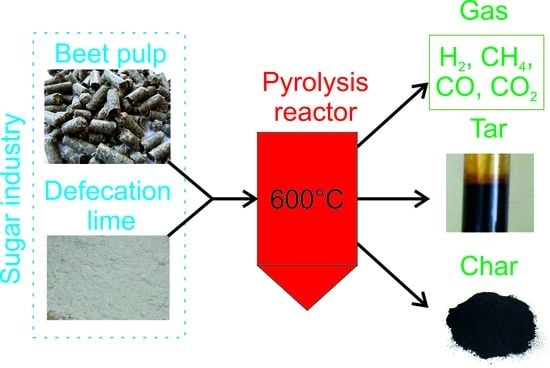
Co-Pyrolysis of Beet Pulp and Defecation Lime in TG-MS System
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Experimental
2.3. Analytical Methods
3. Results and Discussion
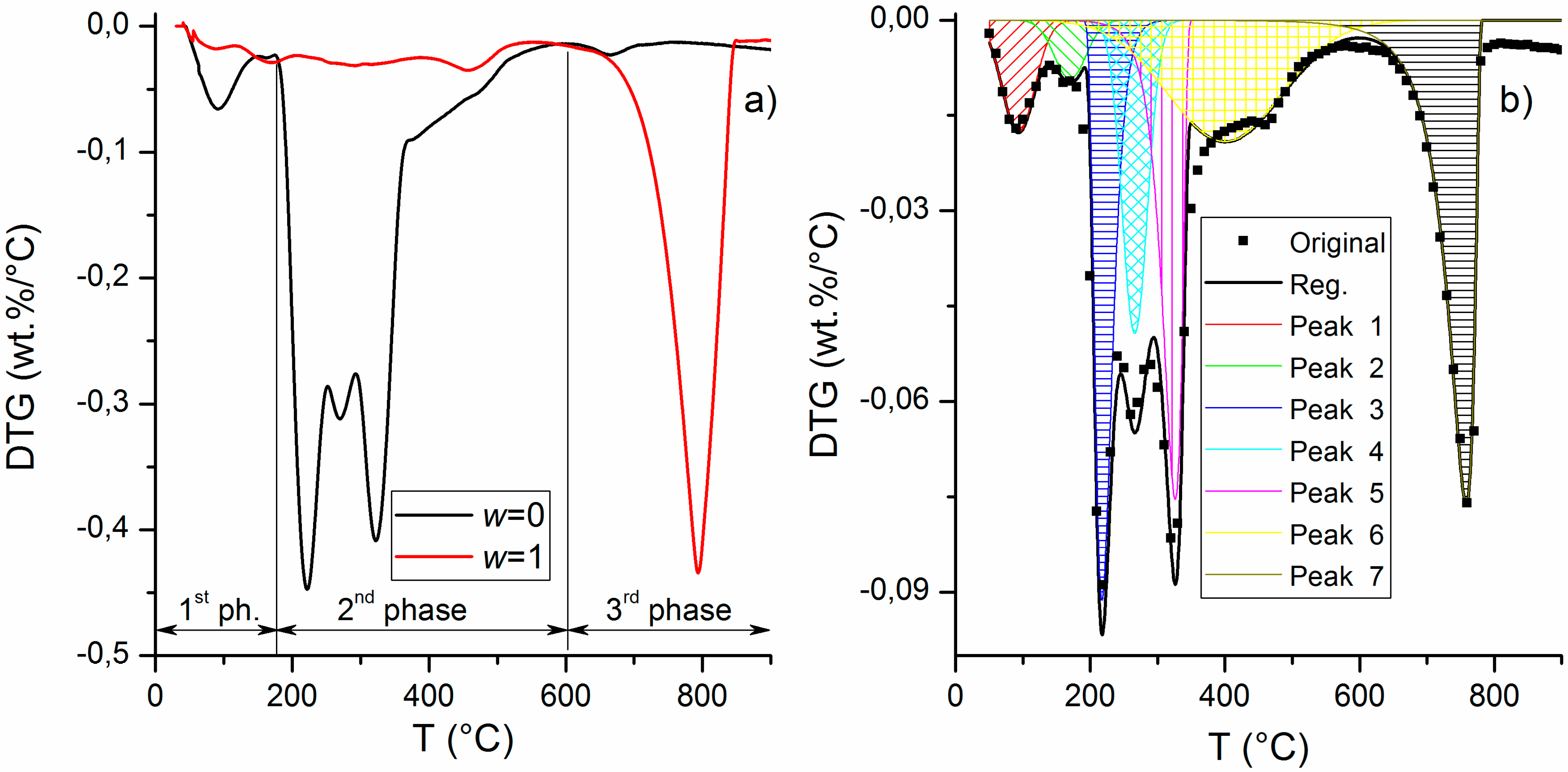
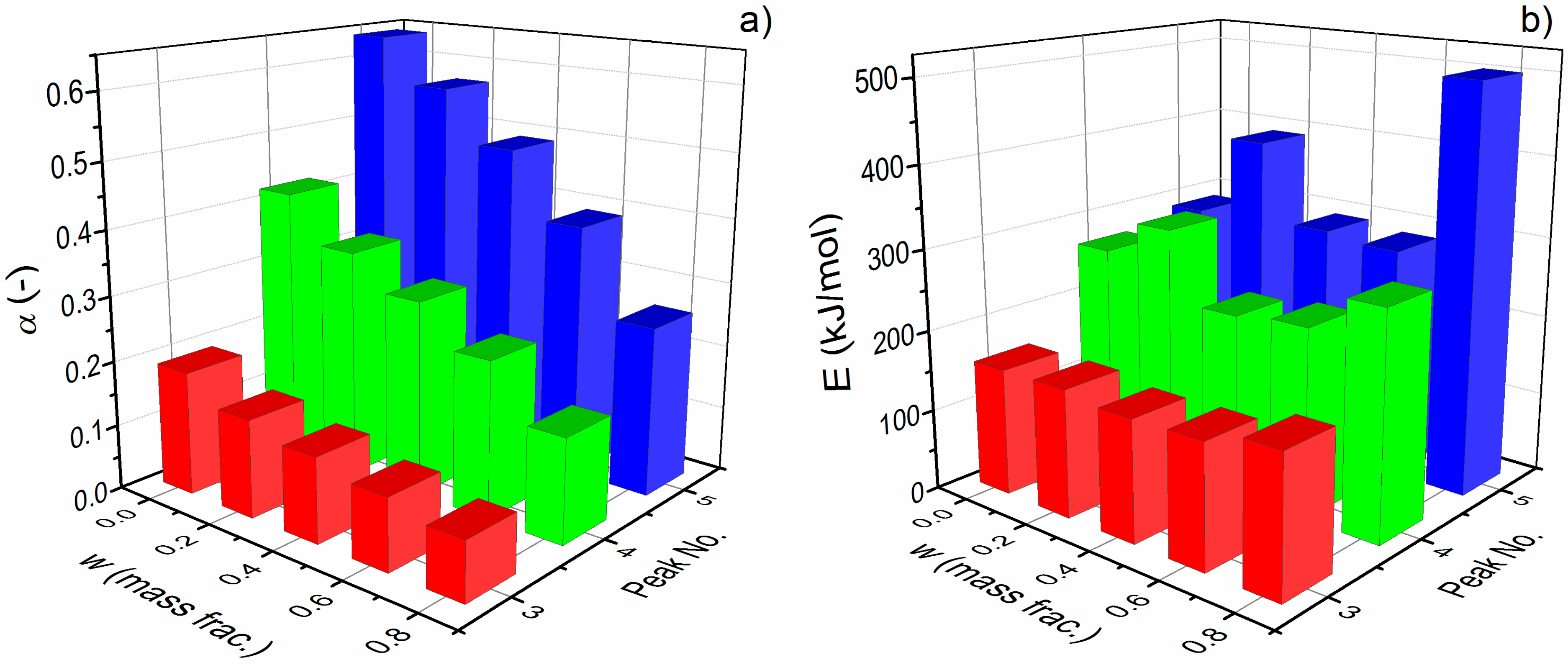
3.1. Kinetic Analysis
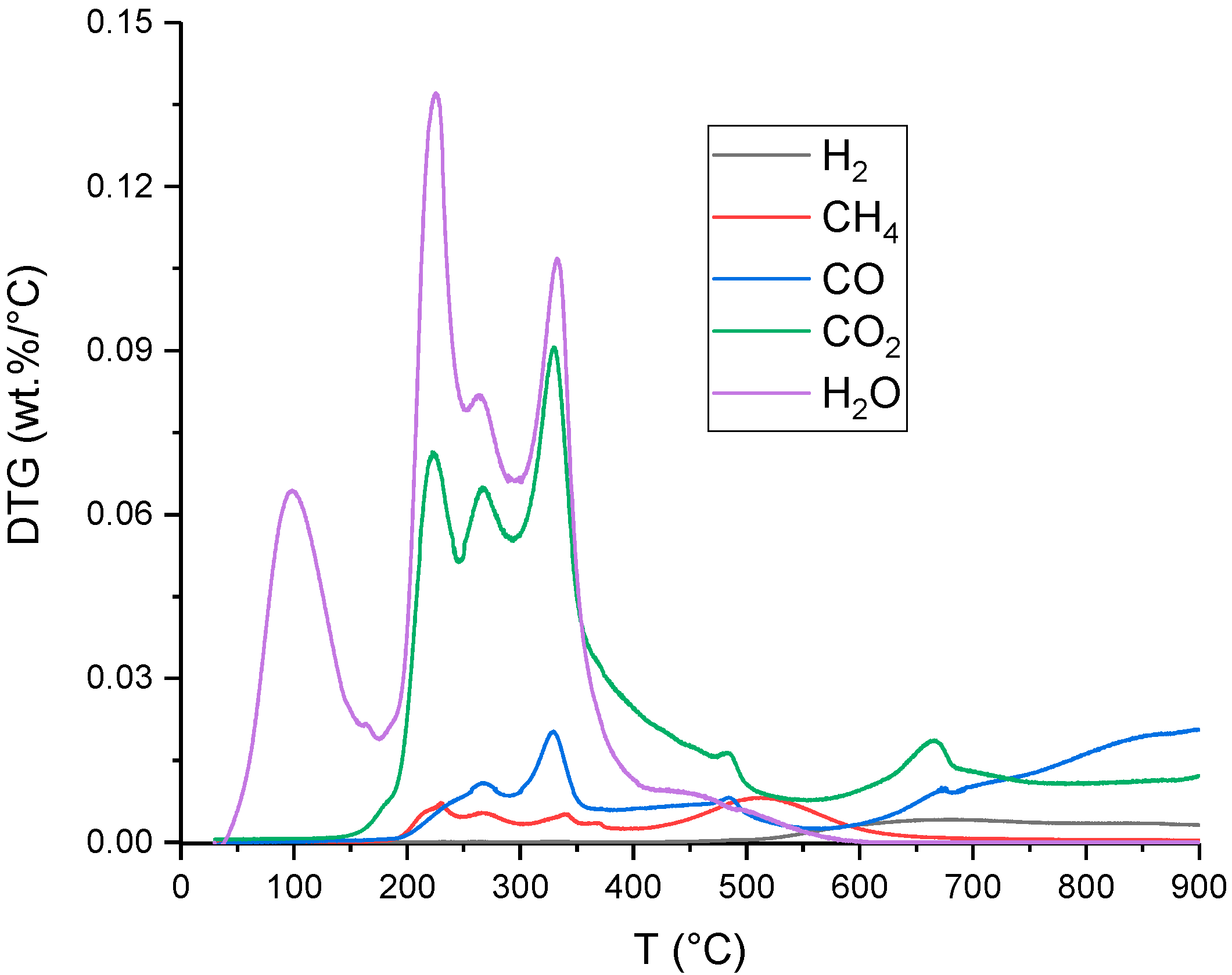
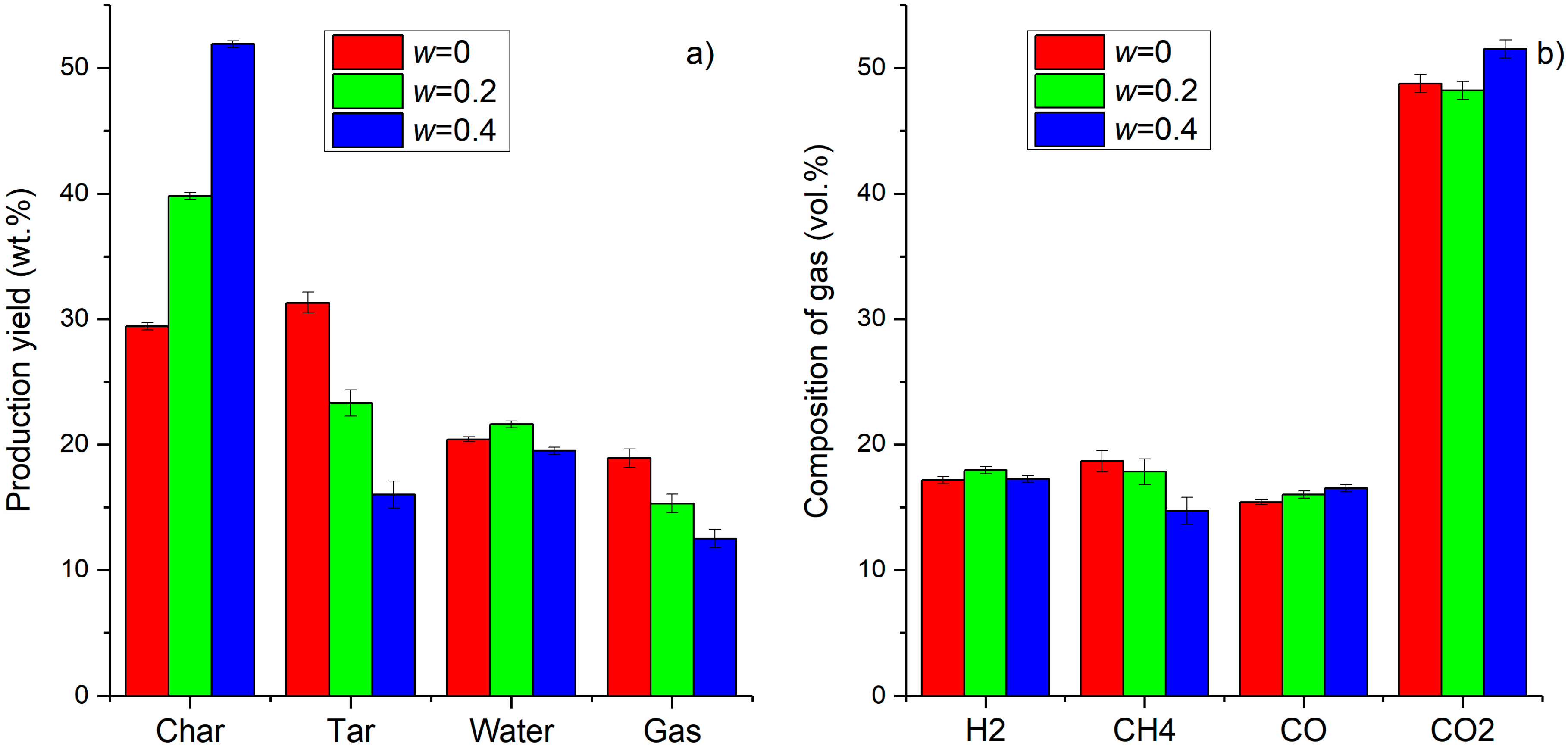
3.2. Production Yield of Char, Tar and Gas
3.3. Char Properties
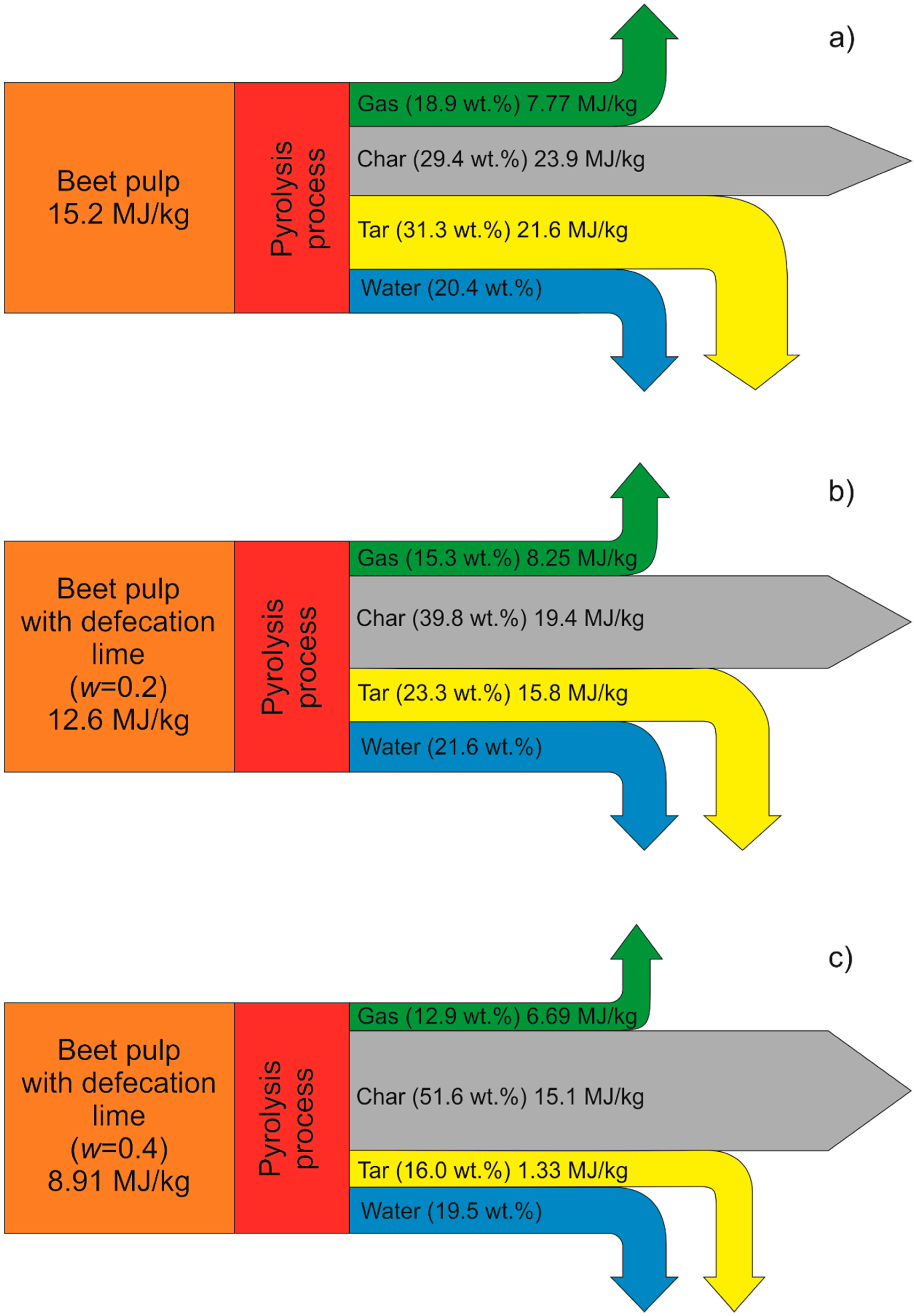
3.4. Energy Balance of Pyrolysis Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Daystar, J.; Treasure, T.; Gonzalez, R.; Reeb, C.; Venditti, R.; Kelley, S. Biofuel path analyses. BioResources 2015, 10, 5096–5116. [Google Scholar]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biomass fast pyrolysis in screw reactors: Prediction of spent coffee grounds bio-oil production through a monodimensional model. Energy Convers. Manag. 2018, 168, 98–106. [Google Scholar] [CrossRef]
- Nicodème, T.; Berchem, T.; Jacquet, N.; Richel, A. Thermochemical conversion of sugar industry by-products to biofuels. Renew. Sustain. Energy Rev. 2018, 88, 151–159. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Asadullah, M.; Rahman, M.A.; Ali, M.M.; Rahman, M.S.; Motin, M.A.; Sultan, M.B.; Alam, M.R. Production of bio-oil from fixed bed pyrolysis of bagasse. Fuel 2007, 86, 2514–2520. [Google Scholar] [CrossRef]
- da Cunha Gonçalves, G.; Pereira, N.C.; Veit, M.T. Production of bio-oil and activated carbon from sugarcane bagasse and molasses. Biomass Bioenergy 2016, 85, 178–186. [Google Scholar]
- Montoya, J.; Pecha, B.; Roman, D.; Janna, F.C.; Garcia-Perez, M. Effect of temperature and heating rate on product distribution from the pyrolysis of sugarcane bagasse in a hot plate reactor. J. Anal. Appl. Pyrolysis 2017, 123, 347–363. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis 2006, 76, 230–237. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Sidi-Yacoub, B.; Oudghiri, F.; Belkadi, M.; Rodríguez-Barroso, R. Characterization of lignocellulosic components in exhausted sugar beet pulp waste by TG/FTIR analysis. J. Therm. Anal. Calorim. 2019, 138, 1801–1809. [Google Scholar] [CrossRef]
- Simkovic, I.; Surina, I.; Mikulášik, R.; Orságová, A.; Ház, A.; Schwarzinger, C. Evaluation of the phytomass source for composite preparation. J. Appl. Polym. Sci. 2013, 127, 508–515. [Google Scholar] [CrossRef]
- DeGroot, W.F.; Kannan, M.P.; Richards, G.N.; Theander, O. Gasification of Agricultural Residues (Biomass): Influence of Inorganic Constituents. J. Agric. Food Chem. 1990, 38, 320–323. [Google Scholar] [CrossRef]
- Sklyadnev, E.V.; Ryazanov, A.N.; Panov, S.Y.; Balabanova, M.Y.; Zinkovskii, A.V. Pyrolytic Processing of Cellulose-Containing Wastes from Food Industry Plants. Chem. Pet. Eng. 2016, 52, 419–424. [Google Scholar] [CrossRef]
- Aho, A.; Käldström, M.; Kumar, N.; Eränen, K.; Hupa, M.; Holmbom, B.; Salmi, T.; Fardim, P.; Murzin, D.Y. Pyrolysis of beet pulp in a fluidized bed reactor. J. Anal. Appl. Pyrolysis 2013, 104, 426–432. [Google Scholar] [CrossRef]
- Devrim, Y.G. Pyrolysis kinetics of blends of yeni Çeltek lignite and sugar beet pulp. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 238–246. [Google Scholar] [CrossRef]
- Yilgin, M.; Deveci Duranay, N.; Pehlivan, D. Co-pyrolysis of lignite and sugar beet pulp. Energy Convers. Manag. 2010, 51, 1060–1064. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.W.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Stolarek, P.; Ledakowicz, S.; Slȩzak, R. Influence of Liming on Kinetics of Sewage Sludge Pyrolysis. Ecol. Chem. Eng. S 2019, 26, 175–188. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, S.; Shen, Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- Ali, I.; Bahaitham, H.; Naebulharam, R. A comprehensive kinetics study of coconut shell waste pyrolysis. Bioresour. Technol. 2017, 235, 1–11. [Google Scholar] [CrossRef]
- Ashraf, A.; Sattar, H.; Munir, S. A comparative applicability study of model-fitting and model-free kinetic analysis approaches to non-isothermal pyrolysis of coal and agricultural residues. Fuel 2019, 240, 326–333. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Wang, L. Characterization of pectin extracted from sugar beet pulp under different drying conditions. J. Food Eng. 2017, 211, 1–6. [Google Scholar] [CrossRef]
- Chryat, Y.; Romdhana, H.; Esteban-Decloux, M. Reducing energy requirement for drying of beet-pulp: Simulation of energy integration between superheated steam and air drying systems. Dry. Technol. 2017, 35, 838–848. [Google Scholar] [CrossRef]
- Nowicki, L.; Ledakowicz, S. Comprehensive characterization of thermal decomposition of sewage sludge by TG-MS. J. Anal. Appl. Pyrolysis 2014, 110, 220–228. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Antal, M.J.; Várhegyi, G. Cellulose pyrolysis kinetics: The current state of knowledge. Ind. Eng. Chem. Res. 1995, 34, 703–717. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass proximate analysis using thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Zhong, C.; Wei, X. A comparative experimental study on the liquefaction of wood. Energy 2004, 29, 1731–1741. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Sfakiotakis, S.; Vamvuka, D. Study of co-pyrolysis of olive kernel with waste biomass using TGA/DTG/MS. Thermochim. Acta 2018, 670, 44–54. [Google Scholar] [CrossRef]
- Amini, E.; Safdari, M.-S.; Weise, D.R.; Fletcher, T.H. Pyrolysis kinetics of live and dead wildland vegetation from the Southern United States. J. Anal. Appl. Pyrolysis 2019, 142, 104613. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E.; Kastanaki, E.; Grammelis, P. Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel 2003, 82, 1949–1960. [Google Scholar] [CrossRef]
- Skreiberg, A.; Skreiberg, O.; Sandquist, J.; Sørum, L. TGA and macro-TGA characterisation of biomass fuels and fuel mixtures. Fuel 2011, 90, 2182–2197. [Google Scholar] [CrossRef]
- Bedyk, T.; Nowicki, L.; Stolarek, P.; Ledakowicz, S. Application of the TG-MS system in studying sewage sludge pyrolysis and gasification. Polish J. Chem. Technol. 2008, 10, 1–5. [Google Scholar] [CrossRef]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Ranzi, E.; Debiagi, P.E.A.; Frassoldati, A. Mathematical Modeling of Fast Biomass Pyrolysis and Bio-Oil Formation. Note I: Kinetic Mechanism of Biomass Pyrolysis. ACS Sustain. Chem. Eng. 2017, 5, 2867–2881. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Hou, X.; Qiu, R.; Chen, Z. Pyrolytic behavior and kinetic of wood sawdust at isothermal and non-isothermal conditions. Renew. Energy 2019, 142, 284–294. [Google Scholar] [CrossRef]
- Kim, P.; Johnson, A.; Edmunds, C.W.; Radosevich, M.; Vogt, F.; Rials, T.G.; Labbé, N. Surface functionality and carbon structures in lignocellulosic-derived biochars produced by fast pyrolysis. Energy Fuels 2011, 25, 4693–4703. [Google Scholar] [CrossRef]
- Li, L.; Quinlivan, P.A.; Knappe, D.R.U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon N. Y. 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional boresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valorization 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, S.S.; Shim, J.W.; Lee, Y.E.; Yoo, Y.S. Pyrolysis characteristics and kinetics of food wastes. Energies 2017, 10, 1191. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I.; Gara, P. Carbonisation of wood residue into charcoal during low temperature process. Renew. Energy 2016, 85, 507–513. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Liu, Z.; Wang, H.; Li, X.; Zhang, P.; Geng, W.; Zhang, G. Experimental and modeling studies on CO2 gasification of biomass chars. Energy 2016, 114, 143–154. [Google Scholar] [CrossRef]
- Król, D.; Poskrobko, Š. Waste and fuels from waste Part I. Analysis of thermal decomposition. J. Therm. Anal. Calorim. 2012, 109, 619–628. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Zondlo, J.W. Pyrolysis of dedicated bioenergy crops grown on reclaimed mine land in West Virginia. J. Anal. Appl. Pyrolysis 2017, 123, 319–329. [Google Scholar] [CrossRef]
- Özbay, G.; Özcifci, A.; Kokten, E.S. The pyrolysis characteristics of wood waste containing different types of varnishes. Turkish J. Agric. For. 2016, 40, 705–714. [Google Scholar] [CrossRef]

| Fraction | Beet Pulp (w = 0 Mass Fraction) | Defecation Lime (w = 1 Mass Fraction) |
|---|---|---|
| Proximate analysis | ||
| Moisture (wt.%) | 3.64 ± 0.08 | 0.52 ± 0.02 |
| Volatiles (wt.%) | 70.89 ± 0.91 | 46.94 ± 0.67 |
| Fixed carbon (wt.%) | 18.94 ± 0.29 | 0.13 ± 0.01 |
| Ash (wt.%) | 6.53 ± 0.12 | 52.41 ± 0.73 |
| Ultimate analysis | ||
| C (wt.%) | 40.47 ± 0.83 | 15.06 ± 0.22 |
| H (wt.%) | 5.84 ± 0.12 | 0.83 ± 0.04 |
| N (wt.%) | 0.77 ± 0.05 | 0.02 ± 0.01 |
| Phase | I | II | III | |||||
|---|---|---|---|---|---|---|---|---|
| Peak No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Position | (°C) | 93.2 | 170.0 | 217.3 | 266.0 | 326.0 | 400.0 | 758.2 |
| Amplitude | (wt.%/°C) | −0.0176 | −0.00922 | −0.0911 | −0.0493 | −0.0754 | −0.01912 | −0.00760 |
| Halfwidth | (°C) | 56.8 | 55.4 | 29.5 | 44.4 | 33.4 | 199.7 | 42.5 |
| Asymmetry | - | 0 | 0 | −0.40 | 0 | 0.60 | 0 | 0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slezak, R.; Krzystek, L.; Dziugan, P.; Ledakowicz, S. Co-Pyrolysis of Beet Pulp and Defecation Lime in TG-MS System. Energies 2020, 13, 2304. https://doi.org/10.3390/en13092304
Slezak R, Krzystek L, Dziugan P, Ledakowicz S. Co-Pyrolysis of Beet Pulp and Defecation Lime in TG-MS System. Energies. 2020; 13(9):2304. https://doi.org/10.3390/en13092304
Chicago/Turabian StyleSlezak, Radosław, Liliana Krzystek, Piotr Dziugan, and Stanisław Ledakowicz. 2020. "Co-Pyrolysis of Beet Pulp and Defecation Lime in TG-MS System" Energies 13, no. 9: 2304. https://doi.org/10.3390/en13092304
APA StyleSlezak, R., Krzystek, L., Dziugan, P., & Ledakowicz, S. (2020). Co-Pyrolysis of Beet Pulp and Defecation Lime in TG-MS System. Energies, 13(9), 2304. https://doi.org/10.3390/en13092304