Cultivation Method Effect on Schizochytrium sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cultivation Conditions
2.3. Materials
2.4. Experimental Station
2.5. Experimental Procedures
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DHA | docosahexaenoic acid |
| TFA | total fatty acids |
| DCW | dry cell weight |
| ATCC | American Type Culture Collection |
| D | dilution rate (dilution factor) |
| F | substrate flow rate |
| V | bioreactor tank volume |
| NADH | nicotinamide adenine dinucleotide |
| FID | flame ionization detector |
| rDHA | rate of DHA production by microalgae |
| rDCW | growth rate of the microalgal biomass |
| rlipids | rate of lipids production |
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Zárate, R.; el Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6. [Google Scholar] [CrossRef]
- Yamagata, K. Dietary docosahexaenoic acid inhibits neurodegeneration and prevents stroke. J. Neurosci. Res. 2020. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Duttaroy, A.K. Maternal Docosahexaenoic Acid Status during Pregnancy and Its Impact on Infant Neurodevelopment. Nutrients 2020, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids focusing on eicosapentaenoic acid and docosahexaenoic acid in the prevention of cardiovascular diseases: A review of the state-of-the-art. Expert Rev. Clin. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hei, A. Fish as a Functional Food in Human Health, Diseases and Well–Being. In Proceedings of the 107th Indian Science Congress, Banglore, India, 3–7 January 2020; Current Status of Researches in Fish and Fisheries, Today and Tomorrow Printers and Publishers: New Delhi, India, 2020. [Google Scholar]
- Rodgers, A.L.; Siener, R. The Efficacy of Polyunsaturated Fatty Acids as Protectors against Calcium Oxalate Renal Stone Formation: A Review. Nutrients 2020, 12, 1069. [Google Scholar] [CrossRef]
- Colombo, S.M.; Rodgers, T.F.M.; Diamond, M.L.; Bazinet, R.P.; Arts, M.T. Projected declines in global DHA availability for human consumption as a result of global warming. Ambio 2020, 49, 865–880. [Google Scholar] [CrossRef]
- Schade, S.; Stangl, G.I.; Meier, T. Distinct microalgae species for food—Part 2: Comparative life cycle assessment of microalgae and fish for eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and protein. J. Appl. Phycol. 2020, 32, 2997–3013. [Google Scholar] [CrossRef]
- Yadav, A.K.; Rossi, W.; Habte-Tsion, H.-M.; Kumar, V. Impacts of dietary eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) level and ratio on the growth, fatty acids composition and hepatic-antioxidant status of largemouth bass (Micropterus salmoides). Aquaculture 2020, 529. [Google Scholar] [CrossRef]
- Tan, K.; Ma, H.; Li, S.; Zheng, H. Bivalves as future source of sustainable natural omega-3 polyunsaturated fatty acids. Food Chem. 2020. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Roy, R.K.; Chadha, A. Docosahexaenoic acid production by a novel high yielding strain of Thraustochytrium sp. of Indian origin: Isolation and bioprocess optimization studies. Algal Res. 2018, 32, 93–100. [Google Scholar] [CrossRef]
- Nazir, Y.; Halim, H.; Al-Shorgani, N.K.N.; Manikan, V.; Hamid, A.A.; Song, Y. Efficient conversion of extracts from low-cost, rejected fruits for high-valued Docosahexaenoic acid production by Aurantiochytrium sp. SW1. Algal Res. 2020, 50. [Google Scholar] [CrossRef]
- Park, W.-K.; Moon, M.; Shin, S.-E.; Cho, J.M.; Suh, W.I.; Chang, Y.K.; Lee, B. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Barclay, W.; Weaver, C.; Metz, J.; Hansen, J. Development of a Docosahexaenoic Acid Production Technology Using Schizochytrium: Historical Perspective and Update. In Single Cell Oils: Microbial and Algal Oils; Cohen, W.Z., Ratledge, C., Eds.; AOCS Press: Urbana, IL, USA, 2010; pp. 82–85. [Google Scholar]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, F.; Zhang, R.; Zhao, L.; Qi, J. Recent progress on biodiesel production from municipal sewage sludge. Renew. Sustain. Energy Rev. 2021, 135. [Google Scholar] [CrossRef]
- Sondhi, S.; Kumar, P. Biodiesel from sewage sludge: An alternative to diesel. In Recent Trends in Biotechnology; MedDocs: Punjab, India, 2020; pp. 1–6. [Google Scholar]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Hydrogenolysis of glycerol to propylene glycol by in situ produced hydrogen from aqueous phase reforming of glycerol over SiO2–Al2O3 supported nickel catalyst. Fuel Process. Technol. 2016, 142, 135–146. [Google Scholar] [CrossRef]
- Bagul, V.P.; Annapure, U.S. Effect of sequential recycling of spent media wastewater on docosahexaenoic acid production by newly isolated strain Aurantiochytrium sp. ICTFD5. Bioresour. Technol. 2020, 306. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wu, S.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. Co-production of high quality hydrogen and synthesis gas via sorption-enhanced steam reforming of glycerol coupled with methane reforming of carbonates. Chem. Eng. J. 2019, 360, 47–53. [Google Scholar] [CrossRef]
- Okhlopkova, E.A.; Serafimov, L.A.; Frolkova, A.V. Methods of Preparing Epichlorohydrin. Theor. Found. Chem. Eng. 2019, 53, 864–870. [Google Scholar] [CrossRef]
- Bouriakova, A.; Mendes, P.S.F.; Katryniok, B.; De Clercq, J.; Thybaut, J.W. Co-metal induced stabilization of alumina-supported copper: Impact on the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Commun. 2020, 146. [Google Scholar] [CrossRef]
- Chiosso, M.E.; Casella, M.L.; Merlo, A.B. Synthesis and catalytic evaluation of acidic carbons in the etherification of glycerol obtained from biodiesel production. Catal. Today 2020. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, J.J.; Shen, J.T.; Zheng, Y.F.; Sun, Y.; Xiu, Z.L. Sequential fed-batch fermentation of 1,3-propanediol from glycerol by Clostridium butyricum DL07. Appl. Microbiol. Biotechnol. 2020, 104, 9179–9191. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Cheng, J.; Hua, J.; Dong, H.; Zhou, J.; Li, Y.-Y. Improving fermentative methane production of glycerol trioleate and food waste pretreated with ozone through two-stage dark hydrogen fermentation and anaerobic digestion. Energy Convers. Manag. 2020, 203. [Google Scholar] [CrossRef]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Fact. 2018, 17, 97. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Optimizing Docosahexaenoic Acid (DHA) Production by Schizochytrium sp. Grown on Waste Glycerol. Energies 2021, 14, 1685. [Google Scholar] [CrossRef]
- Chang, G.; Gao, N.; Tian, G.; Wu, Q.; Chang, M.; Wang, X. Improvement of docosahexaenoic acid production on glycerol by Schizochytrium sp. S31 with constantly high oxygen transfer coefficient. Bioresour. Technol. 2013, 142, 400–406. [Google Scholar] [CrossRef]
- Grayburn, W.; Collins, G.; Hildebrand, D. Fatty acid alteration by a h-9 desaturase in transgenic tabacco tissue. Biotechnology 1992, 10, 675–678. [Google Scholar]
- Lee, C.; Nichols, C.; Blackburn, S.; Dunstan, G.; Koutoulis, A.; Nichols, P. Comparison of Thraustochytrids: Aurantochytrium sp., Schizochytrium sp., Thraustochytrium sp. and Ulkenia sp. for production of biodiesel long-chain omega-3 oils and exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar]
- Nguyen, H.C.; Su, C.-H.; Yu, Y.-K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crop. Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants-A review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Mata, T.; Santosa, J.; Mendesa, A.; Caetanoa, N.; Martinsc, A. Sustainability Evaluation of Biodiesel Produced from Microalgae Chlamydomonas sp. grown in brewery wastewater. Chem. Eng. Trans. 2014, 37, 823–828. [Google Scholar]
- Quilodrán, B.; Cortinez, G.; Bravo, A.; Silva, D. Characterization and comparison of lipid and PUFA production by native thraustochytrid strains using complex carbon sources. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Wang, Q.; Sen, B.; Liu, X.; He, Y.; Xie, Y.; Wang, G. Enhanced saturated fatty acids accumulation in cultures of newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. for large-scale biodiesel production. Sci. Total Environ. 2018, 631–632, 994–1004. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, K.; Kim, J.; Han, J.; Yang, J. Use of organic waste from brewery industry for high-density cultivation of docosahexaenoic acid-rich microalga Aurantochytrium sp. KRS101. Bioresour. Technol. 2012, 129, 351–359. [Google Scholar] [CrossRef]
- Thyagarajan, T.; Puri, M.; Vongsvivut, J.; Barrow, C. Evaluation of bread crumbs as a potential carbon source for the growth of thraustochytrid species for oil and omega-3. Nutrients 2014, 6, 2104–2114. [Google Scholar] [CrossRef]
- Unagul, P.; Assantachai, C.; Phadungruengluij, S.; Suphantharika, M.; Tanticharoen, M.; Verduyn, C. Coconut water as a medium additive for the production of docosahexaenoic acid (C22:6 n3) by Schizochytriummangrovei Sk-02. Bioresour. Technol. 2007, 98, 281–287. [Google Scholar] [CrossRef]
- Hong, W.; Yu, A.; Heo, S.; Oh, B.; Kim, C.; Sohn, J.; Yang, J.W.; Kondo, A.; Seo, J.W. Production of lipids containing high levels of docosahexaenoic acid from empty palm fruit bunches by Aurantiochytrium sp. KRS101. Bioprocess Biosyst. Eng. 2013, 36, 959–963. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y.; Yesuf, J.; Trushenski, J.; Blackburn, J.W. Use of sweet sorghum juice for lipid production by Schizochytriumlimacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [Google Scholar] [CrossRef]
- Fan, K.W.; Chen, F.; Jones, E.B.G.; Vrijmoed, L.L.P. Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J. Ind. Microbiol. Biot. 2001, 27, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Quilodran, B.; Hinzpeter, I.; Quiroz, A.; Shene, C. Evaluation of liquid residues from beer and potato processing for the production of docosahexaenoic acid (C22:6n-3, DHA) by native thraustochytrid strains. World J. Microbiol. Biotechnol. 2009, 25, 2121–2128. [Google Scholar] [CrossRef]
- Sun, X.-M.; Geng, L.-J.; Ren, L.-J.; Ji, X.-J.; Hao, N.; Chen, K.-Q.; Huang, H. Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae. Bioresour. Technol. 2018, 250, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xie, X.; Meesapyodsuk, D. Molecular mechanisms for biosynthesis and assembly of nutritionally important very long chain polyunsaturated fatty acids in microorganisms. Prog. Lipid Res. 2020, 79, 101047. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, Z.; Li, P.; Xia, J.; Chen, B. Formation of triacylglycerol in Nitzschiaclosterium f. minutissima under nitrogen limitation and possible physiological and biochemical mechanisms. J. Exp. Mar. Biol. Ecol. 2012, 418–419, 24–29. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Sun, P.; Ma, X.; Jiang, Y.; Chen, F. Sesamol Enhances Cell Growth and the Biosynthesis and Accumulation of Docosahexaenoic Acid in the Microalga Crypthecodiniumcohnii. J. Agric. Food Chem. 2015, 63, 5640–5645. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Bi, Z.-Q.; Ji, X.-J.; Zhao, Q.-Y.; Huang, H. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresour. Technol. 2018, 267, 438–444. [Google Scholar] [CrossRef]
- Abad, S.; Turon, X. Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: Focus on polyunsaturated fatty acids. Biotechnol. Adv. 2012, 30, 733–741. [Google Scholar] [CrossRef]
- Athalye, S.; Garcia, R.; Wen, Z. Use of biodiesel-derived crude glycerol for producing eicosapentaenoic acid (EPA) by the fungus Pythium irregulare. J. Agric. Food Chem. 2009, 57, 2739–2744. [Google Scholar] [CrossRef]
- Wu, S.; Yu, S.; Lin, L. Effects of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process. Biochem. 2005, 40, 3103–3108. [Google Scholar] [CrossRef]
- Bailey, R.; DiMasi, D.; Hansen, J.; Mirrasoul, P.; Ruecker, C.; Kaneko, T.; Barclay, W. Enhanced Production of Lipids Containing Polyenoic Fatty Acid by Very High Density Cultures of Eukaryotic Microbes in Fermentors. U.S. Patent 6607900 B2, 19 August 2003. [Google Scholar]
- Ganuza, E.; Izquierdo, M. Lipid accumulation in Schizochytrium G13/2S produced in continous culture. Appl. Microbiol. Biotechnol. 2008, 76, 985–990. [Google Scholar] [CrossRef]
- Qu, L.; Ji, X.; Ren, L.; Nie, Z.; Feng, Y.; Wu, W.; Ouyang, P.K.; Huang, H. Enhancement of docosahexaenoic acid production by Schizochytrium sp. using a two-stage oxygen supply control strategy based on oxygen transfer coefficient. Lett. Appl. Microbiol. 2010, 52, 22–27. [Google Scholar] [CrossRef]
- Huang, T.Y.; Lu, W.C.; Chu, I.M. A fermentation strategy for producing docosahexaenoic acid in Aurantiochytriumlimacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour. Technol. 2012, 123, 8–14. [Google Scholar] [CrossRef]
- Yaguchi, T. Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J. Am. Oil Chem. Soc. 1997, 74, 1431–1434. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Pyle, D.; Garcia, R.; Wen, Z. Producing docosahexaenoic acid (DHA)-rich algae from biodiesel- derived crude glycerol: Effects of impurities on DHA production and algal biomass composition. J. Agr. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z. Continous culture of the microalgae Schizochytrium limacinumna biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2010, 102, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Pooksawang, N.; Nangtharat, S.; Yunchalard, S. Batch fermentation of marine microalgae using glycerol obtained from biodiesel plant for docosahexaenoic acid (DHA) production. In Proceedings of the 3rd International Conference on Fermentation Technology for Value-Added Agriculture Production, Khon Kaen, Thailand, 26–28 August 2009; Volume 26, pp. 1–7. [Google Scholar]
- Chi, Z.; Pyle, D.; Wen, Z.; Frear, C.; Chen, S. A laboratory study of producing docosahexaenoic acid form biodiesel-waste glycerol by microalgal fermentation. Process. Biochem. 2007, 42, 1537–1545. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Salley, S. Continous microalgae cultivation in photobioreactor. Biotechnol. Bioeng. 2012, 109, 2468–2474. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, X.; Zhu, X.; Li, Y.; Xu, H.; Zhao, B.; Chen, L.; Zhang, X. Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioener. 2011, 35, 1906–1911. [Google Scholar] [CrossRef]
- Hong, W.; Rairakhwada, D.; Seo, P.; Park, P.; Hur, B.; Kim, C.; Seo, J. Production of lipids containing high levels of docosahexaenoic acid by a new isolated microalga Aurantochytrium sp. KRS101. Appl. Biochem. Botechnol. 2011, 164, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Ren, L.; Huang, H. Scale-up of docosahexaenoic acid production in fed-batch fermentation by Schizochytrium sp. based on volumetric oxygen-transfer coefficient. Biochem. Eng. J. 2013, 77, 82–87. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Sun, J.; Shen, Y.; Wei, D.; Zhu, J.; Chu, J. Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Biores. Technol. 2010, 101, 1961–1967. [Google Scholar] [CrossRef]
- Ratledge, C. Microorganisms as sources of polyunsaturated fatty acids. In Structured and Modified Lipid; Gunstone, W.F., Ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 351–399. [Google Scholar]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, X.; Pei, L.; Luo, Q.; Xu, J. Changes in fatty acids andsterols during batch growth of Pavlova viridis in photobioreactor. J. Appl. Phycol. 2008, 20, 237–243. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Schmidt, F. Optimisation and scale-up of industrial fermentation processes. Appl. Microbiol. Biotechnol. 2005, 68, 425–435. [Google Scholar] [CrossRef]
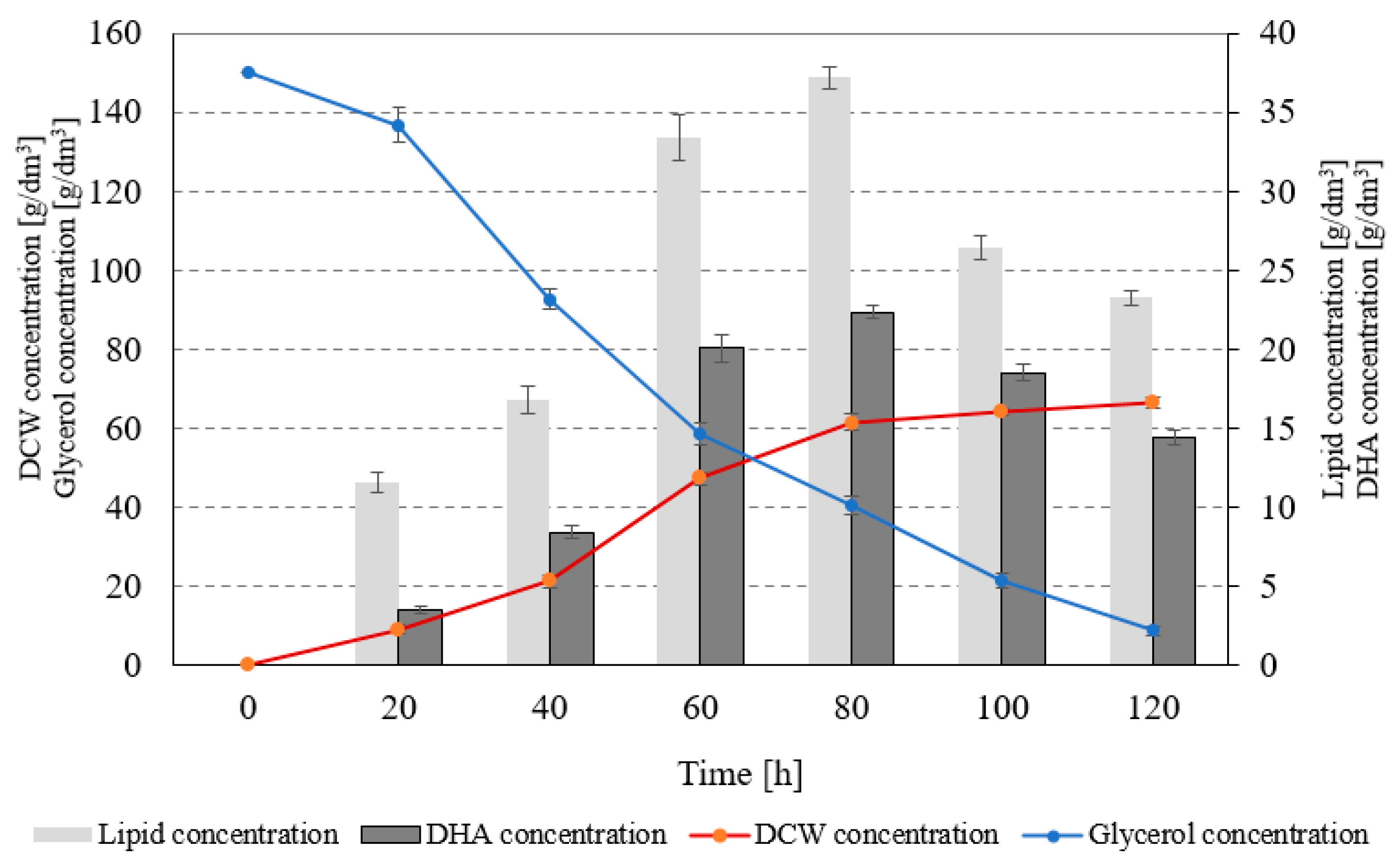
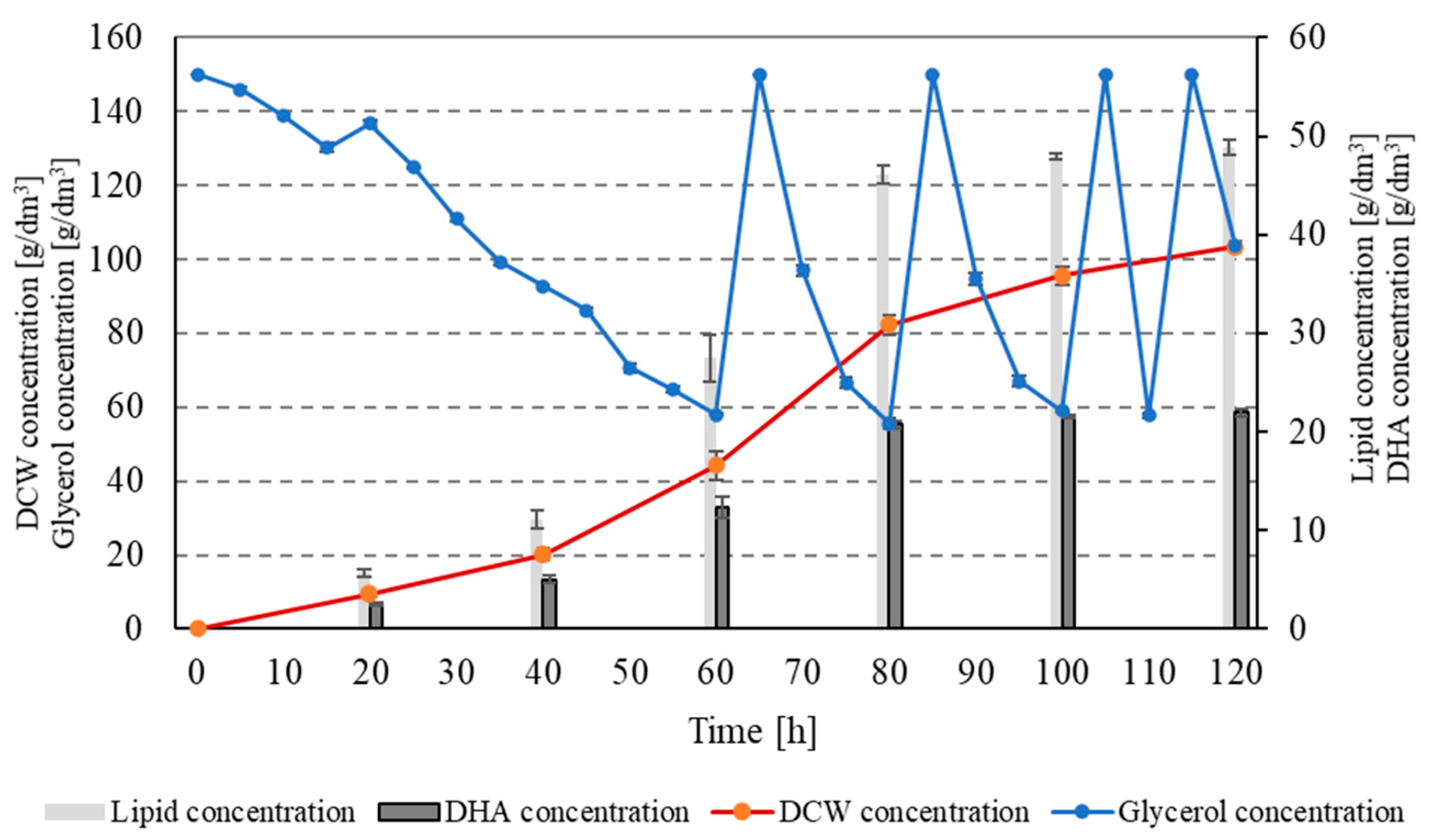
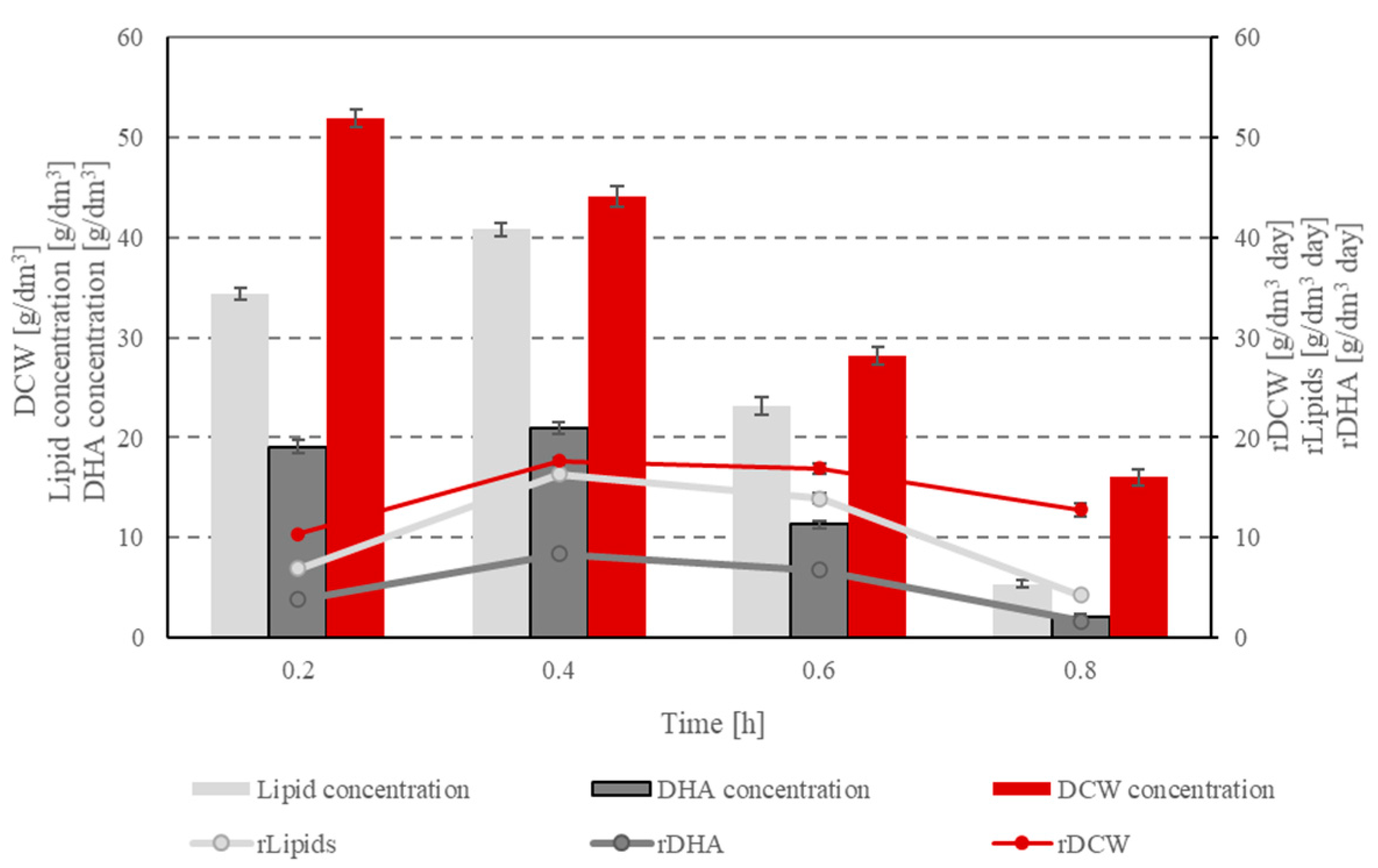
| Component | Unit | Concentration |
|---|---|---|
| Glucose | (g/dm3) | 5.0 |
| Yeast extract | (g/dm3) | 1.0 |
| Peptone | (g/dm3) | 1.0 |
| Artificial seawater | (dm3) | 1.0 |
| Component | Unit | Concentration |
|---|---|---|
| (NH4)2SO4 | (g/dm3) | 1.0 |
| KH2PO4 | (g/dm3) | 3.0 |
| Na2SO4 | (g/dm3) | 12.0 |
| MgSO4 | (g/dm3) | 5.0 |
| K2SO4 | (g/dm3) | 7.0 |
| KCl | (g/dm3) | 2.0 |
| Trace elements | ||
| CaCl2 | (mg/dm3) | 50 |
| MnCl2 | (mg/dm3) | 5.2 |
| ZnSO4 | (mg/dm3) | 5.2 |
| CuSO4 | (mg/dm3) | 0.8 |
| Na2MoO4 | (mg/dm3) | 0.016 |
| NiSO4 | (mg/dm3) | 0.8 |
| FeSO4 | (mg/dm3) | 0.01 |
| CoCl2 | (mg/dm3) | 0.066 |
| thiamine | (mg/dm3) | 0.76 |
| vitamin B12 | (mg/dm3) | 1.2 |
| vitamin B5 (calcium salt of pantothenic acid) | (mg/dm3) | 25.6 |
| Properties | Unit | Concentration |
|---|---|---|
| Color | (-) | light-brown |
| Odor | (-) | characteristic |
| pH | (-) | 5 |
| Glycerol | (% w/w) | 80 |
| Water | (% w/w) | 15 |
| Sulfated ash | (% w/w) | 5 |
| Methanol | (% w/w) | 0.3 |
| MONG (Matter Organic Non-Glycerol) | (% w/w) | 6 |
| Chlorides | (ppm) | 10 |
| Halogen derivatives | (ppm) | 35 |
| Acidity | (cm3) of NaOH consumed | 0.25 |
| Esters | (cm3) of HCl consumed | 8–10 |
| Heavy metals | (ppm) | 5 |
| Aldehydes | (ppm) | 10 |
| Temperature of melting/freezing | (°C) | 18 |
| Temperature of initial boiling | (°C) | 290 |
| Temperature of ignition | (°C) | 177 |
| Temperature of self-ignition | (°C) | 429 |
| Temperature of decomposition | (°C) | >290 |
| Vapor pressure | (mbar) | 0.01 |
| Relative density/density converted to 20 °C | (mg/dm3) | 1.26 |
| Viscosity at 20 °C | (mm2/s) | 1.5 |
| Density at 15 °C | (mg/dm3) | 1.2 |
| Explosive properties | (-) | - |
| Explosive properties | (-) | - |
| Parameter | Unit | Value | |||
|---|---|---|---|---|---|
| Dilution rate D | (1/day) | 0.2 | 0.4 | 0.6 | 0.8 |
| DCW | (g/dm3) | 51.90 ± 0.91 | 44.07 ± 1.06 | 28.19 ± 0.85 | 16.02 ± 0.85 |
| rDCW | (g/dm3·day) | 10.38 ± 0.18 | 17.63 ± 0.42 | 16.92 ± 0.51 | 12.81 ± 0.68 |
| DHA | (g/dm3) | 19.10 ± 0.68 | 20.94 ± 0.55 | 11.29 ± 0.34 | 2.07 ± 0.23 |
| rDHA | (g/dm3·day) | 3.82 ± 0.14 | 8.37 ± 0.22 | 6.78 ± 0.21 | 1.66 ± 0.18 |
| TFA | (g/dm3) | 34.37 ± 0.58 | 40.79 ± 0.63 | 23.16 ± 0.84 | 5.34 ± 0.39 |
| rlipids | (g/dm3·day) | 6.87 ± 0.12 | 16.32 ± 0.25 | 13.89 ± 0.51 | 4.27 ± 0.31 |
| Culture Type | Unit | Batch | Fed-Batch | Continuous (D = 0.4) |
|---|---|---|---|---|
| Culture duration | (h) | 120 | 120 | Continuous culture |
| DCW | (g/dm3) | 66.43 ± 1.29 | 103.44 ± 1.50 | 44.07 ± 1.06 |
| rDCW | (g/dm3·h) | 0.55 ± 0.07 | 0.86 ± 0.12 | 0.37 ± 0.03 |
| rDHA | (g/dm3·h) | 0.12 ± 0.02 | 0.18 ± 0.01 | 0.17 ± 0.01 |
| Culture yield | (gDCW/gglycerol) | 0.47 ± 0.11 | 0.25 ± 0.09 | 0.18 ± 0.03 |
| Glycerol consumption rate | (g/dm3·h) | 1.17 ± 0.21 | 3.47 ± 0.30 | 2.02 ± 0.17 |
| Total fatty acids (TFA) | (g/dm3) | 23.25 ± 0.45 | 48.85 ± 0.81 | 40.79 ± 0.63 |
| DHA | (g/dm3) | 14.36 ± 0.47 | 21.98 ± 0.36 | 20.95 ± 0.55 |
| Growth rate constant in the log phase | (1/h) | 0.027 ± 0.004 | 0.035 ± 0.001 | - |
| Fatty Acid | Unit | Batch Culture | Fed-Batch Culture | Continuous Culture (D = 0.4) |
|---|---|---|---|---|
| C 14:0 | (% TFA) | 2.38 ± 0.21 | 3.51 ± 0.64 | 2.79 ± 0.28 |
| C 16:0 | 27.88 ± 1.37 | 41.01 ± 2.84 | 38.57 ± 1.75 | |
| C 18:0 | 1.47 ± 0.13 | 2.16 ± 0.47 | 2.72 ± 0.43 | |
| C 22:5 (DPA) | 5.52 ± 0.98 | 8.13 ± 0.93 | 7.24 ± 0.94 | |
| C 22:6 (DHA) | 61.76 ± 3.77 | 44.99 ± 2.12 | 51.33 ± 3.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Cultivation Method Effect on Schizochytrium sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon. Energies 2021, 14, 2952. https://doi.org/10.3390/en14102952
Kujawska N, Talbierz S, Dębowski M, Kazimierowicz J, Zieliński M. Cultivation Method Effect on Schizochytrium sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon. Energies. 2021; 14(10):2952. https://doi.org/10.3390/en14102952
Chicago/Turabian StyleKujawska, Natalia, Szymon Talbierz, Marcin Dębowski, Joanna Kazimierowicz, and Marcin Zieliński. 2021. "Cultivation Method Effect on Schizochytrium sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon" Energies 14, no. 10: 2952. https://doi.org/10.3390/en14102952
APA StyleKujawska, N., Talbierz, S., Dębowski, M., Kazimierowicz, J., & Zieliński, M. (2021). Cultivation Method Effect on Schizochytrium sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon. Energies, 14(10), 2952. https://doi.org/10.3390/en14102952









