Characteristics of Plastic Waste Processing in the Modern Recycling Plant Operating in Poland
Abstract
1. Introduction
2. Material and Methods
2.1. Characteristics of the Subject of Research
2.1.1. Production Plant
- production-storage building with equipment for storing, sorting, and compacting of segregated waste brought into the plant
- control room building with control node for the technological installation
- building of the transformer station with voltage ratio of 400/6300 [V] with the main power switchboard of the plant
- shed of pyrolysis node with technological installation for thermal processing of mixed packaging and multilayered waste
- two sets of power generators
- liquefied petroleum gas (propane) and liquid fuels supply station for technological purposes (i.e., heating the reactor during startup or operation of the plant)
- two underground storage tanks for liquefied petroleum gas (propane)
- two underground storage tanks for liquid products forming in technological process
- truck tanker loading station with liquid products forming in technological process
2.1.2. Processed Raw Materials
3. Theory
3.1. Technological Process
3.1.1. Thermal Cracking
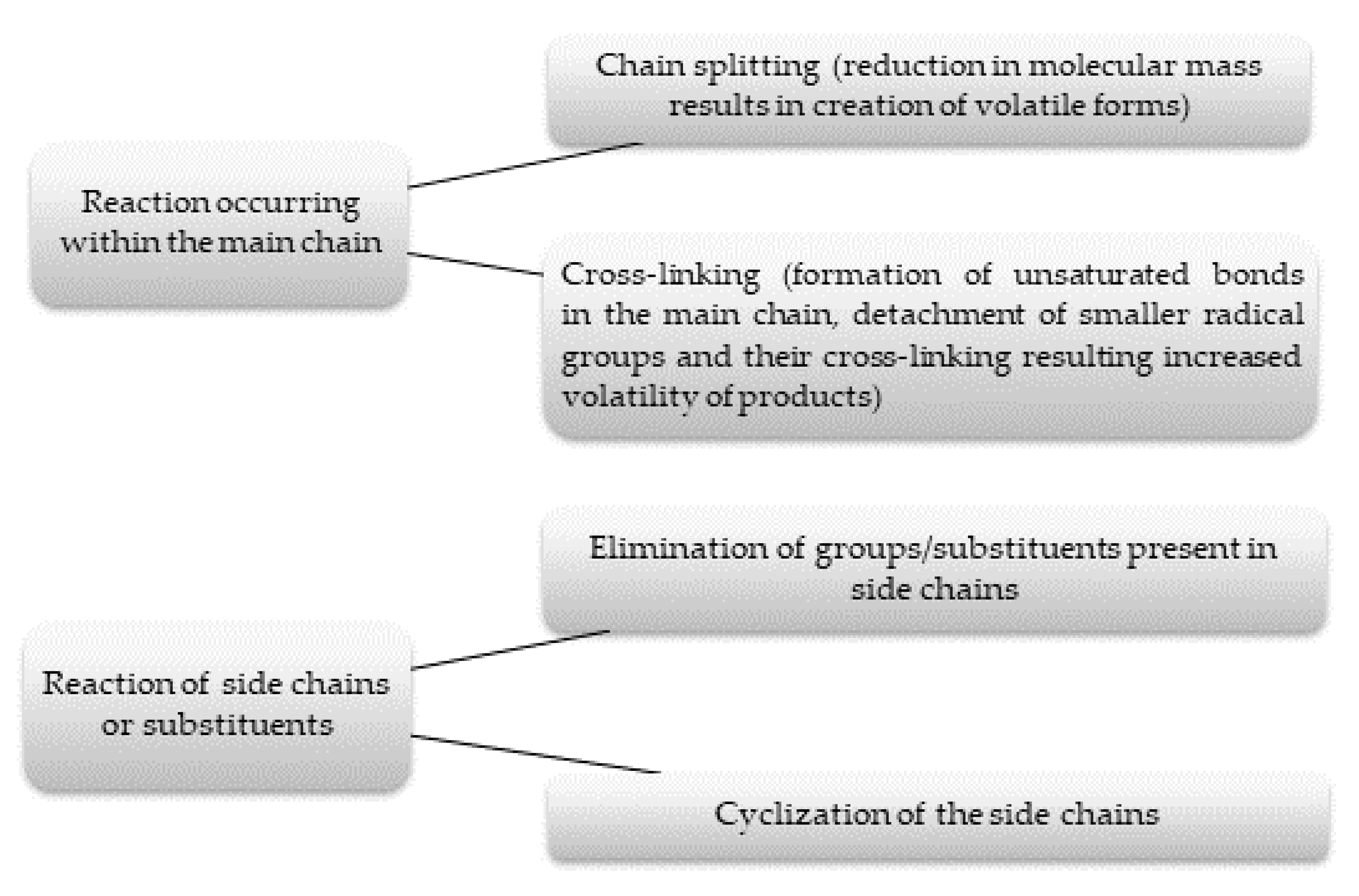
3.1.2. Mechanism of Thermal Decomposition
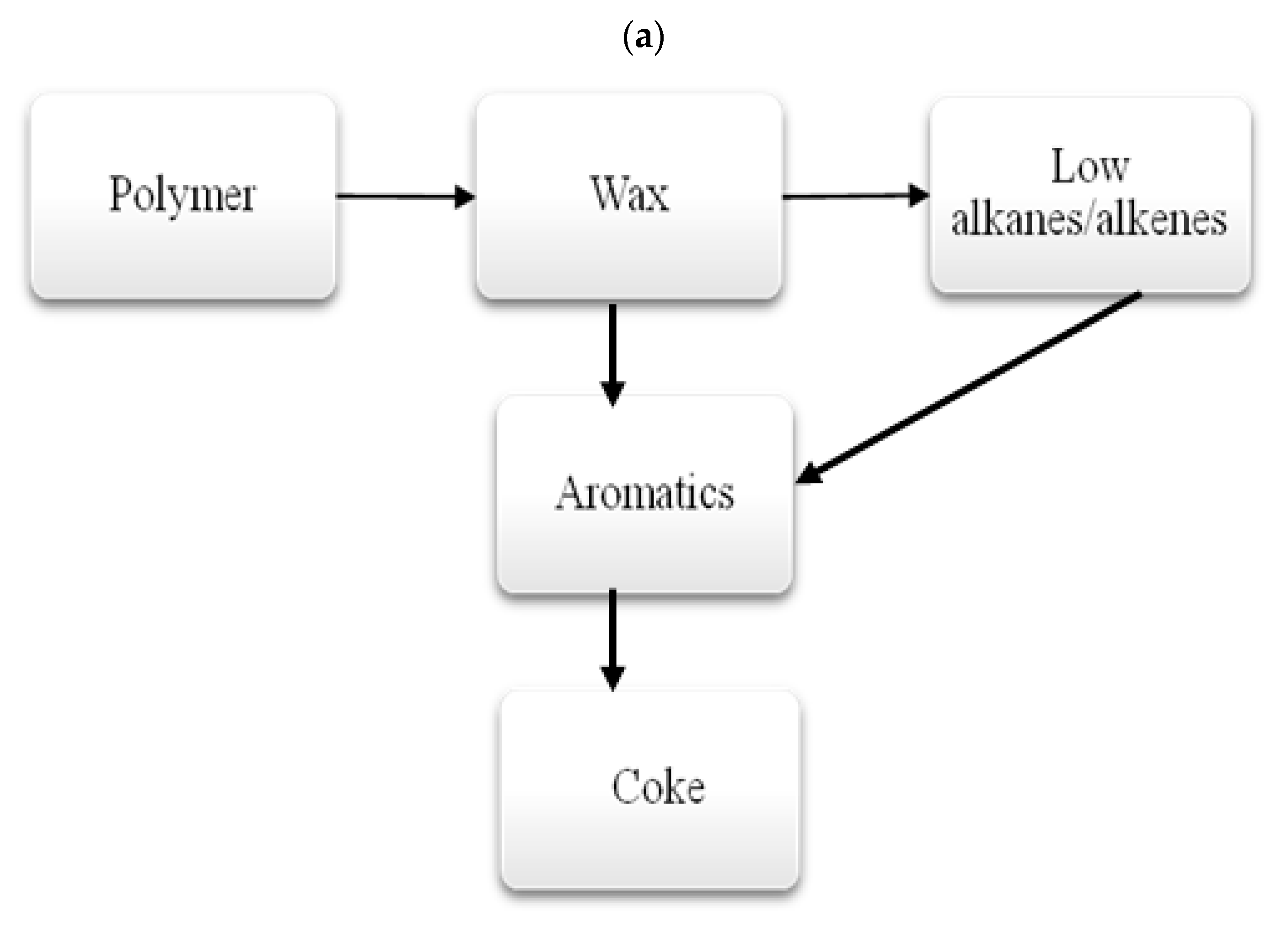
3.1.3. Thermal Decomposition Products of Polymers
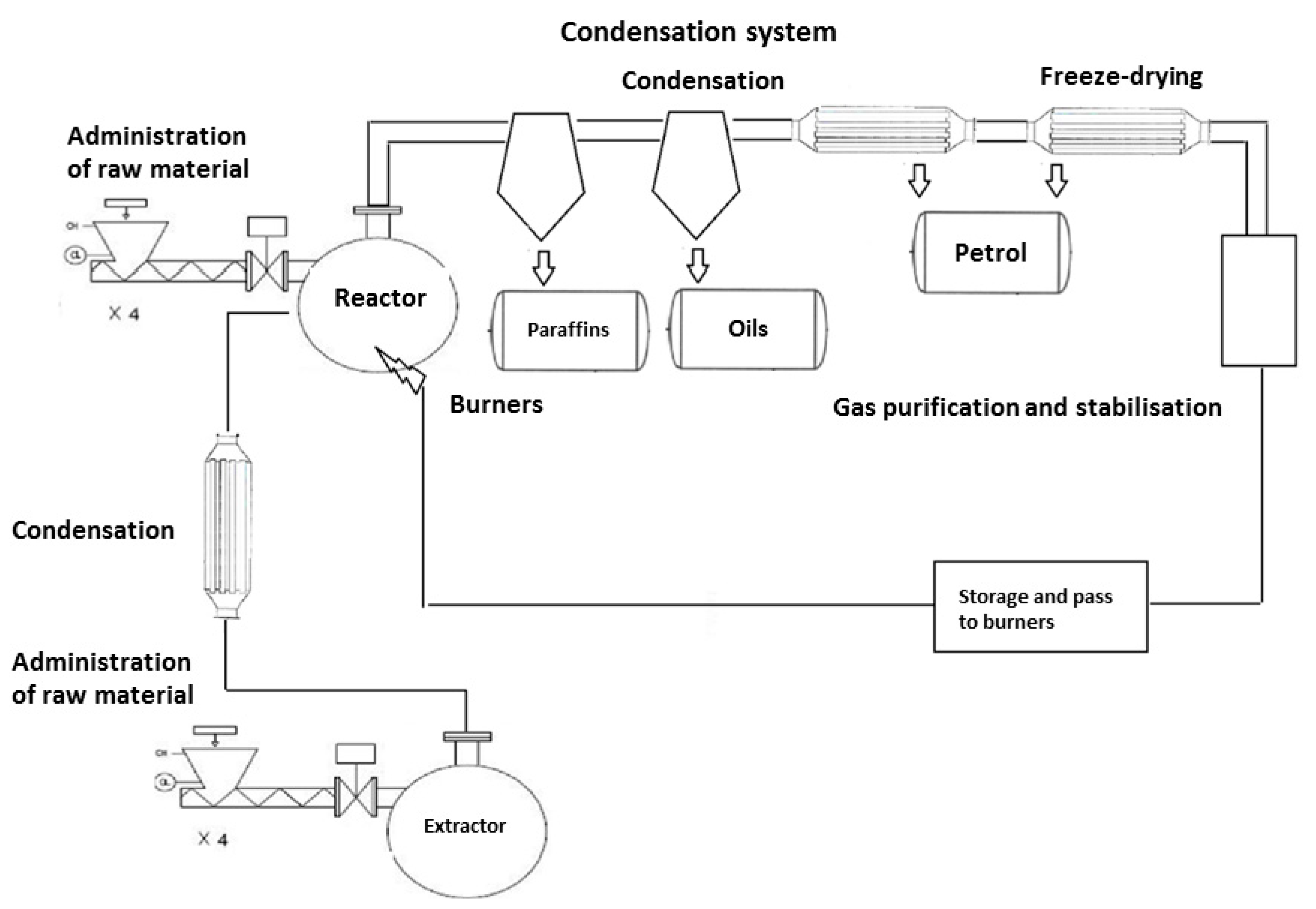
3.1.4. Pyrolysis Installation
- (a)
- The system supplying the production line with a stream of sorted waste (PLW 600/16000 belt conveyor, T1/T4 SIGMA screw conveyors, a feeding system with presses, a system dosing raw material to the R101 reactor and R201 extractor).
- (b)
- A system that ensures proper operation of the extractor (a solvent feed mechanism (heavy fraction from the reactor), a heat exchanger system to maintain temperature of homogenization process in the range of 180–200 °C (E202/W401 jacket-tube exchanger in a dosed solvent-extraction mixture system, by-pass exchangers in a thermal oil-extraction mixture system), a content-mixing system (three circulating pumps P205–207 allowing extraction mass to be exchanged four times per hour), a system for liquefaction and removal of gas created in extractor (E201 heat exchanger combined with a V106 separation tank to separate aqueous and hydrocarbon fraction), a mixture extraction and cleaning system and its dosing to the reactor (P205 dosing pump with F103 filter)).In the extractor operating in the temperature range of 180–200 °C, the mass of solid waste is dissolved in liquid hydrocarbon fractions. These fractions come from the liquefaction of the reactor products and are dosed to the extractor through the pipeline equipped with a pump and automatic valves for flow control. The content of the extractor is mixed with the help of circulation pumps, which accelerates the process of dissolving solid waste and increases the heat transfer between the heating elements and the extraction mixture. After dissolving, the mixture of heavy fraction and liquid polyolefins is taken from the tank lower connector pipes and directed by pumps to the connector pipes located on the reactor side surface.
- (c)
- A system that ensures proper operation of the reactor (a two-level reactor heating system providing the heat necessary to reach and maintain a temperature of 420 °C), set of heating inserts connected by ends to O101–108 gas burners of modulating power of 60–200 kW and an exhaust outlet to a chimney. The gas burners are fed with technological gas created at the pyrolysis stage or propane gas used at the installation start-up stage, a content-mixing system (four circulating pumps P201–204 allowing reaction mass to be exchanged eight times per hour), a reactor and reactive mixture cleaning system (thanks to the extraction system, the solid contamination of the reactor does not exceed 5%). These contaminants are periodically drawn from the bottom of the reactor and then pumped through F101–102 filters and captured by feeders of SF203–204 dryers, which direct dry matter and hydrocarbon vapors present in a filtrate to solid waste storage containers and a condensate-separation system, respectively.A condensate system for the gaseous products of pyrolysis (a system of E101-108 heat exchangers (a stream of vapors of 1000 kg and a temperature of 420 °C is directed to them every hour which, when mixed with injected hydrocarbon mixture at a correspondingly lower temperature, undergoes four-stage condensation. Noncondensing after cooling gas mixture (mainly methane, ethane, propane, and butane and traces of heavier hydrocarbons) flows through an E108 exchanger cooled with aqueous solution of propylene glycol with an inlet temperature of −5 °C). On its way, a stream of condensed vapors goes to intermediate tanks collecting different fractions (V104 tank maintained at a temperature of at least 80 °C—hydrocarbon fraction with boiling temperatures >170 °C, V107 tank maintained at a temperature of at least 60 °C —hydrocarbon fraction with boiling temperatures >120 °C and V103 tank—hydrocarbon fraction with boiling temperatures >30 °C. In the last part of the condensation path there is a V105 tank, which intercepts the gas stream leaving the last fourth stage of condensation. After purification it is used as fuel in burners feeding the technological process)).
- (d)
- A product storage system for sale time or supply of power generator sets (M101 two-chamber tank (K1 chamber, gasoline products (CN–27 10 12 25) from V103 tank, K2 chamber, oil products (CN–27 10 19 29) from V107 chamber); M102 two-chamber tank (K3 chamber, paraffin products (CN–27 10 19 85) from V104 tank, K4 chamber, fuel mixture supplying generator sets which is a mixture of paraffin, oil and gasoline fractions); an installation ensuring the quality and possibility of product design (chambers equipped with apparatus enabling mixing and circulation of liquids, measuring and regulation of its temperature, and assessing the level and acidity of mixture); installed pump units connected with a static mixer enable preparation of fuel mixtures according to specific recipes, e.g., fuel intended for supplying generator sets) – energy product GreenOil [49].
4. Results and Discussion
4.1. Analysis of Recycling Process
4.1.1. Sorting of Waste for Pyrolysis
4.1.2. The Process Heat Demand
- Q1 is the heat required to heat the raw material to the melting point
- Q2 is the heat required to melt polyolefins
- Q3 is the heat required to evaporate the water contained in the waste
- polyolefins (soluble) 75%
- other plastics (insoluble) 20%
- water 5%
- waste throughput 1000 kg/h
- amount of solvent supplied to extractor 2000 kg/h
- input temperature of raw material 15 °C
- the temperature in the extractor 140 °C
- average specific heat of the solvent cps = 2.81 kJ/kgK
- average specific heat of water cpw = 4.18 kJ/kgK (at 50 °C)
- average specific heat of polyolefins cpP = 2.3 kJ/kgK
- heat of fusion of polyolefins HfP = 203 kJ/kg
- heat of water vaporization Hvw = 2264.67 kJ/kg (at 100 °C)
- Q1 is the heat required to heat and decompose the polyolefins
- Q2b is the heat required to evaporate the gasoline fraction
- Q2o is the heat required to evaporate the oil fraction
- Q2p is the heat required to evaporate the paraffin fraction
- gas fraction 10%
- gasoline fraction 10%
- oil fraction 40%
- paraffin fraction 35%
- solid residue 5%
- heat of vaporization of gasolines Hvg = 243.22 kJ/kg (at 200 °C)
- heat of vaporization of oils Hvo = 214.51 kJ/kg (at 300 °C)
- heat of vaporization of paraffins Hvp = 196.93 kJ/kg (at 350 °C)
- cracking heat Hcrac = 1465 kJ/kg
4.1.3. The Oil Fraction Produced in the Process of Thermal Waste Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Energy, Transport and Environment Statistics, Edition 2019. Available online: https://ec.europa.eu/eurostat/documents/3217494/10165279/KS-DK-19-001-EN-N.pdf (accessed on 14 December 2020).
- The EU Environmental Implementation Review 2019, Country Report—Poland. Available online: https://ec.europa.eu/environment/eir/pdf/report_pl_en.pdf. (accessed on 14 December 2020).
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.C.; Karonis, D. Alternative diesel from waste plastics. Energies 2017, 10, 1750. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, M.P. GHG emission and carbon sequestration potential from MSW of Indian metro cities. Urban Clim. 2014, 8, 30–41. [Google Scholar] [CrossRef]
- Another Landfill Burning in Poland. Available online: https://polandin.com/38576028/another-landfill-burning-in-poland (accessed on 18 September 2020).
- Journal of Laws of the Republic of Poland of 2018, Item 1479. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20180001479 (accessed on 14 December 2020).
- Journal of Laws of the Republic of Poland of 2019, Item 2028. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190002028 (accessed on 14 December 2020).
- Plastic Recycling Plants Directory. Available online: https://www.enfrecycling.com/directory/plastic-plant (accessed on 18 September 2020).
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Simon, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Glycolysis of flexible polyurethane wastes containing polymeric polyols. Polym. Deg. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of waste plastics to liquid fuel: A suitable method for plastic waste management and manufacture of value added products—A world prospective. Renew. Sust. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Sadeghi, G.M.M.S.; Shamsi, R.; Sayaf, M. From aminolysis product of PET waste to novel biodegradable polyurethanes. J. Polym. Environ. 2011, 19, 522–534. [Google Scholar] [CrossRef]
- Dou, B.; Wang, K.; Jiang, B.; Song, Y.; Zhang, C.; Chen, H. Fluidized-bed gasification combined continuous sorption-enhanced steam reforming system to continuous hydrogen production from waste plastic. Int. J. Hydrog. Energy 2016, 41, 3803–3810. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Frances, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasolinerange hydrocarbons. J. Anal. Appl. Pyrol. 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Conserv. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef] [PubMed]
- Beyler, C.L.; Hirschler, M.M. Thermal decomposition of polymers. In SFPE Handbook of Fire Protection Engineering 2; Springer: Berlin/Heidelberg, Germany, 2002; Section 1, Chapter 7; pp. 111–131. [Google Scholar]
- Mucha, M. Thermogravimetric studies on polymethylenes and polyethylenes. J. Polym. Sci. Symp. 1976, 25–31. [Google Scholar] [CrossRef]
- Urzendowski, S.R.; Guenther, A.H. Kinetics constants of polymeric materials from thermogravimetric data. J. Anal. 1971, 3, 379–395. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.Y.; Hor, J.L.; Shih, S.M.; Chen, L.W.; Chang, F.W. On the thermal treatment of plastic mixtures of MSW: Pyrolysis kinetics. Waste Manag. 1993, 13, 221–235. [Google Scholar] [CrossRef]
- Westerhout, R.W.J.; Balk, R.H.P.; Meijer, R.; Kuipers, J.A.M.; van Swaaij, W.P.M. Examination and evaluation of the use of screen heaters for the measurement of the high temperature pyrolysis kinetics of polyethylene and polypropylene. Ind. Eng. Chem. Res. 1997, 36, 3360–3368. [Google Scholar] [CrossRef]
- Westerhout, R.W.J.; Waanders, J.; Kuipers, J.A.M.; van Swaaij, W.P.M. Kinetics of the low-temperature pyrolysis of polyethene, polypropene, and polystyrene modelling, experimental determination and comparison with literature models and data. Ind. Eng. Chem. Res. 1997, 36, 1955–1964. [Google Scholar] [CrossRef]
- Sorum, L.; Gronli, M.G.; Hustad, J. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Dickens, B. Thermal degradation study of isotactic polypropylene using factor-jump thermogravimetry. J. Polym. Sci. 1982, 20, 1169–1183. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U. Stepwise pyrolysis for raw material recovery from plastic waste. J. Anal. Appl. Pyrol. 1998, 46, 1–13. [Google Scholar] [CrossRef]
- Gambiroza-Jukic, M.; Cunko, R. Kinetics of the thermal degradation of polypropylene fibres. Acta Polym. 1992, 43, 258–260. [Google Scholar] [CrossRef]
- Sato, A.; Kaneko, K. Differential thermal and chromatographic analysis on pyrolysis of plastics. J. Energy Heat. Mass Trans. 1983, 5, 323–338. [Google Scholar]
- Westerhout, R.W.J.; Waanders, J.; Kuipers, J.A.M.; van Swaaij, W.P.M. Recycling of polyethene and polypropene in a novel bench-scale rotating cone reactor by high-temperature pyrolysis. Ind. Eng. Chem. Res. 1998, 37, 2293–2300. [Google Scholar] [CrossRef]
- Elordi, G.; Lopez, G.; Olazar, M.; Aguado, R.; Bilbao, J. Product distribution modelling in the thermal pyrolysis of high density polyethylene. J. Hazard. Mater. 2007, 144, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- McCaffrey, W.C.; Cooper, D.G.; Kamal, M.R. Tertiary recycling of polyethylene: Mechanism of liquid production from polyethylene by thermolysis/reactive distillation. Polym. Deg. Stab. 1998, 62, 513–521, ISSN 01141-3910. [Google Scholar] [CrossRef]
- McCaffrey, W.C.; Cooper, D.G.; Kamal, M.R. The effect of short-chain branching on the thermolysis/reactive distillation of polyethylene. J. Appl. Polym. Sci. 1999, 73, 1415–1421. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Fluidised bed pyrolysis of low density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrol. 1999, 51, 107–126. [Google Scholar] [CrossRef]
- Williams, P.T.; Stanley, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Res. Conserv. Recyc. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Recovery of hydrocarbon liquid from waste high density polyethylene by thermal pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar] [CrossRef]
- Boundy, B.; Diegel, S.W.; Wright, L.; Davis, S.C. Biomass Energy Data Book, 4th ed.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011. [Google Scholar]
- Ahmad, I.; Ismail Khan, M.; Ishaq, M.; Khan, H.; Gul, K.; Ahmad, W. Catalytic efficiency of some novel nanostructured heterogeneous solid catalysts in pyrolysis of HDPE. Polym. Degrad. Stabil. 2013, 98, 2512–2519. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 2014, 12, 663–671. [Google Scholar] [CrossRef]
- Desai, S.B.; Galage, C.K. Production and analysis of pyrolysis oil from waste plastic in Kolhapur city. Int. J. Energy Res. Gen. Sci. 2015, 3, 590–595, ISSN 2091-2730. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 7th ed; McGraw-Hill: New York, NY, USA, 1997; ISBN 0-07-049841-5. [Google Scholar]
- Fakhrhoseini, S.M.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 1–5. [Google Scholar] [CrossRef]
- McCaffrey, W.C.; Brues, M.I.; Cooper, D.C.; Kamal, M.R. Thermolysis of polyethylene/polystyrene mixtures. J. Appl. Polym. Sci. 1996, 60, 2133–2140. [Google Scholar] [CrossRef]
- Wong, H.W.; Broadbelt, L.J. Tertiary resource recovery from waste polymers via pyrolysis: Neat and binary mixture reactions of polypropylene and polystyrene. Ind. Eng. Chem. Res. 2001, 40, 4716–4723. [Google Scholar] [CrossRef]
- GreenTech Polska, S.A. Green Oil—Safety Data Sheet, version 1.0/EN. Personal Communication. 2020; 1–7, Unpublished. [Google Scholar]
- Rozporządzenie Ministra Klimatu. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/katalog-odpadow-18938969 (accessed on 14 December 2020).
- Klojzy-Karczmarczyk, B.; Staszczak, J. Estimation of the mass of energy fractions in municipal waste produced in areas of different developmental character, Polityka energetyczna. Energy Policy J. 2017, 20, 143–154, ISSN 1429-6675. [Google Scholar]
- Journal of Laws of the Republic of Poland of 2015, Item 1680. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001680 (accessed on 14 December 2020).
- Duda, A.; Fenicki, A.; Molski, P.; Szostak, E.; Duda, P. Design and operation of a modern polish plant for plastic waste recycling through the degradative depolymerization process. A case study. Energies 2020, 13, 6620. [Google Scholar] [CrossRef]


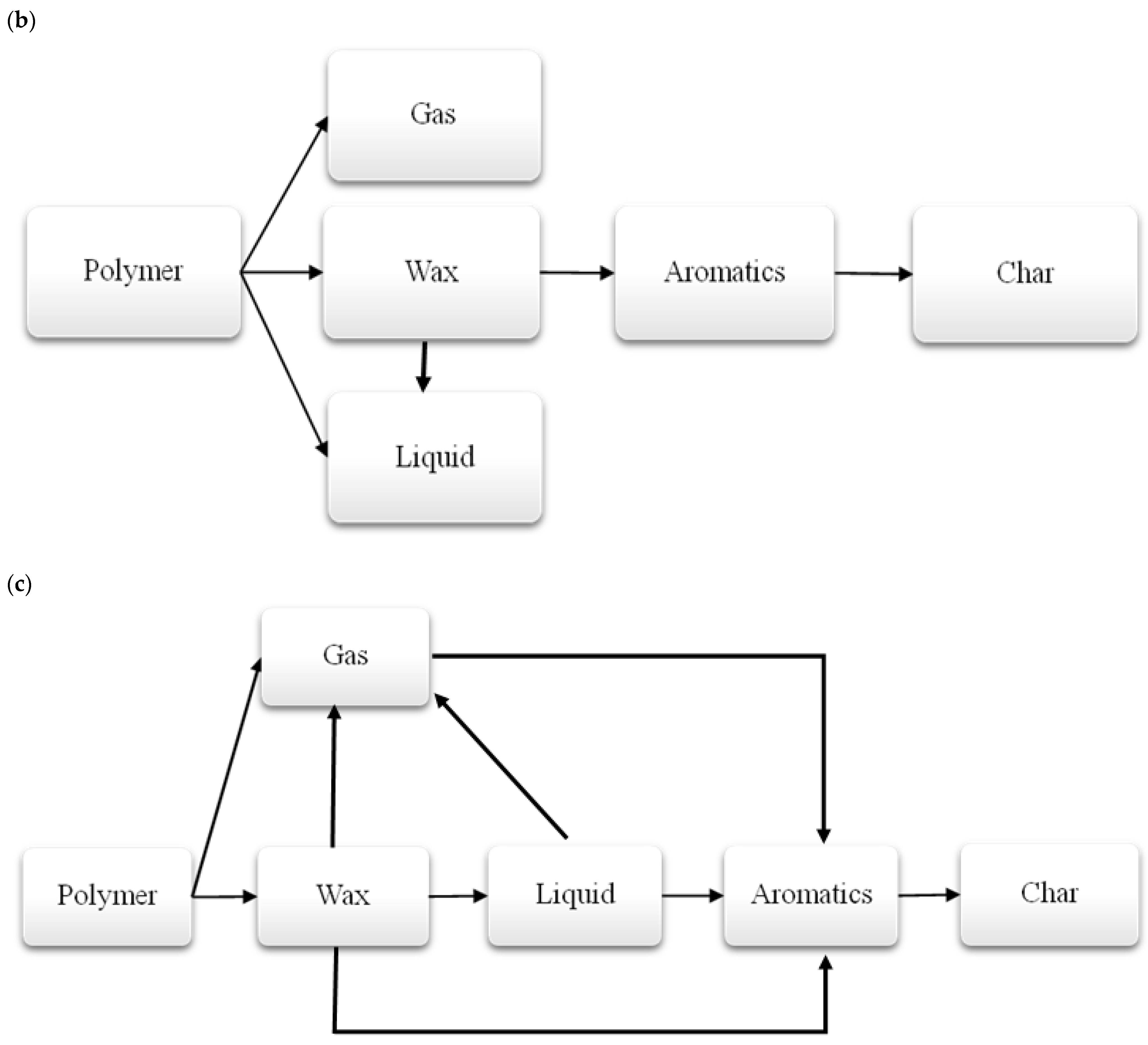
| No. | Waste Code | Type of Waste |
|---|---|---|
| 1. | 03 03 07 | Mechanically separated rejects from processing of waste paper and cardboard |
| 2. | 07 02 13 | Plastic waste |
| 3. | 15 01 02 | Plastic packaging |
| 4. | 15 01 05 | Multimaterial packaging (tetra packs) |
| 5. | 15 01 06 | Mixed packaging waste |
| 6. | 16 01 99 | Other waste not specified |
| 7. | 17 02 03 | Plastics |
| 8. | 19 12 04 | Plastics and rubber |
| 9. | 19 12 12 | Other wastes (including mixed substances and objects) obtained from mechanical waste treatment other than mentioned in 19 12 11 |
| 10. | 20 01 39 | Plastics |
| 11. | 20 01 99 | Other fractions selectively collected not mentioned above |
| Waste Code | Waste Classification by the Company Designation | Average Content in Weight of Waste Delivered to the Plant [%] | Standard Deviation of the Mean Value [%] |
|---|---|---|---|
| 19 12 04 | Plastics for further processing | 60.6 | 20.4 |
| 19 12 09 | Minerals (e.g., sand, stones) | 6.2 | 4.0 |
| 19 12 02 | Ferrous metals | 0.8 | 0.4 |
| 19 12 03 | Nonferrous metals | 0.8 | 0.4 |
| 19 12 12 | Other wastes (including mixed substances and objects) | 25.5 | 19.9 |
| Parameter Determined | Research Method Applied | Content in Sample [wt%] | ||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | ||
| Food waste of plant origin | PN-93/Z-15006 | 0 | 0.09 | 0 |
| Food waste of animal origin | 0 | 0.11 | 0 | |
| Paper and cardboard waste | 30 | 29.9 | 22.20 | |
| Plastic waste | 0 | 60.4 | 74.50 | |
| Textile waste | 0 | 2.80 | 2.40 | |
| Glass waste | 0 | 0 | 0 | |
| Metal waste | 0 | 0.10 | 0.06 | |
| Other organic wastes | 0 | 3.80 | 0.09 | |
| Other mineral wastes | 0 | 2.20 | 0.01 | |
| Fraction <10 mm | 70 | 0.60 | 0.74 | |
| Requirements of Cement Industry | Post-Sorting Ballast (Reject Fraction) | Research Method Applied | |
|---|---|---|---|
| Moisture content [%] | <20 | 31.0 ± 6.2 | CEN/TS 15414-1:2010 |
| Calorific value [MJ kg−1] | >18 | 25.21 ± 2.52 | PN-EN 15400:2011 |
| Sulfur content [%] | <0.5 | 0.06 ± 0.02 | PN-G-04584:2001 |
| Ash content [%] | <20 | 4.1 ± 0.6 | PN-EN 15403:2011 |
| Chlorine content [%] | <0.2 | 0.352 ± 0.088 | PN-EN 15408:2011 |
| No. | Medium and Heavy Fraction | Fuel Oil Standard [x] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Method applied | Sample1 | Sample2 | Sample3 | Sample4 | min | max | |||
| Cetane index | 53.45 ± 0.25 | 46 | |||||||
| 1 | Density at 15 °C | kg/m3 | PN-EN ISO 12185:2002 | 830.5 | 842.5 | 833.2 | 813.5 | 820 | 845 |
| 2 | Polycyclic aromatic hydrocarbons content | % (m/m) | PN-EN 12916:2016-03 | 0.08 | 4.7 | 3.7 | 3.4 | 8 | |
| 3 | Sulfur content | mg/kg | PN-EN ISO 14596:2009 | 278 | 29 | 20 | 63 | 10 | |
| 4 | Flash point | °C | PN-EN ISO 2719:2016-08 | <40 | 58.5 | 45.5 | 49 | >55 | |
| 5 | Water content | mg/kg | PN-EN ISO 12937:2005 | 110 | 40 | 130 | 150 | 200 | |
| 6 | Impurity content | mg/kg | PN-EN 12662:2014-05 | >30 | >30 | >30 | >30 | 24 | |
| 7 | Viscosity at 40 °C | mm2/s | PN-EN ISO 3104:2004 | 1.692 | lack of data | 2.622 | 2.764 | 2 | 4.5 |
| 8 | Distills up to 250 °C | % (v/v) | PN-EN ISO 3405:2012 | 56.1 | 10.00 | 33.1 | 58.9 | <65 | |
| 9 | Distills up to 350 °C | % (v/v) | 91.1 | 98.3 | 97.9 | 97.6 | 85 | ||
| 10 | Distills 95% (v/v) to temperature | °C | * | 344.5 | 333 | 325.9 | 360 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szostak, E.; Duda, P.; Duda, A.; Górska, N.; Fenicki, A.; Molski, P. Characteristics of Plastic Waste Processing in the Modern Recycling Plant Operating in Poland. Energies 2021, 14, 35. https://doi.org/10.3390/en14010035
Szostak E, Duda P, Duda A, Górska N, Fenicki A, Molski P. Characteristics of Plastic Waste Processing in the Modern Recycling Plant Operating in Poland. Energies. 2021; 14(1):35. https://doi.org/10.3390/en14010035
Chicago/Turabian StyleSzostak, Elżbieta, Piotr Duda, Andrzej Duda, Natalia Górska, Arkadiusz Fenicki, and Patryk Molski. 2021. "Characteristics of Plastic Waste Processing in the Modern Recycling Plant Operating in Poland" Energies 14, no. 1: 35. https://doi.org/10.3390/en14010035
APA StyleSzostak, E., Duda, P., Duda, A., Górska, N., Fenicki, A., & Molski, P. (2021). Characteristics of Plastic Waste Processing in the Modern Recycling Plant Operating in Poland. Energies, 14(1), 35. https://doi.org/10.3390/en14010035






