Removal of Cu (II) from Industrial Wastewater Using Mechanically Activated Serpentinite
Abstract
1. Introduction
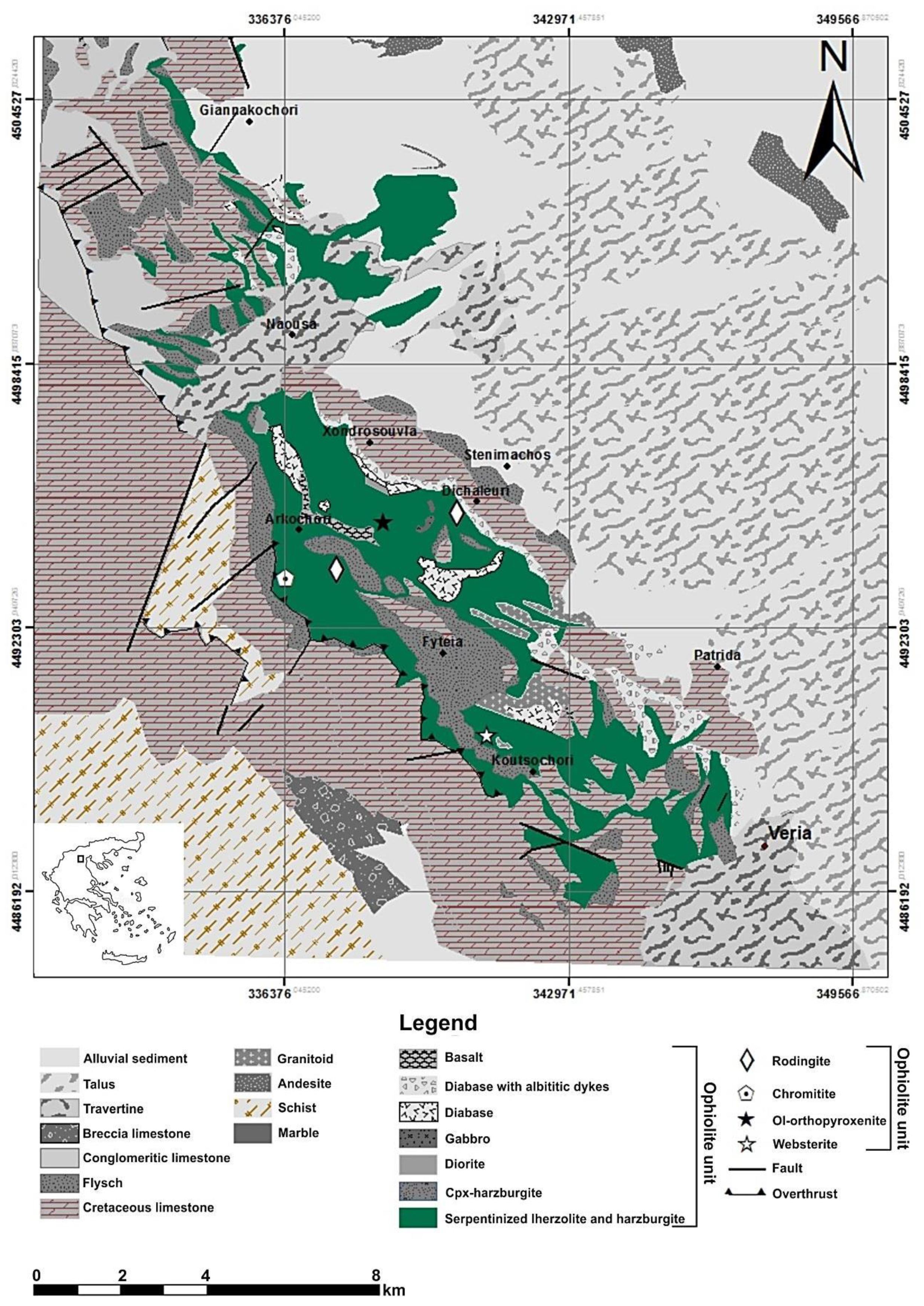
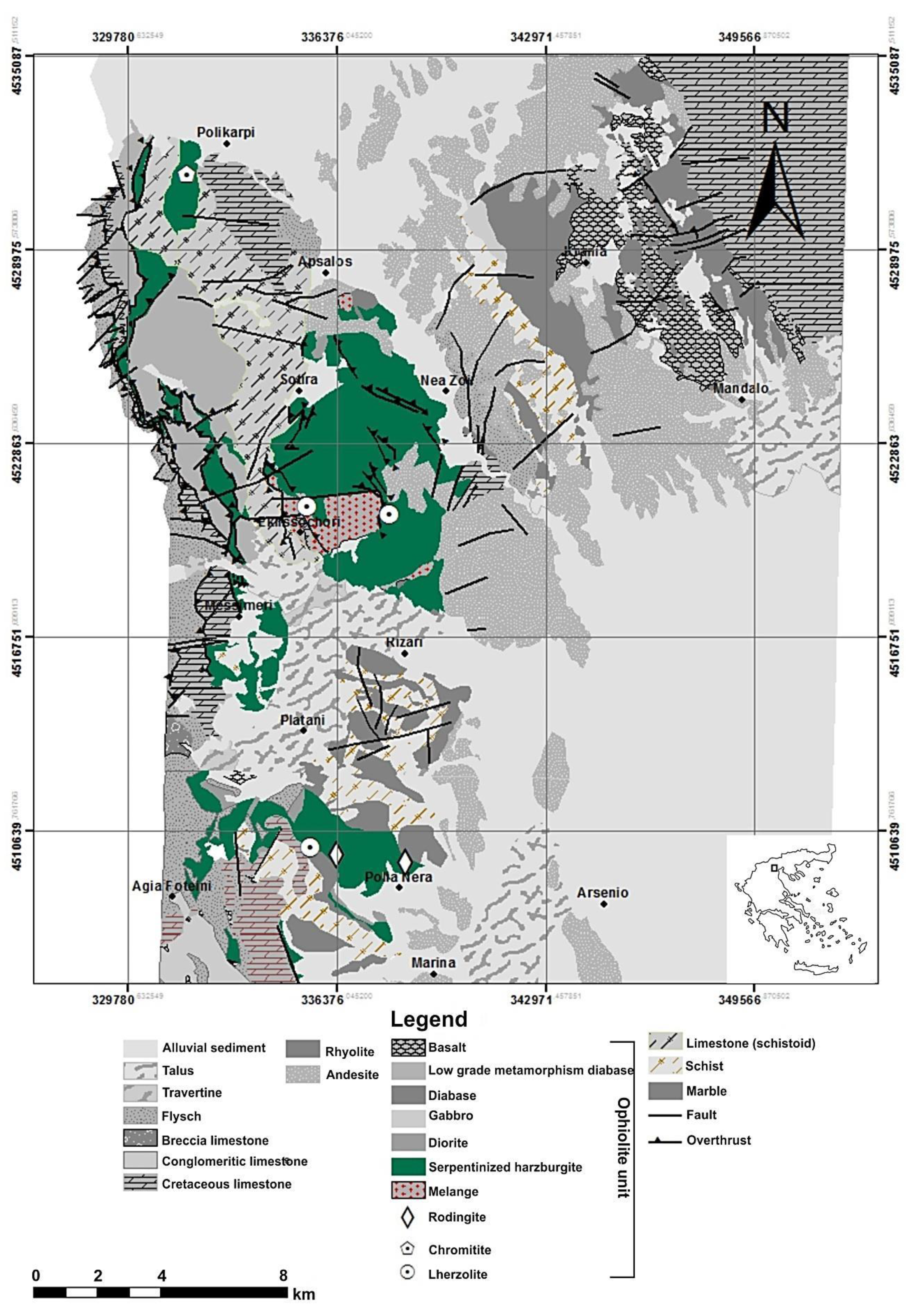
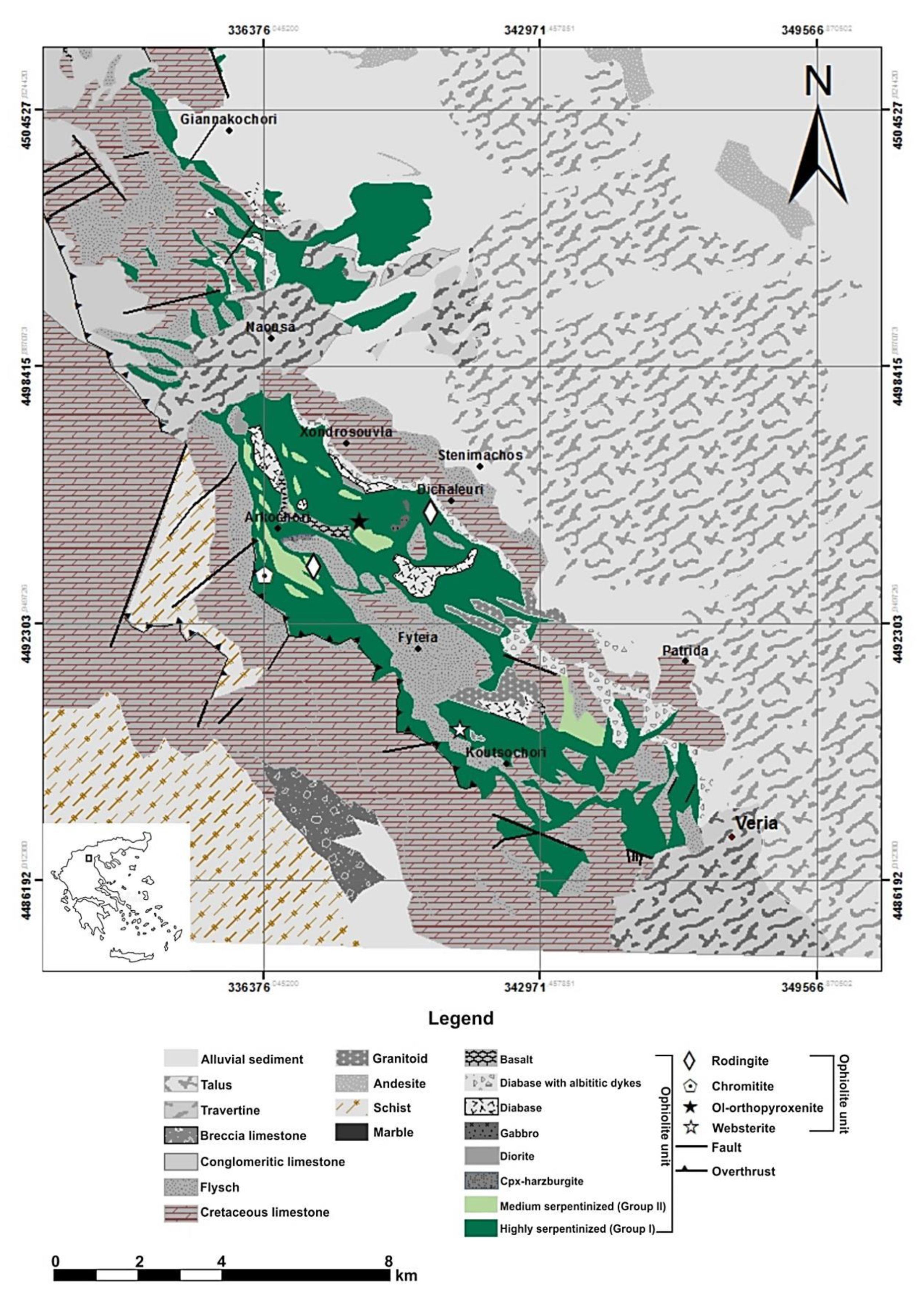
2. Geological Description of Rock Materials Sources
3. Materials and Methods
3.1. Characterization of the Studied Rock Materials
3.2. Methodology for the Cu (II) Removal
4. Results
4.1. Results of the Studied Rock Materials
4.1.1. Petrographic Features of the Studied Rock Materials
4.1.2. XRPD of Mineral Rock Materials
4.1.3. Chemistry of Serpentine Minerals
4.1.4. Geochemical Features of Rock Materials
4.2. Experimental Study Results
4.2.1. Chemical Analysis of the Wastewater
4.2.2. Chemical Analysis of Wastewater after Having Penetrated 4 Times through Columns of Batch Type
4.2.3. Chemical X-ray Diffractometry of Serpentinites after the Experimental Study
5. Discussion
Proposed Areas with Serpentinites for Their Potential Use as Filters for Cu Removal
6. Conclusions
- Two groups of serpentinized ultramafic rocks from the investigated ophiolite complexes which were detected according to their petrographic observations were in accordance with their mineralogical, chemical and geochemical analyses.
- The highly serpentinized rocks (Group I) are more effective in Cu removal in contrast to the medium serpentinized rock samples (Group II).
- The higher mechanical activation of the studied serpentinites (after they have been subjected to a 1500 revolutions LA test) is related to their higher capability to perform Cu removal.
- Selective removal of Cu (II) in the form of the wroewolfeite phase was achieved by using mechanically activated highly serpentinized ultramafic rocks.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ms, A.-R.; Arm, A.-R. Removal of Heavy Metals from Industrial Waste Water by Biomass-Based Materials: A Review. J. Pollut. Eff. Control. 2016, 5. [Google Scholar] [CrossRef]
- Weber, W.J.; McGinley, P.M.; Katz, L.E. Sorption phenomena in subsurface systems: Concepts, models and effects on contaminant fate and transport. Water Res. 1991, 25, 499–528. [Google Scholar] [CrossRef]
- Salmon, S.U.; Oldham, C.E.; Ivey, G.; Salmon, U. Assessing internal and external controls on lake water quality: Limitations on organic carbon-driven alkalinity generation in acidic pit lakes. Water Resour. Res. 2008, 44, 10414. [Google Scholar] [CrossRef]
- Schindler, D.W. The significance of in-lake production of alkalinity. Water Air Soil Pollut. 1986, 30, 931–944. [Google Scholar] [CrossRef]
- Geller, W.; Klapper, H.; Salomons, W. Acidic Mining Lakes; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Geller, W.; Koschorreck, M.; Wendt-Potthoff, K.; Bozau, E.; Herzsprung, P.; Büttner, O.; Schultze, M. A pilot-scale field experiment for the microbial neutralization of a holomictic acidic pit lake. J. Geochem. Explor. 2009, 100, 153–159. [Google Scholar] [CrossRef]
- Lattanzi, P.; Da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite oxidation: A review. Earth-Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Sperling, E.; Grandschamp, C.A.P. Possible water uses in mining lakes: Case study of Agua Claras, Brazil. In Proceedings of the 33rd WEDC International Conference, Accra, Ghana, 7–11 April 2008; WEDC, Loughborough University: Loughborough, UK, 2008; pp. 375–380. [Google Scholar]
- Shevenell, L.; A Connors, K.; Henry, C.D. Controls on pit lake water quality at sixteen open-pit mines in Nevada. Appl. Geochem. 1999, 14, 669–687. [Google Scholar] [CrossRef]
- Meena, A.K.; Kadirvelu, K.; Mishra, G.; Rajagopal, C.; Nagar, P. Adsorptive removal of heavy metals from aqueous solution by treated sawdust (Acacia arabica). J. Hazard. Mater. 2008, 150, 604–611. [Google Scholar] [CrossRef]
- Panayotova, M.; Velikov, B. Influence of zeolite transformation in a homoionic form on the removal of some heavy metal ions from wastewater. J. Environ. Sci. Health Part A 2003, 38, 545–554. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Kamala, C.; Chary, N.; Anjaneyulu, Y. Removal of heavy metals using a plant biomass with reference to environmental control. Int. J. Miner. Process. 2003, 68, 37–45. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Various treatment technologies to remove arsenic and mercury from contaminated groundwater: An overview. In Proceedings of the Southeast Asian Water Environment 1: Selected Papers from the First International Symposium on Southeast Aian Water Environment (biodiversity and Water Environment), Bangkok, Thailand, 1 December 2005. [Google Scholar]
- Coupal, B.; Lalancette, J.-M. The treatment of waste waters with peat moss. Water Res. 1976, 10, 1071–1076. [Google Scholar] [CrossRef]
- McLellan, J.; Rock, C. Pretreating landfill leachate with peat to remove metals. Water Air Soil Pollut. 1988, 37, 203–215. [Google Scholar] [CrossRef]
- Chaney, R.L.; Hundemann, P.T. Use of peat moss columns to remove cadmium from wastewater. J. Water Pollut. Control Fed. 1979, 51, 17–21. [Google Scholar]
- Sharma, D.; Forster, C. Removal of hexavalent chromium using sphagnum moss peat. Water Res. 1993, 27, 1201–1208. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.; Tang, L.; Salvador, J. Copper adsorption by esterified and unesterified fractions of Sphagnum peat moss and its different humic substances. J. Hazard. Mater. 1996, 48, 191–206. [Google Scholar] [CrossRef]
- Zhipei, Z.; Junlu, Y.; Zenghui, W.; Piya, C. A preliminary study of the removal of Pb(2+), Cd(2+), Zn(2+), Ni(2+), and Cr(2+) from wastewater with several Chinese peats. In Proceedings of the Seventh International Peat Congress, Dublin, Ireland, 18–23 June 1984; pp. 147–152. [Google Scholar]
- Dennehy, C.; Lawlor, P.G.; Jiang, Y.; Gardiner, G.E.; Xie, S.; Nghiem, L.D.; Zhan, X. Green-house gas emissions from different pig manure management techniques: A critical analysis. Front. Environ. Sci. Eng. 2017, 11, 11. [Google Scholar]
- Feng, Z.; Zhu, L. Sorption of phenanthrene to biochar modified by base. Front. Environ. Sci. Eng. 2017, 12, 1. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Bai, T.; Tao, J.; Guo, J.; Yang, M.; Wang, S.; Hu, S. Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J. Hazard. Mater. 2016, 320, 386–392. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 2017, 609, 1401–1410. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, J.H.; Ahn, Y.-T.; Chung, J.W. Comparison of Heavy Metal Adsorption by Peat Moss and Peat Moss-Derived Biochar Produced Under Different Carbonization Conditions. Water Air Soil Pollut. 2015, 226, 9. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Stability of calcium carbonate and magnesium carbonate in rainwater and nitric acid solutions. Energy Convers. Manag. 2006, 47, 3059–3068. [Google Scholar] [CrossRef]
- Petrounias, P.; Rogkala, A.; Giannakopoulou, P.P.; Tsikouras, B.; Lampropoulou, P.; Kalaitzidis, S.; Hatzipanagiotou, K.; Lambrakis, N.; Christopoulou, M.A. An Experimental Study for the Remediation of Industrial Waste Water Using a Combination of Low Cost Mineral Raw Materials. Minerals 2019, 9, 207. [Google Scholar] [CrossRef]
- Smith, M. Panasqueira the tungsten giant at 100+. Oper. Focus Int. Min. 2006, 33, 10–14. [Google Scholar]
- Brigatti, M.F.; Galán, E.; Theng, B. Chapter 2 Structures and Mineralogy of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 19–86. ISBN 9780080441832.2006. [Google Scholar]
- Sengupta, A.; Kadam, R.; Rajeswari, B.; Dhobale, A.; Babu, Y.; Godbole, S. Characterization of Indian serpentine by X-ray diffraction, photoacoustic spectroscopy and electron paramagnetic resonance spectroscopy. Appl. Clay Sci. 2010, 50, 305–310. [Google Scholar] [CrossRef]
- Auzende, A.-L.; Pellenq, R.J.-M.; Devouard, B.; Baronnet, A.; Grauby, O. Atomistic calculations of structural and elastic properties of serpentine minerals: The case of lizardite. Phys. Chem. Miner. 2006, 33, 266–275. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Chen, M.; Hu, H.; Lei, Z.; Zhang, Q.; Yuan, W. Mechanochemical activation of serpentine for recovering CU (II) from waste water. Appl. Clay Sci. 2017, 149, 1–7. [Google Scholar] [CrossRef]
- Rogkala, A.; Petrounias, P.; Tsikouras, B.; Hatzipanagiotou, K. New Occurrence of Pyroxenites in the Veria-Naousa Ophiolite (North Greece): Implications on Their Origin and Petrogenetic Evolution. Geoscience 2017, 7, 92. [Google Scholar] [CrossRef]
- Brunn, J.H. Geological Map of Greece, Veroia Sheet, 1:50.000; IGME: Athens, Greece, 1982. [Google Scholar]
- Decourt, J.; Aubouin, J.; Savoyat, E. Le sillon mesohellenique et la zone pelagonienne. Bull. Soc. Geoli. Fr. 1977, 1, 32–70. [Google Scholar]
- Michailidis, K. Zoned chromites with high Mn-contents in the Fe-Ni-Cr-laterite ore deposits from the Edessa area in Northern Greece. Miner. Depos. 1990, 25, 190–197. [Google Scholar] [CrossRef]
- Rogkala, A.; Petrounias, P.; Tsikouras, B.; Giannakopoulou, P.P.; Hatzipanagiotou, K. Mineralogical Evidence for Partial Melting and Melt-Rock Interaction Processes in the Mantle Peridotites of Edessa Ophiolite (North Greece). Minerals 2019, 9, 120. [Google Scholar] [CrossRef]
- Tarney JPe-Piper, G.; Piper, D.J.W. The Igneous Rocks of Greece. The Anatomy of an Orogen. Beiträge zur Regionalen Geologie der Erde (Series). Geol. Mag. 2003, 140, 357. [Google Scholar] [CrossRef]
- Saccani, E.; Photiades, A.; Santato, A.; Zeda, O. New evidence for supra-subduction zone ophiolites in the Vardar zone of northern Greece: Implications for the tectonomagmatic evolution of the Vardar oceanic basin. Ofioliti 2008, 33, 65–85. [Google Scholar]
- Mercier, J.L.; Vergely, P. Geological Map of Greece, Edhessa Sheet, 1:50.000; IGME: Athens, Greece, 1984. [Google Scholar]
- Bish, D.L.; Post, J.E. Quantitative mineralogical analysis using the Rietveld full pattern fitting method. Am. Mineral. 1993, 78, 932–940. [Google Scholar]
- Singh, A.K.; Singh, R.B. Genetic implications of Zn- and Mn-rich Cr-spinels in serpentinites of the Tidding Suture Zone, eastern Himalaya, NE India. Geol. J. 2012, 48, 22–38. [Google Scholar] [CrossRef]
- Chen, M.; Li, Z.; Li, X.; Qu, J.; Zhang, Q. Mechanochemically extracting tungsten through caustic processing of scheelite by controlling calcium dissolution. Int. J. Refract. Met. Hard Mater. 2016, 58, 211–215. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Vdović, N.; Jurina, I.; Škapin, S.D.; Sondi, I. The surface properties of clay minerals modified by intensive dry milling—Revisited. Appl. Clay Sci. 2010, 48, 575–580. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical activation of ultramafic mine waste rock in dry condition for enhanced mineral carbonation. Miner. Eng. 2016, 95, 1–4. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.P.; Rogkala, A.; Stamatis, P.M.; Tsikouras, B.; Papoulis, D.; Lampropoulou, P.; Hatzipanagiotou, K. The Influence of Alteration of Aggregates on the Quality of the Concrete: A Case Study from Serpentinites and Andesites from Central Macedonia (North Greece). Geosciences 2018, 8, 115. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.P.; Rogkala, A.; Stamatis, P.M.; Lampropoulou, P.; Tsikouras, B.; Hatzipanagiotou, K. The Effect of Petrographic Characteristics and Physico-Mechanical Properties of Aggregates on the Quality of Concrete. Minerals 2018, 8, 577. [Google Scholar] [CrossRef]
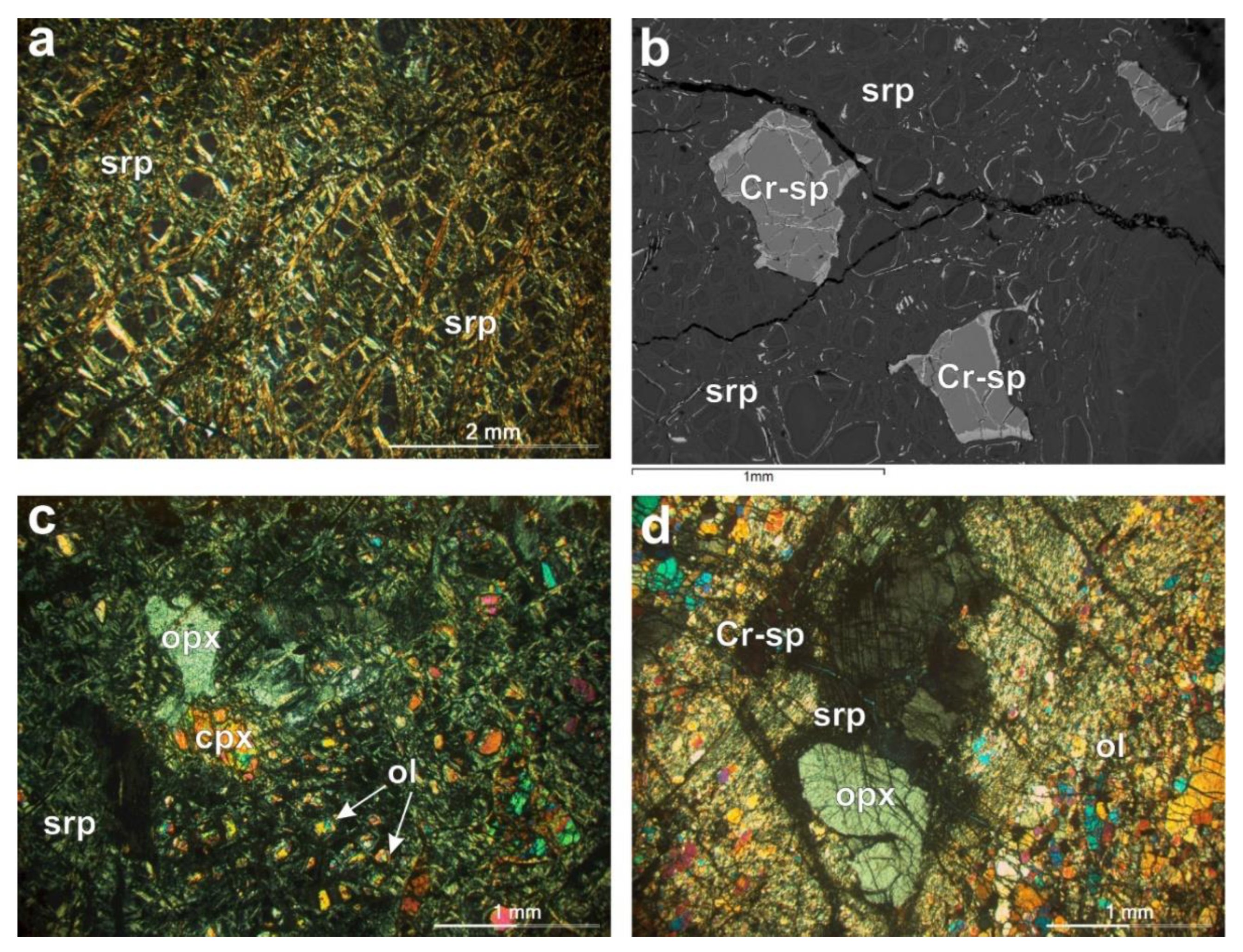
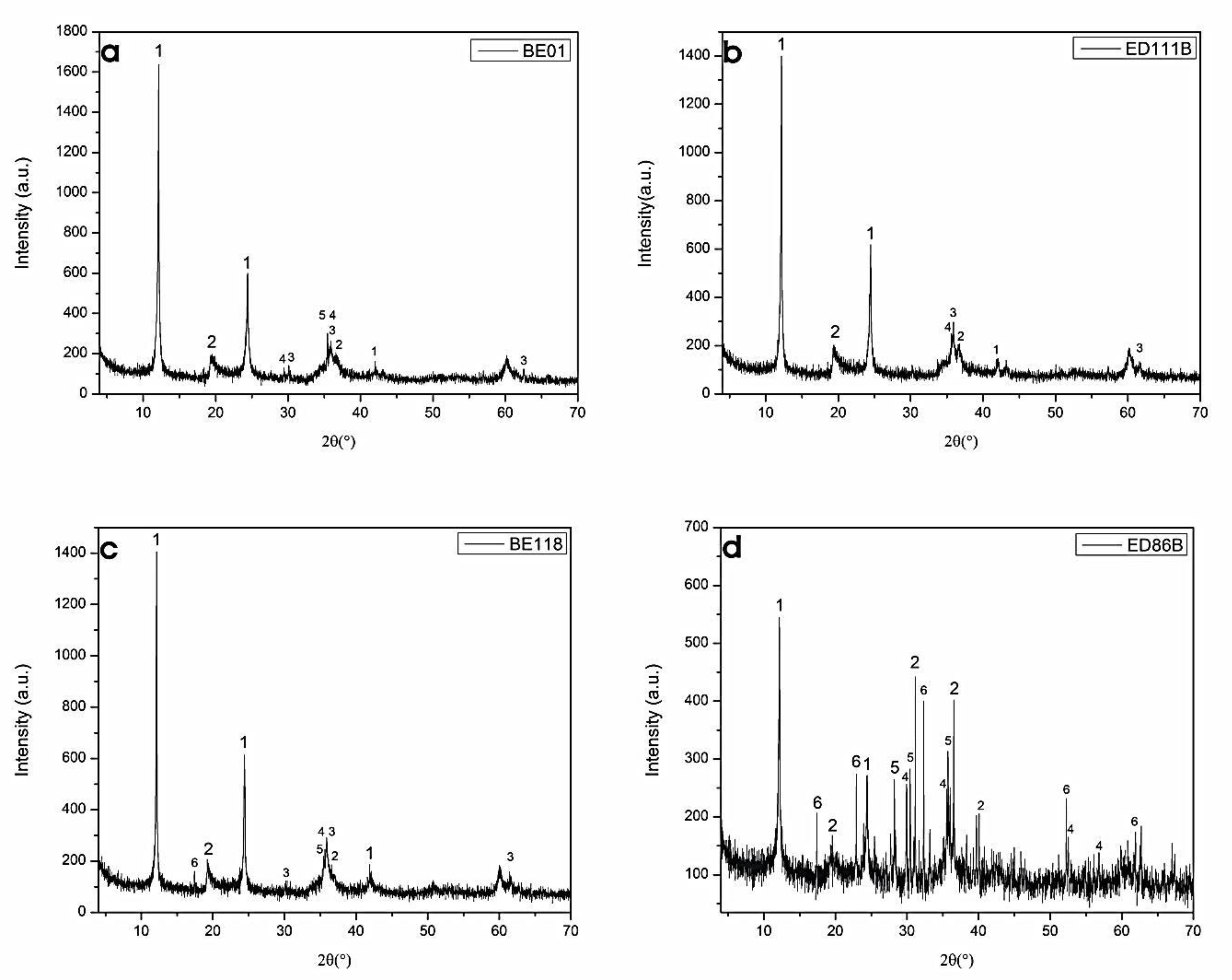



| Samples | ol | opx | cpx | sp | mgt | srp |
|---|---|---|---|---|---|---|
| BE.01 | - | 2.3 | 1.7 | 3.3 | 1.0 | 91.7 |
| ED.111B | - | - | 1.1 | 7.1 | 3.2 | 88.6 |
| BE.118 | 3.0 | 14.0 | 7.0 | 4.5 | 1.5 | 70.0 |
| ED.86B | 11.5 | 15.5 | 11.0 | 3.0 | - | 59.0 |
| Group I | Group II | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | BE.01 | ED.111B | BE.118 | ED.86B | ||||||||||||
| Anal. No | 14 | 18 | 21 | 23 | 3 | 7 | 10 | 14 | 7 | 13 | 17 | 20 | 4 | 5 | 8 | 10 |
| wt.% | ||||||||||||||||
| SiO2 | 46.27 | 44.16 | 44.78 | 44.94 | 43.22 | 43.53 | 44.37 | 45.01 | 42.95 | 44.37 | 42.04 | 42.59 | 43.34 | 42.02 | 43.94 | 43.86 |
| TiO2 | - | - | - | - | - | - | - | - | - | 0.07 | 0.09 | 0.05 | - | - | - | - |
| Al2O3 | 0.43 | - | - | 0.22 | - | - | - | - | - | - | 0.66 | 0.96 | - | 0.70 | 1.67 | 2.58 |
| Fe2O3 | 6.08 | 5.53 | 5.55 | 5.73 | 4.45 | 6.53 | 4.58 | 3.46 | 1.91 | 2.98 | 3.36 | 3.02 | 3.07 | 2.01 | 1.10 | 1.73 |
| MnO | - | - | - | - | - | - | - | - | 0.06 | - | - | - | - | - | - | |
| MgO | 37.55 | 36.58 | 41.50 | 37.85 | 37.96 | 39.65 | 40.38 | 38.72 | 40.66 | 38.14 | 36.45 | 37.17 | 38.05 | 37.06 | 37.17 | 37.84 |
| CaO | - | - | - | - | - | - | - | - | 0.18 | 0.08 | - | 0.14 | - | - | 0.08 | 0.19 |
| Na2O | - | - | - | - | - | - | - | - | - | - | - | 0.06 | - | - | - | - |
| K2O | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NiO | - | - | - | - | - | - | - | - | - | - | - | - | 2.20 | - | 0.39 | 0.45 |
| Cr2O3 | 0.39 | - | - | - | 0.81 | - | - | - | - | 0.05 | - | - | - | 0.64 | 1.35 | 1.93 |
| Sum | 90.72 | 86.27 | 91.83 | 88.74 | 86.44 | 89.71 | 89.33 | 87.19 | 85.70 | 85.75 | 82.60 | 83.99 | 86.66 | 82.43 | 85.70 | 88.58 |
| Formula units based on 7 atoms of oxygens | ||||||||||||||||
| Si | 2.068 | 2.073 | 1.988 | 2.054 | 2.030 | 1.985 | 2.015 | 2.078 | 2.020 | 2.081 | 2.051 | 2.042 | 2.041 | 2.049 | 2.058 | 2.000 |
| Ti | - | - | - | - | - | - | - | - | - | 0.002 | 0.003 | 0.002 | - | - | - | - |
| Al | 0.023 | - | - | 0.012 | - | - | - | - | - | - | 0.038 | 0.054 | - | 0.040 | 0.092 | 0.139 |
| Fe3+ | 0.205 | 0.195 | 0.185 | 0.197 | 0.157 | 0.224 | 0.157 | 0.120 | 0.068 | 0.105 | 0.123 | 0.109 | 0.109 | 0.074 | 0.039 | 0.059 |
| Mn | - | - | - | - | - | - | - | - | - | 0.002 | - | - | - | - | - | - |
| Mg | 2.502 | 2.560 | 2.746 | 2.579 | 2.658 | 2.695 | 2.734 | 2.664 | 2.850 | 2.666 | 2.650 | 2.657 | 2.671 | 2.694 | 2.595 | 2.572 |
| Ca | - | - | - | - | - | - | - | - | 0.009 | 0.004 | - | 0.007 | - | - | 0.004 | 0.009 |
| Na | - | - | - | - | - | - | - | - | - | - | - | 0.006 | - | - | - | - |
| K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ni | - | - | - | - | - | - | - | - | - | - | - | - | 0.083 | - | 0.015 | 0.017 |
| Cr | 0.014 | - | - | - | 0.030 | - | - | - | - | 0.002 | - | - | - | 0.025 | 0.050 | 0.070 |
| Total | 4.811 | 4.829 | 4.919 | 4.842 | 4.876 | 4.903 | 4.906 | 4.862 | 4.947 | 4.863 | 4.866 | 4.877 | 4.905 | 4.882 | 4.852 | 4.866 |
| Group I | Group II | |||
|---|---|---|---|---|
| Sample | BE.01 | ED.111B | BE.118 | ED.86B |
| Major elements (wt.%) | ||||
| SiO2 | 39.82 | 40.21 | 40.17 | 42.01 |
| TiO2 | - | - | 0.01 | 0.13 |
| Al2O3 | 1.01 | 0.44 | 1.48 | 3.57 |
| Fe2O3 | 8.86 | 8.89 | 7.43 | 8.06 |
| MnO | 0.11 | 0.06 | 0.12 | 0.11 |
| MgO | 34.17 | 33.54 | 36.28 | 34.17 |
| CaO | 0.10 | 0.16 | 1.46 | 3.17 |
| Na2O | - | - | - | 0.18 |
| K2O | - | - | - | - |
| P2O5 | - | - | 0.01 | 0.02 |
| LOI | 14.6 | 14.0 | 12.4 | 7.9 |
| Total | 98.67 | 99.30 | 99.36 | 99.32 |
| Trace elements (ppm) | ||||
| Cr | 2963 | 2333 | 2292 | 2607 |
| Co | 91.1 | 88.4 | 100.7 | 92.9 |
| Ni | 2655.8 | 2958.7 | 2185.0 | 1812.2 |
| Cu | 12.9 | 8.2 | 5.8 | 5.1 |
| Zn | 8 | 30 | 24 | 15 |
| Pb | 21.7 | 0.8 | 0.6 | 0.3 |
| As | 7.2 | 2.6 | - | - |
| U | 0.2 | 3.0 | - | - |
| Elements (ppb) | Concentrations |
|---|---|
| Ag | 0.18 |
| As | - |
| Ba | 20.54 |
| Be | 24.11 |
| Cd | 1717.73 |
| Co | 213.11 |
| Cr | - |
| Cs | 13.70 |
| Cu | 8847.21 |
| Ga | 3.90 |
| Li | 25.96 |
| Mn | 70,982.00 |
| Pb | 812.77 |
| Rb | 60.42 |
| Sr | 520.57 |
| V | - |
| U | 111.96 |
| Zn | 285,458.55 |
| Se | 24.91 |
| Ni | 1149.40 |
| Fe | 6149.02 |
| Cu (ppb) | ||||
|---|---|---|---|---|
| Samples/Revolutions | 0 | 500 | 1000 | 1500 |
| BE.01 | 4563.20 | 80.35 | 65.40 | 25.21 |
| ED.111B | 6380.51 | 121.30 | 85.47 | 35.30 |
| BE.118 | 7500.46 | 3670.40 | 1670.40 | 320.48 |
| ED.86B | 8070.31 | 6120.00 | 2720.50 | 670.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrounias, P.; Rogkala, A.; Giannakopoulou, P.P.; Lampropoulou, P.; Koutsovitis, P.; Koukouzas, N.; Laskaris, N.; Pomonis, P.; Hatzipanagiotou, K. Removal of Cu (II) from Industrial Wastewater Using Mechanically Activated Serpentinite. Energies 2020, 13, 2228. https://doi.org/10.3390/en13092228
Petrounias P, Rogkala A, Giannakopoulou PP, Lampropoulou P, Koutsovitis P, Koukouzas N, Laskaris N, Pomonis P, Hatzipanagiotou K. Removal of Cu (II) from Industrial Wastewater Using Mechanically Activated Serpentinite. Energies. 2020; 13(9):2228. https://doi.org/10.3390/en13092228
Chicago/Turabian StylePetrounias, Petros, Aikaterini Rogkala, Panagiota P. Giannakopoulou, Paraskevi Lampropoulou, Petros Koutsovitis, Nikolaos Koukouzas, Nikolaos Laskaris, Panagiotis Pomonis, and Konstantin Hatzipanagiotou. 2020. "Removal of Cu (II) from Industrial Wastewater Using Mechanically Activated Serpentinite" Energies 13, no. 9: 2228. https://doi.org/10.3390/en13092228
APA StylePetrounias, P., Rogkala, A., Giannakopoulou, P. P., Lampropoulou, P., Koutsovitis, P., Koukouzas, N., Laskaris, N., Pomonis, P., & Hatzipanagiotou, K. (2020). Removal of Cu (II) from Industrial Wastewater Using Mechanically Activated Serpentinite. Energies, 13(9), 2228. https://doi.org/10.3390/en13092228












