Abstract
Nowadays, the transport sector is trying to face climate change and to contribute to a sustainable world by introducing modern after-treatment systems or by using biofuels. In sectors such as road freight transportation, agricultural or cogeneration in which the electrification is not considered feasible with the current infrastructure, renewable options for diesel engines such as alcohols produced from waste or lignocellulosic materials with advanced production techniques show a significant potential to reduce the life-cycle greenhouse emissions with respect to diesel fuel. This study concludes that lignocellulosic biobutanol can achieve 60% lower greenhouse gas emissions than diesel fuel. Butanol-diesel blends, with up to 40% butanol content, could be successfully used in a diesel engine calibrated for 100% diesel fuel without any additional engine modification nor electronic control unit recalibration at a warm ambient temperature. When n-butanol is introduced, particulate matter emissions are sharply reduced for butanol contents up to 16% (by volume), whereas NOX emissions are not negatively affected. Butanol-diesel blends could be introduced without startability problems up to 13% (by volume) butanol content at a cold ambient temperature. Therefore, biobutanol can be considered as an interesting option to be blended with diesel fuel, contributing to the decarbonization of these sectors.
1. Introduction
In recent decades, new restrictions on emissions have been legislated in many developed countries, as fast economic growth and urbanization have led to a substantial increase in the size of the vehicle fleet, causing harmful effects on the environment and human health [1]. Specifically, in 2009, European Directive 2009/28/EC [2] proposed a scenario where transport fuels will include up to 10% of biofuels in 2020. In 2015, European Directive (EU) 2015/1513 [3] proposed that at least 0.5% of this renewable fraction should be advanced biofuels (indicative target). A few years ago, European Directive (EU) 2018/2001 [4] promoted the use of biofuels, increasing the mandatory renewable energy in the transport sector up to 14%, including electrification, and the minimum content of advanced biofuels and biogas up to 3.5%, for 2030. The contribution of heavy-duty vehicles in the transport sector, tractors in the agricultural sector and cogeneration sector to greenhouse gas emissions are nowadays increasing but electrification is not yet a viable option for the usual distances and the current infrastructure. Consequently, researchers are focused on the replacement of fossil fuels with renewable fuels, which have lower emissions than greenhouse gases and other pollutants, and are compatible with modern diesel engine technologies [5,6].
Among the renewable options for substituting partially or totally diesel fuels in the mentioned sectors, biodiesel fuel, generally obtained from conventional feedstocks such as vegetable oils or animal fats through a transesterification process with methanol obtaining a mixture of fatty acid methyl esters (FAME), has been widely used for a couple of decades. Several studies concluded that the power output from biodiesel was similar to that of diesel fuel [7,8]. However, the conventional production of biodiesel fuel together with filter plugging problems, caused by some biodiesel components (sterol glycosides and saturated monoacylglycerols), and storage difficulties derived from fast oxidation, has encouraged researchers to focus on the development and the implementation of advanced biofuels to be blended with diesel fuels or even with biodiesel-diesel blends [9,10,11].
Alcohols produced from waste or lignocellulosic materials through advanced production techniques constitute a sustainable alternative. Among alcohols, ethanol and butanol have been proven to reduce the life-cycle greenhouse gas emissions when produced from biomass and waste feedstocks [12]. Advanced processes to produce lignocellulosic biobutanol achieve lower greenhouse gas (GHG) emissions than those from conventional feedstocks (around 40%) [13]. Although biobutanol can be produced from both biological and chemical routes [14], the ABE (acetone-butanol-ethanol) fermentation route in which sugar, glycerol or lignocellulose feedstocks are fermented by microorganisms to produce n-butanol, ethanol and acetone, is the most widely used [15]. The low final butanol concentration, the limitations in the butanol recovery during the fermentation process, the presence of unwanted products such as butyrate and acetate (apart from acetone and ethanol) and high feedstock costs hinder the economic competition of biobutanol with respect to petrochemical synthesis [16]. Therefore, research efforts are focused on these limitations to improve the economic competitiveness of ABE fermentation [17].
Bioalcohols are not only used to replace gasoline in spark-ignition engines but also to replace diesel fuels in diesel engines [18,19]. Although ethanol was traditionally used as a blending component in the transport sector, ethanol shows some problems related to its cold start (high vapor pressure) and its distribution since it cannot be transferred through the existing pipeline infrastructures without corrosion and damage to the rubber seals [20]. Nowadays, the scientific community shows an emerging interest in studying n-butanol as a blending component. The safer character of biobutanol with respect to ethanol for transportation, fuel handling and storage [21], together with its higher cetane number [22], higher heating value [23], lower volatility [21], higher flash point [24], better lubricity [25] and better miscibility with diesel fuels (especially at a low temperature) [26] of n-butanol have contributed to such interest.
Figure 1 shows scientific papers published in the last years regarding alternative fuels in diesel engines, ethanol in diesel engines and butanol in diesel engines. This schematic diagram shows that the scientific community is aware of the interesting opportunities and scenarios derived from the use of biofuels in diesel engines in road freight transportation, in agricultural applications such as tractors, harvesters and self-propelled sprinklers and in cogeneration applications, among others. Furthermore, this figure confirms the increasing interest in n-butanol as a blending component for diesel engines independently of the growing number of publications in the last years. In fact, the black line scaled in the secondary axis represents the increasing number of butanol publications over the total ethanol and butanol publications.
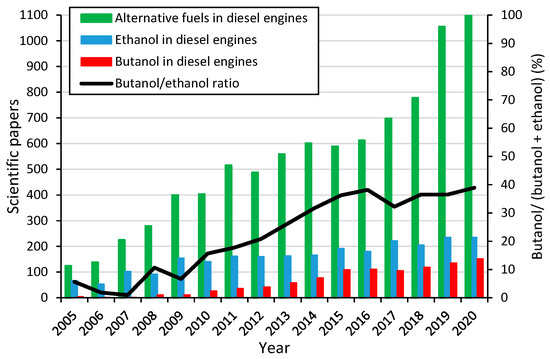
Figure 1.
Bibliometric diagram. ISI web of knowledge [27].
Regarding the use of butanol as a blending component for diesel engines and vehicles, most of the tests found in the literature were carried out under steady conditions [19,28], and only a few of them were conducted following driving cycles [29,30]. The authors of these papers generally concluded that the introduction of n-butanol in diesel fuel sharply decreases particulate matter (PM) emissions due to the oxygen content of the butanol molecule [31,32], and there is an increase in total hydrocarbons (THC) when n-butanol is used [29,33]. However, there was no consensus on carbon monoxide (CO) and nitrogen oxides (NOX) emissions [34,35]. The fuel consumption has been reported to increase for increasing butanol contents due to its lower heating value [36] but without significant penalty in terms of energy consumption for butanol blends with respect to diesel fuel [37]. In terms of startability, cold startability problems have been reported for butanol blends, especially at cold ambient conditions [38].
Since butanol has a significant potential to reduce the life-cycle greenhouse gas emissions with respect to diesel fuel and to introduce a renewable blending component for diesel engines, the aim of this study is to review the properties of butanol, to study their effect on combustion and to compare them with those of ethanol and reference diesel fuel. Additionally, in this review, the different butanol-diesel mixing techniques and their limitations to introduce n-butanol without engine modifications are mentioned. N-butanol benefits in terms of combustion and emissions in diesel engines and vehicles under stationary or transient conditions in the engine test bench or in the chassis dynamometer have been discussed.
The novelty of this review with respect to those previously published regarding biobutanol is mainly focused on: (i) a review of the GHG emissions of biobutanol and bioethanol from different feedstocks compared to those of fossil diesel fuel; (ii) unlike previous studies, this one is focused just on biobutanol as a blending component for diesel engines [39] but has explored this topic in much more detail than was previously carried out, considering a range of different butanol concentrations and the implications on fuel distribution, storage and combustion compared with ethanol blends in diesel engines; (iii) differently to previous reviews about regulated and unregulated emissions from diesel and gasoline engines [40,41], this study focuses on regulated emissions using biobutanol as a blending component, with emphasis on the effect of recent after-treatment technologies.
2. Sustainability of N-Butanol
N-butanol can be produced from biomass via the acetone, butanol, ethanol (ABE) fermentation process. Prior to the development of petrochemical production routes to n-butanol in the 1950s, the majority of n-butanol worldwide was produced through the ABE fermentation of sugars [42]. Today it is understood that the cultivation of food and feed crops for fuel production can cause environmental impacts due to crop cultivation and land-use change [43]. Therefore, the ABE fermentation process is being developed to use waste or lignocellulosic feedstocks. A review of the greenhouse gas (GHG) emissions of bio-butanol illustrates that it can have a substantial GHG reduction compared to fossil diesel, in particular when derived from waste or lignocellulosic materials.
The life-cycle GHG emissions of a fuel are calculated by taking into account emissions from the extraction or cultivation of raw materials, annualized emissions from carbon stock changes caused by land-use change and emissions from processing, transport, distribution and the use of the fuel. Under the methodology laid out in EU Directive (EU) 2018/2001 (RED II) [4], the emissions from fuel use are taken to be zero for biofuels.
Several greenhouse gas assessments of n-butanol produced from sugars can be found in the literature, including [44,45,46], of which the results of Wu et al. [46] provide the most useful comparison with calculations made using the RED II method due to their use of energy allocation. The production of n-butanol from waste and lignocellulosic sugars has only been demonstrated at a pilot scale, but German et al. [13] provide an assessment of the GHG intensity of lignocellulosic butanol if this process was scaled up to a commercial scale. Whilst there is a wide range of results due to the uncertainty in the scale-up and development of the process, they estimate that the GHG emissions of lignocellulosic biobutanol could be as low as 38 gCO2eq./MJ.
The GHG emissions from sugar-based butanol [46] and from lignocellulosic butanol [13] are compared in Figure 2. The carbon intensity of butanol is compared with diesel, corn ethanol and lignocellulosic ethanol based on typical values for these fuels provided in Directive (EU) 2018/2001 [4].
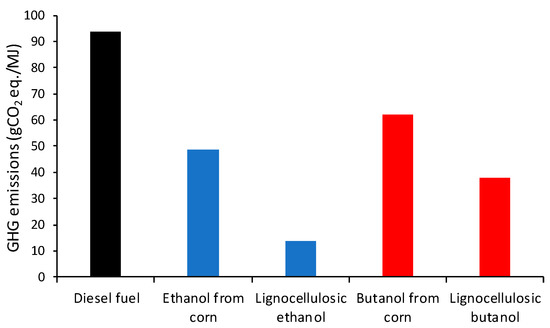
Figure 2.
GHG emissions. Based on [13].
Figure 2 supports the conclusions made across many studies (including [44,47,48]) and reflected in the default values of the RED II [4] that advanced processes to produce lignocellulosic bioethanol and biobutanol can achieve lower GHG emissions than the production of the same fuel from crops. Concretely, lignocellulosic butanol can reduce GHG emissions by 60% with respect to diesel fuel, whereas the reduction for butanol from corn reaches 35%. The higher GHG emissions of lignocellulosic butanol compared to lignocellulosic ethanol are likely due to the earlier stage of development of this technology and the lower yield of butanol compared to ethanol [14].
In addition, oxygenated fuels such as alcohols are an effective way to reduce particle emissions [49]. Nowadays, particle emissions are recognized as one of the most important contributors to climate change. However, these emissions are not taken into account in European Directives, and therefore, the environmental benefit of using alcohols comparatively with fossil fuels could be even greater than suggested by the current method for greenhouse gas assessment.
3. Studies on N-Butanol Properties
Ethanol or n-butanol can be used together with diesel fuel through different mixing techniques. The most common methods are blending and fumigation [39]. In the blending method, alcohol and diesel fuels are premixed before being injected through the diesel fuel injector into the cylinder. In the fumigation method, the alcohol is introduced into the intake air upstream of the manifold either by carbureting, vaporizing or injecting [50].
With the fumigation method, higher alcohol content (up to 50% in energy [51]) can be introduced in the mid-load range without being limited by alcohol miscibility problems or affecting the base diesel fuel properties since it is not directly blended. However, at low loads and high loads, the alcohol content introduced should be reduced. High alcohol content at low loads could lead to misfiring. At high loads, introducing high alcohol content could result in preignition and engine knock.
In terms of combustion benefits, the alcohol evaporation in the intake air reduces the intake temperature increasing its density and, consequently, the air available. Therefore, higher power could be reached. The turbocharger boost pressure can be useful for the atomization of the fumigated alcohol. Nevertheless, potential mechanical problems have been reported in turbocharged diesel engines using the fumigation technique due to the impact of the liquid spray on the turbocharger. The alcohol evaporation is not complete when the alcohol is introduced downstream of the compressor. Furthermore, the fumigation technique requires the addition of a vaporizer or injector and an additional fuel injection system and fuel tank adaption, which increases the engine weight [52,53]. On the contrary, blending alcohols with diesel fuels allows introducing a renewable component in the diesel engine without any engine modification. For the reasons aforementioned, this review is only focused on blending.
Although the alcohol most commonly used as a blending component in the transport sector is ethanol, the higher cetane number of n-butanol, together with its higher heating value, better viscosity, better lubricity, higher flash point and better miscibility with diesel, particularly at a low temperature, suggest that n-butanol is a better renewable component than ethanol in diesel blends [25,54,55].
The main properties of ethanol and n-butanol are listed in Table 1.

Table 1.
Summary of ethanol and n-butanol properties [55].
Although the physicochemical properties of n-butanol are more similar to those of diesel than ethanol, it still cannot replace diesel fuel at 100% [24]. The literature reports that butanol-diesel blends can be tested up to 40% butanol content (volume basis) without engine modifications [37,60].
The following points summarize the properties of n-butanol that make it more attractive from a technical point of view than ethanol as a blend component in diesel engines.
- Higher density. Density affects the spray formation, the injection timing, the atomization and the combustion characteristics, among other effects [21]. The density of n-butanol is lower than that of diesel fuel. Therefore, a smaller amount of alcohol is pressurized and injected by the fuel pump since the dosage is volumetric [35,61]. However, n-butanol density is higher than that of ethanol [55]. Since the excess volume of liquid blends, which is an indication of the presence of molecular interactions, has strong implications on the fuel consumption and on the sizing of fuel tanks, detailed knowledge of the density of blends is required. There is not much literature about butanol-diesel systems. The excess volume of butanol-diesel blends has been observed to be higher than that of ethanol-diesel blends [62]. Since the carbon chain is larger for butanol with respect to ethanol, the non-polar part of the molecule (aliphatic chain) dominates, reducing the polar character of butanol [63]. Consequently, in butanol-diesel blends, the interaction between the hydroxyl group of the alcohol molecule and the aromatic hydrocarbons is weaker (dispersive forces). Particularly, Aissa et al. [64] and Dubey et al. [65] reported positive excess volume for blends of n-butanol and one of the most usual diesel surrogates (n-hexadecane) at 298.15, 303.15, 308.15 and 313.15 K, whereas Mehra et al. [66] concluded negative excess volume in the range from 303.15 to 318.15 K. In the case of alcohol-biodiesel blends since strong interactions are formed between the hydroxyl group of alcohols and the ester group of biodiesels (hydrogen bonds), the excess volume is lower than for alcohol-diesel blends. The positive excess volume is even lower for ethanol-biodiesel blends [67,68].
- Higher viscosity. The viscosity affects the atomization of fuel when it is injected into the combustion chamber, the size of the fuel droplets, the formation of engine deposits and the lubricity of the fuel [69,70]. High-viscosity fuels require more energy in the fuel pump and increase wear in the injection system [71]. On the contrary, fuels with excessively low viscosity may not provide sufficient lubrication for the injection system leading to higher pump and injector leakage, increasing the fuel return and, thus, the fuel consumption associated with the higher pumping power. Viscosity values decrease for increasing alcohol contents in alcohol-diesel blends [55]. Results also show that viscosity is not proportional to the volumetric, mass or molar alcohol content [55]. According to the EN 590 standard of diesel fuels, which establishes that viscosity values should be higher than 2 cSt [72], only ethanol-diesel blends with ethanol content up to 36% (v/v) fulfill this requirement [73]. Since the viscosity of alcohol increases with a longer carbon chain, n-butanol blends from 0% to 100% in diesel fuel would have no restriction [73]. However, in the study carried out by Kuszewski [74], where the viscosity of diesel fuel at 40 °C is closer to the lower limit of the EN 590, only butanol-diesel blends up to 7% (v/v) fulfill this requirement. The reduction in viscosity from blending alcohols (ethanol or n-butanol) with diesel fuel can be compensated by adding biodiesel [75,76]. Although alcohols have been widely used in chemical and petroleum industries, accurate and reliable knowledge of their viscosity is required for the design of transport equipment or pipelines [77]. Therefore, generalized correlations for the prediction of the viscosity of liquid mixtures are needed. Among the different methods, Cano-Gómez et al. [78] studied different butanol-biodiesel blends and reported that Grunberg–Nissan fit better to experimental data than other modeling methods such as Kendall–Monroe or Bingham equations. The Grunberg–Nissan equation has also been used in different studies [55,73] to model the viscosity of different alcohols (methanol, ethanol, propanol, n-butanol and n-pentanol) with diesel and biodiesel fuels, respectively.
- Better lubricity. Controlling the fuel lubricity is essential to protect some engine components with direct contact with fuel, such as injectors, fuel pumps and fuel rails, against wear problems. Pure n-butanol shows better lubricity than pure ethanol [55]. Vinod Babu et al. [60] reported that, in general, the lubricity of pure alcohols improves (leading to a lower wear scar) for increasing molecular weight. For intermediate alcohol concentrations, diesel blends with long carbon chain alcohols (n-butanol and n-pentanol) showed worse lubricity (larger wear scar) than those with a short carbon chain (ethanol and propanol). The lubricity of ethanol-diesel blends at an intermediate ethanol content was shown to be better than expected as a consequence of the alcohol evaporation from the lubricating layer [79,80]. A detailed study about the lubricity of blends of different alcohols (ethanol, propanol, n-butanol and n-pentanol) with diesel fuel [55] reported that, following EN 590 standard [72], which requires a wear scar lower than 460 µm at 60 °C, only those ethanol-diesel blends with an ethanol content higher than 92% or butanol-diesel blends with butanol content above 35%, both volume basis, would not fulfill this standard.
- Higher heating value. Alcohols show lower heating value than diesel fuels. Therefore, a higher amount of alcohol is required to produce the same power output in the engine. However, the heating value increases for increasing carbon atom number. Comparing ethanol and n-butanol, the latter has 25% more energy density in volume than ethanol, reducing the fuel consumption needed to keep a specific load in diesel engines [40,81]. The study carried out by Kuszewski [74], where butanol-diesel blends with 5%, 10%, 15%, 20% and 25% (v/v) butanol content were tested, concluded that introducing 25% butanol content reduced the lower heating value by 6% with respect to that of diesel fuel. Since the lower heating value of diesel fuel often ranges from 41 to 44 MJ/kg, butanol-diesel blends up to 17% (v/v) and ethanol-diesel blends up to 10% can be considered within this range [73].
- Better blend stability. Alcohol-diesel blends can be separated into different phases under specific conditions. This stability strongly depends on the temperature, humidity and fuel composition. In fact, when the temperature decreases, the unstable region becomes wider. Additionally, the presence of moisture negatively affects the miscibility. Alcohols with a long carbon chain show better blending stability than those with a low carbon chain [55]. The polarity of alcohols is induced by the hydroxyl group (R–OH), which is among the most polar chemical groups. Since the carbon chain of butanol is higher than that of ethanol, its global polarity is lower. Therefore, better blending stability is observed between butanol and the mainly non-polar structures of diesel fuels [63]. In particular, low blend stability was reported for ethanol-diesel blends, specifically at intermediate ethanol contents (from 15% to 75% ethanol content) [82,83]. In fact, Kwanchareon et al. [84] reported the appearance of two liquid phases for ethanol-diesel blends with ethanol content from 20% to 80% by volume for temperatures below 10 °C. However, butanol blends showed better blend behavior. In fact, butanol-diesel blends did not show blend stability problems along the whole butanol range for temperatures above 0 °C [26]. Butanol-diesel blends do not need emulsifying agents since the blend does not separate even after several days [85]. Ethanol-diesel blend stability problems can be compensated by additivation or adding biodiesel to the blend [54,84]. Strong interactions are formed between the hydroxyl group of ethanol and the ester group (R-COO-R’) of biodiesel. The intensity of these interactions is even enhanced by the formation of hydrogen bonds [86]. Apart from adding biodiesel to ethanol-diesel blends, miscibility problems in these blends could be conducted by adding an emulsifier or a co-solvent. Emulsifiers allow suspending small droplets of ethanol within the diesel fuel. In order to generate the final blends, emulsification usually requires previous steps such as heating and blending. Co-solvents act as a bridging agent, influencing the molecular bonding and thus leading to a more homogeneous blend [79].
- Better cold-flow properties. Bioalcohols, with a low freezing temperature, have proven to be a sustainable alternative to improve the cold flow properties of diesel fuels (especially biodiesel) [87]. Recent biodiesel filter plugging problems were reported in mild and cold weather countries, causing operating problems mainly attributed to the crystallization of monoacylglycerols of saturated fatty acids, sterol glycosides and other impurities [88,89,90]. As a consequence, additional requirements have been proposed in both European [72] and non-European [91] countries to limit operability problems in diesel engines. In Europe, standard EN 590 [72] establishes some limits for cold filter plugging point (CFPP) and for cloud point (CP). However, there is no standard establishing limits for pour point (PP), and only British and Australian standards establish limits for filterability (FBT). Among alcohols, the benefits of blending light alcohols such as methanol and ethanol with diesel fuels are limited by their aforementioned weak miscibility. The intersolubility of ethanol-diesel blends decreases for decreasing temperatures, with the cold flow properties (CFPP, CP and PP) being consequently affected by the formation of a gelatinous phase or by phase separation [26]. As a consequence of its better blend stability over a wide range of temperatures for the whole concentration range, n-butanol improves the cold flow properties of diesel fuels (especially for high alcohol content). Regarding alcohol-biodiesel blends, Makareviciene et al. [87] reported that the addition of n-butanol to biodiesel resulted in a gradual decrease in the cloud point and the cold filter plugging point. Bouaid et al. [92] justified that n-butanol improves more significantly the cold-flow properties of diesel and biodiesel fuels than ethanol as a consequence of its less polar character.
- Higher cetane number. Among the properties affecting the combustion process, the cetane number is a limiting one. In general, alcohols exhibit low cetane numbers, and therefore, only limited concentrations of these alcohols in the blends are recommended for use in unmodified diesel engines because the cetane number significantly affects the engine efficiency [56]. The higher cetane number of n-butanol with respect to ethanol suggests that its maximum concentration in diesel blends could be increased with respect to that recommended for ethanol [57,61]. Based on the cetane number, the literature reports that butanol-diesel blends with n-butanol content up to 40% (v/v) can be used in diesel engines without any engine alteration [60]. Higher butanol content in the blend leads to excessively high ignition delay [22]. The large, premixed phase derived from the high ignition delay results in excessive heat release rates and in-cylinder pressure peaks [93]. However, taking into account limits proposed by the EN 590 standard, only butanol blends with diesel fuel up to 3% fulfill this limit [22]. This limitation can be compensated with the use of cetane improvers [79]. The mentioned increase in ignition delay for butanol blends is similar when it is blended with diesel or biodiesel fuels. However, some differences appear when ethanol is blended with diesel or biodiesel fuels, with larger delay times in the former case [22].
- Lower enthalpy of vaporization. Since ethanol and butanol have a higher enthalpy of vaporization than diesel fuel, more heat is needed to evaporate the liquid alcohol, resulting in a smaller increase in the gas temperature, which may derive into starting difficulties [61]. Among alcohols, the lower enthalpy of vaporization of n-butanol (620 kJ/kg) with respect to ethanol (944 kJ/kg), suggests that a diesel engine can start more easily operating with butanol than with ethanol at cold ambient conditions [81].
- Better distribution and storage. As n-butanol has a higher flash point and lower volatility than ethanol, butanol blends are safer for transportation, fuel handling and storage than those of ethanol [21,40]. Corrosion in pipelines is mainly attributed to the polarity and the hygroscopic character of the alcohol molecule. Some metals such as magnesium, lead and aluminum are susceptible to chemical attack by alcohol. Furthermore, wet corrosion (mainly caused by the moisture absorption capacity of alcohol) oxidizes most metals. Non-metallic components, especially elastomeric components, are also affected by alcohols [79]. Corrosion acts over the materials used in the fuel delivery and injection systems, among others. Alcohols with high polarity and high water content enhance the corrosive action in the materials. Since ethanol is more polar [62] and more soluble in water than butanol, butanol shows better tolerance to water contamination and, therefore, is more suitable to be distributed through existing pipelines. Furthermore, the less corrosive character of n-butanol with respect to ethanol also contributes to improved storage over longer time periods [20]. Yanai et al. [94] reported that butanol could corrode plastic parts and cause the swelling of rubber components. Nevertheless, the latter could be solved by substituting the rubber sealing material for a material more alcohol tolerant.
The n-butanol properties previously mentioned have a strong influence on combustion parameters. The presence of n-butanol affects the fuel-air mixing process and the injection spray development. The lower density and the lower kinematic viscosity of n-butanol with respect to diesel fuel lead to a better atomization quality for butanol-diesel blends. In addition, the higher volatility of n-butanol leads to a faster evaporation process. Both better atomization and faster evaporation contribute to form more homogeneous fuel-air mixtures, thus decreasing soot formation [35,60].
Cetane number is another important parameter for combustion quality since it can help the optimization of combustion timing. For n-butanol blends, the maximum pressure reached in the combustion chamber during combustion decreases for increasing butanol contents as a consequence of three effects: the energy effect represented through the reduction in the heating value, the chemical effect that is related with the reduction in the equivalence ratio and the dilution effect represented through the over-dilution and caused by their large delay times. Both chemical and dilution effects contribute to reducing the flame velocity, and therefore to enhance the heat transfer to the chamber walls during combustion, making the quality of the combustion poorer [22].
4. Studies on N-Butanol Use in Diesel Engines and Vehicles
This section reviews the use of n-butanol as a blending component in diesel engines and vehicles and reports its effects on combustion, performance and gaseous and particle emissions.
Fossil fuels have often been partially replaced by renewable fuels to reduce both the environmental impact and the dependence on conventional fuels in internal combustion engines. Most of the butanol-diesel emission results found in the literature were tested under steady conditions in a Euro 5 (or inferior) engine test bench under warm ambient conditions [19,85,95].
In general, under steady conditions, the authors observed a sharp decrease in PM emissions for butanol blends (due to the role played by the oxygen content to inhibit soot formation and to enhance soot oxidation) [36,96] with respect to 100% diesel fuel. In terms of gaseous emissions, the literature reports an increase in total hydrocarbons (THC) emissions for butanol blends [34,97]. However, there is no consensus regarding CO and NOX emissions. In the study by Choi et al. [32] CO emissions increased with respect to diesel fuel, whereas in the study presented by Chen et al. [98], the opposite was reported. NOX emissions remained constant in the tests carried out by Siwale et al. [31], whereas in other studies, slight increases [6] and decreases [99] were observed. Rakopoulos et al. [19,100] reported that these blends tend to reduce both particle and NOx emissions simultaneously. Most of the studies observed an increase in fuel consumption for butanol blends associated with its lower heating value [96,98,101,102]. In addition, the lower cetane number of butanol-diesel blends leads to an increase in the ignition delay [103]. The delayed start of combustion will prolong the combustion process, reducing the energy that can be efficiently converted into effective power in the cylinder [104]. This increase reached around 10% for Bu5D (5% butanol 95% diesel, volume basis) and around 14% for Bu25D (25% butanol 75% diesel, volume basis) in tests carried out by Atmanli et al. [99]. The differences in fuel consumption, described above when butanol is introduced, almost disappear in terms of energy consumption [37]. Since the energy consumption (inversely proportional to the engine efficiency) is determined as the product of the fuel consumption by the lower heating value, the lower heating value of butanol blends practically compensates for the increase in fuel consumption [95].
In studies following transient conditions, trends previously described for steady conditions were confirmed. The effect of n-butanol addition on the performance and emissions following the New European Driving Cycle (NEDC) was studied by Armas et al. [33] and Kozak [30] from different Euro 4 diesel engines and by Lapuerta et al. [29,38] in a Euro 6 diesel engine. Some of these studies were carried out in an engine test bench simulating the NEDC driving cycle [29,33], whereas others have studied the use of n-butanol-diesel blends in a chassis dynamometer [30,38]. Only one study was found in the literature testing a Euro 6 vehicle in the chassis dynamometer under NEDC cycle at a cold ambient temperature [38]. Similar to stationary tests, these studies concluded that THC emissions increase and particulate matter sharply decreased for increasing butanol content. Concretely, the literature has reported that in both engine and vehicle tests, the particle number and particle mass emissions were reduced as the blend of butanol increased to 16% (v/v), leading to fewer and finer particles. However, for butanol blends higher than 16% (v/v), particle number and particle mass increased [29,38]. Therefore, particle emissions were found to be minimized for this blend (16% butanol 84% diesel, volume basis). Regarding gaseous emissions, there is no consensus about CO and NOX gaseous emissions for butanol blends. When the engine is fueled with butanol blends, Kozak [30] reported that CO emissions increase, whereas Armas et al. [33] concluded a reduction in CO emissions. NOX emissions remained constant in tests carried out by Lapuerta et al. [38], where a Euro 6 light-duty diesel vehicle was tested following the NEDC at warm and cold ambient conditions and in tests carried out by Kozak [30] testing a Euro 4 passenger car following the NEDC cycle under warm ambient conditions. However, NOX emissions increased in the study carried out by Armas et al. [33], where a Euro 4 engine was tested under the simulated NEDC in the engine test bench at a warm ambient temperature.
For those regulated emissions with no clear trend (CO and NOX emissions), a schematic diagram is shown in Figure 3 summarizing trends described by authors about the use of butanol-diesel blends in diesel engines. In terms of CO emissions, 52% of studies reviewed concluded an increase, whereas 43% of the authors reported a reduction in CO emissions when butanol blends are introduced. Regarding NOX emissions, 46% of the studies concluded that introducing butanol-diesel blends is beneficial, and only 36% of them observed an increasing trend. A total of 18% of the publications reviewed reported that NOX emissions remained constant when butanol blends are introduced.
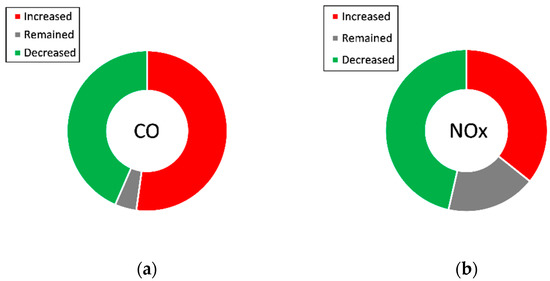
Figure 3.
(a) Summary of CO emissions trends for butanol blends in diesel engines; (b) Summary of NOX emissions trends for butanol blends in diesel engines.
Regarding the cold startability of butanol-diesel blends, Miers et al. [34] studied butanol-diesel blends with 20% and 40% butanol content (v/v) in a light-duty vehicle under transient conditions at a warm ambient temperature, concluding that butanol blends with butanol contents lower than 40% (v/v) could be successfully used in a diesel engine calibrated for 100% diesel fuel without startability problems. However, the vehicle driveability decreases noticeably when Bu40D is introduced, and an ECU recalibration would be needed for a satisfactory engine operation. The vehicle reported increasing roughness occurs during both steady conditions and acceleration events. This study is in agreement with the study carried out by Lapuerta et al. [38], where a Euro 6 light-duty diesel vehicle was tested in a chassis dynamometer following the NEDC under two different ambient conditions (24 and −7 °C). That study concluded that butanol-diesel blends up to 20% butanol content (%v/v) could be introduced without startability and driveability problems at 24 °C, whereas only butanol-diesel blends up to 13% (v/v) could be introduced without startability problems at −7 °C. At −7 °C, some driveability difficulties were also reported for this n-butanol blend (13% n-butanol 87% diesel fuel, volume basis).
Table 2 shows a detailed summary of the different studies found in the literature focused on the performance and regulated emissions of butanol-diesel blends in diesel engines and vehicles under stationary or transient conditions. Since in most of the studies, the reviewed engines were water-cooled, the cooling system is specified only when it is not water-cooled. Since the number of studies found in the literature blending butanol with other fuels or studying unregulated emissions is lower, these are not included in Table 2 but discussed below.

Table 2.
Summary of butanol-diesel blends tested in diesel engines.
Since the butanol content in the blend is mainly limited by the cetane number, the flashpoint and the heating value, only low alcohol contents are interesting because for higher alcohol contents, the heating value and the cetane are decreased. The latter is even below the lower limit established in the EN 590 standard [72]. Following the target established in the last directives promoting biofuels, the optimal range selected could range up to 20% (v/v) butanol content. Table 2 shows that most authors tested butanol-diesel blends up to 16–20% (v/v), reporting no negative effect on energy consumption for these blends with respect to reference diesel fuels. Higher butanol concentrations are generally discarded due to the reasons mentioned above. Although particle emissions find the minimum at 16% (v/v) butanol content, the workable range is reduced up to 13% (v/v) diesel substitution by butanol when startability is studied [38].
Although few studies were found blending butanol with other biofuels, the literature also reports engine tests with butanol-biodiesel blends. Jeevahan et al. [81] studied butanol-biodiesel blends with 10%, 20%, 30%, 40% and 50% of butanol content (%v/v) under four different engine loads concluding that the addition of butanol reduces specific fuel consumption (defined as the fuel consumed by the vehicle per distance traveled), CO, THC and NOX gaseous emissions. Yilmaz et al. [105] also studied butanol-biodiesel blends at 5%, 10% and 20% (volume basis) under different load conditions reporting that n-butanol increases CO and THC and reduces NOX emissions. Cedik, et al. [106] studied ternary blends (butanol-biodiesel-diesel) with 10% n-butanol, 20% biodiesel, 70% diesel and 20% n-butanol, 20% biodiesel and 60% diesel at different stationary conditions. Tests showed an increase in CO and THC emissions and a decrease in NOX and particle emissions.
Apart from regulated gaseous emissions, there are a minor number of studies measuring unregulated pollutants, which are generally emitted from the engine exhaust at much lower concentrations. These emissions are also important because they have potential health effects on humans and animals [41].
Among unregulated emissions, carbonyl compounds have received the highest attention. They are mainly formed by aldehydes and ketones, and they have a carbonyl group (a carbon atom linked to an oxygen atom by a double bond). Formaldehyde and acetaldehyde, which are the predominant carbonyls in the exhaust for vehicles, are toxic contaminants, mutagens and carcinogens [107]. Although few studies were found in the literature regarding unregulated emissions from butanol blends, it was concluded that, in general, alcohol blends with diesel fuels lead to higher carbonyl compound emissions than diesel fuel [41,108]. The high volatility of alcohols makes them partially escape from the combustion chamber mixed with the exhaust gas without being completely oxidized. Ballesteros et al. [109] reported that carbonyl emissions are slightly higher for butanol-diesel blends than for ethanol ones.
Exhaust emissions from diesel vehicles include aromatics such as benzene, toluene and xylene, often called BTX (benzene-toluene-xylene). According to the California Air Resources Board, benzene is a human carcinogen and may cause leukemia [110]. In general, BTX emissions decrease when alcohols are blended with diesel fuels, especially at a high engine load, and consequently, high exhaust temperature [111]. BTX emissions are influenced by the reduction in the combustion temperature when the alcohol is introduced (making BTX oxidation difficult) and by the oxygen in the alcohol molecule, which promotes BTX oxidation and thus contributes to the reduction in benzene emissions [112]. No information regarding BTX was found specifically for butanol-diesel blends.
In terms of the soot reactivity, the literature [113,114] concludes that butanol-diesel blends reduce the soot primary particle diameter and the soot mass density with respect to that of diesel fuel. The regeneration process of the diesel particle filter (DPF) is mainly affected by the exhaust gas composition, the flow rate, the temperature, the flow profiles through the filter channels and the physicochemical characteristics of the soot [115]. Therefore, the particle reduction, together with the better soot reactivity when butanol blends are used [116,117], contributes to a decrease in the DPF regeneration frequency, and therefore, lower oil dilution [118], lower fuel consumption and a longer after-treatment lifetime [119] can be achieved.
After describing and discussing trends derived from the introduction of butanol as a blending component for diesel fuel in diesel engines, reasons used by the authors to explain these trends in terms of regulated gaseous (CO, THC and NOX) and particle emissions, are discussed in the following points.
- CO and THC emissions are highly influenced by the ambient temperature, load, turbocharging and fueling system. Chen et al. [98] reported that CO emissions increase at a low load and they are reduced at a high load.
- Although there is no consensus regarding CO emissions, most of the authors justified the increases in CO and THC emissions for butanol blends with respect to diesel fuel is because of the high enthalpy of vaporization of n-butanol [120]. The fuel evaporation contributes to reducing the in-cylinder temperature, in particular during a cold start.
- Miers et al. [34] reported that n-butanol enhances the diesel oxidation catalyst (DOC) activity. This study concluded that, although the exhaust gas temperature upstream of the DOC was lower for butanol-diesel blends than for reference diesel fuel, the trend downstream of the DOC was reversed due to the oxidation activity inside the catalyst. This trend was confirmed in a Euro 6 diesel engine following an NEDC cycle [29]. These results are very promising for DPF and lean NOX trap (LNT) systems, which need a high temperature for their regeneration.
- Nitrogen oxide emissions are strongly dependent on temperature, local oxygen concentration and combustion duration [61]. Among the different strategies to reduce NO formation, delaying the fuel injection timing (thus affecting engine efficiency) and recirculating the exhaust gas is the most commonly used. In the latter, the introduction of cooled exhaust gas into the combustion chamber results in the dilution of the air charge by replacing O2 with the non-reacting CO2 and H2O. Therefore, the in-cylinder local combustion temperatures are reduced, thus inhibiting the NO formation.
- Regarding NOX emissions from butanol-diesel blends, the literature reports no clear trend because there is compensation between several factors when the engine is tested. Among the factors that contribute to reducing NOX emissions [61]:
- ○
- The higher enthalpy of the vaporization of butanol with respect to diesel fuel, which means that a lower amount of heat is available to increase the gas temperature [33].
- ○
- The low adiabatic flame temperature of n-butanol is derived from its lower C/H ratio [121].
On the other hand, an engine calibrated for diesel fuel operating with butanol-diesel blends requires higher fueling to achieve the demanded power. Since acceleration position is one of the inputs of the engine calibration maps, a decrease in the exhaust gas recirculation (EGR) rate is established in order to increase the air mass flow. Consequently, the NO formation increases.
- N-butanol contributes to significant benefits in particulate matter emissions:
- ○
- The butanol molecule contributes to the increase in oxygen concentration in the butanol-diesel blend, enhancing the soot oxidation process [49].
- ○
- The higher reactivity of n-butanol blends with respect to diesel fuel contributes to improving the soot oxidation process [116].
- ○
- Since the soot formation mainly takes place in the fuel-rich zone at high temperature and pressure conditions, the oxygenated character of n-butanol leads to a local reduction in fuel-rich regions and thus limiting soot formation [61]. In fact, the hydroxyl group of the butanol molecule contributes to reducing soot formation and consequently particulate emissions, even more than other functional groups with similar oxygen content [49]. Molecules with oxygen atoms single-bonded to a carbon atom (such as alcohols and ethers) are more effective at reducing PM emissions than those having double-bonds (such as alkyl esters) because the oxygen in the alcohol or ether is more effective at suppressing soot than the oxygen in the ester for equivalent oxygen content [122]. This was confirmed by Barrientos et al. group contribution method [123], in agreement with other authors [50].
- ○
- Blending diesel fuel with n-butanol reduces the aromatic and sulfur content (the latter does not have a significant influence because of the low sulfur content in current diesel fuels [72]) in the blend leading to a reduction in particulate matter emissions since these compounds are generally considered as soot precursors.
Although n-butanol blends reduce PM emissions, the biological activity of the soluble organic material from the PM emissions of butanol-diesel blends promotes more genotoxicity PM than diesel fuel. This could be a barrier to the butanol penetration in the market. However, further research is necessary to validate this conclusion because limited information was found in the literature. In any case, genotoxicity PM levels are higher for ethanol blends than for butanol blends [124].
Diesel fuels can be blended with bioalcohols, particularly with n-butanol, as a means to introduce a renewable fraction and to provide certain oxygen content. Oxygenated fuels such as alcohols are an effective way to reduce particle emissions. In fact, the butanol molecule contributes to increasing the oxygen concentration in the butanol-diesel blend, enhancing the soot oxidation process and also contributes to reducing the fuel-rich regions limiting the soot formation [123]. Introducing butanol leads to fewer and smaller particles, and thus to slower mean diameters [38]. The reduction in soot is beneficial for users as the frequency of active particulate regeneration is decreased, and thus the extra fuel consumption and the consequent eventual annoyance caused by the after-treatment maintenance.
5. Conclusions
This section summarizes the main conclusions derived from the use of butanol as a biofuel for diesel engines used in road freight transportation, tractors, harvesters and cogeneration. Specifically, the sustainability of lignocellulosic butanol, the properties of butanol-diesel blends and their influence on combustion parameters, transportation, fuel handling and storage, as well as butanol applications as a blending component for diesel fuels in commercial engines are summarized in this section.
N-butanol, produced from biological processes such as ABE fermentation, was reported to have a significant potential to reduce life-cycle greenhouse gas emissions with respect to fossil diesel fuel. Advanced processes to produce lignocellulosic biobutanol can achieve 60% lower GHG emissions than diesel fuel, whereas the reduction for the same fuel from conventional feedstock reaches 35%.
Although the alcohol most commonly used as a fuel component in the transport sector is ethanol, the higher cetane number of n-butanol, together with its higher heating value, better viscosity, better lubricity, better cold-flow properties and better miscibility with diesel, particularly at a low temperature, suggest that n-butanol is a better renewable component than ethanol in diesel blends. Furthermore, since n-butanol has a higher flash point, lower volatility and less corrosive character than ethanol, butanol blends are safer for transportation, fuel handling and storage than those of ethanol.
When butanol is introduced directly by blending with diesel fuel in the fuel tank, additional engine modifications or ECU recalibrations are not needed in a diesel engine calibrated for 100% diesel fuel up to 40% (v/v) butanol content. In most of the studies found in the literature testing butanol-diesel blends in diesel engines and vehicles, tests were made in a Euro 5 (or inferior) diesel engine, under steady conditions and under a warm ambient temperature. In general, the authors concluded that the presence of n-butanol contributes to a sharp decrease in PM emissions up to 16% butanol content (% v/v) and to an increase in THC emissions for increasing butanol content. However, there was no consensus regarding CO and NOX emissions. Most of the studies observed an increase in fuel consumption for butanol blends in line with the lower energy content of butanol compared to diesel fuel. The literature also concludes that a high butanol content in diesel can cause startability problems due to the very low cetane number of butanol. Concretely, startability problems are reported for butanol-diesel blends from 13% butanol onwards at cold ambient temperature, whereas no startability problems up to 40% butanol content are concluded for tests at a warm ambient temperature.
Author Contributions
Conceptualization, D.F.-R. and M.L.; Investigation, D.F.-R., M.L. and L.G.; Resources, D.F.-R.; Data Curation, D.F.-R. and M.L.; Writing—Original Draft Preparation, D.F.-R.; Writing—Review & Editing, M.L.; Visualization, D.F.-R., M.L. and L.G.; Supervision, M.L.; Project Administration, M.L.; Funding Acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European research project “Next Generation Bio-Butanol” (ButaNexT) Project EC-Contract No. 640462, within the European Union Framework Programme for Research and Innovation Horizon 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liaquat, A.M.; Kalam, M.A.; Masjuki, H.H.; Jayed, M.H. Potential emissions reduction in road transport sector using biofuel in developing countries. Atmos. Environ. 2010, 44, 3869–3877. [Google Scholar] [CrossRef]
- European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, 140, 16–62. [Google Scholar]
- European Union. Directive (EU) 2015/1513 of the European Parliament and of the Council of 9 September 2015 amending Directive 98/70/EC relating to the quality of petrol and diesel fuels and amending Directive 2009/28/EC on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2015, 239, 1–29. [Google Scholar]
- European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources (recast). Off. J. Eur. Union 2018, 328, 82–209. [Google Scholar]
- EEA Annual European Union Greenhouse Gas Inventory 1990–2016 and Inventory Report 2018. 2018. Available online: https://www.eea.europa.eu/publications/european-union-greenhouse-gas-inventory-2018 (accessed on 2 February 2021).
- Fayad, M.A.; Tsolakis, A.; Fernández-Rodríguez, D.; Herreros, J.M.; Martos, F.J.; Lapuerta, M. Manipulating modern diesel engine particulate emission characteristics through butanol fuel blending and fuel injection strategies for efficient diesel oxidation catalysts. Appl. Energy 2017, 190, 490–500. [Google Scholar] [CrossRef]
- Fang, T.; Lee, C.F. Bio-diesel effects on combustion processes in an HSDI diesel engine using advanced injection strategies. P. Combust. Inst. 2009, 32, 2785–2792. [Google Scholar] [CrossRef]
- Qi, D.H.; Geng, L.M.; Chen, H.; Bian, Y.Z.; Liu, J.; Ren, X.C. Combustion and performance evaluation of a diesel engine fueled with biodiesel produced from soybean crude oil. Renew. Energy 2009, 34, 2706–2713. [Google Scholar] [CrossRef]
- Dunn, R.O.; Bagby, M.O. Low-temperature properties of triglyceride-based diesel fuels: Transesterified methyl esters and petroleum middle distillate/ester blends. J. Am. Oil Chem. Soc. 1995, 72, 895–904. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Vojtisek-Lom, M.; Beránek, V.; Mikuška, P.; Krumal, K.; Coufalík, P.; Sikorová, J.; Topinka, J. Blends of butanol and hydrotreated vegetable oils as drop-in replacement for diesel engines: Effects on combustion and emissions. Fuel 2017, 197, 407–421. [Google Scholar] [CrossRef]
- Wu, M.; Wang, M.; Liu, J.; Huo, H. Assessment of potential life-cycle energy and greenhouse gas emission effects from using corn-based butanol as a transportation fuel. Biotechnol. Progr. 2008, 24, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- German, L.; Bauen, A. Revised Energy Balance and GHG Assessment; Project Deliverable from EU H2020 ButaNexT project; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Ndaba, B.; Chiyanzu, I.; Marx, S. n-butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 4, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baral, N.R.; Jha, A.K. Acetone-butanol-ethanol fermentation of corn stover by Clostridium species: Present status and future perspectives. World J. Microb. Biot. 2014, 30, 1145–1157. [Google Scholar] [CrossRef]
- Hergueta, C.; Bogarra, M.; Tsolakis, A.; Essa, K. Butanol-gasoline blend and exhaust gas recirculation, impact on GDI engine emissions. Fuel 2017, 208, 662–672. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Dimaratos, A.M.; Kyritsis, D.C. Effects of butanol–diesel fuel blends on the performance and emissions of a high-speed DI diesel engine. Energy Convers. Manag. 2010, 51, 1989–1997. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Lee, C.F.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Fernández-Rodríguez, D.; Cova-Bonillo, A. Autoignition of blends of n-butanol and ethanol with diesel and biodiesel fuels in a constant-volume combustion chamber. Energy 2017, 118, 613–621. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Obwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- No, S.Y. Application of biobutanol in advanced CI engines—A review. Fuel 2016, 183, 641–658. [Google Scholar] [CrossRef]
- Lapuerta, M.; Sánchez-Valdepeñas, J.; Sukjit, E. Effect of ambient humidity and hygroscopy on the lubricity of diesel fuels. Wear 2014, 309, 200–207. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Fernández-Rodríguez, D.; Patiño-Camino, R. Cold flow and filterability properties of n-butanol and ethanol blends with diesel and biodiesel fuels. Fuel 2018, 224, 552–559. [Google Scholar] [CrossRef]
- ISI Web of Knowledge. Available online: https://apps.webofknowledge.com (accessed on 5 April 2021).
- Iannuzzi, S.E.; Valentino, G. Comparative behavior of gasoline-diesel/butanol-diesel blends and injection strategy management on performance and emissions of a light duty diesel engine. Energy 2014, 71, 321–331. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Rodríguez-Fernández, J.; Barba, J.; Ramos, A.; Fernández-Rodríguez, D. Emission benefits from the use of n-butanol blends in a Euro 6 diesel engine. Int. J. Engine Res. 2018, 19, 1099–1112. [Google Scholar] [CrossRef]
- Kozak, M.J. Exhaust Emissions from a Diesel Passenger Car Fueled with a Diesel Fuel-Butanol Blend; SAE Technical Paper 2011-28-0017; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Siwale, L.; Kristóf, L.; Adam, T.; Bereczky, A.; Mbarawa, M.; Penninger, A.; Kolesnikov, A. Combustion and emission characteristics of n-butanol/diesel fuel blend in a turbo-charged compression ignition engine. Fuel 2013, 107, 409–418. [Google Scholar] [CrossRef]
- Choi, B.; Kim, Y.K.; Jung, G.; Lee, C.; Jiang, X.; Choi, I. Effect of diesel fuel blend with biobutanol on the emission of turbocharged CRDI diesel engine. Energy Procedia 2014, 61, 2145–2148. [Google Scholar] [CrossRef][Green Version]
- Armas, O.; García-Contreras, R.; Ramos, A. Pollutant emissions from New European Driving Cycle with ethanol and butanol diesel blends. Fuel Process. Technol. 2014, 122, 64–71. [Google Scholar] [CrossRef]
- Miers, S.A.; Carlson, R.W.; McConnell, S.S.; Ng, H.K.; Wallner, T.; Esper, J.L. Drive Cycle Analysis of Butanol/Diesel Blends in a Light-Duty Vehicle; SAE Technical Paper 2008-01-2381; SAE International: Warrendale, PA, USA, 2008. [Google Scholar] [CrossRef]
- Yao, M.; Wang, H.; Zheng, Z.; Yue, Y. Experimental study of n-butanol additive and multi-injection on HD diesel engine performance and emissions. Fuel 2010, 89, 2191–2201. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Dimaratos, A.M.; Giakoumis, E.G.; Rakopoulos, D.C. Investigating the emissions during acceleration of a turbocharged diesel engine operating with biodiesel or n-butanol diesel fuel blends. Energy 2010, 35, 5173–5184. [Google Scholar] [CrossRef]
- Al-Hasan, M.I.; Al-Momany, M. The effect of iso-butanol-diesel blends on engine performance. Transport 2008, 23, 306–310. [Google Scholar] [CrossRef]
- Lapuerta, M.; Ramos, A.; Barba, J.; Fernández-Rodríguez, D. Cold- and warm-temperature emissions assessment of n-butanol blends in a Euro 6 vehicle. Appl. Energy 2018, 218, 173–183. [Google Scholar] [CrossRef]
- Imran, A.; Varman, N.; Masjuki, H.H.; Kalam, M.A. Review on alcohol fumigation on diesel engine: A viable alternative dual fuel technology for satisfactory engine performance and reduction of environment concerning emission. Renew. Sustain. Energy Rev. 2013, 26, 739–751. [Google Scholar] [CrossRef]
- Trindade, W.; Gonçalves dos Santos, R. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 642–651. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A. Effect of alcohol blend and fumigation on regulated and unregulated emissions of IC engines—A review. Renew. Sustain. Energy Rev. 2016, 57, 1440–1495. [Google Scholar] [CrossRef]
- Moon, H.G.; Jang, Y.; Cho, C.; Lee, J.; Binkley, R.; Lee, S.Y. One hundred years of clostridial butanol fermentation. FEMS Microbiol. Lett. 2016, 363, 1–15. [Google Scholar] [CrossRef]
- Quantifying the Indirect Land Use Change Impact of Biofuels Consumed in the EU (ILUC). International Institute for Applied Systems Analysis (IIASA). Available online: https://iiasa.ac.at/web/home/research/researchPrograms/EcosystemsServicesandManagement/ILUC/ILUC.en.html (accessed on 8 May 2015).
- Brito, M.; Martins, F. Life cycle assessment of butanol production. Fuel 2017, 208, 476–482. [Google Scholar] [CrossRef]
- Pereira, L.G.; Chagas, M.F.; Dias, M.O.S.; Cavalett, O.; Bonomi, A. Life cycle assessment of butanol production in sugarcane biorefineries in Brazil. J. Clean Prod. 2015, 96, 557–568. [Google Scholar] [CrossRef]
- Wu, M.; Wang, M.; Liu, J.; Huo, H. Life Cycle Assessment of Corn-Based Butanol as a Potential Transportation Fuel; ANL/ESD/07-10; Argonne National Laboratory: Lemont, IL, USA, 2007.
- Elgowainy, A.; Han, J.; Ward, J.; Joseck, F.; Gohlke, D.; Lindauer, A.; Ramsden, T.; Biddy, M.; Alexander, M.; Barnhart, S.; et al. Cradle-to Grave Lifecycle Analysis of U.S. Light-Duty Vehicle-Fuel Pathways: A Greenhouse Gas Emissions and Economic Assessment of Current (2015) and Future (2025–2030) Technologies; ANL/ESD-16/7; Argonne National Laboratory: Lemont, IL, USA, 2016.
- Müller-Langer, F.; Majer, S.; O’Keeffe, S. Benchmarking biofuels–a comparison of technical, economic and environmental indicators. Energy Sustain. Soc. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakumis, E.G. Impact of properties of vegetable oil, ethanol and n-butanol on the combustion and emissions of turbocharged HDDI diesel engine operating under steady and transient conditions. Fuel 2015, 156, 1–19. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Cadrazco, M.; López, A.F.; Sanchez-Valdepeñas, J.; Agudelo, J.R. Impact of dual-fuel combustion with n-butanol or hydrous ethanol on the oxidation reactivity and nanostructure of diesel particulate matter. Fuel 2015, 161, 18–25. [Google Scholar] [CrossRef]
- Eugene, E.E.; Bechtold, R.L.; Timbario, T.J.; McCallum, P.W. State of the art report on the use of alcohols in diesel engines. SAE Tech. Pap. 1984, 93, 840118. [Google Scholar]
- López, A.F.; Cadrazco, M.; Agudelo, A.F.; Corredor, L.A.; Vélez, J.A.; Agudelo, J.R. Impact of n-butanol and hydrous ethanol fumigation on the performance and pollutant emissions of an automotive diesel engine. Fuel 2015, 153, 483–491. [Google Scholar] [CrossRef]
- Gowthan, M.; Mohan, C.G.; Prakash, R. Effect of n-butanol fumigation on the regulated and unregulated emission characteristics of a diesel engine. Fuel 2019, 242, 84–95. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; García-Contreras, R. Effect of ethanol on blending stability and diesel engine emissions. Energy Fuel 2009, 23, 4343–4354. [Google Scholar] [CrossRef]
- Lapuerta, M.; García-Contreras, R.; Campos Fernández, J.; Dorado, M.P. Stability, lubricity, viscosity and cold flow properties of alcohol-diesel blends. Energy Fuel 2010, 24, 4497–4502. [Google Scholar] [CrossRef]
- Zelenka, P.; Kapus, P.; Mikulic, L.A. Development and optimization of methanol fueled compression ignition engines for passenger cars and light duty trucks. SAE Tech. Pap. 1991, 910851. [Google Scholar]
- Xiaolu, L.; Xinqi, Q.; Liang, Z.; Junhua, F.; Zhen, H.; Huimin, X. Combustion and emission characteristics of a two-stroke diesel engine operating on alcohol. Renew. Energy 2005, 30, 2075–2084. [Google Scholar]
- Huang, J.; Wang, Y.; Li, S.; Roskilly, A.P.; Yu, H.; Li, H. Experimental investigation on the performance and emissions of a diesel engine fuelled with ethanol–diesel blends. Appl. Therm. Eng. 2009, 29, 2484–2490. [Google Scholar] [CrossRef]
- Abo-Rachid, H.; El Marrouni, K.; Kaliaguine, S. DFT studies of the hydrogen abstraction from primary alcohols by O2 in relation with cetane number data. J. Mol. Struct. Theochem. 2003, 631, 241–250. [Google Scholar] [CrossRef]
- Vinod Babu, M.; Madhu Murthy, K.; Amba Prasad Rao, G. Butanol and pentanol: The promising biofuels for CI engines-a review. Renew. Sustain. Energy Rev. 2017, 78, 1068–1088. [Google Scholar]
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions with ethanol or n-butanol diesel fuel blends during transient operation: A review. Renew. Sustain. Energy Rev. 2013, 17, 170–190. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Siddiquah, M. Partial molar volume of mefenamic acid in alcohol at temperatures between T=293.15 and T=313.15 K. J. Brazil Chem. Soc. 2006, 17, 851–858. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; García-Contreras, R.; Bogarra, M. Molecular interactions in blends of alcohols with diesel fuels: Effect on stability and distillation. Fuel 2015, 139, 171–179. [Google Scholar] [CrossRef]
- Aissa, M.A.; Radovic, I.R.; Kijevcanin, M.L. A systematic study on volumetric and transport properties of binary systems 1-propanol + n-hexadecane, 1-butanol + n-hexadecane and 1-propanol + ethyl oleate at different temperatures: Experimental and modeling. Fluid Phase Equilibr. 2018, 473, 1–16. [Google Scholar] [CrossRef]
- Dubey, G.P.; Sharma, M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalene. J. Chem. Eng. 2008, 53, 1032–1038. [Google Scholar] [CrossRef]
- Mehra, R.; Israni, R. Effect of temperature on excess molar volumes of binary mixtures of hexadecane and butanol. Indian J. Pure Appl. Phys. 2000, 38, 81–83. [Google Scholar]
- Barabás, I. Liquid densities and excess molar volumes of ethanol + biodiesel binary system between the temperatures 273.15 K and 333.15 K. J. Mol. Liq. 2015, 204, 95–99. [Google Scholar] [CrossRef]
- Djojoputro, H.; Ismadji, S. Density and viscosity of binary mixtures of ethyl-2-methylbutyrate and ethyl hexanoate with methanol, ethanol, and 1-propanol at (293.15, 303.15, and 313.15) K. J. Chem. Eng. Data 2005, 50, 1343–1347. [Google Scholar] [CrossRef]
- Korres, D.M.; Anastopoulos, G.; Lois, E.; Alexandridis, A.; Sarimveis, H.; Bafas, G. A neural network approach to the prediction of diesel fuel lubricity. Fuel 2002, 81, 1243–1250. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compound. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Sci. Technol. 2009, 6, 677–694. [Google Scholar] [CrossRef]
- Automotive Fuels-Diesel-Requirements and Test Methods; European Committee for Standarization: Brussels, Belgium, 2013.
- Lapuerta, M.; Rodríguez-Fernández, J.; Fernández-Rodríguez, D.; Patiño-Camino, R. Modeling viscosity of butanol and ethanol blends with diesel and biodiesel fuels. Fuel 2017, 199, 332–338. [Google Scholar] [CrossRef]
- Kuszewski, H. Physical and Chemical Properties of 1-Butanol–Diesel Fuel Blends. Energy Fuel 2018, 23, 11619–11631. [Google Scholar] [CrossRef]
- Chotwichien, A.; Luengnaruemitchai, A.; Jai-In, S. Utilization of palm oil alkyl esters as an additive in ethanol-diesel and butanol-diesel blends. Fuel 2009, 88, 1618–1624. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Huang, Y.; Huang, S.; Zhou, P.; Chen, X.; Qin, T. Experimental study on combustion characteristics of an n-butanol-biodiesel droplet. Energy 2018, 160, 490–499. [Google Scholar] [CrossRef]
- Habibi, H.; Hekmat-Nazemi, A.; Kamran-Pirzaman, A.; Mohammadi, A.H. Modeling viscosity of alcohols based on the CPA-EoS + f-theory. J. Mol. Liq. 2016, 220, 558–565. [Google Scholar] [CrossRef]
- Cano-Gómez, J.J.; Iglesias-Silva, G.A. A new correlation for the prediction of kinematic viscosities of biodiesel + higher alcohols blends at atmospheric pressure. Fuel 2018, 237, 1254–1261. [Google Scholar] [CrossRef]
- Hansen, A.C.; Zhang, Q.; Lyne, P.W. Ethanol-diesel fuel blends—A review. Bioresour. Technol. 2005, 96, 277–285. [Google Scholar] [CrossRef]
- Hernández, J.P.; Lapuerta, M.; García-Contreras, R.; Agudelo, J.R. Modelling of evaporative losses in n-alcohol/diesel fuel blends. Appl. Therm. Eng. 2016, 102, 302–310. [Google Scholar] [CrossRef]
- Jeevahan, J.; Sriramanjaneyulu, S.; Durairaj, R.B.; Mageshwaran, G. Experimental investigation of the suitability of 1-butanol blended with biodiesel as an alternative biofuel in diesel engines. Biocatal. Agric. Biotechnol. 2018, 15, 72–77. [Google Scholar] [CrossRef]
- Chamdra, R.; Kumar, R. Fuel properties of some stable alcohol–diesel microemulsions for their use in compression ignition engines. Energy Fuel 2007, 21, 3410–3414. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jaworski, A.; Ustrzycki, A. Lubricity of ethanol–diesel blends–Study with the HFRR method. Fuel 2017, 208, 491–498. [Google Scholar] [CrossRef]
- Kwanchareon, P.; Luengnaruemitchai, A.; Jai-In, S. Solubility of a diesel-biodiesel-ethanol blend, its fuel properties and its emission characteristics from diesel engine. Fuel 2007, 86, 1053–1061. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Papagiannakis, R.G.; Kyritsis, D.C. Combustion heat release analysis of ethanol or n-butanol diesel fuel blends in heavy-duty DI diesel engine. Fuel 2011, 90, 1855–1867. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanism and Structure; John Wiley and Sons: Hoboken, NJ, USA, 2020; ISBN 978-0-470-46259-1. [Google Scholar]
- Makareviciene, V.; Kazancev, K.; Kazanceva, I. Possibilities for improving the cold flow properties of biodiesel fuel by blending with butanol. Renew. Energy 2015, 75, 805–807. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, N.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef]
- Dunn, R.O. Effects of minor constituents on cold flow properties and performance of biodiesel. Prog. Energy Combust. 2009, 35, 481–489. [Google Scholar] [CrossRef]
- De Torres, M.; Jiménez-Osés, G.; Mayoral, J.A.; Pires, E. Fatty acid derivatives and their use as CFPP additivies in biodiesel. Bioresour. Technol. 2001, 102, 2590–2594. [Google Scholar] [CrossRef]
- Regulating Australian Fuel Quality. Available online: www.environment.gov.au/topics/environment-protection/fuel-quality/standards/diesel (accessed on 26 October 2020).
- Bouaid, A.; El Boulifi, N.; Hahati, K.; Martinez, M.; Aracil, J. Biodiesel production from biobutanol. Improvement of cold flow properties. Chem. Eng. J. 2014, 238, 234–241. [Google Scholar] [CrossRef]
- Hernández, J.J.; Lapuerta, M.; Cova-Bonillo, A. Autoignition reactivity of blends of diesel and biodiesel fuels with butanol isomers. J. Energy Inst. 2019, 92, 1223–1231. [Google Scholar] [CrossRef]
- Yanai, T.; Han, X.; Reader, A.T.; Zheng, M.; Tjong, J. Preliminary investigation of direct injection neat n-butanol in a diesel engine. J. Energy Resour. Technol. 2015, 137, 1–10. [Google Scholar] [CrossRef]
- Nabi, M.D.; Zare, A.; Hossain, F.M.; Bodisco, T.A.; Ristovski, Z.D.; Brown, R.J. A parametric study on engine performance and emissions with neat diesel and diesel-butanol blends in the 13-Mode European Stationary Cycle. Energy Convers. Manag. 2017, 184, 251–259. [Google Scholar] [CrossRef]
- Dogan, O. The influence of n-butanol/diesel fuel blends utilization on a small diesel engine performance and emissions. Fuel 2011, 90, 2467–2472. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, D.; Naseer Siddiquee, M.; Nagpal, A.; Kumar, N. Performance and Emission Characteristics of n-Butanol and ISO-Butanol Diesel Blend Comparison; SAE Technical Paper 2015-01-2819; SAE International: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Han, Z.; Du, B.; Liu, Y.; Lee, C. Study of performance and emissions of a passenger-car diesel engine fueled with butanol-diesel blends. Energy 2013, 55, 638–646. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. A comparative analysis of n-butanol/diesel and pentanol/diesel blends in a compression ignition engine. Fuel 2018, 234, 161–169. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Hountalas, D.T.; Kakaras, E.C.; Giakoumis, E.G.; Papagiannakis, R.G. Investigation of the performance and emissions of bus engine operating on butanol/diesel fuel blends. Fuel 2010, 89, 2781–2790. [Google Scholar] [CrossRef]
- Choi, B.; Jiang, X.; Kim, Y.K.; Jung, G.; Lee, C.; Choi, I.; Song, C.S. Effect of diesel fuel blend with n-butanol on the emission of a turbocharged common rail direct injection diesel engine. Appl. Energy 2015, 146, 20–28. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Teng, W.; Huang, R.; Liu, Q.; Wang, Y. Effects of EGR rates on combustion and emission characteristics in a diesel engine with n-butanol/PODE3-4/diesel blends. Appl. Therm. Eng. 2019, 146, 212–222. [Google Scholar] [CrossRef]
- Mack, J.H.; Schuler, D.; Butt, R.H.; Dibble, R.W. Experimental investigation of butanol isomer combustion in homogeneous charge compression ignition (HCCI) engines. Appl. Energy 2016, 165, 612–626. [Google Scholar] [CrossRef]
- Satsangi, D.P.; Tiwari, N. Experimental investigation on combustion, noise, vibrations, performance and emissions characteristics of diesel/n-butanol blends driven genset engine. Fuel 2018, 221, 48–60. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Benalil, K.; Davis, S.M.; Calva, A. Effect of biodiesel-butanol fuel blends on emissions and performance characteristics of a diesel engine. Fuel 2014, 135, 46–50. [Google Scholar] [CrossRef]
- Čedík, J.; Pexa, M.; Holúbek, M.; Aleš, Z.; Pražan, R.; Kuchar, P. Effect of diesel fuel-coconut oil-butanol blends on operational parameters of diesel engine. Energies 2020, 13, 3796. [Google Scholar] [CrossRef]
- Yang, P.; Lin, Y.; Lin, K.C.; Jhang, S.; Chen, S.; Wang, C.; Lin, Y. Comparison of carbonyl compound emissions from a diesel engine generator fueled with blends of n-butanol, biodiesel and diesel. Energy 2015, 90, 266–273. [Google Scholar] [CrossRef]
- Song, C.L.; Zhao, Z.A.; Lv, G.; Song, J.N.; Liu, L.D.; Zhao, R.F. Carbonyl compound emissions from a heavy-duty diesel engine fueled with diesel fuel and ethanol–diesel blend. Chemosphere 2010, 79, 1033–1039. [Google Scholar] [CrossRef]
- Ballesteros, R.; Hernández, J.J.; Guillén-Flores, J. Carbonyls speciation in a typical European automotive diesel engine using bioethanol/butanol–diesel blends. Fuel 2012, 95, 136–145. [Google Scholar] [CrossRef]
- Törnqvist, M.; Ehrenberg, L. On cancer risk estimation of urban air. Environ. Health Persp. 1994, 102, 173–182. [Google Scholar]
- Merritt, P.M.; Ulmet, V.; McCormick, R.L.; Mitchell, W.E.; Baumgard, K.J. Regulated and Unregulated Exhaust Emissions Comparison for Three Tier Nonroad Diesel Engines Operating on Ethanol Diesel Blends; SAE Technical Paper 2005-01-2193; SAE International: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Cheung, C.S.; Di, Y.; Huang, Z. Experimental investigation of regulated and unregulated emissions from a diesel engine fueled with ultralow-sulfur diesel fuel blended with ethanol and dodecanol. Atmos. Environ. 2008, 42, 8843–8851. [Google Scholar] [CrossRef]
- Yan, F.; Cheng, X.; Qiu, L.; Huang, R.; Huang, S.; Liu, B. Spray flame soot sampling and morphology analysis of butanol–diesel blends. J. Energy Inst. 2017, 90, 855–863. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Zheng, Z.; Xu, J.; Yao, M. Effects of n-butanol, 2-butanol, and methyl octynoate addition to diesel fuel on combustion and emissions over a wide range of exhaust gas recirculation (EGR) rates. Appl. Energy 2013, 112, 246–256. [Google Scholar] [CrossRef]
- Song, J.; Alam, M.; Boehman, A.L. Impact of alternative fuels on soot properties and DPF regeneration. Combust. Sci. Tecnol. 2007, 179, 1997–2037. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Hernández, J.J.; Sánchez-Valdepeñas, J. Effect of oxygenated and paraffinic alternative diesel fuels on soot reactivity and implications on DPF regeneration. Fuel 2016, 185, 460–467. [Google Scholar] [CrossRef]
- Lapuerta, M.; Sánchez-Valdepeñas, J.; Barba, J.; Fernández-Rodríguez, D.; Andrés, J.P.; García, T. Analysis of soot from the use of butanol blends in a Euro 6 diesel engine. Energy Fuel 2019, 33, 2265–2277. [Google Scholar] [CrossRef]
- Thornton, M.; Alleman, T.; Luecke, J.; McCormick, R. Impacts of biodiesel fuel blends oil dilution on light-duty diesel engine operation. SAE Int. J. Fuels Lubr. 2009, 2, 781–788. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Lapuerta, M.; Sánchez-Valdepeñas, J. Regeneration of diesel particulate filters: Effect of renewable fuels. Renew. Energy 2017, 104, 30–39. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Liu, J.; Lee, C. Combustion and emissions characteristics of high n-butanol/diesel ratio blend in a heavy-duty diesel engine and EGR impact. Energy Convers. Manag. 2014, 78, 787–795. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0521154215. [Google Scholar]
- William, A.; Black, S.; McCormick, R.L. Biodiesel fuel property effects on particulate matter reactivity. In Proceedings of the 6th International Exhaust Gas and Particulate Emissions Forum, Ludwigsburg, Germany, 9 March 2010. NREL/CP-540-74286 1-9. [Google Scholar]
- Barrientos, E.J.; Lapuerta, M.; Boehman, A.L. Group additivity in soot formation for the example of C-5 oxygenated hydrocarbon fuels. Combust. Flame 2013, 160, 1484–1498. [Google Scholar] [CrossRef]
- Cadrazco, M.; Agudelo, J.R.; Orozco, L.Y.; Estrada, V. Genotoxicity of diesel particulate matter emitted by port-injection of hydrous ethanol and butanol. J. Energy Resour. Technol. 2017, 139, 042207. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Akasyah, M.K.; Jamol, M.F.; Yusop, A.F. Evaluation of engine combustion and exhaust emissions characteristics using diesel/butanol blended fuel. Appl. Therm. Eng. 2019, 156, 209–219. [Google Scholar] [CrossRef]
- Nour, M.; Attia, A.M.A.; Nada, S.A. Combustion, performance and emission analysis of diesel engine fueled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers. Manag. 2019, 185, 313–329. [Google Scholar] [CrossRef]
- Joy, N.; Balan, K.N.; Nagappan, B.; Abraham Baby, S.J. Emission analysis of diesel and butanol blends in research diesel engine. Pet. Sci. Tecnol. 2020, 38, 289–296. [Google Scholar] [CrossRef]
- Pan, M.; Tong, C.; Qian, W.; Lu, F.; Yin, J.; Huang, H. The effect of butanol isomers on diesel engine performance, emission and combustion characteristics under different load conditions. Fuel 2020, 277, 118188. [Google Scholar] [CrossRef]
- Siva-Prasad, K.; Srinivasa Rao, S.; Raju, V.R.K. Effect of compression ratio and fuel injection pressure on the characteristics of a CI engine operating with butanol/diesel blends. Alex. Eng. J. 2021, 60, 1183–1197. [Google Scholar] [CrossRef]
- Li, J.; Yu, W.; Yang, W. Evaluating performance and emissions of a CI engine fueled with n-octanol/diesel and n-butanol/diesel blends under different injection strategies. Fuel 2021, 284, 119085. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).