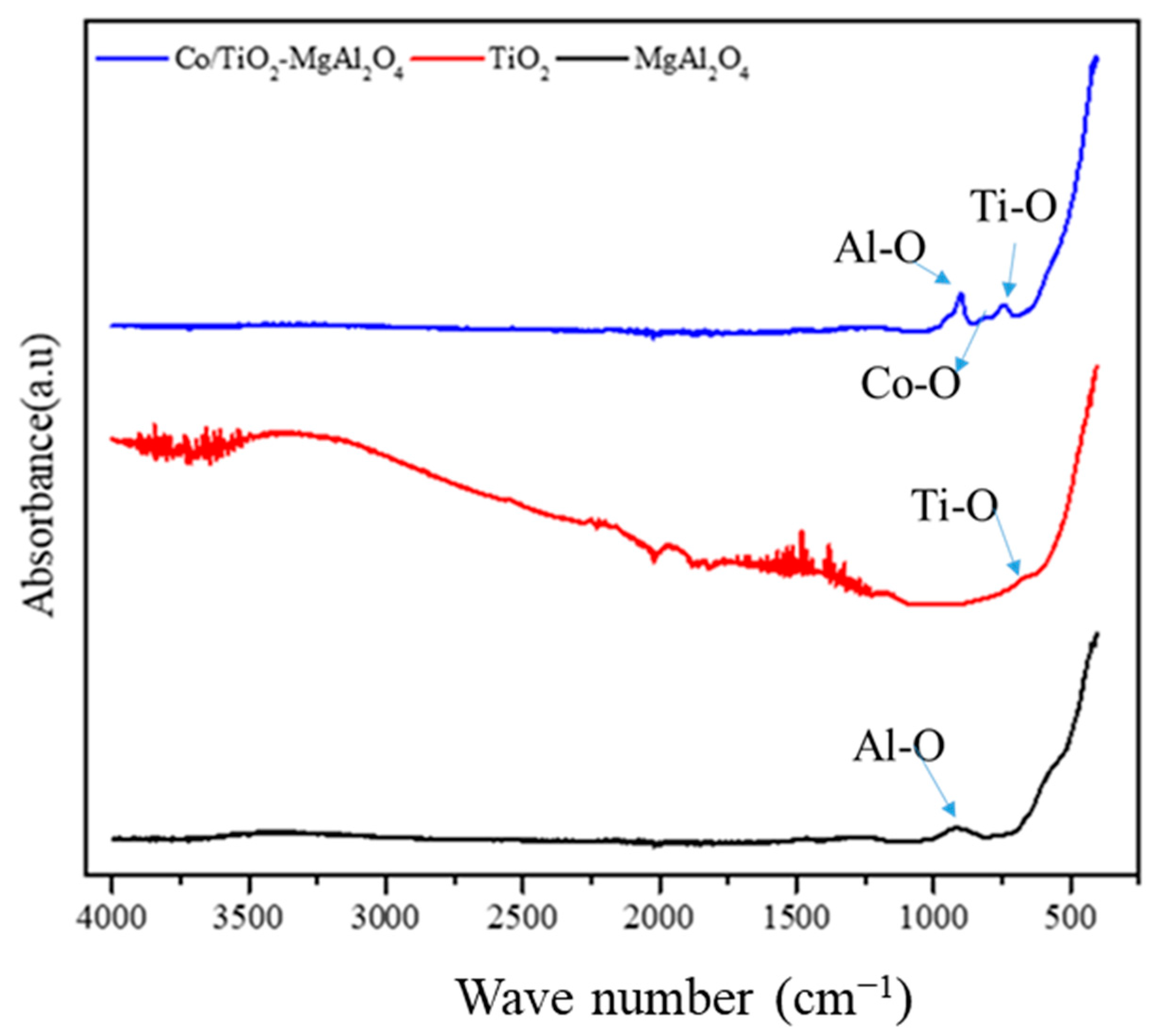
3.1. Characterization of Catalyst
3.2. Dry Reforming of Methane (DRM)
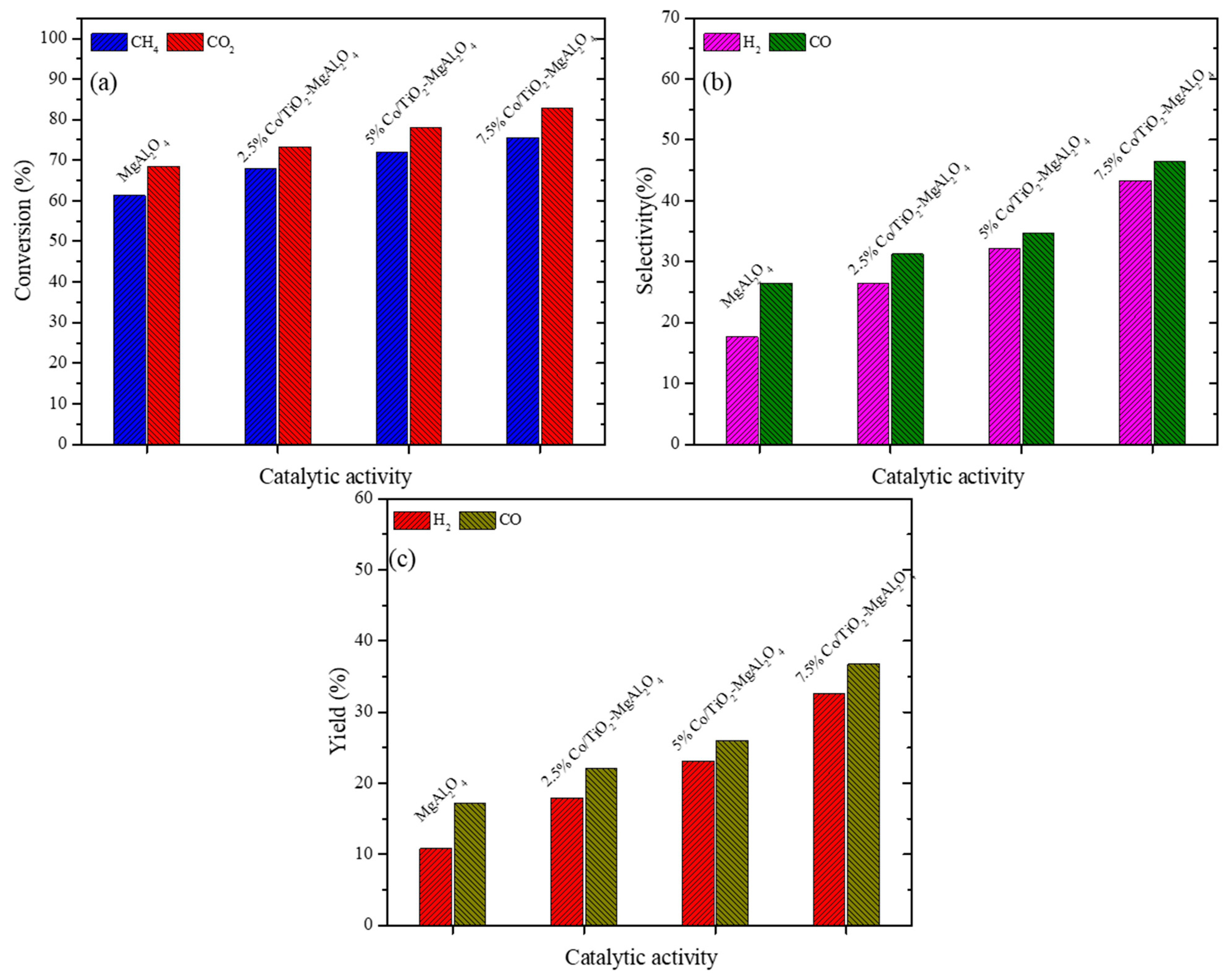
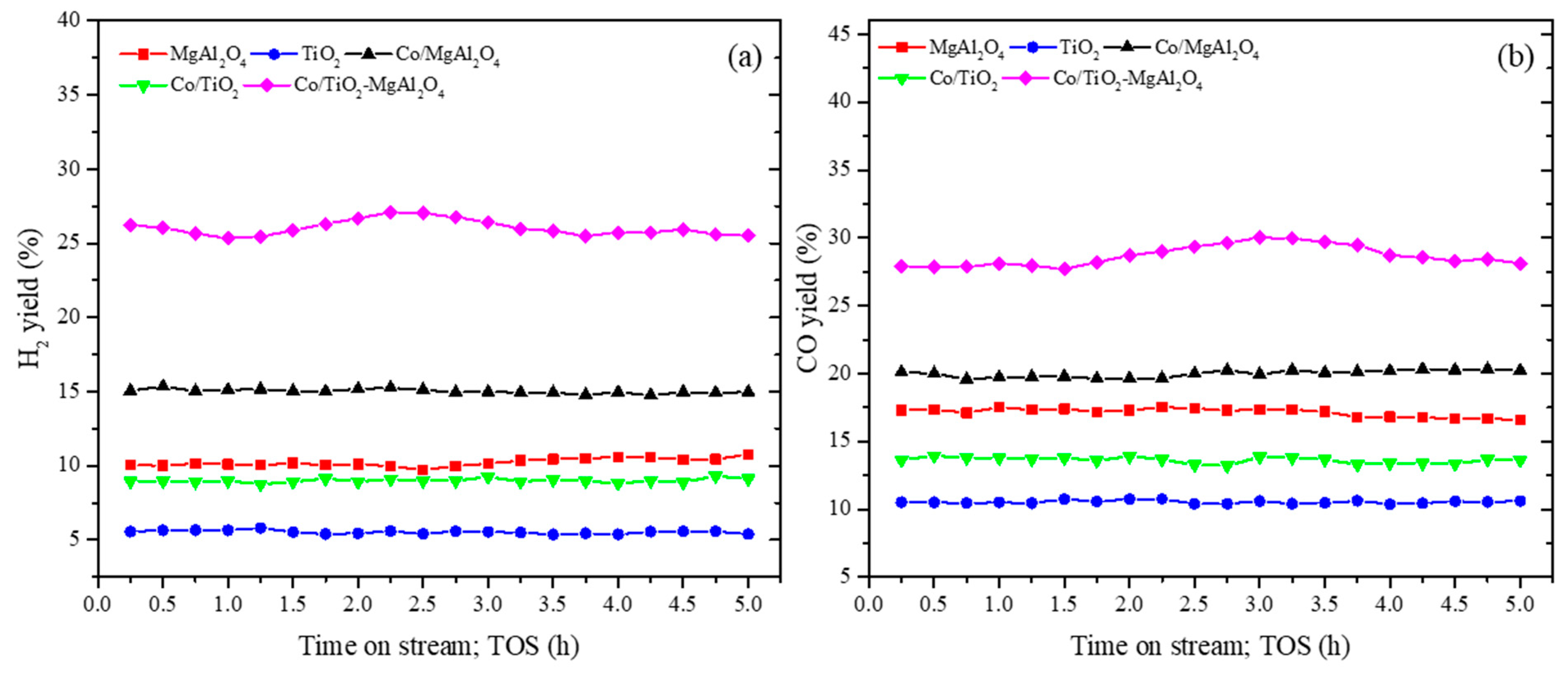
3.2.1. Catalyst Activity Test
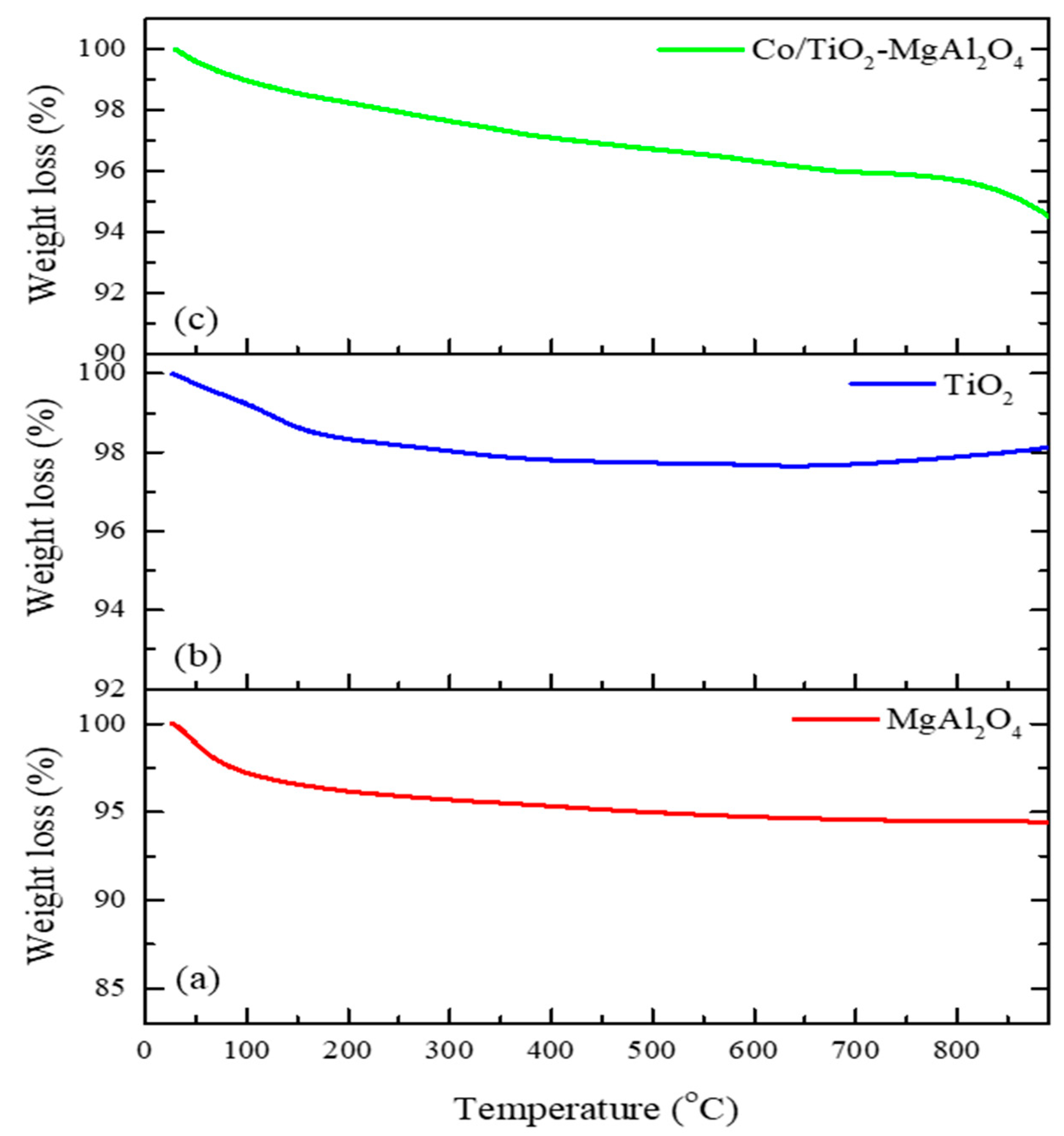
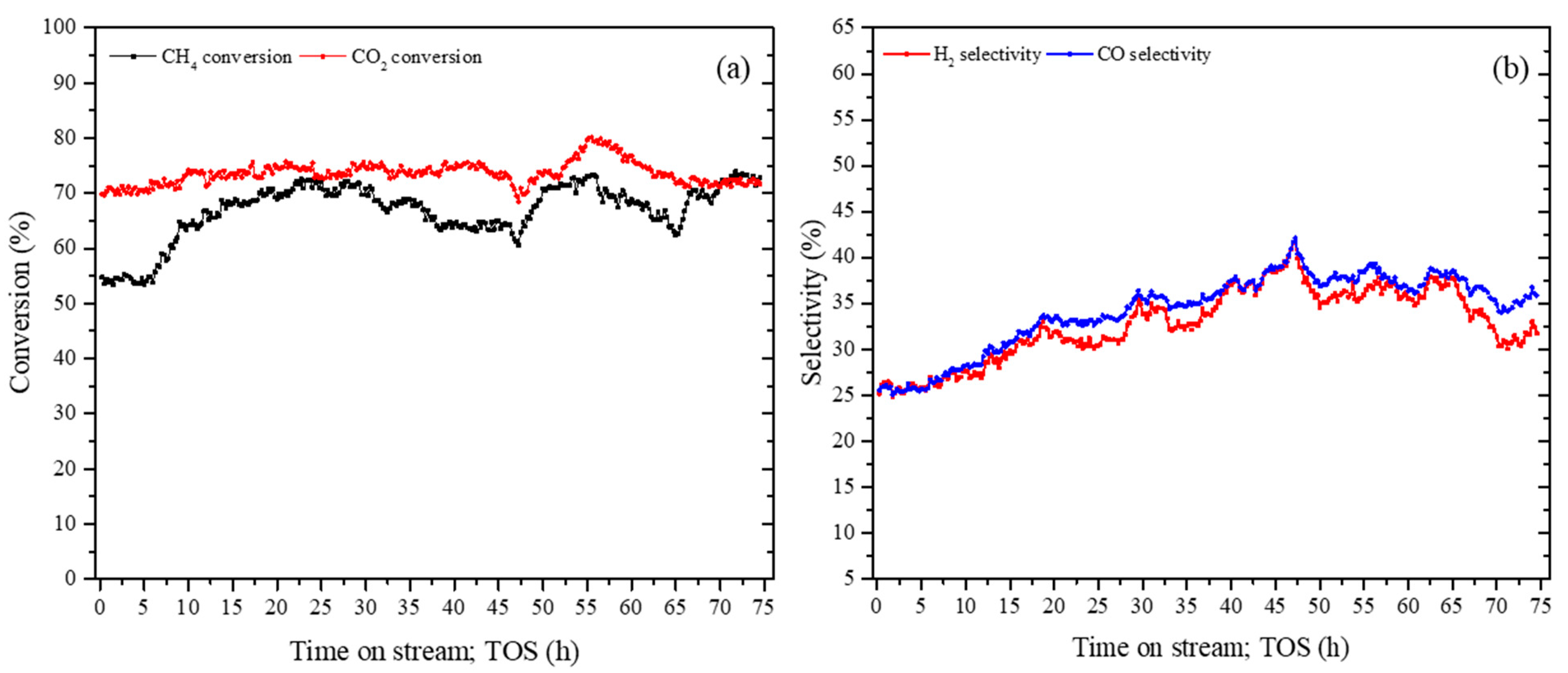
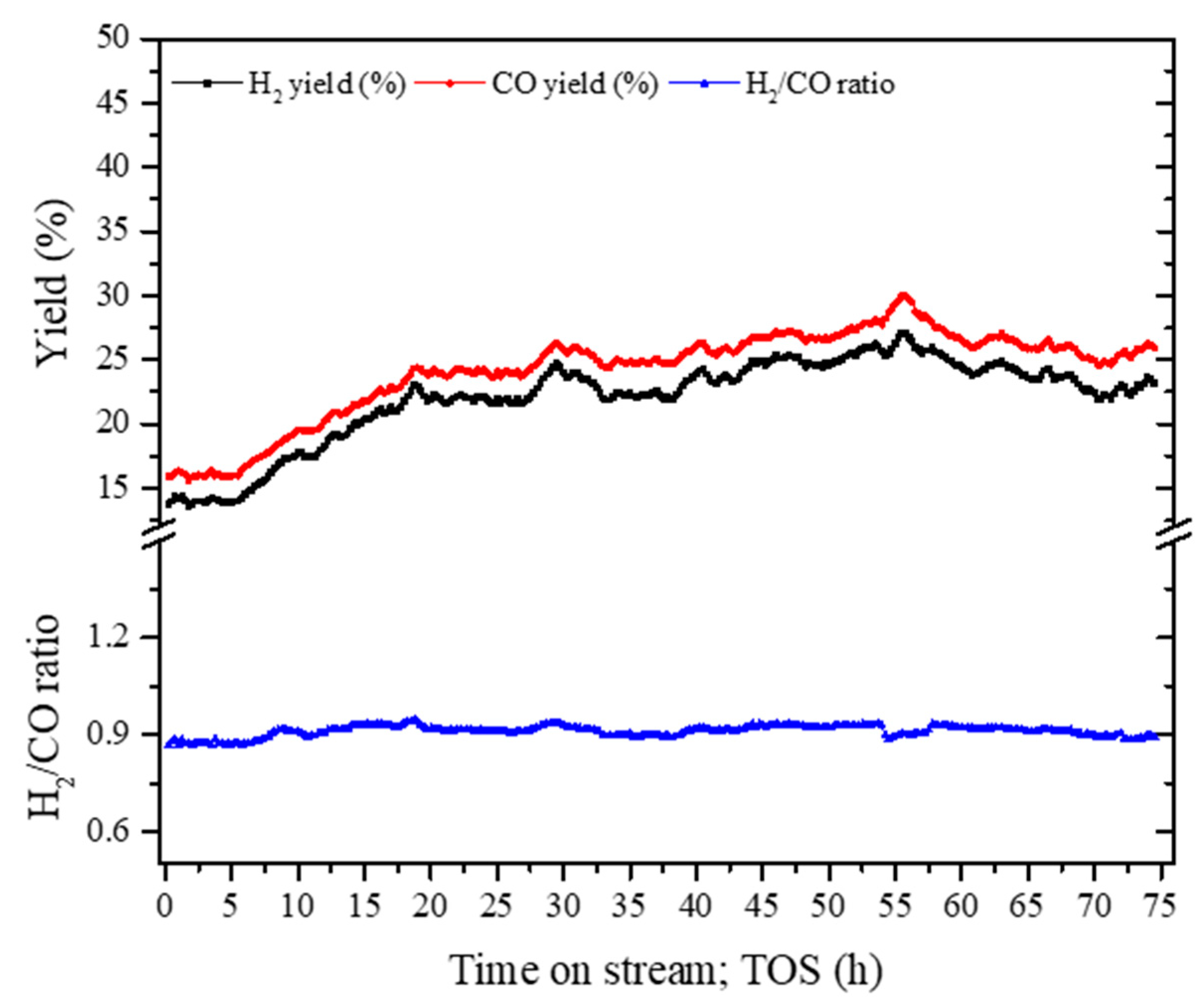
3.2.2. Stability Analysis of Composite
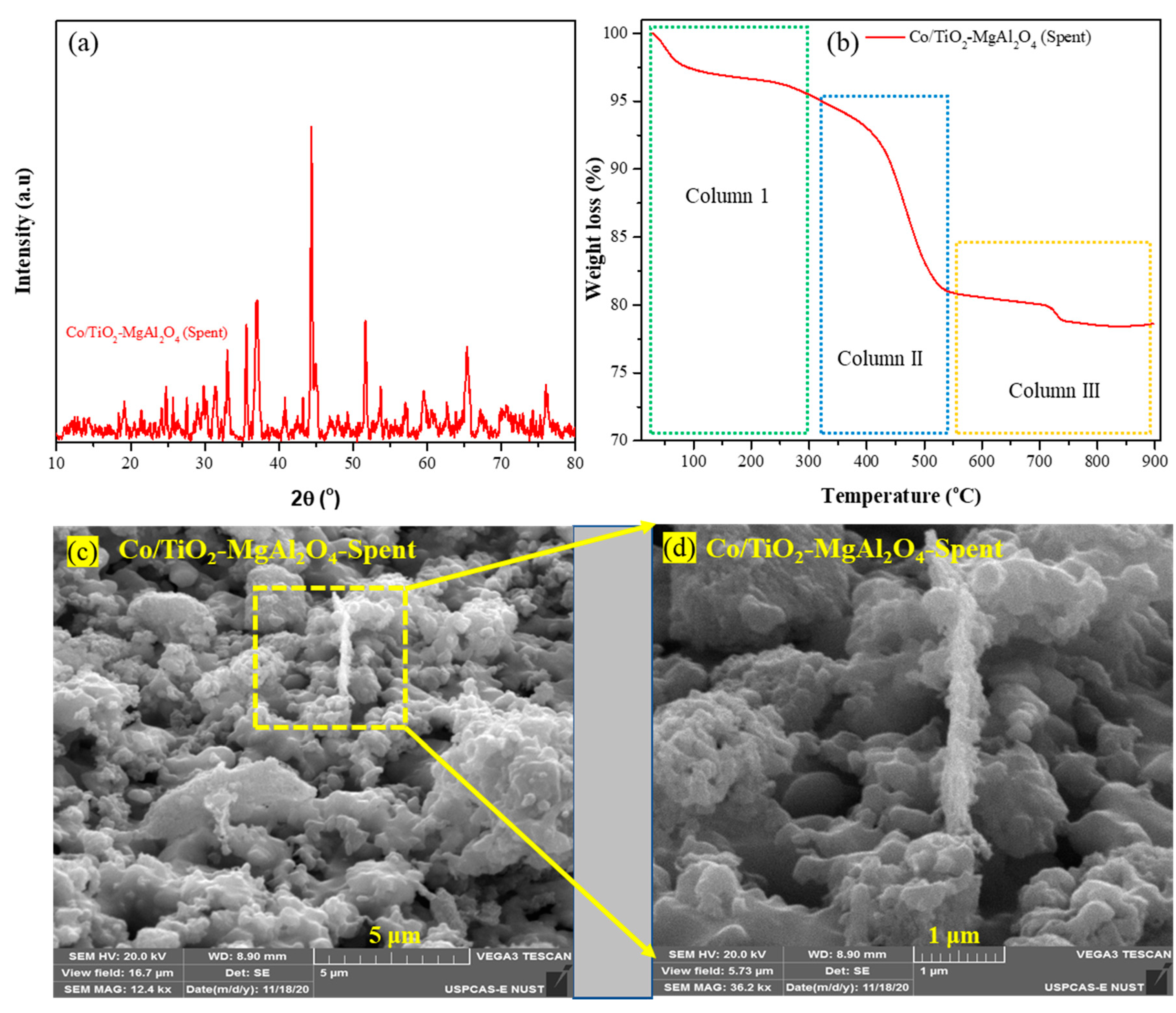
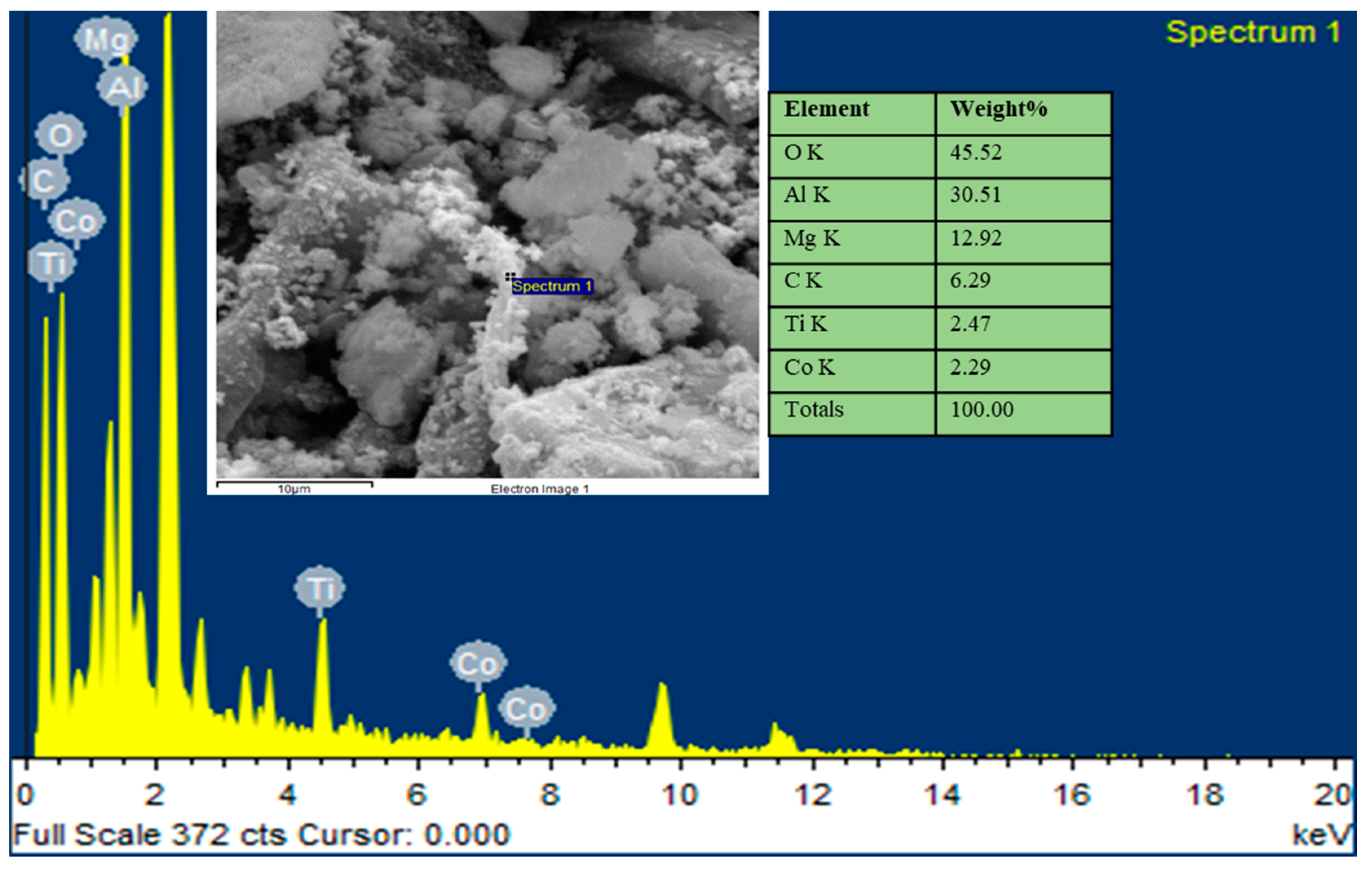
3.3. Characterisation of Spent Catalyst
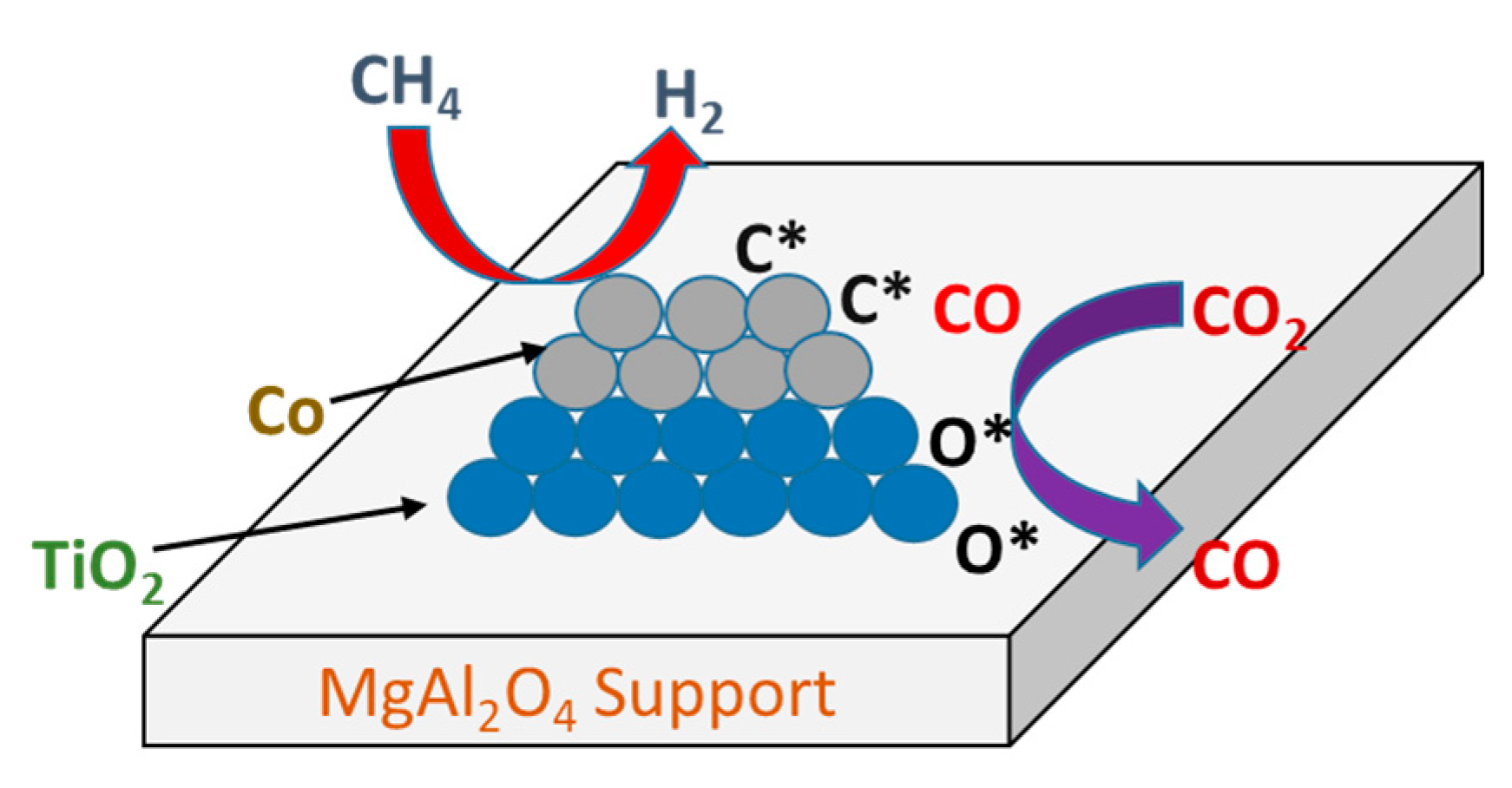
3.4. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Anderson, P.; Jhong, H.-R.M.; Paster, M.; Stubbins, J.F.; Kenis, P.J. Greenhouse gas emissions, energy efficiency, and cost of synthetic fuel production using electrochemical CO2 conversion and the Fischer–Tropsch process. Energy Fuels 2016, 30, 5980–5989. [Google Scholar] [CrossRef]
- Nawar, A.; Ali, M.; Khoja, A.H.; Waqas, A.; Anwar, M.; Mahmood, M. Enhanced CO2 capture using organic acid structure modified waste eggshell derived CaO sorbent. J. Environ. Chem. Eng. 2021, 9, 104871. [Google Scholar] [CrossRef]
- Khoja, A.H.; Anwar, M.; Shakir, S.; Mehran, M.T.; Mazhar, A.; Javed, A.; Amin, N.A.S. Thermal dry reforming of methane over La2O3 co-supported Ni/MgAl2O4 catalyst for hydrogen-rich syngas production. Res. Chem. Intermed. 2020, 46, 3817–3833. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Zaffar, A.; Khan, B.A.; Khoja, A.H.; Khan, U.M.; Sarmad, Q.; Mehran, M.T.; Naqvi, S.R.; Ali, M. Synthesis of Ash Derived Co/Zeolite Catalyst for Hydrogen Rich Syngas Production via Partial Oxidation of Methane. Bull. Chem. React. Eng. Catal. 2021. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Lazar, M.D. Hydrogen and/or syngas production by combined steam and dry reforming of methane on nickel catalysts. Int. J. Hydrogen Energy 2020, 45, 26254–26264. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Dry reforming of methane using different dielectric materials and DBD plasma reactor configurations. Energy Convers. Manag. 2017, 144, 262–274. [Google Scholar] [CrossRef]
- Khoja, A.H.; Azad, A.K.; Saleem, F.; Khan, B.A.; Naqvi, S.R.; Mehran, M.T.; Amin, N.A. Hydrogen Production from Methane Cracking in Dielectric Barrier Discharge Catalytic Plasma Reactor Using a Nanocatalyst. Energies 2020, 13, 5921. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Aziz, M.; Setiabudi, H.; Teh, L.; Annuar, N.; Jalil, A. A review of heterogeneous catalysts for syngas production via dry reforming. J. Taiwan Inst. Chem. Eng. 2019, 101, 139–158. [Google Scholar] [CrossRef]
- Selvarajah, K.; Phuc, N.H.H.; Abdullah, B.; Alenazey, F.; Vo, D.-V.N. Syngas production from methane dry reforming over Ni/Al2O3 catalyst. Res. Chem. Intermed. 2016, 42, 269–288. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/ɤ-Al2O3 -MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Usman, M.; Daud, W.M.A.W. Microemulsion based synthesis of Ni/MgO catalyst for dry reforming of methane. RSC Adv. 2016, 6, 38277–38289. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhou, X.D. Catalytic conversion of CO2 to value added fuels: Current status, challenges, and future directions. Chin. J. Catal. 2016, 37, 999–1015. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.; Osatiashtiani, A. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.; Herrera, M.; Larrubia, M.; Alemany, L. Nanostructured Pt-and Ni-based catalysts for CO2-reforming of methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.; Bizkarra, K.; Gutierrez, A.; Arraibi, J.; Gartzia, L.; Bañuelos, J.; Lopez-Arbeloa, I.; Cambra, J. Ni and RhNi catalysts supported on Zeolites L for hydrogen and syngas production by biogas reforming processes. Chem. Eng. J. 2014, 238, 178–188. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Muhammad, A. Photocatalytic CO2 methanation over NiO/In2O3 promoted TiO2 nanocatalysts using H2O and/or H2 reductants. Energy Convers. Manag. 2016, 119, 368–378. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.M. Effect of NiAl2O4 formation on Ni/Al2O3 stability during dry reforming of methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef]
- Das, S.; Thakur, S.; Bag, A.; Gupta, M.S.; Mondal, P.; Bordoloi, A. Support interaction of Ni nanocluster based catalysts applied in CO2 reforming. J. Catal. 2015, 330, 46–60. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Budiman, A.W.; Song, S.-H.; Chang, T.-S.; Shin, C.-H.; Choi, M.-J. Dry reforming of methane over cobalt catalysts: A literature review of catalyst development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Ewbank, J.L.; Kovarik, L.; Kenvin, C.C.; Sievers, C. Effect of preparation methods on the performance of Co/Al2O3 catalysts for dry reforming of methane. Green Chem. 2014, 16, 885–896. [Google Scholar] [CrossRef]
- Abasaeed, A.E.; Al-Fatesh, A.S.; Naeem, M.A.; Ibrahim, A.A.; Fakeeha, A.H. Catalytic performance of CeO2 and ZrO2 supported Co catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrogen Energy 2015, 40, 6818–6826. [Google Scholar] [CrossRef]
- Ferencz, Z.; Baán, K.; Oszkó, A.; Kónya, Z.; Kecskés, T.; Erdőhelyi, A. Dry reforming of CH4 on Rh doped Co/Al2O3 catalysts. Catal. Today 2014, 228, 123–130. [Google Scholar] [CrossRef]
- el Hassan, N.; Kaydouh, M.; Geagea, H.; el Zein, H.; Jabbour, K.; Casale, S.; el Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef]
- Usman, M.; Daud, W.M.A.W.; Abbas, H.F. Dry reforming of methane: Influence of process parameters-A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Process optimization of DBD plasma dry reforming of methane over Ni/La2O3MgAl2O4 using multiple response surface methodology. Int. J. Hydrogen Energy 2019, 44, 11774–11787. [Google Scholar] [CrossRef]
- di Michele, A.; Dell’Angelo, A.; Tripodi, A.; Bahadori, E.; Sanchez, F.; Motta, D.; Dimitratos, N.; Rossetti, I.; Ramis, G. Steam reforming of ethanol over Ni/MgAl2O4 catalysts. Int. J. Hydrogen Energy 2019, 44, 952–964. [Google Scholar] [CrossRef]
- Medeiros, R.L.; Macedo, H.P.; Melo, V.R.; Oliveira, Â.A.; Barros, J.M.; Melo, M.A.; Melo, D.M. Ni supported on Fe-doped MgAl2O4 for dry reforming of methane: Use of factorial design to optimize H2 yield. Int. J. Hydrogen Energy 2016, 41, 14047–14057. [Google Scholar] [CrossRef]
- Safari, J.; Akbari, Z.; Naseh, S. Nanocrystalline MgAl2O4 as an efficient catalyst for one-pot synthesis of multisubstituted imidazoles under solvent-free conditions. J. Saudi Chem. Soc. 2016, 20, S250–S255. [Google Scholar] [CrossRef]
- Hadian, N.; Rezaei, M.; Mosayebi, Z.; Meshkani, F. CO2 reforming of methane over nickel catalysts supported on nanocrystalline MgAl2O4 with high surface area. J. Nat. Gas Chem. 2012, 21, 200–206. [Google Scholar] [CrossRef]
- Seo, H.O.; Sim, J.K.; Kim, K.-D.; Kim, Y.D.; Lim, D.C.; Kim, S.H. Carbon dioxide reforming of methane to synthesis gas over a TiO2–Ni inverse catalyst. Appl. Catal. A Gen. 2013, 451, 43–49. [Google Scholar] [CrossRef]
- Abbas, T.; Tahir, M.; Saidina Amin, N.A. Enhanced metal–support interaction in Ni/Co3O4/TiO2 nanorods toward stable and dynamic hydrogen production from phenol steam reforming. Ind. Eng. Chem. Res. 2018, 58, 517–530. [Google Scholar] [CrossRef]
- Kho, E.T.; Scott, J.; Amal, R. Ni/TiO2 for low temperature steam reforming of methane. Chem. Eng. Sci. 2016, 140, 161–170. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Mosayebi, Z.; Rezaei, M.; Hadian, N.; Kordshuli, F.Z.; Meshkani, F. Low temperature synthesis of nanocrystalline magnesium aluminate with high surface area by surfactant assisted precipitation method: Effect of preparation conditions. Mater. Res. Bull. 2012, 47, 2154–2160. [Google Scholar] [CrossRef]
- Nor, A.M.; Achoi, M.F.; Mamat, M.H.; Zabidi, M.M.; Abdullah, S.; Mahmood, M.R. Synthesis of TiO2 nanowires via hydrothermal method. Jpn. J. Appl. Phys. 2012, 51, 06FG08. [Google Scholar] [CrossRef]
- Charisiou, N.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Avraam, D.; Goula, M. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Hassan, M.; Liaquat, R.; Naqvi, S.R.; Mehran, M.T.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Saberi, A.; Golestani-Fard, F.; Sarpoolaky, H.; Willert-Porada, M.; Gerdes, T.; Simon, R.; Liebscher, C. Development of MgAl2O4 spinel coating on graphite surface to improve its water-wettability and oxidation resistance. Ceram. Int. 2009, 35, 457–461. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, T.; Wang, L.; Yao, P.; Zhang, J.; Chen, Y.; Chen, Y.; Wang, J. Synthesis of a High-Stability Nanosized Pt-Loaded MgAl2O4 Catalyst for n-Decane Cracking with Enhanced Activity and Durability. Ind. Eng. Chem. Res. 2020, 59, 4338–4347. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Eswaran, L.; Luong, J.H.; Gedanken, A. Sonochemical preparation of polyaniline@ TiO2 and polyaniline@ SiO2 for the removal of anionic and cationic dyes. Ultrason. Sonochem. 2020, 62, 104864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Yu, X.; Tang, S.; Han, S.; Yang, L. Irradiation synthesis and characterization of CoAl2O4: Ce and Mn-codoped CoAl2O4: Ce phosphors. Optik 2020, 210, 164508. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, J.; Lin, L.; Wu, X.; He, D. Controlled synthesis and characterization of hybrid Sn-doped Co3O4 nanowires for supercapacitors. Mater. Lett. 2018, 216, 248–251. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Q.; Wang, L.; Li, B. The flux growth of single-crystalline CoTiO3 polyhedral particles and improved visible-light photocatalytic activity of heterostructured CoTiO3/gC3N4 composites. Dalton Trans. 2016, 45, 17748–17758. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Andre, R.; Sahoo, J.K.; Jochum, F.D.; Theato, P.; Natalio, F.; Berger, R.; Branscheid, R.; Kolb, U.; Tremel, W. Hydrogen peroxide sensors for cellular imaging based on horse radish peroxidase reconstituted on polymer-functionalized TiO2 nanorods. Nanoscale 2011, 3, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Thanabodeekij, N.; Sathupunya, M.; Jamieson, A.M.; Wongkasemjit, S. Correlation of sol–gel processing parameters with microstructure and properties of a ceramic product. Mater. Charact. 2003, 50, 325–337. [Google Scholar] [CrossRef]
- Adak, A.; Saha, S.; Pramanik, P. Synthesis and characterization of MgAl 2 O 4 spinel by PVA evaporation technique. J. Mater. Sci. Lett. 1997, 16, 234–235. [Google Scholar] [CrossRef]
- Sanjabi, S.; Obeydavi, A. Synthesis and characterization of nanocrystalline MgAl2O4 spinel via modified sol–gel method. J. Alloys Compd. 2015, 645, 535–540. [Google Scholar] [CrossRef]
- Tai, J.Y.; Leong, K.H.; Saravanan, P.; Aziz, A.A.; Sim, L.C. Dopant-free oxygen-rich titanium dioxide: LED light-induced photocatalysis and mechanism insight. J. Mater. Sci. 2017, 52, 11630–11642. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Oxygen rich titania: A dopant free, high temperature stable, and visible-light active anatase photocatalyst. Adv. Funct. Mater. 2011, 21, 3744–3752. [Google Scholar] [CrossRef]
- Sim, L.C.; Tan, W.H.; Leong, K.H.; Bashir, M.J.; Saravanan, P.; Surib, N.A. Mechanistic characteristics of surface modified organic semiconductor g-C3N4 nanotubes alloyed with titania. Materials 2017, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, D.D.M.; Sadaiyandi, K.; Mahendran, M.; Sagadevan, S. Precipitation method and characterization of cobalt oxide nanoparticles. Appl. Phys. A 2017, 123, 264. [Google Scholar] [CrossRef]
- Domínguez, A.; Fernández, Y.; Fidalgo, B.; Pis, J.; Menéndez, J. Biogas to syngas by microwave-assisted dry reforming in the presence of char. Energy Fuels 2007, 21, 2066–2071. [Google Scholar] [CrossRef]
- Tavasoli, A.; Sadaghiani, K.; Nakhaeipour, A.; Ahangari, M. Cobalt loading effects on the structure and activity for Fischer-Tropsch and water-gas shift reactions of Co/Al2O3 catalysts. Iran. J. Chem. Chem. Eng. 2007, 26, 9–16. [Google Scholar]
- Wang, W.-J.; Chen, Y.-W. Influence of metal loading on the reducibility and hydrogenation activity of cobalt/alumina catalysts. Appl. Catal. 1991, 77, 223–233. [Google Scholar] [CrossRef]
- Zhang, G.; Su, A.; Du, Y.; Qu, J.; Xu, Y. Catalytic performance of activated carbon supported cobalt catalyst for CO2 reforming of CH4. J. Colloid Interface Sci. 2014, 433, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Baamran, K.S.; Tahir, M. Ni-embedded TiO2-ZnTiO3 reducible perovskite composite with synergistic effect of metal/support towards enhanced H2 production via phenol steam reforming. Energy Convers. Manag. 2019, 200, 112064. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Seo, H.O. Recent scientific progress on developing supported Ni catalysts for dry (CO2) reforming of methane. Catalysts 2018, 8, 110. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K. Influence of reduction temperature on the catalytic behavior of Co/TiO2 catalysts for CH4/CO2 reforming and its relation with titania bulk crystal structure. J. Catal. 2005, 230, 75–85. [Google Scholar] [CrossRef]
- Nagaoka, K.; Takanabe, K.; Aika, K. Modification of Co/TiO2 for dry reforming of methane at 2 MPa by Pt, Ru or Ni. Appl. Catal. A Gen. 2004, 268, 151–158. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Wang, F.; Yuan, K.; Wang, L.; Wu, K.; Xu, G.; Chen, W. One-step synthesis of ordered mesoporous CoAl2O4 spinel-based metal oxides for CO2 reforming of CH4. RSC Adv. 2015, 5, 48256–48268. [Google Scholar] [CrossRef]
- Salvador, F.; Sánchez-Montero, M.J.; Izquierdo, C. C/H2O reaction under supercritical conditions and their repercussions in the preparation of activated carbon. J. Phys. Chem. C 2007, 111, 14011–14020. [Google Scholar] [CrossRef]
- Guler, M.; Dogu, T.; Varisli, D. Hydrogen production over molybdenum loaded mesoporous carbon catalysts in microwave heated reactor system. Appl. Catal. B Environ. 2017, 219, 173–182. [Google Scholar] [CrossRef]
- Chong, C.C.; Setiabudi, H.D.; Jalil, A.A. Dendritic fibrous SBA-15 supported nickel (Ni/DFSBA-15): A sustainable catalyst for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18533–18548. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. Role of La2O3 as promoter and support in Ni/γ-Al2O3 catalysts for dry reforming of methane. Chin. J. Chem. Eng. 2014, 22, 28–37. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Topalidis, A.; Petrakis, D.; Ladavos, A.; Loukatzikou, L.; Pomonis, P. A kinetic study of methane and carbon dioxide interconversion over 0.5% Pt/SrTiO3 catalysts. Catal. Today 2007, 127, 238–245. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazhar, A.; Khoja, A.H.; Azad, A.K.; Mushtaq, F.; Naqvi, S.R.; Shakir, S.; Hassan, M.; Liaquat, R.; Anwar, M. Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production. Energies 2021, 14, 3347. https://doi.org/10.3390/en14113347
Mazhar A, Khoja AH, Azad AK, Mushtaq F, Naqvi SR, Shakir S, Hassan M, Liaquat R, Anwar M. Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production. Energies. 2021; 14(11):3347. https://doi.org/10.3390/en14113347
Chicago/Turabian StyleMazhar, Arslan, Asif Hussain Khoja, Abul Kalam Azad, Faisal Mushtaq, Salman Raza Naqvi, Sehar Shakir, Muhammad Hassan, Rabia Liaquat, and Mustafa Anwar. 2021. "Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production" Energies 14, no. 11: 3347. https://doi.org/10.3390/en14113347
APA StyleMazhar, A., Khoja, A. H., Azad, A. K., Mushtaq, F., Naqvi, S. R., Shakir, S., Hassan, M., Liaquat, R., & Anwar, M. (2021). Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production. Energies, 14(11), 3347. https://doi.org/10.3390/en14113347